Abstract
The Notch signaling pathway is highly conserved in all animal metazoa: upon Notch receptor activation, transcription of Notch target genes is turned on by an activator complex that centers on the transcription factor CSL. In the absence of signal, CSL assembles transcriptional repression complexes that display remarkable evolutionary diversity. The major antagonist of Notch signaling in insects named Hairless was originally identified in Drosophila melanogaster. It binds to the Drosophila CSL homologue Suppressor of Hairless [Su(H)] and recruits the two general co-repressors, Groucho and C-terminal binding protein. Whereas the majority of Notch signaling components is conserved between insects and vertebrates, Hairless is found only in insects. Here, we present the analysis of the Hairless gene from Daphnia pulex and, hence, for the first time from a crustacean. Daphnia and Drosophila Hairless protein sequences are highly diverged. Known functional domains, however, the Su(H), Groucho and the C-terminal binding protein interactions domains, are well conserved. Moreover, direct binding of the Daphnia Hairless protein and the respective Drosophila interaction partners was detected, demonstrating the conservation at the molecular level. In addition, interaction between Daphnia Hairless and Drosophila Su(H) was demonstrated in vivo, as co-overexpression of the respective genes during Drosophila development resulted in the expected downregulation of Notch activity in the fly. Structural models show that the Hairless-Su(H) repressor complexes from Daphnia and Drosophila are almost indistinguishable from one another. Amino acid residues in direct contact within the Hairless-Su(H) complex are at absolutely identical positions in the two homologues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the animal kingdom, there are several highly conserved pathways important for establishing the adult organism from undifferentiated cells. In a complex interplay, these pathways together allow cellular differentiation and specification. A prime example is the Notch signaling pathway, dysfunction of which leads to fatal disorders and oncogenic development, respectively (reviewed in Hori et al. 2013, Bray 2016). The name giving molecule Notch functions as the receptor in the signal receiving cell. Mutation of the corresponding Notch gene in Drosophila results in a haplo-insufficient phenotype characterized by nicks in the margin of the wings. Homozygous Notch mutants are embryonic lethal; the most prominent phenotype is a hypertrophy of the nervous system at the expense of hypoderm, apart from cell differentiation defects in almost any other tissue (Hartenstein et al. 1992). Activation of the Notch receptor results from binding of membrane-tethered ligands on neighboring cells and cumulates in intra-membrane cleavage of Notch (reviewed in Hori et al. 2013). Subsequently, the intracellular domain of Notch (ICN or NICD) engages in transcriptional activation of its target genes by the assembly of an activator complex (reviewed in Kovall and Blacklow 2010, Bray 2016). The central component of this activator complex is the DNA binding protein CSL, an abbreviation combining the orthologues from Homo sapiens, Drosophila melanogaster, and Caenorhabditis elegans, respectively (human C-promoter Binding Factor 1 [CBF1] or RBPJ, Suppressor of Hairless [Su(H)], and lin-12 and Glp-1 phenotype [Lag1]) (reviewed in Kovall and Blacklow 2010; Contreras-Cornejo et al. 2016).
In vertebrates and insects, Notch target genes are silenced in the absence of signal by repressor complexes that again are assembled by the CSL protein (reviewed in Borggrefe and Oswald 2009; Maier 2006). Vertebrate co-repressors include, for example, SHARP (SMRT/HDAC1 associated repressor protein) (Oswald et al. 2002), KyoT2 (Collins et al. 2014), or RITA (Tabaja et al. 2017) that bind the beta-trefoil domain of the CSL protein, thereby probably competing with NICD (Tabaja et al. 2017). In contrast, the major Notch antagonist in Drosophila named Hairless binds Su(H) at the C-terminal domain (CTD) (Maier et al. 2011, Yuan et al. 2016). Hairless recruits two general co-repressors, Groucho (Gro) and C-terminal binding protein (CtBP), to eventually silence Notch target genes in Drosophila (Morel et al. 2001, Barolo et al. 2002, Nagel et al. 2005). Structural analyses of the Su(H)-H repressor complex revealed that Hairless binding enforces a structural change that precludes the binding of NICD (Yuan et al. 2016). SHARP has been suggested to be the functional homologue of Hairless, since it binds directly to CSL and recruits CtBP and other co-repressors (Oswald et al. 2005). There is in fact a Drosophila SHARP homologue called split ends (spen) that shares a number of domains with SHARP, however, lacks a CSL binding motif (Oswald et al. 2005). Hence, although the molecules and the molecular mechanisms of Notch target gene activation are extremely well conserved in several species throughout the higher animal kingdom (reviewed in Bray 2016), Notch target gene repression seems not. Despite the high degree of conservation within CSL molecules—about 80% of its residues are identical between mouse and fly—no Hairless molecule has been found so far outside of insects (Maier 2006).
To gather additional information on the evolution of Hairless, we have functionally analyzed the orthologues from Drosophila hydei (DmH) (Marquart et al. 1999) and from Apis mellifera (AmH) (Maier et al. 2008). It turned out that Hairless is a rather fast evolving gene and that the Hairless proteins from dipteran flies, DmH and DhH, are about three times the size of their honeybee homologue AmH (Marquart et al. 1999, Maier et al. 2008). Nevertheless, the tiny AmH protein largely fulfilled Hairless function in D. melanogaster, as it could even rescue a loss of function phenotype to some extent (Maier et al. 2008). Sequence comparisons revealed the presence of small stretches of high conservation corresponding to the interaction domains in Drosophila Hairless, SBD (for Su(H) binding domain), GBD (for Groucho binding domain), and CBD (for CtBP binding domain) that had been defined experimentally already (Maier 2006, Maier et al. 2008). Accordingly, AmH bound to the D. melanogaster protein partners caused gain of function phenotypes when overexpressed in the fly (Maier et al. 2008).
In the meanwhile, the genomes of several additional animal species have been sequenced. While scanning the databases, we now identified a Hairless orthologue outside of insects for the first time, in the crustacean Daphnia pulex. This allows further insights into Hairless evolution. The Crustacea and Insecta separated more than 400 million years ago, adding another approximately 150 million years of evolution since the split of Hymenoptera and Diptera (Fig. 1) (Honeybee Genome Sequencing Consortium 2006). In the course of evolution, mainly the domains of known function have been conserved. We show that Daphnia Hairless still binds to the Drosophila interaction partners and retains some biological activity in the fly. The functional performance in D. melanogaster is reduced compared to the A. mellifera orthologue. Comparison of the various Hairless orthologues may hence help to define the actual requirements for a Hairless protein to fully fulfill its function in transcriptional repression of Notch target genes.
Phylogenic tree of the species with experimentally studied Hairless genes. Daphnia is the first species outside of insects where the Hairless gene was studied. The crustacean lineage separated from the Insecta approximately 420 million years ago. The phylogenic tree is adapted from the Honeybee Genome Sequencing Consortium (2006)
Material and methods
Cloning of Daphnia Hairless and generation of DpH constructs
A clone of D. pulex animals, collected from slimy-log pond (GPS coordinates N 43.830013, W −124.148152), was used for phenol extraction of total genomic DNA according to Preiss et al. (1988). The same clone of animals has been used also for the D. pulex genome project (Colbourne et al. 2011). Genomic DNA covering the Daphnia Hairless gene (DpH gDNA) was PCR amplified with upper primer 5′ CGG AAT CAA TTG GAA AAT TAT GTC AGA 3′ and lower primer 5′ GGT TAG GAG TGC TCT CTA GCT CGT CTA A 3′, cloned into pSC-A-amp/kan vector (Agilent Technologies) and sequence verified (Macrogen GmbH Amsterdam, Netherlands). Notably, we were not able to amplify the Hairless gene from a European population of D. pulex with the same primer pair. The Daphnia Hairless cDNA (DpH cDNA) was likewise cloned by PCR amplification with the same primer pair, using cDNA synthesized from the same source as above. To this end, total RNA was isolated from 50 adult D. pulex animals using TRIzol, and cDNA prepared with RevertAid First Strand cDNA Synthesis Kit and oligo-dT18 primers according to the manufacturers’ protocol (Thermo Fischer Scientific, Dreieich, Germany). Sequencing revealed a codon exchange (D117G) in the conserved CT box, presumably as a result from the PCR amplification. It was reverted in the DpH cDNA used for further cloning by exchanging a Pvu II fragment with the corresponding genomic fragment.
The Daphnia Hairless gDNA (DpH gDNA) was digested with Xho I/Xba I and cloned into likewise digested transformation vector pUAST-attB, whereas for the cloning of the cDNA (DpH cDNA) Eco RI digested products were utilized. The DNA was integrated into the D. melanogaster genome on chromosome 3L at 68E using the Phi C31 system (Bischof et al. 2007). For a rescue experiment the Eco RI digested gDNA was ligated into pCaSper-hsRX8 vector (Maier et al. 1997). D. melanogaster transgenic lines where obtained from classical P element-mediated germ line transformation.
For tagging DpH protein with a myc tag, the DpH cDNA was first shuttled as Eco RI fragment into pBT vector (Agilent Technology, Santa Clara CA, USA). For the subsequent steps, a clone was selected with the pBT polylinker oriented with Kpn I at the 5′ and Sac I at the 3′ side of the sequence. Then, an Aat II restriction site was introduced with the QuickChange II XL Site directed Mutagenesis Kit (Agilent Technology, Santa Clara CA, USA) at codon position D433, V434 without changing the meaning of the codons. The following primers were used: 5′ ATG ATG GCG ATG ACG ACG TCC CCC TCA ATT TGA 3′ and 5′ TCA AAT TGA GGG GGA CGT CGT CAT CGC CAT CAT 3′. The myc tag was introduced into the opened Aat II site with annealed primers coding for the tag. The Aat II site was restored at the 5′ end but not at the 3′ end. In addition an Eco RV restriction site was created in the primer duplex coding for the myc tag as a test for correct orientation (sequence verified). Primers for the myc tag were: 5′ CGA ACA GAA GTT GAT ATC CGA AGA AGA CCT CCA CGT 3′ (Eco RV site underlined) and 5′ GGA GGT CTT CTT CGG ATA TCA ACT TCT GTT CGA AGT 3′. As a consequence of this strategy, the myc tag was integrated 5′ of the CBD. The Daphnia Hairless-myc cDNA (DpH cDNA-myc) was cloned via the Eco RI site into pUAST-attB and integrated in the D. melanogaster genome at 3L 68E as outlined above. Constructs were sequence verified.
Protein-protein interaction studies
Generation of the Daphnia H cDNA bait construct: The sticky ends of Eco RI digested pEG202 vector (Gyuris et al. 1993) were filled in with Klenow polymerase, followed by a Bam HI digest and used for the integration of the Eco RV/Bam HI digested DpH cDNA. Generation of the Daphnia H GBD (codons 289 to 361) construct: DpH cDNA was used as template for PCR with upper primer 5′ CGG CCG AAT TCG CTC ATT CAC T 3′ containing an Eco RI site, and lower primer M13-20. The amplicon was digested with Eco RI at the 5′ and at an internal Xho I site at the 3′ end and cloned into Eco RI/Xho I digested pEG202 vector (Gyuris et al. 1993). Constructs were sequence verified. The yeast two-hybrid experiments were performed with the yeast strain EGY48 as outlined before (Gyuris et al. 1993, Nagel et al. 2005). As bait, full-length Hairless genes DmH (pEG-HFL; Nagel et al. 2005) and DpH as well as the GBD of DpH cloned in pEG202 vector were used. Expression of the pEG constructs was examined by Western blot analysis using rabbit anti-LexA antibody (Bio Academia; Osaka, Japan). The other constructs contained the D. melanogaster full-length protein coding region of Su(H) in pJG4-5 vector and Gro and CtBP in VP16 vector (Nagel et al. 2005).
Fly work
For tissue-specific overexpression, the Gal4/UAS system was employed (Brand and Perrimon 1993). UAS-lines were D. melanogaster full-length Hairless at 68E and full-length Su(H) at 96E (Maier et al. 2011), and the newly generated Drosophila lines containing either the genomic D. pulex Hairless gene, UAS-DpHgDNA (i.e., containing the intron), or the untagged or myc-tagged respective cDNAs, UAS-DpHcDNA, and UAS-DpHcDNA-myc, all located at 68E on 3L like the DmH control. For the combined overexpression with fly Su(H), the respective strains were recombined by standard genetics followed by PCR genotyping. As driver lines, we used omb-Gal4, gmr-Gal4, and Bx-Gal4 (http://flybase.org). vgBE-lacZ served as reporter for Notch activity as outlined before (Kim et al. 1996; Maier et al. 2011). Crosses were performed at 25 °C. Rescue experiments were done as described before (Maier et al. 1997, Maier et al. 2008) using the respective full-length Hairless genes under heat shock control hs-DmH and hs-DpH. To this end, flies carrying either transgene were crossed to H P8/TM6B (Maier et al. 1992), and the offspring was raised at ambient temperature to be analyzed for bristle phenotypes that were assessed statistically. Offspring from a cross of H P8/TM6B and y 1 w 67c23 served as control.
Phenotypic analyses
Adult flies were pictured with a table top scanning electron microscope (Neoscope JCM-5000; Nikon, Tokyo, Japan). Wings were mounted in Euparal (Roth, Karlsruhe, Germany) and photographed with an ES120 camera using Pixera Viewfinder software version 2.0 (Optronics, Goleta CA, USA). Imaginal discs were dissected from the third wandering instar larvae in PBS and fixed in 4% paraformaldehyde as described in Zimmermann et al. (2015). Incubations were done in PBS plus 0.3% Triton X-100 and 3% normal goat or donkey serum. The following antibodies were used: guinea pig anti-H-A and rat anti-Su(H) (Maier et al. 2013), rabbit anti-Su(H) and rabbit anti-myc A4-1 (Santa Cruz Biotechnology, Santa Cruz CA, USA), mouse anti-beta-galactosidase, and mouse anti-Cut (both from the DSHB Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa, Dept. of Biology, Iowa City, IA, USA). Secondary antibodies from donkey or goat were coupled to either Cy3, Cy5 or FITC (minimal cross reactivity; Jackson Immuno-Research Laboratories, purchased from Dianova, Hamburg, Germany). Discs were mounted in Vectashield (Vector labs, Biozol, Eching, Germany) and analyzed on a Zeiss Axiophot linked to a BioRad MRC 1024 confocal microscope (Zeiss, Jena, Germany).
Computational analysis
Databases for identifying the Hairless homologues as well as other Notch signaling pathway components in D. pulex and Litopenaeus vannamei (white shrimp) were Ensembl metazoa (http://metazoa.ensembl.org/index.html) and NCBI (http://www.ncbi.nlm.nih.gov/genome/browse/). To analyze the sequences, we used the HUSAR service of the DKFZ (http://genius.embnet.dkfz-heidelberg.de/menu/w2h/w2hdkfz/) as outlined earlier (Schlatter and Maier 2005). The sequence of the DpH gene was submitted to GenBank (accession MF678832; BankIt2040817 DpH).
The structures of the Apis (G62–P99), Daphnia (G56–Y90) and shrimp (G130–P168) Hairless peptides, as well as that of the Daphnia CSL homologue (K261–Y690, isoform 1) were generated with the SWISS-MODEL protein structure homology-modeling server (https://swissmodel.expasy.org/) and visualized using PyMOL, licensed to DM.
Results and discussion
In D. melanogaster, Hairless (H) acts as the major antagonist in the highly conserved Notch signaling pathway (reviewed in Maier 2006). Yet, no Hairless homologue has been found so far outside of insect species. To extend our current understanding on the evolution of this important modulator of Notch activity, we made use of available genome sequences to isolate and functionally characterize the Hairless gene from the common water flea D. pulex.
Identification of the D. pulex Hairless homologue
Availability of the D. pulex genomic sequence (http://www.ncbi.nlm.nih.gov/genome/browse/ http://genome.jgi.doe.gov/Dappu1/Dappu1.home.html) (Colbourne et al. 2011) allowed us to identify in silico a presumptive Hairless orthologue in a crustacean arthropod, i.e., outside of insects for the first time.Footnote 1 After contacting the Daphnia Genomic Consortium, we were informed of the isolation of cDNAs that encoded peptide sequences aligning convincingly with the D. melanogaster Hairless protein sequence (personal communication of John K. Colbourne and Donald Gilbert). Based on this information, we started a more rigorous study of the presumptive Hairless gene from D. pulex, DpH.
The sequence alignment revealed high conservation of the functional domains known in the fly Hairless protein: the SBD, i.e., the Su(H) binding domain, containing N-terminal NT and C-terminal CT boxes (Maier et al. 2008), the GBD, i.e. the Groucho binding domain and the CBD, i.e., the CtBP binding domain (Figs. 2 and 3 and online resource Supplemental Fig. 1). In addition, we noted high similarities in three presumptive nuclear localization signals (NLS). The Daphnia Hairless DpH protein is rather small with a total of 448 residues. It is less than half the size of D. melanogaster Hairless DmH (1059 aa), but about 60 amino acids bigger than the A. mellifera AmH homologous protein (392 aa) (Fig. 2 and online resource Supplemental Fig. 1). The overall amino acid composition and charge is very similar in all three Hairless proteins, being very basic overall with pIs above 10 (DmH 10.35, AmH 10.85, DpH 10.63). Apparently, Hairless gene size has increased dramatically in the course of dipteran evolution. We still know little about the function of the extra sequences in DmH. Systematic deletion analyses, however, revealed the requirement of most of these extra stretches as their loss impairs DmH activity in the fly (Maier et al. 1997). In addition, DmH contains an acidic stretch that dampens its activity, which is not conserved in either honeybee or Daphnia (Maier et al. 2008).
Comparison of the DmH and DpH proteins. a Alignment of the Hairless amino acids sequence from Drosophila melanogaster (DmH) and Daphnia pulex (DpH). The three domains of known function are Su(H) interaction domain consisting of a N-terminal and a C-terminal box NT and CT, the Gro binding domain GBD, and the CtBP binding domain CBD—they are highly conserved (underlined). In addition, three putative nuclear localization signals NLS1-3 are well conserved (colored in cyan). Arrows indicate the positions of the five introns in DmH. In contrast, there is only one intron in DpH (blue sharp arrow) and two in AmH (orange arrowheads); they share position with DmH. The Daphnia Hairless protein is only 448 amino acids long and, hence, much smaller than the Drosophila DmH homologue with 1077 amino acids; not all the residues could be aligned (indicated by dots in the Daphnia sequence). Blue depicts identical, red highly similar, yellow similar residues. b The overall identity between the two proteins is rather low with about 20% for sequences outside of functional domains. The interaction domains, however, stand out by their conservation: the NT domain is 75% and the CT domain 50% identical; GBD is 90% and the CBD is 75% identical between the two species, respectively. DmH = D. melanogaster Hairless, DpH = D. pulex Hairless. c The genomic organization of the DpH gene is depicted. The sequence was retrieved from DAPPUscaffold_49_Cont2683 (sequence ID: ACJG01002683.1); the DpH locus is positioned within 47609–49153
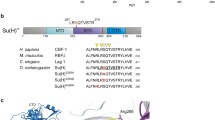
Sequence alignment of the Hairless interaction domains. Amino acid sequences of the Hairless interaction domains were aligned for Drosophila melanogaster (DmH), Apis mellifera (AmH), and Daphnia pulex (DpH). As second crustacean member, the white shrimp Litopenaeus vannamei (LvH) sequence was included. It was taken from the NCBI genome database. a Su(H) binding domain, SBD, with N- and C-terminals boxes, NT and CT. Residues identified in DmH to directly contact Dm Su(H) are marked with asterisks. Note their high conservation in all studied Hairless proteins. b Groucho binding domain, GBD, contains an eh1 sequence; the consensus shown below conforms to that of Pax8 proteins (Buscarlet and Stifani 2007). c CtBP binding domain, CBD, contains a binding motif typical of hZEB-2 proteins (shown below), conforming to the general consensus for a CtBP binding motif qdqPXDLSxkkk (Turner and Crossley 2001). Numbers indicate the first and last positions of the respective sequence. Blue are identical, red highly similar, and yellow similar residues
There is some variation in gene structure with respect to the number of introns: DmH has five introns, AmH has two, and DpH has only one intron. Whereas the intron position is conserved between DmH and AmH or DpH, the intron positions in the Daphnia and Apis Hairless genes are different (Fig. 2a, c). This unexpected pattern may result from independent events of intron loss from a larger number of introns earlier in evolution (mostly occurring in Daphnia ancestors), or independent events of gain (mainly after the crustacean/Insecta radiation) and losses.
Cloning of the Daphnia Hairless gene
First, the genomic region of Daphnia Hairless was PCR amplified from total DNA derived from D. pulex animals that had been used for the genome sequencing project. Sequence analysis revealed that the complete Hairless coding region was included. We sequenced four independent isolates with together seven codon changes compared with the sequence of the genome project (ID249527) (Colbourne et al. 2011). Since each change has been found in at least two independent clones, we suppose that they reflect naturally occurring polymorphisms. Further isolates with changes not found elsewhere were discarded.
The D. pulex Hairless cDNA clone was PCR amplified out of a pool of cDNA made from poly(A)+ RNA isolated from the same D. pulex strain used for genomic DNA extraction. We were able to isolate one D. pulex Hairless cDNA clone. In addition to six polymorphisms also found in the isolated genomic DNA clones, there was one additional exchange which we classified as PCR artifact: it was at codon position 117 (G instead of D) and considered problematic being localized in the highly conserved CT box of the Hairless SBD. This position is invariable in all species studied so far, including another crustacean member, L. vannamei (white shrimp) (Fig. 3a). Although the function of this conserved region is still undetermined, we decided to replace a part of the cDNA containing this substitution by a genomic Pvu I fragment (codons D73 to D299) to match gDNA and cDNA DpH sequences.
Protein-protein interaction studies
Although the three interaction domains, SBD, GBD and CBD, that have been identified in DmH before, are well conserved in Daphnia (Fig. 2b), there is quite some variation in detail as revealed by the interspecies comparison (Fig. 3). The most remarkable conservation is observed for the GBD: only a single amino acid residue is exchanged between DmH and DpH (Fig. 3b). Accordingly, both match the defined eh1 consensus for a Gro binding site (Buscarlet and Stifani 2007), as do the GBDs of AmH and of Hairless from white shrimp (LvH) (Fig. 3b). We therefore would expect a high conservation of the respective Gro homologues as well and also good binding between DpH and D. melanogaster Gro.
The CBD shows a likewise high conservation with regard to the consensus for a CtBP binding site (Turner and Crossley 2001). In contrast to DmH, however, several extra amino acids are present at the C-terminus of DpH and also of AmH and LvH compared to the Drosophila homologue (Fig. 3c). There are more examples in the literature describing proteins with extended C-termini that bind to CtBP proteins (Turner and Crossley 2001). It is conceivable that co-evolution of the two partner proteins has occurred to adjust for optimal binding, in which case DpH may show a lowered affinity to D. melanogaster CtBP.
The SBD is the largest of the three domains; it comprises a N-terminal part, the NT box which is essential and sufficient for the binding of Su(H) (Maier et al. 2008, Maier et al. 2011, Yuan et al. 2016). The strict requirement of this domain is reflected by its high conservation (Fig. 3a). Moreover, those amino acids known from structural analyses to directly contact Su(H) (Yuan et al. 2016) are absolutely invariant in the studied species (Fig. 3a). The C-terminal part of the SBD, named CT box, is more divergent overall, albeit again some positions are absolutely conserved. Although we still have no indications regarding its function, its high conservation during evolution makes some specific requirement very likely.
We next approached the structural conservation of DpH experimentally, by asking the question, whether the presumptive interaction domains were able to bind to the respective D. melanogaster protein partners. To this end, we performed yeast two-hybrid experiments with DpH and Su(H), Gro and CtBP from D. melanogaster. DmH was included for a comparison. As shown in Fig. 4, DpH bound well to both Su(H) and CtBP proteins, in a manner similar to DmH. We might have expected reduced binding to CtBP based on the extended C-terminus of DpH, which was, however, not observed. In contrast, no binding to Gro was detected, despite the high conservation of the binding consensus. We note, however, that the binding of the cognate fly protein partners is also rather weak, which may be attributed to the large size of the proteins (Fig. 4). We therefore generated a smaller construct of DpH comprising the GBD (codons 289–361): indeed interaction with Gro was now well detected in the yeast two-hybrid assay (Fig. 4). As predicted by the sequence conservation of the GBD and CBD binding sites and the interaction of DpH with the respective melanogaster orthologues, both Gro (ID129280) and CtBP (ID304733) are well conserved in D. pulex by in silico analysis (Colbourne et al. 2011; http://genome.jgi.doe.gov/Dappu1/Dappu1.home.html).
Protein interaction between Daphnia Hairless and Drosophila protein partners. Protein interactions were assessed by yeast two-hybrid interaction assays. Hairless full length protein from D. melanogaster (DmH) and D. pulex (DpH) (both in pEG vectors) were tested for their interactions with D. melanogaster Su(H) (in pJG vector), Gro and CtBP (both in VP16 vectors). Empty vectors served as controls. Strong interactions were observed between DpH and Su(H) as well as CtBP, whereas that with Gro was much weaker. Binding between Dm Gro and DpH was confirmed by using the isolated GBD from D. pulex. A scheme of the respective constructs, shown to scale, is depicted on the right; interaction domains are highlighted
In vivo activity of Daphnia Hairless in D. melanogaster
Would the Hairless gene from Daphnia display biological activity in the fly? We asked this question despite the fact that Daphnia Hairless is quite diverged from the Drosophila orthologue, not only by amino acid sequence but also by size. As we had shown before that the tiny Hairless gene from honeybee is remarkably potent in replacing the D. melanogaster orthologue (Maier et al. 2008), we likewise wanted to test Daphnia Hairless activity in a first analysis. Moreover, the yeast two-hybrid experiments demonstrated the ability of DpH to bind to the fly interaction partners.
We generated a gDNA construct of DpH under the control of the Drosophila hsp70 heat shock promoter and established transgenic Drosophila lines. An equivalent hsp-DmH construct is able to rescue the haplo-insufficient phenotype of heterozygous Hairless mutants, if expressed at ambient temperatures, due to the leaky expression of the hsp70 promoter (Bang and Posakony 1992, Maier et al. 1997, Maier et al. 2002). The offspring from a cross of the hsp-DpH fly line with the Hairless null mutation H P8 was evaluated and compared with that from a hsp-DmH cross. Drosophila flies bear a total of 40 large mechano-sensory bristles at invariant positions: there are 14 on the head and 26 on the thorax (Fig. 5). These bristles are sensitive to the Hairless gene dose, and heterozygous H mutants exhibit a specific loss of several macrochaetae (Bang et al. 1991, Maier et al. 1992, Praxenthaler et al. 2015, Smylla et al. 2016). Bristle numbers were counted on head and thorax and related to H P8 heterozygotes derived from a control cross with y 1 w 63c23 flies (Fig. 5). Crosses were set up in parallel to minimize environmental influences. H P8 heterozygotes lack about ten macrochaetae on average, four on the head and six on the thorax. Notably, the thoracic bristle loss was nearly completely rescued by hsp-DmH. Correspondingly, the biggest rescue effect with the hsp-DpH transgene was seen on the thorax: on average four macrochaetae were lacking. Although the difference appears subtle, it was highly significant. Rescue of bristle loss on the head was only achieved with hsp-DmH, as the small rescue effects of hsp-DpH were not significant (Fig. 5). Altogether, we note that the Hairless gene of Daphnia is active in the fly to some degree, however, by far not as potent as the Apis homologue (Maier et al. 2008). This observation may open an avenue to learn more on Hairless function by comparing Apis and Daphnia proteins in more detail.
Daphnia Hairless is functional in the fly. Rescue experiment with transgenic Drosophila (hsp-DmH) and Daphnia (hsp-DpH) Hairless genes under heat shock promoter control at ambient temperatures. A wild-type fly has 40 mechano-sensory macrochaetae at invariant positions on head (14) and thorax (26), as highlighted on the right (hemi-fly is shown as flies are bilaterally symmetrical). Heterozygous H null mutants (here H P8/+ in a y 1 w 63c23 background) display a typical loss of about four macrochaetae on the head and six on the thorax (Bang et al. 1991, Praxenthaler et al. 2015), which is well rescued by one copy of a wild type hsp-DmH transgene (Bang and Posakony 1992, Maier et al. 2002, Maier et al. 2008). Note that rescue is nearly complete for thoracic bristles, but only partial for the ones on the head. The hsp-DpH transgene also rescued some of the H P8 bristle loss on the thorax, demonstrating its biological activity in the fly. 20 female flies were analyzed each and the significance was determined (*** p ≤ 0.001, ns not significant; standard error is indicated). The average count of missing bristles is shown within the bars
Ectopic expression of the Daphnia Hairless gene in Drosophila
Next, we wanted to address the functionality of DpH in antagonizing Notch signaling activity during Drosophila development. To this end, we generated three transgenic DpH constructs under UAS-control that allow tissue specific overexpression in the fly (Brand and Perrimon 1993), one containing genomic DNA (i.e., with intron): UAS-DpHgDNA, one with the cDNA: UAS-DpHcDNA, and one with a myc-tagged cDNA: UAS-DpHcDNA-myc. In order to avoid position effects, all three were placed at 68E using the PhiC31 method (Bischof et al. 2007), the identical position as the UAS-DmH control (Maier et al. 2011). We first addressed the development of mechano-sensory bristles, which strictly depends on the Notch signaling pathway in Drosophila. At first, a sensory mother cell is selected from a proneural field by lateral inhibition. Then, after two rounds of cell division, four different cells, the outer shaft and socket cells and the inner sheath cell and neuron, are determined by dichotomy (reviewed in Schweisguth 2015). All steps are highly sensitive to the dose of Notch activity. The time point of dysfunction, therefore, dictates the phenotypic consequences (Schweisguth 2015).
The UAS-DpH lines were ectopically expressed during imaginal development with the Bx-Gal4 driver line and compared with UAS-DmH and UAS-GFP as controls (Fig. 6). As has been observed before, overexpression of UAS-DmH is semilethal, and the emerging flies have severe bristle defects lacking many micro- and macrochaetae (Maier et al. 1997, Maier et al. 2011). This phenotype is caused by a transformation of outer into inner cell fate during earlier developmental stages (Nagel et al. 2000, Schweisguth 2015). In addition, split bristles are observed, resulting from a transformation of socket into shaft cell fate (Fig. 6). Overexpression of any of the three Daphnia Hairless constructs resulted in very similar bristle defects: bristle loss and split bristles. Overall, the phenotypes were variable from very mild to strong as the ones shown in Fig. 6; no lethality was observed. The weakest influence on bristle development was seen with UAS-DpHcDNA-myc (Fig. 6). In this construct, a myc tag was added in frame 5′ of the CBD, not to tamper with CtBP binding affinity. Otherwise, the construct is identical to UAS-DpHcDNA. Hence, the apparent milder effects are suggestive of structural defects impairing DpHcDNA-myc activity.
Overexpression of Daphnia Hairless affects bristle development in Drosophila. Tissue specific expression of the given UAS-construct was induced during thorax development with Bx-Gal4. UAS-GFP served as control and flies with a normal pattern of macro- and microchaetae emerge. The Drosophila Hairless construct contains cDNA (DmHcDNA), and its overexpression results in a dramatic loss of bristles, as well as a transformation of bristle sockets into shafts. For Daphnia Hairless, three different transgenes were tested: genomic DNA (DpHgDNA), cDNA (DpHcDNA), and a myc-tagged cDNA (DpHcDNA-myc). Overexpression of either of the three Daphnia Hairless constructs induced bristle loss and socket to shaft transformations, albeit the effects of DpHcDNA-myc were rather mild. Arrows point to bristle loss and arrowheads to examples of socket to shaft transformations, resulting in split bristles when complete. Female flies are shown. Size bar in the upper panel corresponds to 200 μm and to 50 μm in the lower panel
In general, ectopic expression of the Daphnia Hairless gene caused little phenotypic changes compared to the controls with one notable exception. Using omb-Gal4 to drive expression in the distal wing anlagen, UAS-DmH results in smaller wings with pronounced wing margin defects (Nagel et al. 2005, Maier et al. 2008) (Fig. 7a, b). Whereas margin defects were rarely observed upon omb-mediated overexpression of any Daphnia Hairless construct (about 2% with DpHcDNA, n = 40), additional bristles formed on the distal part of the L2 wing vein (Fig. 7c, d). A similar phenotype has been described in flies where scute was mis-expressed specifically in developing L2 veins (Lunde et al. 2003), as otherwise overexpression of scute during wing development results in wings decorated with bristles all over (Chien et al. 1996). The scute gene is strictly required for the generation of sensory organ precursor cells and is important for establishing proneural fate also in Daphnia (Simpson 1990, Ungerer et al. 2011, Hartenstein and Stollewerk 2015, Klann and Stollewerk 2017). As the role of Hairless is to protect presumptive proneural cells from lateral inhibition by Notch signals (Maier 2006), its activity follows that of proneural genes. To this point, we do not know of factor(s) generating proneural potential in the omb-expression domain, but we think that it is unlikely DpH itself. Ectopic bristles notably on the L2 wing vein are also observed in viable hairy mutant alleles. The hairy gene encodes a transcriptional repressor that recruits the Gro co-repressor and is required for the correct segmentation of the Drosophila embryo (http://flybase.org). Perhaps, the binding of ectopic DpH to Gro specifically limits Gro availability for Hairy protein during wing development, affecting Hairy activity, thereby causing hairy mutant phenotypes. In this case, reducing gro gene dosage should aggravate the phenotype. Although this hypothesis is tempting, we have no explanation, why in this case DmH or AmH act differently.
Overexpression of Daphnia Hairless causes ectopic bristles on the wings of Drosophila. UAS-Hairless transgenes as indicated were overexpressed in the distal wing anlagen using omb-Gal4. a UAS-GFP served as control, and the emerging flies develop a normal pattern of veins and mechano- and chemosensory bristles along the anterior margin (anterior is to the top, distal to the left). b Overexpression of Drosophila Hairless (DmHcDNA) leads to gaps in the wing margin (arrow). Overexpression of either c genomic (DpHgDNA) or d cDNA (DpHcDNA) Daphnia Hairless constructs only rarely affected the margin. Many wings, however, developed additional bristles along the distal portion of the second longitudinal vein (arrowheads in the enlargements)
Repression activity of Daphnia Hairless on Notch target genes
In contrast to either DmH or AmH (Maier et al. 2008), ectopic DpH protein was barely affecting the development of the wing margin (Fig. 7). We therefore wondered whether DpH was able to repress Notch target genes during Drosophila wing development, which is most easily studied in wing imaginal discs at the dorso-ventral boundary. Here, the Notch signaling pathway is specifically activated, and in consequence target and downstream genes, wingless, vestigial, and cut (Neumann and Cohen 1996). As a read out for Notch activity, we used the expression of Cut (Fig. 8) and of a vgBE-lacZ reporter gene (online resource Supplemental Fig. 2), that contains the Notch-responsive vg boundary enhancer (Kim et al. 1996). Overexpression of a control UAS-GFP construct with the omb-Gal4 driver line had no influence, as expected (Fig. 8 and online resource Supplemental Fig. 2). The expression domain of omb-Gal4 is within a central field of the wing anlagen and overlaps the dorso-ventral boundary, allowing to address the effects on Notch gene expression within the same tissue directly. As shown before, overexpression of DmH results in repression of Notch targets Cut or vgBE-lacZ, as well as in a marked decrease of tissue size (Nagel et al. 2005, Maier et al. 2011) (Fig. 8 and online resource Supplemental Fig. 2). Similar results had been obtained with the ectopic induction of honeybee AmH (Maier et al. 2008). In contrast, overexpression of Daphnia Hairless constructs had no effect on tissue size, whereas a mild repression was seen for Cut or vgBE-lacZ (Fig. 8 and online resource Supplemental Fig. 2). Lacking an antibody specific for Daphnia Hairless protein, we could not be sure whether it was expressed at all. Instead, we used the myc-tagged version, despite its weaker activity revealed by the phenotypic analyses (see Figs. 6 and 7). Using anti-myc antibodies, expression of UAS-DpHcDNA-myc was confirmed (Fig. 8 and online resource Supplemental Fig. 2). Again vgBE-lacZ or Cut repression was seen in few cases as a small gap in target gene expression (examples are shown in Fig. 8 and online resource Supplemental Fig. 2). To better visualize the repression of Cut by DpHcDNA-myc, we performed a Z-stack analysis (online resource Supplemental Fig. 2). In the UAS-GFP control, Cut is present in all the nuclei, such that the signal spans the entire epithelium. In contrast, repression of Cut by DpHcDNA-myc appears as a depression of the apical-basal signal, since not all nuclei are stained with the same intensity. Nevertheless, the weak loss of Cut expression is in good agreement with the intactness of the wing margin upon DpH overexpression overall (Fig. 7c, d).
Overexpression of Daphnia Hairless inhibits Notch activity in Drosophila. a Drosophila (DmHcDNA) and Daphnia (DpHcDNA, DpHcDNA-myc) UAS-Hairless constructs as indicated were overexpressed in the central wing anlagen using omb-Gal4. As a read out for Notch activity, Cut expression was monitored (red). UAS-GFP served as control (green), showing the normal expression of Cut protein in a stripe along the dorso-ventral border of the wing imaginal disc. Drosophila Hairless (green) antagonizes Notch activity within the overexpression domain, inhibiting Cut expression (arrow) and affecting tissue growth as well. Overexpression of either Daphnia Hairless construct had only little effect on wing disc size, which was indistinguishable from wild type; however, Cut expression was slightly inhibited (arrows). As the anti-DmH antiserum unfortunately did not recognize DpH protein, DpHcDNA-myc was overexpressed and detected with anti-myc antibodies (green). Size bar corresponds to 100 μm in all panels
Daphnia Hairless forms a super-repressor together with Drosophila Su(H)
Our yeast two-hybrid assays demonstrated that Daphnia Hairless protein is well able to bind to Drosophila Su(H) protein in vitro (Fig. 4), which we wanted to address also in vivo. Earlier work has shown that a combined overexpression of Drosophila Hairless and Su(H) causes an extreme downregulation of Notch activity due to excessive repressor complex formation (Morel et al. 2001, Maier et al. 2011, Maier et al. 2013, Yuan et al. 2016). The effects are very drastic and cause lethality to a large degree, but can be studied for example in wing imaginal discs on Notch target gene expression and tissue growth (Maier et al. 2011, Maier et al. 2013, Yuan et al. 2016). In order to perform a combined overexpression of Daphnia Hairless with Drosophila Su(H), the transgenic UAS-DpH strains were recombined with transgenic UAS-Su(H) flies by genetic means. Overexpression was induced with the omb-Gal4 driver line, and Notch activity recorded by analyzing Cut and vgBE-lacZ reporter gene expression as outlined above (Fig. 9 and online resource Supplemental Fig. 3). As described earlier, co-expression of DmH and Drosophila Su(H) caused a dramatic downregulation of the Notch target genes and also massive tissue loss within the overexpression domain (Fig. 9 and online resource Supplemental Fig. 3). Indeed, overexpression of any of the Daphnia Hairless constructs in combination with Drosophila Su(H) caused dramatic repression of either Cut (Fig. 9), or the vgBE-lacZ reporter (online resource Supplemental Fig. 3). In addition, a marked tissue loss was a consequence of the combined overexpression of Su(H) with both UAS- DpHgDNA and UAS-DpHcDNA, but least with UAS-DpHcDNA-myc in support of its reduced activity (Fig. 9 and online resource Supplemental Fig. 3). These strong effects were unexpected given the results of the sole overexpression (Fig. 8 and online resource Supplemental Fig. 2). We conclude that Drosophila wing development is extremely sensitive to an excess of Su(H)-H repression complexes.
Daphnia Hairless interacts with Drosophila Su(H) to assemble a super-repressor. Co-overexpression of Hairless and Su(H) in Drosophila results in the formation of excessive repressor complexes, leading to an extreme repression of Notch activity. This effect was visualized by combined overexpression of the indicated Drosophila (DmHcDNA) or Daphnia (DpHgDNA, DpHcDNA, DpHcDNA-myc) constructs with Drosophila UAS-Su(H) in the central wing anlagen using omb-Gal4. As a read out for Notch, Cut expression (red) was monitored. Su(H) protein is shown in blue, Hairless protein in green, and was detected with anti-H antiserum and with anti-myc as indicated. The super-repressor effect is clearly seen with DmHcDNA: Cut expression is completely lost from the overexpression domain (arrow), which is very small due to tissue loss. Likewise, the three Daphnia Hairless constructs lead to a strong super-repressor phenotype, albeit the defects are weakest for DpHcDNA-myc. In all cases, Cut expression is completely inhibited within the overexpression domain (arrows). Moreover, the wing blade is smaller and split into two halves, similar to what is seen with DmHcDNA in combination with Drosophila Su(H). Size bar corresponds to 100 μm in all panels
In order to analyze super-repressor phenotypes in adult flies, we turned to the eye as an unessential tissue for fly survival. A combined overexpression of Drosophila Su(H) with either Drosophila or Daphnia Hairless constructs was performed using the gmr-Gal4 driver line which drives expression in the differentiating eye field (http://flybase.org). Whereas GFP overexpression did not influence eye development, DmH overexpression causes smaller eyes with a rough surface (Fig. 10) due to misarrangement of ommatidia and cell death, as described before (Müller et al. 2005, Protzer et al. 2008, Nagel and Preiss 2011). A combined overexpression of Drosophila Hairless and Su(H) is lethal: the eyes of pharate adults do not differentiate any ommatidia and display a glazed surface (Kurth et al. 2011, Yuan et al. 2016). The single overexpression of the Daphnia DpH cDNA in the gmr pattern caused primarily the loss of interommatidial bristles (Fig. 10). Upon combined overexpression of DpH cDNA with Drosophila Su(H), however, the eyes were dramatically reduced and rough (Fig. 10). In fact, the phenotype was stronger than the one achieved with Drosophila Hairless overexpression alone (Fig. 10). Overall, these data demonstrate the residual biological activity of the Daphnia Hairless gene during the development of various fly tissues.
Interaction of Daphnia Hairless with Drosophila Su(H) during eye development. Single or combined overexpression of Drosophila and Daphnia Hairless and Drosophila Su(H) was induced with gmr-Gal4. UAS-GFP served as control, resulting in eyes with the typical regular arrangement of the facets and interommatidial bristles. Overexpression of DmHcDNA causes smaller eyes that appear rough due to loss and irregular arrangement of the ommatidia. Many interommatidial bristles are missing (arrowhead), or split (asterisk marks example). Overexpression of DpHcDNA has little effect on eye size or architecture; however, many bristles are lacking (arrow). Overexpression of Dm Su(H) results in bigger eyes with irregular ommatidia and lacking bristles. The combined overexpression of Drosophila Hairless and Su(H) is lethal; animals develop to pharate stage, however, with no eyes (arrow). Overexpression of both, Drosophila Su(H) and Daphnia Hairless cDNA leads to smaller eyes, with multiple ectopic bristles, typical of a repression of Notch activity. Size bar corresponds to 100 μm in all panels
Modeling the structure of the Daphnia CSL-H repressor complex
Recently, the crystal structure of the Drosophila Su(H)-H repressor complex was published [PDB ID: 5E24] (Yuan et al. 2016). We used these data to model the structure of CSL binding domain of Hairless proteins from several species including the honeybee A. mellifera, the water flee D. pulex, and the white shrimp L. vannamei (Fig. 11a), employing the Swiss-Model database for alignments (https://swissmodel.expasy.org/). Despite an evolutionary distance of several hundred millions years, the predicted protein structures are remarkably similar albeit not identical, placing those amino acids, known from the DmH homologue to directly contact Su(H), at the exact same position independent of the species (Fig. 11a).
Structure modeling of Su(H)-H repressor complexes in arthropods. a The Apis mellifera, Daphnia pulex, and white shrimp Hairless amino acid sequences were modeled using the Swiss-Model database. The sequences were aligned to the Drosophila structure of the Su(H)-H repressor complex (PDB ID: 5E24). Despite some apparent differences in the ribbon diagrams and the molecular surface models, the positions of the conserved interacting residues (orange; L235, F237, L245, L247, W258 in DmH) are remarkably similar. b Open book representation of the molecular surface of the repressor complex from D. melanogaster and D. pulex. The D. pulex CSL homologue was modeled based on the structure of D. melanogaster Su(H) in the Su(H)-H complex using the Swiss-Model database. Residues in the Hairless protein that contact Su(H)/CSL are marked in orange, and likewise the ones in Su(H)/CSL contacting Hairless are marked in green and in pink. The latter marks residues essential for the interaction
Next, we wanted to model the presumptive Daphnia CSL-H repressor complex. To this end, the sequence of CSL from D. pulex was retrieved by scanning the genomic DNA using NCBI tblastn with the Drosophila Su(H) protein sequence (http://www.ncbi.nlm.nih.gov/genome/browse/). We found a single homologue in the Daphnia genome that contains at least six introns versus three introns in the D. melanogaster Su(H) gene (online resource Supplemental Fig. 3). Computational prediction of intron/exon boundaries was not difficult in the conserved parts of the coding region, whereas those for the 5′ non-conserved region are highly speculative. A glutamine-rich open reading frame was detected close to the first obvious exon, however, lacking a start codon. We predict an extension of the coding region including a start codon by adding a small further intron. Further upstream, a second glutamine-rich exon with a start codon was noted (online resource Supplemental Fig. 4). We propose that both start sites are used, resulting in two different CSL proteins by differential splicing, as both predicted introns obey the GT-AG exon/intron rule (online resource Supplemental Fig. 4). In this case, the CSL Daphnia gene structure would match that of vertebrates that use alternative splicing in the 5′ region of the gene to generate different CSL isoforms (Kawaichi et al. 1992; https://www.ncbi.nlm.nih.gov/protein/). Off note, our predictions for Daphnia CSL proteins differ from the ones in the database (http://genome.jgi.doe.gov/Dappu1/Dappu1.home.html: ID98470, ID127544, ID237385, ID313669, ID45248; various predicted isoforms) mostly with regard to the 5′ end.
Structure models of the two presumptive Daphnia CSL proteins were generated using the Swiss-Model database (https://swissmodel.expasy.org/) and the D. melanogaster Su(H)-H structure [PDB ID: 5E24] (Yuan et al. 2016). No difference in structure prediction for the two presumptive Daphnia CSL proteins were seen, however, subtle differences compared with Drosophila Su(H) (Fig. 11b). The open book representation of the CSL-Hairless interface, however, shows that all the amino acids in CSL known to make specific contacts with Hairless are at identical positions in both species. In fact, despite the apparent differences in the individual structures, there is no doubt that the two Daphnia proteins Hairless and CSL fit perfectly to form a repressor complex matching the one from Drosophila. These data support our experimental data showing that Daphnia Hairless is able to build a functional repressor complex together with Drosophila Su(H) protein.
Conclusions
For the first time, we have identified and studied a Hairless homologue outside of insects, extending the conservation of this gene into the phylum of Arthropoda. We note, however, that we were unable to find a Hairless homologue in published sequences of spider to date, restricting its presence to certain classes of Mandibulata within this phylum (Hartenstein and Stollewerk 2015). The functional domains of the Hairless gene have been conserved for a period of at least 400 million years, despite the fact that the gene is evolving fast, resulting in considerable sequence divergence overall. The minimal requirements for a functional Hairless protein are contained within roughly 500 amino acids comprising binding domains for its interaction partners Su(H)/CSL, Gro and CtBP, as well as several nuclear localization signals. Complex formation with Su(H)/CSL is the primary role of Hairless, building the centre of the repressor complex that recruits the two general co-repressors Gro and CtBP for subsequent silencing of Notch target genes (Maier 2006). All these factors show a much higher degree of conservation than Hairless, suggesting a surprisingly high degree of evolutionary flexibility for this central component. If one considers the fact that the interaction domains within Hairless are rather small and that the rest of the Hairless protein is unstructured, Hairless may be considered a hinge-joint connecting Su(H)/CSL with its co-repressor partners. Vertebrate CSL proteins directly interact with co-repressors, as shown recently by crystal structure analysis for KyoT2 and RITA, respectively (Collins et al. 2014, Tabaja et al. 2017). Perhaps, this ability appeared in the course of bilaterian evolution specifically within deuterostomes, or perhaps was lost in protostomes to be replaced by Hairless protein. Likewise possible is that the vertebrate Hairless homologue has not yet been identified due to its rapid evolutionary change.
Notes
Flybase (http://flybase.org/reports/FBgn0001169.html) refers to five H orthologues in the centipede Strigamia maritima. Only one of the deposited sequences, however, shares weak homology with the H gene maybe representing a true H homologue. We have notified flybase of our observations.
References
Bang AG, Hartenstein V, Posakony JW (1991) Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development 111:89–104
Bang AG, Posakony JW (1992) The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev 6:1752–1769
Barolo S, Stone T, Bang AG, Posakony JW (2002) Default repression and Notch signaling: Hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev 16:1964–1976
Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104:3312–3317
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 66:1631–1646
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415
Bray SJ (2016) Notch signalling in context. Nat Rev Mol Cell Biol 17:722–735
Buscarlet M, Stifani S (2007) The ‘Marx’ of Groucho on development and disease. Trends Cell Biol 17:353–361
Chien CT, Hsiao CD, Jan LY, Jan YN (1996) Neuronal type information encoded in the basic-helix–loop–helix domain of proneural genes. Proc Natl Acad Sci U S A 93(23):13239–13244
Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK et al (2011) The ecoresponsive genome of Daphnia pulex. Science 33:555–561
Collins KJ, Yuan Z, Kovall RA (2014) Structure and function of the CSL-KyoT2 corepressor complex: a negative regulator of Notch signaling. Structure 22:70–81
Contreras-Cornejo H, Saucedo-Correa G, Oviedo-Boyso J, Valdez-Alarcón JJ, Baizabal-Aguirre VM, Cajero-Juárz M, Bravo-Patiño A (2016) The CSL proteins, versatile transcription factors and context dependent corepressors of the Notch signaling pathway. Cell Div 11:12
Gyuris J, Golemis E, Chertkov H, Brent R (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with cdk2. Cell 75:791–803
Hartenstein AY, Rugendorf A, Tepass U, Hartenstein V (1992) The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 116:1203–1220
Hartenstein V, Stollewerk A (2015) The evolution of early neurogenesis. Dev Cell 32:390–407
Honeybee Genome Sequencing Consortium (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443:931–949
Hori K, Sen A, Artavanis-Tsakonas S (2013) Notch signaling in glance. J Cell Sci 126:2135–2140
Kawaichi M, Oka C, Shibayama S, Koromilas AE, Matsunami N, Hamaguchi Y, Honjo T (1992) Genomic organization of Mouse J kappa recombination signal binding protein (RBP-J kappa) gene. J Biol Chem 267:4016–4022
Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB (1996) Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382:133–138
Klann M, Stollewerk A (2017) Evolutionary variation in neural gene expression in the developing sense organs of the crustacean Daphnia magna. Dev Biol 424:50–61
Kovall RA, Blacklow SC (2010) Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol 92:31–71
Kurth P, Preiss A, Kovall RA, Maier D (2011) Molecular analysis of the Notch repressor-complex in Drosophila: characterization of potential Hairless binding sites on Suppressor of Hairless. PLoS One 6(11):e27986
Lunde K, Trimble JL, Guichard A, Guss KA, Nauber U, Bier E (2003) Activation of the knirps locus links patterning to morphogenesis of the second wing vein in Drosophila. Development 130:235–248
Maier D (2006) Hairless: the ignored antagonist of the Notch signalling pathway. Hereditas 143:212–221
Maier D, Stumm D, Kuhn K, Preiss A (1992) Hairless, a Drosophila gene involved in neural development, encodes a novel, serine rich protein. Mech Dev 38:143–156
Maier D, Marquart J, Thompson-Fontaine A, Beck I, Wurmbach E, Preiss A (1997) In vivo structure-function analysis of Drosophila Hairless. Mech Dev 67:97–106
Maier D, Nagel AC, Preiss A (2002) Two isoforms of the Notch antagonist Hairless are produced by differential translation initiation. Proc Natl Acad Sci U S A 99:15480–15485
Maier D, Chen AX, Preiss A, Ketelhut M (2008) The tiny Hairless protein from Apis mellifera: a potent antagonist of Notch signalling in Drosophila melanogaster. BMC Evol Biol 8:175
Maier D, Kurth P, Schulz A, Russell A, Yuan Z, Gruber K, Kovall RA, Preiss A (2011) Structural and functional analysis of the repressor complex in the Notch signaling pathway of Drosophila melanogaster. Mol Biol Cell 22:3242–3252
Maier D, Praxenthaler H, Schulz A, Preiss A (2013) Gain of function Notch phenotypes associated with ectopic expression of the Su(H) C-terminal domain illustrate separability of Notch and Hairless-mediated activities. PLoS One 8:e81578
Marquart J, Alexief-Damianof C, Preiss A, Maier D (1999) Rapid divergence in the course of Drosophila evolution reveals structural important domains of the Notch antagonist Hairless. Dev Genes Evol 209:155–164
Morel V, Lecourtois M, Massiani O, Maier D, Preiss A, Schweisguth F (2001) Transcriptional repression by Suppressor of Hairless involves the binding of a Hairless-dCtBP complex in Drosophila. Curr Biol 11:789–792
Müller D, Kugler SJ, Preiss A, Maier D, Nagel AC (2005) Genetic modifier screens on Hairless gain-of-function phenotypes reveal genes involved in cell differentiation and cell death. Genetics 171:1137–1152
Nagel AC, Maier D, Preiss A (2000) Su(H) independent activity of Hairless during mechano-senory organ formation in Drosophila. Mech Dev 94:3–12
Nagel AC, Krejci A, Tenin G, Bravo-Patiño A, Bray S, Maier D, Preiss A (2005) Hairless-mediated repression of notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol Cell Biol 25:10433–10441
Nagel AC, Preiss A (2011) Fine tuning of Notch signaling by differential co-repressor recruitment during eye development of Drosophila. Hereditas 148:77–84
Neumann CJ, Cohen SM (1996) A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122:347–3485
Oswald F, Kostezka U, Astrahantseff K, Bourteele S, Dillinger K, Zechner U, Ludwig L, Wilda M, Hameister H, Knöchel W, Liptay S, Schmid RM (2002) SHARP is a novel component of the Notch/RBP-Jkappa signalling pathway. EMBO J 21:5417–5426
Oswald F, Winkler M, Cao Y, Astrahantseff K, Bourteele S, Knöchel W, Borggrefe T (2005) RBP-Jkappa/SHARP recruits CtIP/CtBP corepressors to silence Notch target genes. Mol Cell Biol 25:10379–10390
Praxenthaler H, Smylla TK, Nagel AC, Preiss A, Maier D (2015) Generation of new Hairless alleles by genomic engineering at the Hairless locus in Drosophila melanogaster. PLoS One 10(10):e0140007
Preiss A, Hartley DA, Artavanis-Tsakonas S (1988) The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J 7:3917–3927
Protzer CE, Wech I, Nagel AC (2008) Hairless induces cell death by downregulation of EGFR signalling activity. J Cell Sci 121:3167–3176
Schlatter R, Maier D (2005) The Enhancer of split and Achaete-Scute complexes of Drosophilids derived from simple ur-complexes preserved in mosquito and honeybee. BMC Evol Biol 5:67
Schweisguth F (2015) Asymmetric cell division in the Drosophila bristle lineage: from the polarization of sensory organ precursor cells to Notch-mediated binary fate decision. WIREs Dev Biol 4:299–309
Simpson P (1990) Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development 109:509–519
Smylla TK, Preiss A, Maier D (2016) In vivo analysis of internal ribosome entry at the locus by genome engineering in Drosophila. Sci Rep. https://doi.org/10.1038/srep34881
Tabaja N, Yuan Z, Oswald F, Kovall RA (2017) Structure-function analysis of RBPJ-interacting and tubulin-associated (RITA) reveals regions critical for repression of Notch target genes. J Biol Chem 292(25):10549–10563
Turner J, Crossley M (2001) The CtBP family: enigmatic and enzymatic transcriptional co-repressors. BioEssays 23:683–690
Ungerer P, Eriksson BJ, Stollewerk A (2011) Neurogenesis in the water flea Daphnia magna (Crustacea, Branchiopoda) suggests different mechanisms of neuroblast formation in insects and crustaceans. Dev Biol 357:42–52
Yuan Z, Praxenthaler H, Tabaja N, Torella R, Preiss A, Maier D, Kovall RA (2016) Structure and function of the Su(H)-Hairless repressor complex, the major antagonist of Notch signalling in Drosophila melanogaster. PLoS Biol 14(7):e1002509
Zimmermann M, Kugler SJ, Schulz A, Nagel AC (2015) Loss of putzig activity results in apoptosis during wing imaginal development in Drosophila. PLoS One 10(4):e0124562
Acknowledgments
We greatly acknowledge U. Gigengack, H. Mastel, S. Mohaved, and H. Reichle for the technical assistance and L. Fedoseeva for the help during a Master module. We thank the DSHB for the antisera. We are grateful to J.K. Colbourne and D. Gilbert for sharing unpublished data and R.O. Schill for classifying the European Daphnia pulex species. We thank A.C. Nagel for carefully reading the manuscript. This work was supported by Deutsche Forschungsgemeinschaft (DFG) grants MA1328/10 and MA1328/11-1 to DM.
Funding
This study was funded by the German Research Foundation DFG to DM (MA1328/10 and MA1328/11-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Siegfried Roth
Electronic supplementary material
ESM 1
(PDF 1418 kb)
Rights and permissions
About this article
Cite this article
Zehender, A., Bayer, M., Bauer, M. et al. Conservation of the Notch antagonist Hairless in arthropods: functional analysis of the crustacean Daphnia pulex Hairless gene. Dev Genes Evol 227, 339–353 (2017). https://doi.org/10.1007/s00427-017-0593-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-017-0593-4