Abstract
The nervous system of the antenna of the grasshopper Schistocerca gregaria consists of two nerve tracts in which sensory cells project their axons to the brain. Each tract is pioneered early in embryogenesis by a pair of identified cells located apically in the antennal lumen. The pioneers are thought to originate in the epithelium of the antenna and then delaminate into the lumen where they commence axogenesis. However, unambiguous molecular identification of these cells in the epithelium, of an identifiable precursor, and of their mode of generation has been lacking. In this study, we have used immunolabeling against neuron-specific horseradish peroxidase and against Lachesin, a marker for differentiating epithelial cells, in combination with the nuclear stain DAPI, to identify the pioneers within the epithelium of the early embryonic antenna. We then track their delamination into the lumen as differentiated neurons. The pioneers are not labeled by the mesodermal/mesectodermal marker Mes3, consistent with an epithelial (ectodermal) origin. Intracellular dye injection, as well as labeling against the mitosis marker phospho-histone 3, identifies precursor cells in the epithelium, each associated with a column of cells. Culturing with the S-phase label 5-ethynyl-2′-deoxyuridine (EdU) shows that both a precursor and its column have incorporated the label, confirming a lineage relationship. Each set of pioneers can be shown to belong to a separate lineage of such epithelial cells, and the precursors remain and are proliferative after generating the pioneers. Analyses of mitotic spindle orientation then enable us to propose a model in which a precursor generates its pioneers asymmetrically via self-renewal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pioneer neurons can be defined as the cells whose axons establish a future pathway in a developing nervous system. Ramón y Cajal (1890) first observed pioneer axons in the central nervous system of the chicken embryo, and since then, the mechanisms regulating growth cone guidance during axogenesis have been intensively studied and shown to be conserved across a wide range of species from flatworms to mammals (see Bentley and O’Connor 1992; Goodman 1996; Tessier-Lavigne and Goodman 1996; Mueller 1999; Dickson 2002). In insects, pioneer neurons were first described by Bate (1976) in appendages of the grasshopper such as the antenna and leg. In the antenna, these cells were located in the lumen near the tip of the appendage and at its border with the epithelium and subsequently shown to establish two nerve tracts—one ventral and one dorsal—extending along the epithelial/lumenal border (see Anderson and Tucker 1988), first to the antennal base (Ho and Goodman 1982; Berlot and Goodman 1984; Seidel and Bicker 2000; Boyan and Williams 2004; Ehrhardt et al. 2015a) then via the deutocerebrum to the protocerebrum of the brain (Boyan and Ehrhardt 2015).
Earlier cellular studies proposed that the apical pioneers of the grasshopper antenna (Ho and Goodman 1982; Berlot and Goodman 1984) and leg (Keshishian 1980; Keshishian and Bentley 1983) originate in the epithelium and subsequently delaminate into the lumen prior to formation of the basal lamina, after which they commence axogenesis. However, in the absence of molecular data identifying these pioneers as neurons in the epithelium, and without a proliferative marker identifying a putative precursor cell, their mode of generation has remained unclear. For instance, it was not clear whether a single identifiable precursor delaminated and subsequently underwent mitosis to generate the pioneers, or whether the pioneers were generated in the epithelium and delaminated into the lumen as already differentiated neurons. This compared to an extensive literature on the generation of sensory cells in the epithelia of the grasshopper, moth, and Drosophila, and where both individual precursors were identified (Heathcote 1981; Keil 1992; Keil and Steiner 1990; Bodmer and Jan 1987) and regulation of the differentiation and fate of each cell in the sensory cluster via genes such as atonal, numb, and pox-neuro documented (e.g., Uemura et al. 1989; Jan and Jan 1990; Bier et al. 1990; Dambey-Chaudiere et al. 1992; Jarman et al. 1995; Jarman 2014).
In keeping with sensory epithelial cells in general (see Campos-Ortega and Hartenstein 1985; Hartenstein 1987), the somata of sensory cells remain within the antennal epithelium and do not delaminate (Chapman 2002; Ehrhardt et al. 2015b). It was therefore unclear whether they and the pioneer neurons originate via a conserved cellular pathway. To clarify these aspects of pioneer cell life history, we performed intracellular dye injection as well as immunolabeling in wholemount embryos very early in embryogenesis. We are able to identify separate putative stem cells for each set of pioneers and demonstrate the delamination of these pioneers as differentiated neurons into the lumen. Analysis of mitotic spindle orientation in the stem cells allows us to propose a model of how the lineage containing the pioneers might be generated.
Materials and methods
Animals and preparation
Eggs were produced by a crowded colony of Schistocerca gregaria with a 12/12-h light/dark regime, 35% air humidity, a day temperature of 30 °C, and a night temperature of 20 °C. Eggs were incubated in moist, aerated containers under this same regime. Embryos were staged according to Bentley et al. (1979). For immunolabeling, staged embryos were removed from the egg and embryonic membranes in 0.1 M phosphate buffered saline (PBS: 2 mM NaH2PO4 monohydrate; 16 mM Na2HPO4 anhydrous; 175 mM NaCl, adjusted to pH 7.4 with NaOH).
Whole embryo culture
The protocol followed was that of Ehrhardt et al. (2015a). Briefly, eggs were disinfected in 70% ethanol for 1 min before dissection. Unfixed embryos were removed from the egg and placed in a sterile culture medium which consisted of Grace’s Insect TC Medium (Bio & SELL 2.12G07J), 600 mg/l l-glutamine (Roth), 20 μl/ml 20-hydroecdysone (Sigma), 25 μg/ml juvenile hormone III (Sigma-Aldrich), 120 units/ml penicillin, 120 units/ml streptomycin, and 2.5 μg/ml Fungizone (Gibco). Cultured embryos grew an average of 4% over the course of the experiment (2 days) compared with 10% normally. Embryos were subsequently processed for HRP labeling as below.
Immunolabeling
Unless otherwise stated, the basic tissue preparation and protocols for primary antibody labeling were as described in Ehrhardt et al. (2015a, 2016).
Cell identification markers
Anti-1C10
The 1C10 (Lachesin) antibody recognizes a cell surface molecule belonging to the Ig superfamily (Karlstrom et al. 1993). In grasshopper, expression of the molecule occurs initially on all differentiating epithelial cells, but only cells involved in neurogenesis such as neuroblasts and precursors continue to express the molecule later. The 1C10 antibody (mouse, gift of M. Bastiani) was diluted 1:5000 in preincubation medium.
Horseradish peroxidase
The antibody against horseradish peroxidase (HRP) is used as a specific neuronal marker in insects (Jan and Jan 1982). The polyclonal anti-HRP antibody in rabbit (Dianova, 323-005-021) was diluted 1:200 in preincubation medium (0.1 M PBS, 0.5% Triton-X, 1% NGS, 3% BSA).
Cell proliferation markers
Anti-phospho-histone H3 (Ser10)
The PH3 antibody recognizes and binds to the phosphorylated form of the amine terminal of histone H3. This binding is only possible when the chromatin lies dissociated from the nucleosome complex, as occurs during mitotic chromosome condensation (see Hendzel et al. 1997; Adams et al. 2001 for details). The PH3 antibody (rabbit, Millipore) was diluted 1:250 in preincubation medium.
5-Ethynyl-2′-deoxyuridine
5-Ethynyl-2′-deoxyuridine (EdU) is a thymidine analog which is incorporated into the DNA of proliferating cells during the S phase of the cell cycle (Sousa-Nunes et al. 2011; Takagi et al. 2012). A Click-iT® EdU imaging kit (Invitrogen, C10337) was used for EdU incorporation experiments. Unfixed embryos were incubated in a 50 μM solution of EdU in PBS for 2–4 h at room temperature on a shaker in the dark. The embryos were then fixed in 3.7% paraformaldehyde (PFA in PBS), washed, and incubated in the Click-iT® reaction solution according to the kit instructions.
Ontogenetic marker
Mes3
The anti-Mes3 antibody binds to a cell surface epitope expressed by cells of mesodermal/mesectodermal origin (Kotrla and Goodman 1984; Boyan and Williams 2007; Ehrhardt et al. 2015a). Prior to Mes3 immunolabeling, wholemount embryos were fixed in PIPES-FA (3.7% paraformaldehyde in 100 mM PIPES, 2 mM EGTA, 1 mM MgSO4) for 1 h at room temperature then washed in PBS for 1 h. Embryos were preincubated in PBS with 0.1% Triton-X and 5% fetal calf serum for 1 h at room temperature. The Mes3 antibody raised in mouse (gift of C. Goodman) was diluted 1:4 in the preincubation medium.
Secondary antibodies
Protocols for secondary antibodies were as described in Ehrhardt et al. (2015a, 2016).
Controls for the specificity of all antibodies employed involved (a) application of the relevant secondary in the absence of the primary antibody (in no case was a staining pattern observed) and (b) comparison of our experimental results with previously published data (see above). In all cases, an identical staining pattern to that first description was observed.
Nuclear marker
4,6-Diamidino-2-phenylindole
4,6-Diamidino-2-phenylindole (DAPI, Sigma) is a cell-permeable fluorescent probe which binds to the minor groove of double-stranded DNA (Naimski et al. 1980). DAPI was diluted 1:100 in 0.1 M PBS. Embryos were exposed to DAPI for 30 min at room temperature, and this was followed by 6 washing cycles each with a 20-min duration in 0.1 M PBS.
Intracellular dye injection
Staged embryos (ca. 40%) were dissected out of the egg into embryonic Ringer solution (125 mM NaCl, 3 mM KCl, 10 mM CaCl2, 1 mM MgSO4, 5 mM Hepes buffer, pH 7.4) and freed from embryonic membranes. A living antenna was then dissected from the head and positioned on a glass slide coated with HistoBond® (Marienfeld). The slide bearing the preparation was transferred to a fixed stage microscope (Zeiss Axioskop 2) equipped with epifluorescence and DIC optics. A fine chlorided silver wire acted as a reference electrode. Thin-walled glass capillary microelectrodes (Clarke Instruments) having resistances of 30–40 MΩ when filled with 5% Lucifer Yellow dissolved in 1 M LiCl were connected to the head stage of a Getting 5 DC Amplifier. Intracellular penetrations of target cells were monitored optically using a Zeiss ceramic ×63 Achromat water immersion objective, a 1.3 MP Color CCD camera (Scion Corp.), and Scion Visicapture™ software. Iontophoretic dye injection into a penetrated cell was via constant hyperpolarizing current (2 nA). Fixation was in situ for 30 min in 2% paraformaldehyde followed by washing in Ringer and coating in Vectashield® (Vector Laboratories).
Imaging
Optical sections of preparations were acquired with a Leica TCS SP5 confocal laser scanning microscope with ×20 and ×63 oil immersion objectives. Fluorophores were visualized using excitation wavelengths of 405 nm for DAPI, 488 nm for Alexa® 488 and EdU, 520 nm for lucifer yellow, and 561 nm for Cy3. Fluorescence photomicrographs were obtained as described above. All images were processed using public domain software (ImageJ). False colors were applied where necessary and adjustments made to brightness and contrast. Final figures were formatted using Canvas™ X software (ACD Systems).
Results
Early axogenesis by apical pioneer neurons
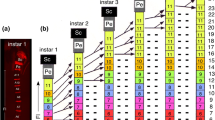
Immunolabeling against the neuron-specific marker HRP at 31% of embryogenesis reveals a pair of ventral pioneer neurons in the lumen at the apical tip of an antenna from a cultured embryo (Fig. 1a). The ventral pioneers are the only HRP-positive neurons in the lumen at this time and have just commenced axogenesis. Subsequently, the ventral pioneers extend a growth cone further towards the antennal base while HRP labeling identifies the dorsal pioneers at the border of the epithelium and lumen (Fig. 1b). Culturing reveals that at 35% of embryogenesis, the dorsal pioneers are now also present in the lumen and that both sets of pioneers have extended axons on opposite sides of the antenna towards its base (Fig. 1c). No other pioneers will appear apically in the lumen after this stage.
Early axogenesis by apical pioneer neurons of the antenna as revealed by confocal images following immunolabeling against the neuron-specific marker HRP. a At 31% of embryogenesis, the ventral pioneers (vA1p, white asterisks) are seen in the lumen (Lu) near the apex of the antenna. The A1 terminology refers to the cells belonging to the most apical (first) meristal annulus of the antenna (see Chapman 2002). The pioneers have generated growth cones and extend the first filopodia (open white arrowhead) towards the antennal base (to the left in all panels). Dashed line indicates the approximate border between the epithelium (Ep) and Lu. b At 31–32% of embryogenesis, the filopodia (open white arrowhead) from the vA1p cells extend further towards the antennal base, while the dorsal pioneers (dA1p) have appeared at the apical border of the Ep and Lu. c At 35% of embryogenesis, both sets of pioneers occupy equivalent positions on opposite sides of the antennal lumen. Axons with growth cones (open white arrowheads) extend towards the antennal base. a, c Derive from whole embryo culture. Scale bar represents 20 μm in a, 15 μm in b, and 20 μm in c
Apical pioneers have an ectodermal origin
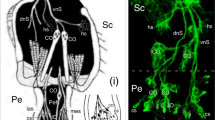
The data presented above show apical pioneers in the antennal lumen, which derives from a mesodermal germ band (see Anderson 1973), but do not reveal whether the pioneers also originate there. This can be resolved at the gross level using double labeling against HRP and the mesoderm marker Mes3 (Fig. 2). Here, we consider the ventral pioneers (vA1p) to be representative of both sets of apical pioneers. Double labeling at 35% of embryogenesis shows (Fig. 2a) the HRP-positive vA1p neurons in the lumen to have extended axons towards the antennal base and to be surrounded by a number of HRP-negative/Mes3-positive cells. The latter are likely to be hemocyte precursors (see Ball et al. 1987). The HRP-positive pioneers, on the other hand, are Mes3-negative indicating an ectodermal origin, such as the antennal epithelium (see Anderson 1973). A repeat experiment in which DAPI has been additionally employed to reveal all nuclei present (Fig. 2b) again shows the sibling vA1p cells labeled by HRP among non-neuronal nuclei representing undifferentiated cells of unknown identity, plus a single unidentified Mes3-positive cell in the vicinity. The data are consistent with an ectodermal origin for the apical pioneers.
Ontogeny of the apical pioneers. a Confocal image following double immunolabeling against neuron-specific HRP (green) and the mesoderm-specific label Mes3 (magenta) at 35% of embryogenesis. Dashed white line indicates the border between the epithelium (Ep) which is of ectodermal origin and the lumen (Lu) which is of mesodermal origin. The ventral pioneers (vA1p) have extended axons (open white arrowhead) towards the antennal base (top of image) and are clearly HRP-positive but Mes3-negative, consistent with an ectodermal and not mesodermal/mesectodermal origin. Other Mes3-positive cells in the lumen are all HRP-negative and are most likely hemocyte precursors. b Confocal image from a different preparation and at higher magnification to a following triple labeling against HRP (green), Mes3 (pink), and DAPI (blue) at 35% of embryogenesis. The vA1p cells (white stars) are HRP-positive and Mes3-negative and extend axons (open white arrowhead) towards the antennal base. One prominent Mes3-positive/HRP-negative cell is present in the vicinity of the pioneers, which are surrounded by DAPI-stained cells that are HRP-negative/Mes3-negative and so represent undifferentiated cells of unknown identity. Scale bar represents 25 μm in a and 8 μm in b
Molecular identification and delamination of apical pioneer neurons
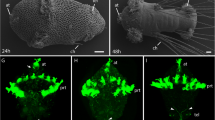
If the apical pioneers originate in the epithelium and then delaminate into the lumen as previously proposed (Ho and Goodman 1982; Berlot and Goodman 1984), they should be molecularly identifiable in both these locations. Immunolabeling against neuron-specific HRP does reveal cells closely apposed in the epithelium early in embryogenesis and resembling the ventral and dorsal apical pioneers (Fig. 3a, d). The label Lachesin, which is expressed by differentiating epithelial cells, identifies these same pairs of cells (Fig. 3b, e). At a slightly later stage of development, the ventral pioneers are observed in the lumen (Fig. 3f), while the dorsal cells are still within the epithelium. These data corroborate the findings of previous studies which report a staggered appearance of pioneers in the lumen—the ventral cells first, followed by the dorsal cells (Ho and Goodman 1982; Berlot and Goodman 1984). All labeled cells extend HRP- and Lachesin-positive dendritic projections centrifugally towards the outer edge of the antennal epithelium. Since the cells express the neuron-specific label HRP while still within the epithelium, we conclude that these cells represent already differentiated neurons and are likely to have originated within the epithelium itself and not from a precursor that has previously delaminated into the lumen. An appropriate precursor, however, remains to be identified (but see below).
Molecular identification of apical pioneer neurons (a–c, vA1p, white asterisks; d–f, dA1p, open white stars) in the early embryonic antenna. Photomicrographs following immunolabeling against neuron-specific horseradish peroxidase (HRP, green) identify the ventral (a, white asterisks) and one dorsal (d, open white star) apical pioneers within the epithelium (Ep) prior to their delamination into the lumen (Lu) of the antenna. Pioneers direct HRP-positive processes (white arrowheads) centrifugally to the outer edge of the epithelium (white dashed lines delimit the epithelium). Confocal images following immunolabeling against Lachesin, a marker for differentiating epithelial cells (green), reveal the ventral (b, c, f, vA1p, white asterisks) and dorsal (e, f, dA1p, open white stars) pioneer neurons. Counterstaining with the DNA label DAPI (c, f) highlights the cell nuclei. Both sets of pioneers (b, c, vA1p; e, f, dA1p) direct Lachesin-positive processes (white arrowheads) centrifugally to the outer edge of the epithelium. At 29% of embryogenesis (c), the ventral pioneers (white asterisks) are in the process of delaminating into the antennal lumen. At 30% of embryogenesis (f), the ventral pioneers (white asterisks) have fully delaminated, whereas the dorsal pioneers (white stars) are still within the epithelium and delaminate later (not shown). Scale bar in f represents 10 μm in a, b, d, e and 12 μm in c, f
Identification of proliferative precursor cells
Proliferative cells can be identified during the late meta- to early anaphase of their cell cycle by the appearance of condensed chromosomes labeled using anti-phospho-histone 3 (PH3, see “Materials and methods” section). In the antennal epithelium, PH3 labeling shows that proliferative cells are found at stereotypic sites in the epithelium early in embryogenesis (see Fig. 7). At the apex of the antenna, PH3 labeling in combination with the differentiating epithelial cell label Lachesin and the nuclear stain DAPI identifies proliferative cells associated with a column of epithelial cells that includes a pair of pioneer neurons (Fig. 4). Imaging the optical z-stacks at the appropriate depth in the antenna reveals the proliferative cell associated with a column that contains the ventral pioneers (Fig. 4a–d). These pioneers are still in the epithelium and have yet to delaminate into the lumen. In a repeat experiment involving these same labels but at a slightly later age, proliferative cells were found at the same locations in the epithelium (Fig. 4e–h). Splitting the z-stack of optical images into dorsal and ventral substacks made it possible to identify both the dorsal (Fig. 4e) and ventral (Fig. 4g) proliferative cells, each associated with an epithelial cell column which included the respective apical pioneers. The images show that the dorsal pioneers had not yet fully delaminated, while both ventral pioneers were now located in the lumen. 3D imaging and rotation of the respective confocal optical stacks (Fig. 4f, h) confirm the presence of two proliferative cells, one dorsal and one ventral, each associated with a column of epithelial cells including the respective pioneers.
Identification of a putative precursor cell for the apical pioneers. a–d Confocal images following triple labeling with the epithelial cell marker Lachesin (a, green), the DNA marker DAPI (b, blue), and the mitosis marker PH3 (c, magenta), at 29% of embryogenesis, identify a proliferative cell (white star) at the periphery associated with a column of Lachesin-positive epithelial cells (white crosses) (d, merge). The column includes the ventral apical pioneers (vA1p, white asterisks) which have yet to delaminate into the lumen (Lu). Dashed white lines delimit the epithelium (Ep) from the lumen (Lu) throughout. e–h Each pair of apical pioneers has its own precursor cell. Confocal images (e, g) and 3D imaging (f, h) following triple labeling against the epithelial cell marker Lachesin (green), the mitosis marker PH3 (magenta), and the DNA marker DAPI (blue), at 30% of embryogenesis, reveal a column of cells (white crosses) and a proliferative precursor cell (PC, white star) associated with each pair of dorsal (e, f; white asterisks) and ventral (g, h; white asterisks) apical pioneers. The dorsal pioneers (e) have not yet fully delaminated, while the ventral pioneers (g) are now in the lumen. 3D images are z-projections of optical sections with the antenna rotated dorsally (f) and ventrally (h) to reveal the respective putative precursor cell (white star) and its column of Lachesin-positive (green) epithelial cells. Scale bar represents 12 μm throughout
Identification of supporting cells and processes
Neural stem cells (neuroblasts) in the ventral nerve cord (VNC) of the embryonic grasshopper are surrounded by supporting cells which, during early embryogenesis, are dye-coupled to the stem cell and also extend processes that encase its lineage of progeny (Doe and Goodman 1985a, b). We performed intracellular dye injection into candidate stem cells and supporting cells in the antenna of a living 39% embryo to examine whether the cellular organization described for the VNC also applies to the antennal epithelium (Fig. 5). Intracellular penetration and dye-filling revealed a large cell at a location consistent with it being a stem cell based on our earlier experiments using DAPI and the proliferative cell label anti-PH3 (c.f. Fig. 4). Lucifer yellow dye injected into a putative supporting cell surrounding this large ventral stem cell spread to a further supporting cell and revealed a process extending through the epithelium towards the lumen (Fig. 5c, d). Three minutes after commencing dye injection into the one supporting cell, both supporting cells were observed to be dye-coupled to the stem cell they accompany (Fig. 5e). This cellular association is therefore consistent with that reported for the VNC of the early embryonic grasshopper (see Doe and Goodman 1985a, b).
Intracellular dye injection identifies the stem cell and its supporting cells in the antenna of a living 39% embryo. a Photomicrograph of the ventral antennal surface viewed with interference contrast optics (DIC) shows glass capillary containing lucifer yellow (LY) dye penetrating the putative stem cell (white star) for the ventral pioneer cells at the antennal tip. A supporting cell (black asterisk) is located near the stem cell. Thick dashed white line delimits the lumenal border (Lu) from the epithelium (Ep) in all panels. Apex of the antenna is to the top in all panels. b Fluorescence photomicrograph following iontophoretic injection of LY into the penetrated cell from a reveals the large stem cell (open black star) at the outer edge of the epithelium. Glass capillary is still in place. c Fluorescence photomicrograph of the antennal tip (outlined by thin dashed white line) from another living 39% embryo during intracellular dye injection reveals LY dye-coupling between two putative supporting cells (black asterisks) associated with the large ventral stem cell (open white star). One of the supporting cells extends a process (white arrowheads) towards the epithelial border with the Lu. The stem cell is not yet stained, but its outline is discernible. Glass capillary is out of focus here. d Photomicrograph as above but with combined transmitted and fluorescence optics 1 min after commencing dye injection shows the dye-coupled supporting cells (black asterisks) surrounding the putative stem cell (open white star) in the epithelium. The process (white arrowhead) from one supporting cell is visible. e Same preparation as in c, d but 3 min after commencing dye injection shows that LY has now spread from the supporting cells (black asterisks) into the stem cell (open black star). Scale bar in e represents 10 μm in a, b and 8 μm in c–e
Columns of antennal epithelial cells represent cell lineages
Association of a proliferative cell with a column of epithelial cells does not alone demonstrate a lineage relationship, so that we cannot yet refer to these cells as precursors for the pioneers. This required confirmation from embryos cultured in the presence of the thymidine analog EdU which is incorporated into the DNA of a proliferating cell during the S phase of the cell cycle and is then passed on to all its progeny (see “Materials and methods” section).
Examination of the cellular organization of the antennal epithelium early in embryogenesis using the nuclear stain DAPI (Fig. 6a) reveals columns of cells oriented radially around the tip of the antenna and projecting in the apical/basal antennal axis towards the lumen. Immunolabeling against Lachesin shows the differentiated apical pioneers located basally in one of these columns and at the edge of the lumen (Fig. 6a). The column of cells located apically to the pioneers is delimited from neighboring cells by Lachesin-positive processes which we speculate are from supporting cells such as those identified via intracellular dye injection (see Fig. 5). Culturing in the presence of EdU followed by immunolabeling against EdU and Lachesin, and application of DAPI, reveals that all the epithelial cells in a given column incorporate EdU and so represent actual lineages (Fig. 6b). The question remained as to whether the pioneers belong to such a lineage.
Columnar organization of antennal epithelial cells. a Confocal image following double labeling with Lachesin, a marker for differentiating epithelial cells (green) and DAPI (blue) at 31% of embryogenesis, reveals a column of nuclei (white asterisks) in the epithelium terminating in the ventral apical pioneers (open white stars) at the border of the epithelium (Ep) and Lumen (Lu) (dashed white line) and which are in the process of delaminating. Lachesin-positive processes (white arrowheads) separate the columns. b Confocal image following triple labeling against Lachesin (green, white arrowheads), the S phase (proliferative) cell marker EdU (magenta), and DAPI (blue) at 31% of embryogenesis reveals columns of EdU-positive cells (white asterisks) delimited by Lachesin-positive processes (white arrowheads) in the epithelium (Ep). c, d. Both members of a pioneer cell pair belong to the same column of epithelial cells. Substacks of confocal images in the dorsal and ventral planes following triple labeling with Lachesin (cyan), DAPI (blue), and EdU (magenta), at 29% of embryogenesis, identify the two members of a ventral pioneer pair: vA1p1 (white asterisk, c) and vA1p2 (white asterisk, d) which derive from the precursor cell indicated (white star). Black asterisk in d indicates the vA1p1 pioneer. Scale bar represents 12 μm in a, 20 μm in b, and 15 μm in c, d
The dorsal and ventral apical pioneers are each found as pairs initially in the epithelium and later in the lumen (see Figs. 1 and 3 above). In order to confirm a lineage association and establish whether both members of a pair (e.g., vA1p1, vA1p2) belong to the same lineage, we repeated the triple labeling after culturing embryos in a medium containing EdU and then split the confocal z-stacks of optical images into dorsal and ventral substacks to isolate in this case the ventral pair (Fig. 6c, d). We found that the vA1p1 (Fig. 6c) and vA1p2 (Fig. 6d) cells belong to the same column of epithelial cells, all of whom have incorporated EdU, and that these cells are lineage-related to the same proliferative cell, which we propose is their precursor. Analysis of z-stacks for the dorsal pair of pioneers provided equivalent results (not shown). No other neurons are present in the apical lumen prior to appearance of the pioneers, suggesting that they are at least early-born cells of the lineage which are progressively pushed towards the lumenal border by younger progeny.
Mitotic spindle orientation identifies precursors for pioneer neurons
Knowledge about the mitotic axis can assist in establishing the mode of cell division and hence the propagation direction of progeny from a given proliferative cell (see Doe 2008). In the grasshopper antenna, this information is obtainable from the orientation of condensed chromosomes during the metaphase of the cell cycle (Fig. 7). Confocal images following triple labeling (Lachesin, PH3, DAPI) at 31% of embryogenesis identify the respective dorsal and ventral pioneers in the antennal epithelium and reveal a stereotypic spindle orientation in their mitotically active precursors. Chromosomal organization at metaphase in the precursors for the dorsal (Fig. 7a) and ventral (Fig. 7b) apical pioneers suggests that the mitotic spindle is consistently oriented in line with the apical/basal axis of the antenna. The fact that the precursors remain proliferative, and that the pioneers are located at the lumenal end of their respective columns, indicates that the mode of cell division is asymmetric. The data are consistent with each precursor dividing repeatedly via self-renewal resulting in a column of progeny directed centripetally towards the lumen where the pioneer neurons are subsequently found.
Mitotic spindle orientation distinguishes precursors for pioneer neurons from those for putative sensory cells in the antennal epithelium. Confocal images following triple labeling (Lachesin, green; PH3, magenta; DAPI, blue) at 31% of embryogenesis reveal stereotypic differences in the spindle orientation of mitotically active precursors in the antennal epithelium (Ep). Insets show data from each panel at higher magnification. a Chromosomal organization in the precursor (dPC) for the dorsal apical pioneers (dA1p, white asterisks) at metaphase indicates that the mitotic spindle is oriented along the apical/basal axis of the antenna (double white arrows in inset). b Chromosomal organization in the precursor (vPC) for the ventral apical pioneers (vA1p, black asterisks) at metaphase indicates that the mitotic spindle is also oriented along the apical/basal axis of the antenna (double white arrows in inset). Preparation is the same as in a, but the confocal stack has been expanded to include the ventral surface of the antenna (white asterisks label the vA1p cells). The dPC and vPC would therefore each generate a column of progeny directed towards the lumen (Lu). c Chromosomal organization in a precursor (white arrowhead) for putative epithelial sensory cells indicates that the mitotic spindle is oriented orthogonally to the apical/basal axis of the antenna (double white arrows in inset). d Chromosomal organization in a precursor (white arrowhead) for putative epithelial sensory cells in a different preparation to c confirms the orientation of the mitotic spindle as being orthogonal to the apical/basal axis of the antenna (double white arrows in inset). Scale bar in d represents 10 μm in all panels and 5 μm in all insets
Proliferative cells are also found elsewhere along the antennal epithelium (Fig. 7c, d), and chromosomal organization in such proliferative cells indicates a mitotic spindle perpendicular to the antennal axis so that progeny would not form a single column directed towards the lumen. Since delaminated neurons are also never found in the lumen at such locations, we speculate that these proliferative cells may be the precursors of sensory cells which remain in the epithelium.
Discussion
Two tissues have been identified as generating proliferative cells in arthropod appendages: the ectodermal epithelium and the mesoderm/mesectoderm, which is represented by the lumen (Keshishian 1980; Heathcote 1981; Keil and Steiner 1990; Boyan and Williams 2004, 2007; Ehrhardt et al. 2015b). The epithelium is the source of sensory cells whose development has been extensively studied at both cellular and molecular levels (Bodmer and Jan 1987; Uemura et al. 1989; Jan and Jan 1990; Bier et al. 1990; Keil and Steiner 1990; Dambey-Chaudiere et al. 1992; Keil 1992, 1997; Meier et al. 1991; Gupta and Rodrigues 1997; Bellaïche and Schweisguth 2001). The lumen, on the other hand, is the source of hemocytes, one of whose functions in the grasshopper is to generate the basal lamina (Ball et al. 1987) upon which pioneer neuron growth cones navigate to the brain (Anderson and Tucker 1988, 1989). The lumen is also the source of the basal pioneers which have now been shown to be of mesodermal/mesectodermal origin (Ehrhardt et al. 2015a) and act as guidepost cells for the outgrowing axons of the apical pioneers (Ho and Goodman 1982; Berlot and Goodman 1984; Seidel and Bicker 2000).
The adult antenna contains two nerve tracts—one dorsal and one ventral—both located in the lumen (see Chapman 1982, 2002; Bentley and O’Connor 1992; Goodman 1996). The tracts are founded at around 30–31% of embryogenesis by separate sets of pioneers which appear in the lumen at slightly staggered times and are the first cells to direct growth cones towards the brain (Ho and Goodman 1982; Berlot and Goodman 1984; Seidel and Bicker 2000). The axons of differentiated sensory cells within the epithelium subsequently project onto those of the pioneers (Boyan and Williams 2004; Ehrhardt et al. 2015a, b). In contrast to the sensory cells, the pioneers have been proposed to delaminate from the epithelium at the apex of the antenna, but as their origins have not been investigated in detail at the molecular level, it is not clear if individual precursors equivalent to those reported for putatively homologous cells in leg appendages (Keshishian 1980; Lefcort and Bentley 1989) or to the SOPs of sensory cells (see Jarman 2014) are involved.
In this study, we have used immunolabeling and whole embryo culture in the grasshopper S. gregaria to demonstrate that the pairs of apical pioneers which later delaminate into the lumen can be identified by both the neuron-specific label horseradish peroxidase (see Jan and Jan 1982) and the differentiating epithelial cell marker Lachesin (see Karlstrom et al. 1993) while still in the epithelium (Fig. 3). We have previously shown that they are also labeled by Lazarillo, a cell-surface glycoprotein which is expressed by a subset of neurons including pioneers and sensory neurons and which regulates guided axogenesis in both the central (Ganfornina et al. 1995; Sánchez et al. 1995) and antennal (Ehrhardt et al. 2015a) nervous systems. While still in the epithelium, pioneers are interconnected with other epithelial cells via thin dendritic processes which are retained even once the pioneers are located in the lumen (Fig. 3). Such cytoskeletal extensions, termed epidermal feet, have been described for the putatively equivalent pioneers in the leg (Lefcort and Bentley 1989) and are characteristic of epithelial cells across insect species (Locke and Huie 1981). Finally, the pioneers are negative to the mesodermal/mesectodermal label Mes3 (Fig. 2; Kotrla and Goodman 1984), consistent with this epithelial (ectodermal) origin.
Origin and lineage of pioneers
Their molecular profile, comprising the neuron-specific labels HRP and Lazarillo and the label for differentiating epithelial cells, Lachesin, has allowed us to follow the development of the apical pioneers during early embryonic development. The data confirm that these cells originate in the epithelium and then delaminate into the lumen as previously proposed (Ho and Goodman 1982; Berlot and Goodman 1984). As in the leg (Lefcort and Bentley 1989), they do so as molecularly differentiated neurons, which implies a derivation from a progenitor in the epithelium. In the leg, this progenitor has been termed the pioneer mother cell (PMC, Keshishian 1980; Lefcort and Bentley 1989), but the mechanism in the antenna has remained unclear till now.
The cells of the antennal epithelium are characteristically organized into columns (Fig. 6), and we have employed the mitosis marker phospho-histone 3 to identify two proliferative cells at stereotypic locations in the antennal tip, each associated with a column of epithelial cells including the pioneers (Figs. 4 and 7). Intracellular dye injection reveals that these columns are delimited by the processes of so-called supporting cells which accompany the stem cell (Fig. 5) and which also express the label Lachesin (Figs. 3, 4, 6, and 7). Culturing experiments involving the S-phase label EdU show that both the proliferative cell and the pioneer cells in its column have incorporated EdU, confirming a lineage relationship (Fig. 6). The proliferative cells can therefore be considered to be pioneer cell precursors, and the ventral and dorsal pairs of pioneers can be shown to belong to the lineages of separate precursors (Fig. 6). In the leg, only one pair of pioneers is generated at the apical tip, and these derive from a single pioneer mother cell (Lefcort and Bentley 1989).
A model for the generation of the antennal apical pioneer neurons
Several observations point to a possible mechanism for the generation of pioneer neurons in the antenna. First, pioneers within a pair characteristically maintain a single-file spatial relationship to one another both within the epithelium and then subsequently in the lumen (Figs. 1, 2, and 3, and see Ehrhardt et al. 2016). Second, the precursors remain in the epithelium and are proliferative even after delamination of the pioneers (Figs. 4 and 7). Third, their proliferative nature allowed us to determine a cleavage plane, deduced from the pattern of condensed chromosomes at the metaphase of the cell cycle (Fig. 7). Spindle orientation in stem cells has been shown to regulate cell fate in the developing grasshopper (Yamashiki 1981; Yamashiki and Kawamura 1986a, b), Drosophila (Doe 2008), and vertebrate (Higginbotham and Gleeson 2007) nervous systems. We found that the spindle axis in the precursors of the apical pioneers was stereotypically oriented parallel to the column of its progeny and so in line with the apical/basal axis of the antenna (Fig. 7). This was not the case in proliferative cells elsewhere in the antennal epithelium where pioneer cells do not differentiate, but may involve the generation of sensory cells. An examination of this latter aspect is beyond the scope of the present study. Taken together, these data suggest a model in which each precursor divides asymmetrically and so generates its lineage sequentially via self-renewal (Fig. 8). In the absence of other identifiable cells in the epithelium during the timespan of our study, we cannot demonstrate that the pioneers represent the first-born cells of their lineage. However, the prior absence of any differentiated cells other than the delaminated pioneers in the antennal lumen at these locations makes this plausible.
Schematic summarizes the known molecular profile of the A1 pioneers and proposes a model for their origin from a precursor. Model applies to both ventral and dorsal pioneers. Codes for the various labels are indicated underneath the schematic. a A PH3-positive/EdU-positive mitotically active precursor cell (PC) in the epithelium (Ep) generates paired pioneers which are labeled by the neuron-specific markers HRP (green) and Lazarillo (cyan), Lachesin, the differentiating epithelial cell label (brown), and the DNA label DAPI (blue). The presence of the S-phase label EdU (red dots) in both the PC and the pioneers suggests their common ontogeny. The apical pioneers subsequently delaminate from the epithelium into the lumen (Lu) and commence axogenesis (green arrow). b The model proposes that the PC generates a columnar array of progeny, the first-born ones being the apical pioneers. The resulting column of cells is organized linearly through the epithelium, and the pioneers are always connected in single file even after delamination into the lumen. Since the PC is maintained throughout this period, the parsimonious explanation is that the PC divides asymmetrically via a self-renewal mode (recurring arrow)
A major implication of our model is that the pioneers are not siblings, but are proposed to derive from successive cell cycles of the precursor. If so, the developmental pattern would differ from that reported for putatively homologous pioneers in the leg (Keshishian 1980; Lefcort and Bentley 1989). These authors found that the Ti1 cells are generated in the epithelium by a large PMC. Analysis of centrosome location, spindle microtubule orientation, and cleavage furrow all suggested that the PMC divides conventionally to generate the pair of pioneers. Cleavage in the PMC differed from that in other proliferative epithelial cells of the leg in that the PMC’s spindle was oriented perpendicular to the epithelium and at the basal surface, rather than parallel to the plane of the epithelium and at the apical surface. This may represent a significant difference to what we observe in the antenna (Fig. 7) and explain the different cell division modes. Further, during the mitotic process, Lefcort and Bentley (1989) report that the PMC maintains contact via cellular processes with another large epithelial cell located more distally at the apical surface and which also commences mitosis. It is not clear which cells derive from this second proliferative cell, but we have not observed such a cellular constellation in the antenna.
Further experiments are obviously required to clarify the division modes in the respective mother cells for the antennal and leg pioneers. This could involve establishing the segregation of cell polarity determinants such as Par-3, Par-6, and aPKC, scaffolding proteins such as Miranda, and cargo proteins such as Prospero and Brat (see Doe 2008). Further, the regulation of Numb and Notch proteins, whose cellular distribution differs in progeny deriving from asymmetric as opposed to symmetric divisions, could provide valuable insights into the cell division modes involved (see Neumüller and Knoblich 2009). In vertebrates, the transcription factor Pax6 has been shown to promote asymmetric cell divisions in neuroepithelial precursors which then uniquely express the molecular marker Tis21 (Götz and Huttner 2005).
It is possible, given the serially homologous nature of insect appendages (see Gibson and Gehring 1988; Casares and Mann 1998), that the precursors for each set of pioneers are nevertheless homologous, but are regulated in different ways to generate their respective pioneering progeny. Similar differences with respect to the way pioneer neurons are generated have been observed in the CNS of both the grasshopper and Drosophila (Bastiani et al. 1985; Goodman and Doe 1994; Boyan and Williams 2008). In this regard, we see distinct parallels between our model for the apical pioneers in the antenna (Figs. 7 and 8) and neuronal development in other epithelia such as the optic lobe. In the medulla of Drosophila, there is a coordinated spatio-temporal conversion of neuroepithelial cells to neuroblasts involving a switch from symmetric to asymmetric cell divisions (see Apitz and Salecker 2014). The precursors in the optic lobe then generate lineages of postmitotic progeny organized in radial columns (c.f. our data for the antennal epithelium, Figs. 4 and 7). These chains of progeny subsequently become subdivided into neuronal subtypes by a cascade of temporal identity transcription factors. A similar mechanism might regionalize the columns of somata in the antennal epithelium and so regulate the differentiation of the apical pioneers.
References
Adams RR, Maiato H, Earnshaw W, Carmena M (2001) Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol 153:865–879
Anderson DT (1973) Embryology and phylogeny in annelids and arthropods. Pergamon Press, Oxford, New York
Anderson H, Tucker RP (1988) Pioneer neurones use basal lamina as a substratum for outgrowth in the embryonic grasshopper limb. Development 104:601–608
Anderson H, Tucker RP (1989) Spatial and temporal variation in the structure of the basal lamina in embryonic grasshopper limbs during pioneer neurone outgrowth. Development 106:185–194
Apitz H, Salecker I (2014) A challenge of numbers and diversity: neurogenesis in the Drosophila optic lobe. J Neurogen 28:233–249
Ball EE, de Couet HG, Horn PL, Quinn JMA (1987) Haemocytes secrete basement membrane components in embryonic locusts. Development 99:255–259
Bastiani MJ, du Lac S, Goodman CS (1985) The first growth cones in insect embryos: model system for studying the development of neuronal specificity. In: Selverston AI (ed) Model neural networks and behaviour. Plenum Press, New York, pp. 149–174
Bate CM (1976) Embryogenesis of an insect nervous system I. A map of the thoracic and abdominal neuroblasts in Locusta migratoria. J Embryol Exp Morphol 35:107–123
Bellaïche Y, Schweisguth F (2001) Lineage diversity in the Drosophila nervous system. Curr Opin Gen Dev 11:418–423
Bentley D, O’Connor TP (1992) Guidance and steering of peripheral growth cones in grasshopper embryos. In: Letourneau C, Kater SB, Macagno ER (eds) The nerve growth cone. Raven Press, New York, pp. 265–282
Bentley D, Keshishian H, Shankland M, Torian-Raymond A (1979) Quantitative staging of embryonic development of the grasshopper, Schistocerca nitens. J Embryol Exp Morphol 54:47–74
Berlot J, Goodman CS (1984) Guidance of peripheral pioneer neurons in the grasshopper: adhesive hierarchy of epithelial and neuronal surfaces. Science 223:493–496
Bier E, Jan LY, Jan YN (1990) rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev 4:190–203
Bodmer R, Jan YN (1987) Morphological differentiation of the embryonic peripheral neurons in Drosophila. Roux’s Arch Dev Biol 196:69–77
Boyan GS, Ehrhardt EE (2015) Pioneer neurons of the antennal nervous system project to protocerebral pioneers in the grasshopper Schistocerca gregaria. Dev Genes Evol 225:377–382
Boyan GS, Williams JLD (2004) Embryonic development of the sensory innervation of the antenna of the grasshopper Schistocerca gregaria. Arthr Struct Dev 33:381–397
Boyan GS, Williams JLD (2007) Embryonic development of a peripheral nervous system: nerve tract associated cells and pioneer neurons in the antenna of the grasshopper Schistocerca gregaria. Arthr Struct Dev 36:336–350
Boyan GS, Williams JLD (2008) Evidence that the peripheral brain commissure is pioneered by neurons with a peripheral-like ontogeny in the grasshopper Schistocerca gregaria. Arth Struct Dev 37:186–198
Campos-Ortega JA, Hartenstein V (1985) The embryonic development of Drosophila melanogaster. Springer, Berlin
Casares F, Mann RS (1998) Control of antennal versus leg development in Drosophila Nature 392:723–726
Chapman RF (1982) The insects: structure and function. Hodder and Stoughton, London
Chapman RF (2002) Development of phenotypic differences in sensillum populations on the antennae of a grasshopper, Schistocerca americana. J Morphol 254:186–194
Dambey-Chaudiere C, Jamet E, Burri M, Bopp D, Basler K, Hafen E, Dumont N, Spielmann P, Ghysen A, Noel M (1992) The paired box gene pox-neuro—a determinant of poly-innervated sense organs in Drosophila. Cell 69:159–172
Dickson BJ (2002) Molecular mechanisms of axon guidance. Science 298:1959–1964
Doe CQ (2008) Neural stem cells: balancing self-renewal with differentiation. Development 135:1575–1587
Doe CQ, Goodman CS (1985a) Early events in insect neurogenesis. I. Development and segmental differences in the pattern of neuronal precursor cells. Dev Biol 111:193–205
Doe CQ, Goodman CS (1985b) Early events in insect neurogenesis. II. The role of cell interactions and cell lineage in the determination of neuronal precursor cells. Dev Biol 111:206–219
Ehrhardt E, Kleele T, Boyan GS (2015b) A method for immunolabeling neurons in intact cuticularized insect appendages. Dev Genes Evol 225:187–194
Ehrhardt E, Liu Y, Boyan GS (2015a) Axogenesis in the antennal nervous system of the grasshopper Schistocerca gregaria revisited: the base pioneers. Dev Genes Evol 225:39–45
Ehrhardt EE, Graf P, Kleele T, Liu Y, Boyan GS (2016) Fates of identified pioneer cells in the developing antennal nervous system of the grasshopper Schistocerca gregaria. Arth Struct Dev 45:23–30
Ganfornina MD, Sánchez D, Bastiani MJ (1995) Lazarillo, a new GPI-linked surface lipocalin, is restricted to a subset of neurons in the grasshopper embryo. Development 121:123–134
Gibson G, Gehring WJ (1988) Head and thoracic transformation caused by ectopic expression of Antennapedia during Drosophila development. Development 102:657–675
Goodman CS (1996) Mechanisms and molecules that control growth cone guidance. Ann Rev Neurosci 19:341–377
Goodman CS, Doe CQ (1994) Embryonic development of the Drosophila central nervous system. In: Bate M, Martinez-Arias A (eds) The development of Drosophila, vol 1. Cold Spring Harbor Press, New York, pp. 1131–1206
Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788
Gupta BP, Rodrigues V (1997) Atonal is a proneural gene for a subset of olfactory sense organs in Drosophila. Genes Cells 2:225–233
Hartenstein V (1987) The influence of segmental compartmentalization on the development of the larval peripheral nervous system in Drosophila melanogaster. Roux’s Arch Dev Biol 196:101–112
Heathcote RD (1981) Differentiation of an identified sensory neuron (SR) and associated structures (CTO) in grasshopper embryos. J Comp Neurol 202:1–18
Hendzel M, Wie Y, Mancini MA, Van Hooser A, Ranali T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360
Higginbotham HR, Gleeson JG (2007) The centrosome in neuronal development. Trends Neurosci 30:276–283
Ho RK, Goodman CS (1982) Peripheral pathways are pioneered by an array of central and peripheral neurones in grasshopper embryos. Nature 297:404–406
Jan LY, Jan YN (1982) Antibodies to horseradish-peroxidase as specific neuronal markers in Drosophila and grasshopper embryos. Proc Natl Acad Sci U S A 79:2700–2704
Jan YN, Jan LY (1990) Genes required for specifying cell fates in Drosophila embryonic sensory nervous system. Trends Neurosci 13:493–498
Jarman AP (2014) Development of the auditory organ (Johnston’s organ) in Drosophila. In: Romand R, Varela-Nieto I (eds) Development of auditory and vestibular systems. Academic Press, pp 31–63
Jarman AP, Sun Y, Jan LY, Jan YN (1995) Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development 121:2019–2030
Karlstrom RO, Wilder LP, Bastiani MJ (1993) Lachesin: an immunoglobulin superfamily protein whose expression correlates with neurogenesis in grasshopper embryos. Development 118:509–522
Keil TA (1992) Fine structure of a developing insect olfactory organ: morphogenesis of the silkmoth antenna. Microsc Res Tech 22:351–371
Keil TA (1997) Comparative morphogenesis of sensilla: a review. Int J Insect Morphol Embryol 26:151–160
Keil TA, Steiner C (1990) Morphogenesis of the antenna of the male silkmoth, Antheraea polyphemus. II. Differential mitoses of ‘dark’ precursor cells create the Anlagen of sensilla. Tissue Cell 22:705–720
Keshishian H (1980) The origin and morphogenesis of pioneer neurons in the grasshopper metathoracic leg. Dev Biol 80:388–397
Keshishian H, Bentley D (1983) Embryogenesis of peripheral nerve pathways in grasshoppers legs. I. The initial nerve pathway to the CNS. Dev Biol 96:89–102
Kotrla KJ, Goodman CS (1984) Transient expression of a surface antigen on a small subset of neurones during embryonic development. Nature 311:151–153
Lefcort F, Bentley D (1989) Organization of cytoskeletal elements and organelles preceding growth cone mergence from an identified neuron in situ. J Cell Biol 108:1737–1749
Locke M, Huie P (1981) Epidermal feet in insect morphogenesis. Nature 293:733–735
Meier T, Chabaud F, Reichert H (1991) Homologous patterns in the embryonic development of the peripheral nervous system in the grasshopper Schistocerca gregaria and the fly Drosophila melanogaster. Development 112:241–253
Mueller BK (1999) Growth cone guidance: first steps towards a deeper understanding. Ann Rev Neurosci 22:351–388
Naimski P, Bierzyimageski A, Fikus M (1980) Quantitative fluorescent analysis of different conformational forms of DNA bound to the dye 4′,6-diamidine-2-phenylindole, and separated by gel electrophoresis. Anal Biochem 106:471–475
Neumüller RA, Knoblich JA (2009) Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23:2675–2699
Ramón y Cajal S (1890) A quelle epoque aparaissent les expansion des cellule neurveuses de la moelle epinere du poulet. Anat Anz 5:609–613
Sánchez D, Ganfornina MD, Bastiani MJ (1995) Developmental expression of the lipocalin Lazarillo and its role in axonal pathfinding in the grasshopper embryo. Development 121:135–147
Seidel C, Bicker G (2000) Nitric oxide and cGMP influence axogenesis of antennal pioneer neurons. Development 127:4541–4549
Sousa-Nunes R, Yee LL, Gould AP (2011) Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471:508–513
Takagi A, Kurita K, Terasawa T, Nakamura T, Bando T, Moriyama Y, Mito T, Noji S, Ohuchi H (2012) Functional analysis of the role of eye absent and sine oculis in the developing eye of the cricket Gryllus bimaculatus. Develop Growth Differ 54:227–240
Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274:1123–1133
Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN (1989) numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 58:349–360
Yamashiki N (1981) The role of the spindle body in unequal division of the grasshopper neuroblast. Zool Mag 90:93–101
Yamashiki N, Kawamura KY (1986a) Microdissection studies on the polarity of unequal division in grasshopper neuroblasts. I. Subsequent divisions in neuroblast-type cells produced against the polarity by micromanipulation. Exp Cell Res 166:127–138
Yamashiki N, Kawamura KY (1986b) Microdissection studies on the polarity of unequal division in grasshopper neuroblasts. II. Cell division in binucleate neuroblasts. Develop Growth Differ 28:603–609
Acknowledgements
We thank Drs. Sanchez and Ganfornina for the gift of the Lazarillo antibody, Dr. Goodman for the gift of the Mes3 antibody, and Dr. Bastiani for the gift of the Lachesin antibody. We thank Dr. Yu Liu for the critical reading of the manuscript and Karin Fischer for the excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the guidelines for animal welfare as laid down by the Deutsche Forschungsgemeinschaft.
Competing interests
The authors declare that they have no competing interests.
Funding
Both authors received support for this study from the Graduate School of Systemic Neuroscience, University of Munich.
Additional information
Communicated by Claude Desplan
Both authors contributed equally to this study.
Rights and permissions
About this article
Cite this article
Boyan, G., Ehrhardt, E. Ontogeny of pioneer neurons in the antennal nervous system of the grasshopper Schistocerca gregaria . Dev Genes Evol 227, 11–23 (2017). https://doi.org/10.1007/s00427-016-0565-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-016-0565-0