Abstract
Prolonged fixation can lead to the generation of tiny and fast eye movements called microsaccades, whose dynamics can be associated with higher cognitive mechanisms. Saccade preparation is also reflected in microsaccadic activity, but the few studies on this topic provided mixed results. For instance, fewer microsaccades have been observed when participants were asked to prepare for an anti-saccade (i.e., a saccade in the opposite direction to the target) as compared to a pro-saccade (i.e., a saccade executed towards a target), but null results have also been reported. In the attempt to shed new light on this topic, two experiments were carried out in which the context of presentation of pro- and anti-saccade trials was manipulated. Pupil size was also recorded, as a further index of cognitive load. In Experiment 1, participants were asked to prepare and perform pro- and anti-saccades in response to a peripheral target, according to a central instruction cue provided at the beginning of each trial (intermixed condition). In Experiment 2, the same task was employed, but pro- and anti-saccade trials were delivered in two distinct blocks (blocked condition). In both experiments, greater saccadic latencies and lower accuracy emerged for anti- than for pro-saccades. However, in the intermixed condition, a lower microsaccadic rate and a greater pupil size emerged when participants prepared for anti- rather than pro-saccades, whereas these differences disappeared in the blocked condition. These results suggest that contextual factors may play a key role in shaping oculomotor dynamics linked to saccade preparation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microsaccades are tiny and fast eye movements that we tend to produce during prolonged fixation (see Martinez-Conde, Macknik, Troncoso, & Dyar, 2013; Martinez-Conde & Macknik, 2017). Even if a shared consensus concerning the mechanisms underlying microsaccade generation is still missing (e.g., Collewijn & Kowler, 2008; Martinez-Conde et al., 2013; Poletti & Rucci, 2016; Rolfs, 2009), increasing evidence has been reported, showing that microsaccades are shaped not only by vision-related processes (e.g., Costela et al., 2014; Engbert & Kliegl, 2004; Ko, Poletti, & Rucci, 2010; Martinez-Conde, Macknik, Troncoso, & Dyar, 2006; McCamy, Macknik, & Martinez-Conde, 2014; McCamy, Otero-Millan, Di Stasi, Macknik, & Martinez-Conde, 2014) but also by higher order cognitive mechanisms (e.g., Betta, Galfano, & Turatto, 2007; Engbert & Kliegl, 2003; Kliegl, Rolfs, Laubrock, & Engbert, 2009; Lange, Zweck, & Sinn, 2017; Rolfs, Engbert, & Kliegl, 2005; Valsecchi and Turatto, 2009).
Interestingly, the preparation of a motor response is also reflected in microsaccadic dynamics. Preparing for an action is an essential ability to establish effective interactions with the environment, and the study of any behavioural index that could track motor preparation is, therefore, of great interest. Nonetheless, so far, only a few studies explored the potential link between microsaccades and motor preparation. In a pioneering study, Betta and Turatto (2006) focused on manual response preparation, reporting a decrement in the absolute frequency of microsaccades when participants were asked to provide a manual response to an upcoming signal, as compared to a condition in which no manual response was required. Saccade preparation has also been explored by adopting the anti-saccade task (Hermens, Zanker, & Walker, 2010; Watanabe, Matsuo, Zha, Munoz, & Kobayashi, 2013; see also Jainta, Vernet, Yang, & Kapoula, 2011). In the classic version of the anti-saccade task, participants are required to maintain fixation on a central spot and to perform a saccade immediately after the onset of a target that can appear leftwards or rightwards with respect to the centre of the screen. Depending on the instruction, saccades have to be executed either towards the target (i.e., pro-saccade; for instance, if the target appears on the right, then the saccade has to be executed towards the right as well, landing on the target) or away from the target (i.e., anti-saccade; for instance, if the target appears on the right, then the saccade has to be executed towards the left, landing on the opposite location to that of the target). Typically, smaller latencies and a greater accuracy are observed for pro- than for anti-saccades, in line with the idea that pro-saccades would be strongly reflexive, whereas anti-saccades would require the implementation of more volitional processes (see Everling & Fischer, 1998; Munoz & Everling, 2004). Intriguingly, the two studies that explored microsaccadic dynamics in pro-/anti-saccade preparation reported mixed results. Indeed, while in Hermens et al. (2010, Experiment 1B), no differences in microsaccadic rate emerged when participants were asked to prepare for and execute pro- and anti-saccades immediately after target onset, Watanabe et al. (2013) reported that the preparation of pro-saccades was associated with a greater microsaccadic rate as compared to anti-saccades.
Several methodological aspects could account for the divergent results reported by Hermens et al. (2010) and Watanabe et al. (2013), such as differences in stimuli, timing or sample size. Nevertheless, we hypothesise that one of the main reasons for this inconsistency could be identified in the way pro- and anti-saccade trials were delivered. Indeed, while Hermens et al. (2010) adopted a blocked design, in which pro- and anti-saccades were executed in two distinct blocks, Watanabe et al. (2013) adopted a mixed design, in which pro- and anti-saccades were executed in a random fashion in accordance with an instruction cue provided at the beginning of each trial (see also Zeligman & Zivotofsky, 2017). Hence, while in Hermens et al. (2010) the oculomotor behaviour remained constant within each block, in Watanabe et al. (2013), participants were required to constantly update their oculomotor behaviour. This, in turn, likely resulted in a different impact on cognitive load and its reflection on working memory which is involved in the maintenance of task-related information (e.g., Miyake & Shah, 1999). Intriguingly, increasing evidence is showing that cognitive load, manipulated through a differential involvement of working memory, is reflected in several eye movement parameters, such as saccadic latency (e.g., Schaeffer et al., 2015) and trajectory (e.g., Theeuwes, Olivers, & Chizk, 2005) and even microsaccadic rate (Dalmaso, Castelli, Scatturin, & Galfano, 2017; Gao, Yan, & Sun, 2015; Pastukhov & Braun, 2010; Siegenthaler et al., 2014; Valsecchi, Betta, & Turatto, 2007). For instance, in Siegenthaler et al. (2014) participants were asked to mentally count forward (low load) vs. backward (high load) while maintaining fixation on a central spot. Similarly, in Gao et al. (2015), participants were asked to make an addition or a subtraction between a given number and another small (low load) vs. large (high load) number. In both Gao et al. (2015) and Siegenthaler et al. (2014), high load conditions led to a decrement in microsaccadic rate. In the same vein, in Dalmaso et al. (2017), participants were asked to remember two digits (low load) vs. five digits (high load). A reduction in microsaccadic rate was associated with the high load condition as compared to the low load condition. Taken together, these studies suggest that a stronger involvement of working memory could be associated with a decrease of microsaccade generation (see also Xue et al., 2017, for similar results with a perceptual load manipulation). We believe that a similar rationale could also be applied to explain the different pattern of microsaccadic rate associated with immediate pro- and anti-saccade preparation reported by the previous studies, since it is reasonable to hypothesise that the reduced cognitive load in the blocked design of Hermens et al. (2010) may have contributed to decrease the differences in microsaccadic rate during pro- and anti-saccade preparation; on the other hand, the higher cognitive load in the intermixed design of Watanabe et al. (2013) may have contributed to reveal differences in microsaccadic rate during pro- and anti-saccade preparation. Importantly, the divergent results reported by Hermens et al. (2010) and Watanabe et al. (2013) could also be explained by considering more specific mechanisms known to be involved in pro-/anti-saccades, such as task switching (e.g., Cherkasova, Manoach, Intriligator, & Barton, 2002; Pierce, McCardel, & McDowell, 2015) or proactive inhibitory control (e.g., Albares et al., 2011; Wardak, Ramanoël, Guipponi, Boulinguez, & Ben Hamed, 2011). Moreover, Zeligman and Zivotofsky (2017), who explored the behavioural effects of intermixed vs. blocked pro-/anti-saccade trial administration, and reported a lower accuracy for both pro- and anti-saccades when these were performed in an intermixed fashion, also invoked cognitive load as the possible determinant of their findings. Following Zeligman and Zivotofsky (2017), in the present context, we make reference to cognitive load as the broader factor differentiating the two trial-administration conditions.
In more detail, we directly explored the impact of intermixed vs. blocked pro-/anti-saccade trial administration on microsaccadic dynamics analysed during the preparatory phase of saccadic eye movements. In Experiment 1, pro- and anti-saccade trials were administered in a random fashion (intermixed condition), while in Experiment 2, they were provided in two distinct blocks (blocked condition). Furthermore, unlike previous studies (Hermens et al., 2010; Watanabe et al., 2013), in both experiments, pupil size was also recorded. Indeed, converging evidence is accumulating, showing that pupil size can be employed to track both cognitive control and working memory load dynamics (for reviews, see Just, Carpenter, & Miyake, 2003; van der Wel & van Steenbergen, 2018). In more detail, stimuli—or experimental conditions—associated with an increased task demand are typically associated with an increment in pupil size as compared to a pre-stimulus baseline period (e.g., Hyönä, Tommola, & Alaja, 1995; Klinger, Tversky, & Hanrahan, 2011; Lisi, Bonato, & Zorzi, 2015; Piquado, Isaacowitz, & Wingfield, 2010). For instance, pupil size would tend to increase with the number of information (e.g., digits or colours) that participants are asked to keep in memory (e.g., Kahneman & Beatty, 1966; Unsworth & Robison, 2018). In the same vein, Wang, Brien, and Munoz (2015) reported a greater pupil size associated with the preparation of an anti-saccade than a pro-saccade, delivered in a random fashion. Similarly, pupil size increments have been observed when participants prepared for a more complex manual response as compared to an easier one (e.g., Richer & Beatty, 1985). Hence, pupil size can be considered as a reliable, independent and online physiological index to estimate the amount of cognitive resources required to perform a given task (see van der Wel & van Steenbergen, 2018). In the present context, we reasoned that pupil size analyses might provide converging evidence for the role of cognitive load in shaping microsaccadic dynamics. In line with this latter notion, a recent study by Krejtz, Duchowski, Niedzielska, Biele and Krejtz (2018) extended the study of Siegenthaler et al. (2014), showing that—in a forward (low load) vs. backward (high load) mental counting task—both pupil size and microsaccades reflected differences in cognitive load. Overall, we expected to observe a lower microsaccadic rate and greater pupil size when participants were asked to prepare an anti- than a pro-saccade. Moreover, we also expected these differences to be greater in the intermixed condition (Experiment 1) as compared to the blocked condition (Experiment 2), in line with the view of a greater cognitive load in the former than in the latter case.
Experiment 1: intermixed pro- and anti-saccade trials
In this experiment, a centrally placed instruction cue informed participants on whether to prepare for a pro-saccade or an anti-saccade. The instruction cue was randomly selected on each trial, so that participants were constantly asked to update their oculomotor behaviour for the whole duration of the experiment.
Materials and methods
Participants
Twenty-two naïve students (mean age = 23 years; SD = 1.43, 2 males) with normal or corrected-to-normal vision were tested. The study was approved by the Ethics Committee for Psychological Research at the University of Padova and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Apparatus
Eye movements were recorded binocularly at 500 Hz using an EyeLink 1000 Plus (SR-Research)Footnote 1. Stimuli were presented through Experiment Builder (SR-Research) on a 24-in monitor (1280 × 1024 px, 120 Hz) placed at 65 cm from the participant. A chinrest was used to prevent head movements. Room luminance and screen background (grey coloured; R = 180, G = 180, B = 180) were kept constant throughout the experiment and they were identical for all participants.
Procedure
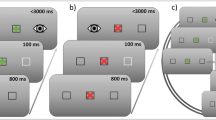
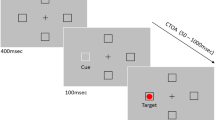
The task was designed by following the main guidelines of the anti-saccade task protocol proposed by Antoniades et al. (2013). A nine-point calibration was followed by a validation procedure. Before each trial, participants were asked to look at a centrally placed ring (external diameter 0.4°, black coloured; internal diameter 0.14°, grey coloured), and then, the experimenter initiated the trial by pressing the spacebar on the host PC (Fig. 1, drift checking frame). In this manner, it was possible to both perform a drift checking procedure and control that participants were looking at the centre of the screen. A successful drift checking was followed by a brief tone that informed the participant of the imminent start of the trial. Each trial started with a centrally placed ring (external diameter 0.4°, black coloured; internal diameter 0.14°, grey coloured) for 1500 ms (Fig. 1, fixation frame) which was then replaced by the instruction cue, which consisted of either a square (side 0.73°; black coloured) or a diamond (i.e., the square rotated of 45°) with a small circle at the centre (diameter 0.14°, grey coloured; Fig. 1, instruction cue frame). Half of the participants were instructed to prepare a pro-saccade in the presence of a square and an anti-saccade in the presence of a diamond, the other half learned the opposite association. After a random duration of 2000–2500 ms (100-ms steps), a target circle (diameter 0.5°; black coloured) appeared 10° either rightwards or leftwards of the instruction cue (Fig. 1, target frame). Depending on the instruction cue, participants were asked to perform, as fast and accurate as possible, a pro-saccade towards the target or an anti-saccade in the opposite direction. Finally, a visual feedback appeared for 1000 ms inviting the participant to blink, if needed. Importantly, in both the fixation and the instruction frames, participants were asked to maintain their eyes at the centre of the screen avoiding blinking; otherwise, an error visual feedback appeared for 1000 ms, and the trial was aborted and appended at the end of the session. This allowed us to collect a reasonable number of epochs without blinks while preventing an excessive duration of the experiment. Ten randomly selected practice trials were followed by 200 experimental trials presented in random order. Hence, there were potentially 100 data points per experimental cell (pro- vs. anti-saccade). A short break was provided every 40 trials. The whole experiment lasted about 1 h.
Stimuli (not drawn to scale) and sequence of events employed in both Experiments 1 (intermixed condition) and 2 (blocked condition). a, b Two trials in which the instruction cue is the square and the diamond, respectively. Depending on the association between the instruction cue and the requested eye movement, participants were asked to prepare and perform a pro-saccade towards the target or an anti-saccade towards the opposite location to that of the target
Results
Saccadic errors and latencies: data pre-processing
DataViewer (SR-Research) was used to generate two saccade reports (one for each eye), time-locked to target onset, containing saccadic latency and direction. Saccadic latency was measured as the time elapsing from target onset to the initiation of the first saccade. The first saccade was identified as the first eye movement with a velocity and acceleration exceeding 30°/s and 8000°/s2, respectively, and with a minimum amplitude of 1°. After that, saccadic latencies were obtained by averaging the data of the left and the right eyes. Trials in which participants blinked during the first saccade were discarded from the analyses (2.45% of trials). Saccadic directional errors (i.e., saccades directed towards the spatial location opposite to that requested by the instruction cue) were analysed separately (6.93% of trials). Finally, saccadic latencies less than 80 ms or greater than 800 ms were interpreted as outliers and discarded from the analyses (0.77% of trials).
Saccadic errors and latencies: data analyses
The mean percentage of saccadic directional errors was analysed through a two-tailed paired t test between pro-saccade and anti-saccade trials. The results indicated that participants committed less errors during pro-saccade trials (M = 3.90%, SE = 0.767) as compared to anti-saccade trials (M = 10.75%, SE = 1.796; t(21) = 3.542, p = 0.002, d = 0.754; see Fig. 2; Table 1).
Mean saccadic latencies of correct trials were analysed through a two-tailed paired t test between pro-saccade and anti-saccades. The results indicated that participants were faster to perform a pro-saccade (M = 288 ms, SE = 13.67) as compared to an anti-saccade [M = 342 ms, SE = 15.18, t(21) = 9.606, p < 0.001, d = 2.048].
Overall, these results confirmed that the anti-saccade task worked as expected, since pro-saccades led to faster and more accurate responses as compared to anti-saccades (see also Everling & Fischer, 1998).
Microsaccadic rate: data pre-processing
Microsaccades were analysed binocularly and only the correct trials identified in saccade analyses were considered. DataViewer (SR-Research) was used to generate a sample report starting at the onset of the instruction and ending after 2000 ms. This sample report contained X and Y coordinates of both eyes, sampled every 2 ms, and was used to detect microsaccades by employing a modified version of the algorithm proposed by Engbert and Kliegl (2003). This algorithm was adapted for the 500-Hz sampling frequency and implemented in MATLAB (MathWorks). The velocity threshold was set to λ = 4, the minimum duration threshold was set to four samples, and only binocular microsaccades were considered. Moreover, only microsaccades with a maximum amplitude of 1° were included in the analyses (see also Dalmaso et al., 2017; Engbert & Kliegl, 2003; Martinez-Conde et al., 2013).
Microsaccadic rate: data analyses
First, we verified that the algorithm detected microsaccades accurately. Since it is known that microsaccades, similar to saccades, are characterized by a positive correlation between peak velocity and amplitude known as “main sequence” (see Zuber, Stark, & Cook, 1965), we performed a correlation analysis between these two parameters. A positive correlation emerged, r = 0.697 p < 0.001, suggesting that we identified microsaccades correctly (see Fig. 3).
Correlation between peak velocity and amplitude in Experiment 1. This “main sequence” (see Zuber et al., 1965) established that microsaccades were correctly detected
Then, we calculated the microsaccadic rate within the 2000-ms temporal epoch that started at the onset of the instruction cue. This was achieved by calculating microsaccadic rate separately for each participant and experimental condition, and then averaging these data across participants. As depicted in Fig. 4, after the onset of the instruction cue (t = 0), microsaccadic rate showed a period of inhibition followed by period of rebound, a pattern that is fully consistent with the previous evidence (e.g., Engbert & Kliegl, 2003; Hafed & Ignashchenkova, 2013; see also Rolfs, 2009). To note, the two previous studies that explored microsaccade generation before pro- and anti-saccades found differences in microsaccadic rate about 400 ms before target onset (Hermens et al., 2010; Watanabe et al., 2013). Here, to explore microsaccades behaviour in more detail, ten comparisons [false discovery rate (fdr) corrected] between mean microsaccadic rate for pro- and anti-saccades were performed through a 200-ms moving window that started at the onset of the instruction cue (see, e.g., Dalmaso et al., 2017; Hermens et al., 2010; Valsecchi & Turatto, 2009, for a similar approach). Overall, fewer microsaccades emerged for the preparation of anti-saccades as compared to pro-saccades, and this was evident in most of the 200-ms time windows (indicated by green bars in Fig. 4; ts > 2.281, ps < 0.047) apart from three, in which the comparisons did not reach the canonical levels of significance (i.e., 0–200, 400–600, and 1000–1200, indicated by grey bars in Fig. 4; ts < 2.048, ps > 0.067). In sum, these findings are in line with the previous evidence in which fewer microsaccades were associated with the preparation of an anti-saccade rather than a pro-saccade (Hermens et al., 2010, Experiment 1A; Watanabe et al., 2013).
Mean microsaccadic rate calculated within the 2000-ms temporal epoch starting from the instruction cue onset (i.e., t = 0) and ending at the target onset (i.e., t = 2000), in Experiment 1. Shaded areas indicate the standard error of the mean. Green and grey rectangles below x-axis indicate the ten 200-ms time windows (fdr corrected) used for statistical testing. Asterisk denotes a significant difference between the two experimental conditions, while “ns” means that the difference was non-significant. Fewer microsaccades emerged for the preparation of anti-saccades as compared to pro-saccades, and this was evident in most of the 200-ms time windows apart from three (i.e., 0–200, 400–600, and 1000–1200 ms)
Pupil size: data pre-processing
Pupil size was analysed binocularly averaging the data of left and right eyes and only the correct trials identified in saccade analyses were considered. DataViewer (SR-Research) was used to generate two sample reports: one considering the 100-ms time window just before the onset of the instruction (i.e., the baseline temporal epoch), and another one starting at the onset of the instruction and ending after 2000 ms (i.e., the experimental temporal epoch). These two sample reports contained the average pupil size sampled every 2 ms. Because the EyeLink 1000 Plus reports pupil size in arbitrary integer units, these units were converted in mm by making a comparison with the arbitrary integer units corresponding to an artificial eye of 7 mm. Each data point within the experimental temporal epoch was then transformed to differences relative to a baseline level, namely, the average pupil size in the 100-ms baseline temporal epoch before instruction onset (see Mathôt, Fabius, Van Heusden, & Van der Stigchel, 2018). This baseline-correction procedure was applied separately for each participant and trial.
Pupil size: data analyses
First, no differences emerged between mean pupil size baseline for pro- and anti-saccade trials, t(21) = 0.516, p = 0.611, d = 0.110 (see Fig. 5). Then, pupil size within the 2000-ms epoch was analysed following the same procedure adopted for microsaccades, namely, ten comparisons (fdr corrected) between mean pupil size for pro- and anti-saccades were performed through a 200-ms moving window that started at the onset of the instruction. None of the comparisons between 0 and 600 ms (indicated by grey bars in Fig. 5) were statistically significant (ts < 1.810, ps > 0.106). More interestingly, all the comparisons between 600 and 2000 ms (indicated by green bars in Fig. 5) were significant (ts > 2.276; ps < 0.047), reflecting a greater pupil size in the preparation of anti-saccades than pro-saccade trials (also see Wang et al., 2015).
Top-left inset illustrates mean values of pupil size at baseline, calculated 100 ms before target onset. The main plot illustrates mean pupil size calculated within the 2000-ms temporal epoch starting from the instruction cue onset (i.e., t = 0) and ending at the target onset (i.e., t = 2000). Shaded areas indicate the standard error of the mean. Green and grey rectangles below x-axis indicate the ten 200-ms time windows (fdr corrected) used for statistical testing. Asterisk denotes a significant difference between the two experimental conditions, while “ns” means that the difference was non-significant. Starting from t = 600 ms, a greater pupil size was associated with the preparation of anti-saccades as compared to pro-saccades
Discussion
Experiment 1 results can be summarised as follows. As for saccades, smaller latencies and a greater accuracy emerged for pro- than for anti-saccades, thus confirming that the pro-/anti-saccade task worked properly (see also Everling & Fischer, 1998; Munoz & Everling, 2004). More interestingly, preparation of an immediate pro-saccade was reflected in a greater number of microsaccades as compared to preparation of an immediate anti-saccade. This finding can be interpreted as evidence that microsaccade rate can provide a reliable index of cognitive load. This interpretation is supported by the data stemming from the analyses of pupil size. Indeed, pupil size is known to be modulated by the engagement of cognitive resources (e.g., Kahneman & Beatty, 1966; Piquado et al., 2010), and accordingly, the present findings show a larger dilation during the preparation of an immediate anti-saccade, as compared to the preparation of an immediate pro-saccade. The overall pattern of results aligns with the previous studies using an intermixed presentation of trials (Wang et al., 2015; Watanabe et al., 2013).
Experiment 2: blocked pro- and anti-saccade trials
In Experiment 2, the same task of Experiment 1 was employed with the following exception: Pro- and anti-saccade trials were delivered in two distinct blocks rather than in a random fashion. In so doing, participants were not requested to constantly update their oculomotor behaviour on each trial, as an attempt to reduce the impact of cognitive load on pro-/anti-saccade preparation. In other words, because the participants had to reiterate the very same oculomotor response across the whole block of trials, the differences in both microsaccades and pupil size associated with pro-/anti-saccade preparation, were expected to decrease.
Materials and methods
Participants
A new sample of 22 naïve students (mean age = 22 years; SD = 2.33, 1 male) with normal or corrected-to-normal vision were tested. The study was approved by the Ethics Committee for Psychological Research at the University of Padova and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Apparatus
The apparatus was the same as that used in Experiment 1.
Procedure
The procedure was identical to that employed in Experiment 1, with the following exception: Pro- and anti-saccade trials were presented separately—rather than intermixed—in two distinct blocks. Block order was counterbalanced across participants.
Results and discussion
Data pre-processing procedures were the same as in the previous experiment.
Saccadic errors and latencies
Saccades were analysed as in Experiment 1. Trials with blinks were removed (3.30% of trials). Directional errors (4.66% of trials) were analysed separately. Trials with latencies less than 80 ms and greater than 800 ms were also removed (0.17% of trials).
The mean percentage of saccadic directional errors was analysed through a two-tailed paired t test between pro-saccade and anti-saccade trials. The results indicated that participants committed less errors on pro-saccade trials (M = 1.43%, SE = 0.293) as compared to anti-saccade trials [M = 8.67%, SE = 1.281; t(21) = 5.782, p < 0.001, d = 1.232].
Mean saccadic latencies of correct trials were analysed through a two-tailed paired t test between pro- and anti-saccades. The results indicated that participants were faster to perform a pro-saccade (M = 255 ms, SE = 9.55) as compared to an anti-saccade (M = 319 ms, SE = 13.16, t(21) = 7.905, p < 0.001, d = 1.685; see Fig. 6).
Overall, as for Experiment 1, these results confirmed that the anti-saccade task worked properly.
Microsaccadic rate
Microsaccades were analysed as in Experiment 1. A positive correlation emerged between peak velocity and amplitude, r = 0.614, p < 0.001 (see Fig. 7), suggesting that microsaccades were identified correctly.
Correlation between peak velocity and amplitude in Experiment 2. As for Experiment 1, this “main sequence” (see Zuber et al., 1965) established that microsaccades were correctly detected
Microsaccadic rate analyses were performed within the 2000-ms temporal epoch starting at the instruction cue onset (t = 0; see Fig. 8). Similar to Experiment 1, an inhibition phase was followed by a rebound phase. More importantly, none of the ten fdr-corrected comparisons showed significant differences between pro- and anti-saccade trials (indicated by grey bars in Fig. 8; ts < 2.724, ps > 0.06), a result in line with Hermens et al. (2010; Experiment 1B).
Mean microsaccadic rate, in Experiment 2, calculated within the 2000-ms temporal epoch starting from the instruction cue onset (i.e., t = 0) and ending at the target onset (i.e., t = 2000). Shaded areas indicate the standard error of the mean. Grey rectangles below x-axis indicate the ten 200-ms time windows (fdr corrected) used for statistical testing. “ns” denotes a non-significant difference between the two experimental conditions. Overall, no differences emerged for the preparation of anti-saccades as compared to pro-saccades
Pupil size
Pupil size was analysed as in Experiment 1. No differences emerged between mean pupil size baseline for pro- and anti-saccade trials, t(21) = 1.2, p = 0.244, d = 0.256 (see Fig. 9). Moreover, none of the ten fdr-corrected comparisons computed within the 2000-ms epoch were significant (ts < 1.866, ps > 0.527; see Fig. 9). Hence, these results mimicked the pattern reported in microsaccadic rate.
Top-left inset illustrates mean values of pupil size at baseline, calculated 100 ms before target onset. The main plot illustrates mean pupil size calculated within the 2000-ms temporal epoch starting from the instruction cue onset (i.e., t = 0) and ending at the target onset (i.e., t = 2000). Shaded areas indicate the standard error of the mean. Grey rectangles below x-axis indicate the ten 200-ms time windows (fdr corrected) used for statistical testing. “ns” denotes a non-significant difference between the two experimental conditions. Overall, no differences emerged for the preparation of anti-saccades as compared to pro-saccades
Discussion
Experiment 2 led to two main results. First, pro-saccades were associated with lower latencies and a greater accuracy, in line with Experiment 1 and previous literature (e.g., Munoz & Everling, 2004). Second, and more importantly, the pattern of microsaccadic rate was consistent with the results reported by Hermens et al. (2010) for immediate pro- and anti-saccades in a blocked design. Indeed, no significant differences emerged in microsaccadic rate as a function of whether participants were requested to prepare for and execute a pro- or an anti-saccade. No effects emerged for pupil size either. The overall pattern is consistent with our hypothesis that the discrepant findings reported in the literature might stem from a differential cognitive load induced by the specific experimental procedures adopted by Hermens et al. (2010) and Watanabe et al. (2013). These findings thus confirm that contextual factors can be highly involved in shaping oculomotor behaviour.
General discussion
Despite microsaccades are highly involved in many mechanisms related to low-level vision (e.g., Collewijn & Kowler, 2008; Hafed, Chen, & Tian, 2015; Martinez-Conde et al., 2013), evidence is accumulating showing that these tiny eye movements are also sensitive to higher order cognitive mechanisms, such as attention (e.g., Engbert & Kliegl, 2003) or motor preparation (e.g., Betta & Turatto, 2006). The main aim of the current study was to investigate microsaccadic rate associated with the preparation of pro- vs. anti-saccades to shed light on the conflicting results in the literature. Unlike the few previous studies on this topic (Hermens et al., 2010; Watanabe et al., 2013), here, we directly manipulated the way pro- and anti-saccade trials were delivered, which was either intermixed (Experiment 1) or blocked (Experiment 2). Moreover, pupil size was also recorded as an indirect additional way to assess cognitive control and working memory load (van der Wel & van Steenbergen, 2018). Overall, fewer microsaccades and a greater pupil size emerged when participants prepared for anti-saccades as compared to pro-saccades, but these differences emerged only when pro- and anti-saccade trials were administered in an unpredictable fashion.
One of the main differences between intermixed vs. blocked conditions was that while in the first case, participants were constantly asked to update their oculomotor behaviour in accordance with the instruction cue provided at the beginning of each trial, in the second case, this updating behaviour was unnecessary and unlikely. This would be associated—in turn—with a different cognitive load, which was reasonably higher in Experiment 1 (intermixed design) as compared to Experiment 2 (blocked design). Importantly, an increasing number of studies reported a decrement in microsaccadic rate when individuals are engaged in highly demanding tasks, such as difficult arithmetic processing and mental counting (Gao et al., 2015; Siegenthaler et al., 2014; Valsecchi et al., 2007) or maintenance of information in memory (Dalmaso et al., 2017). Hence, it seems highly likely that cognitive load played a key role in shaping microsaccadic dynamics in our two experiments. Importantly, the potential involvement of cognitive load finds also support—albeit indirect—in pupil size analyses. Indeed, it is known that a greater pupil size is typically associated with increasing processing complexity (e.g., Hyönä et al., 1995) and greater cognitive/working memory load (e.g., Kahneman & Beatty, 1966; Klinger et al., 2011; Piquado et al., 2010; Unsworth & Robison, 2018; for reviews see Just et al., 2003; van der Wel & van Steenbergen, 2018). This pattern emerged also in the preparation of anti-saccades as compared to pro-saccades, but only in Experiment 1 (intermixed condition), thus mirroring the results observed for microsaccadic rate. It is important to note that the potential relationship between executive control and the oculomotor system finds also support at the neural level, since there is evidence of a broad neural pathway between these two domains (Shen, Bezgin, Selvam, McIntosh, & Ryan, 2016). Moreover, some studies suggest that the dorsolateral prefrontal cortex, a fundamental cortical region for executive functioning (e.g., Gilbert et al., 2006), would directly project to the Superior Colliculus (SC; Johnston & Everling, 2009), a subcortical structure which seems highly involved in both microsaccade generation (e.g., Hafed, Goffart, & Krauzlis, 2009; Krauzlis, Goffart, & Hafed, 2017; see also Peel, Hafed, Dash, Lomber, & Corneil, 2016) and pupillary response (Wang, Boehnke, White, & Munoz, 2012).
The observation that a greater microsaccadic rate was associated with the preparation of a pro-saccade rather than an anti-saccade does not seem to be entirely consistent with the theoretical model proposed by Rolfs, Kliegl and Engbert (2008). According to this model, saccadic eye movements would be mainly generated by the activity within a motor map localized in the SC. The central site of this map—likely located on the rostral SC—would encode for fixations, while the more peripheral sites of this map would encode for saccades. When the firing rate activation within this motor map would exceed a hypothetical upper-bound threshold, one of these two oculomotor behaviours (i.e., fixation vs. saccade) would take place, according to the activation locus (i.e., central vs. peripheral). Moreover, due to a local excitation mechanism, this activation would also spread to adjacent areas within the map. Consequently, the central activation underlying a fixation would also slightly extend to neighbouring sites encoding saccades. Thus, microsaccade generation would be the consequence of a spread activation of the central motor map site. Hence, according to Rolfs et al. (2008), microsaccade generation should be more pronounced in oculomotor tasks associated with an enhanced fixational activity, such as the anti-saccade task, in accordance with physiological evidence that showed a greater activation of the rostral SC before anti-saccades than pro-saccades (Everling, Dorris, Klein, & Munoz, 1999). However, both our data and the few studies on this topic (Hermens et al., 2010; Watanabe et al., 2013) reported opposite—or even null—results.
The available findings with anti- and pro-saccade tasks seem to be consistent with the model proposed by Otero-Millan, Macknik, Serra, Leigh and Martinez-Conde (2011), who described a threshold-free neural mechanism for microsaccade generation. In more detail, Otero-Millan et al. (2011) hypothesised the presence of reciprocal inhibitory circuits between the omnipause neurons (OPN), responsible for the fixation behaviour, and the long-lead burst neurons (LLBN), responsible for saccade generation. During a fixation, a greater activation in the rostral SC would activate OPN which, in turn, would inhibit the LLBN and, as a result, the tendency to generate microsaccades. Occasionally, SC activation would move from the rostral to the caudal lobe, for reasons likely due to neuron noise or fixational errors (or both) rather than to threshold exceeding. As a consequence, the caudal SC would activate the LLBN which, in turn, would inhibit the OPN, thus increasing the likelihood to generate a microsaccade. According to this model, anti-saccade preparation could be associated with a decrement in microsaccade production, because, in this case, SC activation would be stronger in the rostral lobe (see also Everling et al., 1999). Complementarily, pro-saccade preparation could activate more strongly the caudal SC or, at least, the activation could be more spread within the SC, thus explaining the greater production of microsaccades (see also Watanabe et al., 2013). Hence, one could speculate that the context in which pro- and anti-saccades were executed in the current study (i.e., intermixed vs. blocked administration) may have played a role in shaping neuron noise, fixational errors, or both, which may have been higher in the intermixed condition (Experiment 1) as compared to the blocked condition (Experiment 2). Given the recent finding that microsaccade deployment could also be shaped by cortical activity in the frontal eye fields (FEF; Peel et al., 2016), and given that FEF are highly involved in pro-/anti-saccade tasks (e.g., Everling & Munoz, 2000; see also Zhou & Constantinidis, 2017), future studies are, nonetheless, necessary to elucidate in more detail the actual mechanisms underlying microsaccade generation under these specific circumstances.
Similar to the modern “rediscovery” of microsaccades (see Rolfs, 2009), pupillometry has also gained a renewed interest in vision science in recent years. More specifically, new potential links between pupil size and both physiological and cognitive mechanisms have been reported (e.g., Sirois & Brisson, 2014). In accordance with a recent theoretical framework, attention orienting would be served by a neural network which would include, other than areas such as the SC and the FEF, even the autonomic nervous system, that is known to control pupil size (see Corneil & Munoz, 2014). Indeed, physiological evidence showed that pupil size modulations can be evoked by stimulating the SC (Wang et al., 2012), and combined effects on both pupil size and saccades (including microsaccades) have been reported in attention-related tasks (Privitera, Carney, Klein, & Aguilar, 2014; Wang, Blohm, Huang, Boehnke, & Munoz, 2017). Anatomically, this pupil orienting response would be regulated by indirect projections from the SC to the autonomic system with no involvements of the OPN. This would explain why, in some cases, the pupil orienting response can be elicited without generating saccades (Wang et al., 2012). Taken together, all of these results suggest that the pupillary response could be expression of the orienting reflex mechanism, and future studies could track both pupil and (micro)saccades to get a wider picture of visuo-attentional mechanisms.
Conclusions
In two experiments, we investigated microsaccadic rate and pupil size dynamics in the preparation of pro- vs. anti-saccades. Overall, our results showed that a smaller microsaccadic rate and a greater pupil size are associated with the preparation of an anti-saccade as compared to a pro-saccade, but these differences emerged only when these two saccadic eye movements had to be performed in a random (Experiment 1) rather than blocked (Experiment 2) fashion. We suggest that cognitive load might have played a key role in shaping our results, in line with the notion that the functioning of human oculomotor system would be highly sensitive to ongoing high-level cognitive mechanisms.
Notes
Some authors reported that, in video-based eye-tracking systems, changes in pupil size can influence the computation of eye-gaze direction, leading to potential artefactual results (e.g., Choe, Blake, & Lee, 2016; Nyström, Hooge, & Andersson, 2016). However, Gautier, Bedell, Siderov and Waugh (2016; Appendix C)—who recorded pupil size and microsaccades through an EyeLink 1000—concluded that pupil size is unlikely to affect microsaccade detection.
References
Albares, M., Criaud, M., Wardak, C., Nguyen, S. C. T., Ben Hamed, S., & Boulinguez, P. (2011). Attention to baseline: Does orienting visuospatial attention really facilitate target detection? Journal of Neurophysiology, 106, 809–816.
Antoniades, C., Ettinger, U., Gaymard, B., Gilchrist, I., Kristjánsson, A., Kennard, C., et al. (2013). An internationally standardised antisaccade protocol. Vision Research, 84, 1–5.
Betta, E., Galfano, G., & Turatto, M. (2007). Microsaccadic response during inhibition of return in a target-target paradigm. Vision Research, 47, 428–436.
Betta, E., & Turatto, M. (2006). Are you ready? I can tell by looking at your microsaccades. Neuroreport, 17, 1001–1004.
Cherkasova, M. V., Manoach, D. S., Intriligator, J. M., & Barton, J. J. (2002). Antisaccades and task-switching: Interactions in controlled processing. Experimental Brain Research, 144, 528–537.
Choe, K. W., Blake, R., & Lee, S. H. (2016). Pupil size dynamics during fixation impact the accuracy and precision of video-based gaze estimation. Vision Research, 118, 48–59.
Collewijn, H., & Kowler, E. (2008). The significance of microsaccades for vision and oculomotor control. Journal of Vision, 8, 1–21.
Corneil, B. D., & Munoz, D. P. (2014). Overt responses during covert orienting. Neuron, 82, 1230–1243.
Costela, F. M., Otero-Millan, J., McCamy, M. B., Macknik, S. L., Troncoso, X. G., & Jazi, A. N., et al. (2014). Fixational eye movement correction of blink-induced gaze position errors. PLoS One, 9, e110889.
Dalmaso, M., Castelli, L., Scatturin, P., & Galfano, G. (2017). Working memory load modulates microsaccadic rate. Journal of Vision, 17, 1–12. https://doi.org/10.1167/17.3.6.
Engbert, R., & Kliegl, R. (2003). Microsaccades uncover the orientation of covert attention. Vision Research, 43, 1035–1045.
Engbert, R., & Kliegl, R. (2004). Microsaccades keep the eyes’ balance during fixation. Psychological Science, 15, 431–436.
Everling, S., Dorris, M. C., Klein, R. M., & Munoz, D. P. (1999). Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience, 19, 2740–2754.
Everling, S., & Fischer, B. (1998). The antisaccade: A review of basic research and clinical findings. Neuropsychologia, 36, 885–899.
Everling, S., & Munoz, D. P. (2000). Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience, 20, 387–400.
Gao, X., Yan, H., & Sun, H.-J. (2015). Modulation of microsaccade rate by task difficulty revealed through between- and within-trial comparisons. Journal of Vision, 15, 1–15. https://doi.org/10.1167/15.3.3.
Gautier, J., Bedell, H. E., Siderov, J., & Waugh, S. J. (2016). Monocular microsaccades are visual-task related. Journal of Vision, 16, 1–16.
Gilbert, S. J., Spengler, S., Simons, J. S., Steele, J. D., Lawrie, S. M., Frith, C. D., et al. (2006). Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience, 18, 932–948.
Hafed, Z. M., Chen, C. Y., & Tian, X. (2015). Vision, perception, and attention through the lens of microsaccades: Mechanisms and implications. Frontiers in Systems Neuroscience, 9, 167.
Hafed, Z. M., Goffart, L., & Krauzlis, R. J. (2009). A neural mechanism for microsaccade generation in the primate superior colliculus. Science, 323, 940–943.
Hafed, Z. M., & Ignashchenkova, A. (2013). On the dissociation between microsaccade rate and direction after peripheral cues: Microsaccadic inhibition revisited. Journal of Neuroscience, 33, 16220–16235.
Hermens, F., Zanker, J. M., & Walker, R. (2010). Microsaccades and preparatory set: A comparison between delayed and immediate, exogenous and endogenous pro-and anti-saccades. Experimental Brain Research, 201, 489–498.
Hyönä, J., Tommola, J., & Alaja, A. M. (1995). Pupil dilation as a measure of processing load in simultaneous interpretation and other language tasks. Quarterly Journal of Experimental Psychology, 48A, 598–612.
Jainta, S., Vernet, M., Yang, Q., & Kapoula, Z. (2011). The pupil reflects motor preparation for saccades—Even before the eye starts to move. Frontiers in Human Neuroscience, 5, 97.
Johnston, K., & Everling, S. (2009). Task-relevant output signals are sent from monkey dorsolateral prefrontal cortex to the superior colliculus during a visuospatial working memory task. Journal of Cognitive Neuroscience, 21, 1023–1038.
Just, M. A., Carpenter, P. A., & Miyake, A. (2003). Neuroindices of cognitive workload: Neuroimaging, pupillometric and event-related potential studies of brain work. Theoretical Issues in Ergonomics Science, 4, 56–88.
Kahneman, D., & Beatty, J. (1966). Pupil diameter and load on memory. Science, 154, 1583–1585.
Kliegl, R., Rolfs, M., Laubrock, J., & Engbert, R. (2009). Microsaccadic modulation of response times in spatial attention tasks. Psychological Research Psychologische Forschung, 73, 136–146.
Klinger, J., Tversky, B., & Hanrahan, P. (2011). Effects of visual and verbal presentation on cognitive load in vigilance, memory, and arithmetic tasks. Psychophysiology, 48, 323–332.
Ko, H.-K., Poletti, M., & Rucci, M. (2010). Microsaccades precisely relocate gaze in a high visual acuity task. Nature Neuroscience, 13, 1549–1553.
Krauzlis, R. J., Goffart, L., & Hafed, Z. M. (2017). Neuronal control of fixation and fixational eye movements. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160205.
Krejtz, K., Duchowski, A. T., Niedzielska, A., Biele, C., & Krejtz, I. (2018). Eye tracking cognitive load using pupil diameter and microsaccades with fixed gaze. PLoS One, 13, e0203629.
Lange, E. B., Zweck, F., & Sinn, P. (2017). Microsaccade-rate indicates absorption by music listening. Consciousness and Cognition, 55, 59–78.
Lisi, M., Bonato, M., & Zorzi, M. (2015). Pupil dilation reveals top-down attentional load during spatial monitoring. Biological Psychology, 112, 39–45.
Martinez-Conde, S., & Macknik, S. L. (2017). Unchanging visions: The effects and limitations of ocular stillness. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160204.
Martinez-Conde, S., Macknik, S. L., Troncoso, X. G., & Dyar, T. A. (2006). Microsaccades counteract fading during fixation. Neuron, 49, 297–305.
Martinez-Conde, S., Otero-Millan, J., & Macknik, S. L. (2013). The impact of microsaccades on vision: Towards a unified theory of saccadic function. Nature Reviews Neuroscience, 14, 83–96.
Mathôt, S., Fabius, J., Van Heusden, E., & Van der Stigchel, S. (2018). Safe and sensible preprocessing and baseline correction of pupil-size data. Behavior Research Methods, 50, 94–106.
McCamy, M. B., Macknik, S. L., & Martinez-Conde, S. (2014). Different fixational eye movements mediate the prevention and the reversal of visual fading. Journal of Physiology, 592, 4381–4394.
McCamy, M. B., Otero-Millan, J., Di Stasi, L. L., Macknik, S. L., & Martinez-Conde, S. (2014). Highly informative natural scene regions increase microsaccade production during visual scanning. Journal of Neuroscience, 34, 2956–2966.
Miyake, A., & Shah, P. (Eds.). (1999). Models of working memory: Mechanisms of active maintenance and executive control. Cambridge: Cambridge University Press.
Munoz, D. P., & Everling, S. (2004). Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience, 5, 218–228.
Nyström, M., Hooge, I., & Andersson, R. (2016). Pupil size influences the eye-tracker signal during saccades. Vision Research, 121, 95–103.
Otero-Millan, J., Macknik, S. L., Serra, A., Leigh, R. J., & Martinez-Conde, S. (2011). Triggering mechanisms in microsaccade and saccade generation: A novel proposal. Annals of the New York Academy of Sciences, 1233, 107–116.
Pastukhov, A., & Braun, J. (2010). Rare but precious: Microsaccades are highly informative about attentional allocation. Vision Research, 50, 1173–1184.
Peel, T. R., Hafed, Z. M., Dash, S., Lomber, S. G., & Corneil, B. D. (2016). A causal role for the cortical frontal eye fields in microsaccade deployment. PLoS Biology, 14, e1002531.
Pierce, J. E., McCardel, J. B., & McDowell, J. E. (2015). Trial-type probability and task-switching effects on behavioral response characteristics in a mixed saccade task. Experimental Brain Research, 233, 959–969.
Piquado, T., Isaacowitz, D., & Wingfield, A. (2010). Pupillometry as a measure of cognitive effort in younger and older adults. Psychophysiology, 47, 560–569.
Poletti, M., & Rucci, M. (2016). A compact field guide to the study of microsaccades: Challenges and functions. Vision Research, 118, 83–97.
Privitera, C. M., Carney, T., Klein, S., & Aguilar, M. (2014). Analysis of microsaccades and pupil dilation reveals a common decisional origin during visual search. Vision Research, 95, 43–50.
Richer, F., & Beatty, J. (1985). Pupillary dilations in movement preparation and execution. Psychophysiology, 22, 204–207.
Rolfs, M. (2009). Microsaccades: Small steps on a long way. Vision Research, 49, 2415–2441.
Rolfs, M., Engbert, R., & Kliegl, R. (2005). Cross- modal coupling of oculomotor control and spatial attention in vision and audition. Experimental Brain Research, 166, 427–439.
Rolfs, M., Kliegl, R., & Engbert, R. (2008). Toward a model of microsaccade generation: The case of microsaccadic inhibition. Journal of Vision, 8, 1–23.
Schaeffer, D. J., Chi, L., Krafft, C. E., Li, Q., Schwarz, N. F., & McDowell, J. E. (2015). Individual differences in working memory moderate the relationship between prosaccade latency and anti- saccade error rate. Psychophysiology, 52, 605–608.
Shen, K., Bezgin, G., Selvam, R., McIntosh, A. R., & Ryan, J. D. (2016). An anatomical interface between memory and oculomotor systems. Journal of Cognitive Neuroscience, 28, 1772–1783.
Siegenthaler, E., Costela, F. M., McCamy, M. B., Di Stasi, L. L., Otero-Millan, J., Sonderegger, A., et al. (2014). Task difficulty in mental arithmetic affects microsaccadic rates and magnitudes. European Journal of Neuroscience, 39, 287–294.
Sirois, S., & Brisson, J. (2014). Pupillometry. Interdisciplinary Reviews: Cognitive Science, 5, 679–692.
Theeuwes, J., Olivers, C. N., & Chizk, C. L. (2005). Remembering a location makes the eyes curve away. Psychological Science, 16, 196–199.
Unsworth, N., & Robison, M. K. (2018). Tracking working memory maintenance with pupillometry. Attention, Perception, & Psychophysics, 80, 461–484.
Valsecchi, M., Betta, E., & Turatto, M. (2007). Visual oddballs induce prolonged microsaccadic inhibition. Experimental Brain Research, 177, 196–208.
Valsecchi, M., & Turatto, M. (2009). Microsaccadic responses in a bimodal oddball task. Psychological Research Psychologische Forschung, 73, 23–33.
van der Wel, P., & van Steenbergen, H. (2018). Pupil dilation as an index of effort in cognitive control tasks: A review. Psychonomic Bulletin and Review(in press). https://doi.org/10.3758/s13423-018-1432-y.
Wang, C. A., Blohm, G., Huang, J., Boehnke, S. E., & Munoz, D. P. (2017). Multisensory integration in orienting behavior: Pupil size, microsaccades, and saccades. Biological Psychology, 129, 36–44.
Wang, C. A., Boehnke, S. E., White, B. J., & Munoz, D. P. (2012). Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. Journal of Neuroscience, 32, 3629–3636.
Wang, C. A., Brien, D. C., & Munoz, D. P. (2015). Pupil size reveals preparatory processes in the generation of pro-saccades and anti-saccades. European Journal of Neuroscience, 41, 1102–1110.
Wardak, C., Ramanoël, S., Guipponi, O., Boulinguez, P., & Ben Hamed, S. B. (2012). Proactive inhibitory control varies with task context. European Journal of Neuroscience, 36, 3568–3579.
Watanabe, M., Matsuo, Y., Zha, L., Munoz, D. P., & Kobayashi, Y. (2013). Fixational saccades reflect volitional action preparation. Journal of Neurophysiology, 110, 522–535.
Xue, L., Huang, D., Wang, T., Hu, Q., Chai, X., Li, L., et al. (2017). Dynamic modulation of the perceptual load on microsaccades during a selective spatial attention task. Scientific Reports, 7, 16496.
Zeligman, L., & Zivotofsky, A. Z. (2017). Back to basics: The effects of block vs. interleaved trial administration on pro-and anti-saccade performance. PLoS ONE, 12, e0172485.
Zhou, X., & Constantinidis, C. (2017). Fixation target representation in prefrontal cortex during the antisaccade task. Journal of Neurophysiology, 117, 2152–2162.
Zuber, B. L., Stark, L., & Cook, G. (1965). Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science, 150, 1459–1460.
Acknowledgements
This work was funded by the Italian Ministry of Education, University, and Research (Futuro in Ricerca 2012, Grant number RBFR12F0BD to Giovanni Galfano) and by the University of Padova (Bando Giovani Ricercatori 2015 “Assegno Senior”, Grant number GRIC15QDDH to Mario Dalmaso). The authors are grateful to Ralf Engbert for his valuable suggestions on data analysis, to Daniela Toffoletto for her help during data collection, and to two anonymous reviewers for their advice and constructive criticisms on a previous version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dalmaso, M., Castelli, L. & Galfano, G. Microsaccadic rate and pupil size dynamics in pro-/anti-saccade preparation: the impact of intermixed vs. blocked trial administration. Psychological Research 84, 1320–1332 (2020). https://doi.org/10.1007/s00426-018-01141-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-018-01141-7