Abstract
Over the course of six sessions, 24 young (M = 19.40 years, SD 1.61) and 24 older participants (M = 71.48 years, SD 3.86) performed simple, repetitive tapping tasks at 300 and 600 ms target durations concurrently with two cognitive tasks under non-switch or switch conditions. Despite substantial improvements, over sessions, reliable switch costs remained, which were pronounced in older adults. Young and older adults alike showed increased drift in the tapping tasks under dual-task conditions. Under dual-task non-switch conditions, older adults maintained the same timing accuracy (variability) as in the single-task condition. However, variability increased when concurrent cognitive task-set switching was required, while young adults even improved timing accuracy relative to the single-task condition. Being at odds with extant models of timing, our findings demonstrate that control of simple repetitive movements is far from automatic even at intervals below 1 s. Interference with timing in older adults is not caused by multi-tasking per se, but depends on the cognitive control demands of the concurrent task. We argue that our findings suggest a critical role of cognitive control processes for the maintenance of representations of target durations during interval production. This hypothesis received further support from patterns of local interference in the timing of individual intervals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex tasks like gourmet cooking involve precise sequencing and timing of individual actions when implementing the steps of an ambitious recipe. Diversions caused by alternative tasks (multi-tasking) are assumed to increase cognitive control demands and to impair performances in concurrent tasks. In contrast, familiar, simple actions like walking in a straight direction in even terrain seem to proceed rather automatically and we regularly combine them with cognitive tasks like planning the day ahead. However, recent studies revealed that even seemingly automatic tasks like maintaining an upright posture interfere with cognitive processing in older adults (for a review; Boisgontier et al., 2013). Timing processes have enjoyed considerable attention in multi-tasking research. While perceptual tasks using duration judgement paradigms pointed to interference between timing processes and concurrent cognitive tasks, timing accuracy in movement production is considered to be automatic unless it involves sequencing, complex movement trajectories, or long target durations. Related control processes have been labelled low-level timing in the literature (Krampe, Mayr, & Kliegl, 2005) and their neural underpinnings are typically located in subcortical regions. In this study, we investigate adult-age differences in multi-task settings involving low-level timing and cognitive tasks differing in their cognitive control demands by presenting them in switch vs. non-switch formats. We provide analyses of perturbations in timing during concurrent processing, which elucidate critical differences between duration judgement and movement production and their susceptibilities for interference with higher level cognitive processes.
Measures of timing performance
Many studies investigating movement timing use isochronous tapping, that is, participants are asked to repeatedly produce a single target duration. In isochronous tapping at below 1 s target durations, participants typically have little problems to match the appropriate tempo on average and timing accuracy is typically measured in terms of the variability of produced intervals. This variability increases systematically with target durations following Weber’s law (Gibbon, 1977; Krampe et al., 2005; Wing, 1980). When timing is assessed at multiple target durations, this relation is taken into account using variation coefficients, that is, standardizing variance or SD by mean produced intervals. This method has been used in single-task timing studies with children and young adults (Drake, Jones, & Baruch, 2000), healthy young and older adults (Bangert & Balota, 2012), and also in dual-task timing studies including older adults (Krampe, Doumas, Lavrysen, & Rapp, 2010). Systematic speeding up or slowing down within a trial is referred to as drift. Drift increases observed tapping variability and this effect is typically pronounced at longer target durations. Drift is, therefore, usually taken out by a linear detrending before analyzing tapping variability. In itself, drift is found to reflect individual and group differences and is, therefore, taken into account as a measure of tapping performance (Collier & Ogden, 2004). In the present study, we analyze timing accuracy in isochronous tapping in terms of drift and variation coefficients.
Models of timing
One classic approach to the timing of repetitive, discrete movements is the two-level timing model by Wing and Kristofferson (1973). This model distinguishes central-timer variability and variability due to motor delays. The central timer is conceived as a clock-like device that delineates time intervals with a variability that is duration-dependent. It sends triggers to the motor system to initiate movement production with duration-independent motor delays. The other classic model, the pacemaker–accumulator model (Buhusi & Meck, 2005), has its roots in duration judgment paradigms. According to this model, a pacemaker regularly emits pulses, which are then passed into the accumulator via an attention-controlled switch. The accumulator registers and counts the incoming pulses and stores this information as a reference duration that is used for duration judgments or reproductions (Creelman, 1962; Treisman, 1963).
Both models have been applied with some success to the study of timing. Besides their different popularity in perceptual (pacemaker–accumulator) and movement production (two-level timing) domains, the models also differ with respect to the range of target durations attributed to their component processes. While the Wing–Kristofferson model has been mostly applied as a model of variability in repetitive tapping tasks with target durations in the 200–2000 ms range, pacemaker–accumulator models have been for the most part used to account for the accuracy of single reproductions or duration judgments in the second, minute, or even day range (for a review, see Buhusi & Meck, 2005). The two-level timing model does not foresee any role for higher level cognitive processes in repetitive timing. In contrast, the pacemaker–accumulator model emphasizes the role of attention during the accumulation of pulses.
In their hierarchical model of timing control, Krampe et al. (2005) emphasized the differences between tasks requiring the sequencing of multiple target durations (rhythm production) and simple, isochronous tapping tasks with basically no sequencing demands. According to their model, rhythm production rests on the selection, maintenance, and updating of task sets (rhythm programs; Vorberg & Wing, 1996), processes involving considerable cognitive control and working memory demands. In contrast, isochronous tapping tasks can be controlled by the low-level timing mechanism, which executes the target intervals provided by higher level processes without requiring cognitive control by itself. One implication of this model is that timing tasks with minimal requirements on sequencing or target interval specification processes should be performed in an automatic fashion with a little interference from or to higher level cognitive processes. This assumption is in line with empirical evidence coming from studies investigating the neural underpinnings of duration judgement and movement production. In duration judgment tasks, brain imaging studies point to subcortical brain activation in putamen and basal ganglia (Buhusi & Meck, 2005). Target duration has a critical role in low-level timing of movement production tasks, as was highlighted in a study by Lewis and Miall (2003). Based on fMRI evidence, they concluded that only production of intervals with a duration above 1 s required cognitive processes like internal counting, whereas timing of sub-second intervals rested on automatic processes supported by subcortical brain regions such as the basal ganglia and putamen (Johannsen et al., 2013; Wiener, Turkeltaub, & Coslett, 2010) and the cerebellum (Ivry & Keele, 1989).
Low-level timing, adult-aging, and multi-tasking
Studies investigating adult-age-related changes in isochronous tapping typically found little or no differences between healthy older to young adults (Duchek, Balota, & Ferraro, 1994; Greene & Williams, 1993; Krampe et al., 2005; Salthouse, Wright, & Ellis, 1979). Considering the well-documented age-related changes in processes involving frontal lobe mechanisms (West, 1996), like cognitive control and working memory (Fisk & Sharp, 2004), these findings suggest that low-level timing is not involving related mechanisms to a substantial degree. In duration judgment tasks, older adults have been found to show larger errors than young adults (Anderson, Rueda, & Schmitter-Edgecombe, 2014; Pütz, Ulbrich, Churan, Fink, & Wittmann, 2012). At the same time, the role of higher level cognition in estimation tasks appeared to be the same for young and older adults, since multi-tasking studies showed young and older adults to be equally affected by cognitive load during duration judgments (Block, Hancock, & Zakay, 2010).
The pacemaker–accumulator model has inspired numerous duration judgement studies using dual-task manipulations, because it provides a straightforward account for this situation. Presumably, under multi-task conditions, pacemaker pulses are missed, because attention is drawn away by the concurrent task, such that it takes longer for the accumulator to reach the number of pulses initially stored as a reference duration. As a result, intervals reproduced under concurrent task load will be lengthened compared with target durations learned under single-task conditions. This prediction was largely confirmed in several studies (Brown, 2006; Brown, Collier, & Night, 2013; Droit-Volet, Wearden, & Zelanti, 2015; Fortin & Masse, 2000), but some studies also failed to find effects of multi-tasking in the first place (Fortin & Breton, 1995; Ogden, Salominaite, Jones, Fisk, & Montgomery, 2011), or showed a shortening of intervals when concurrent cognitive load was increased (Maes, Wanderley, & Palmer, 2015).
The only study to our knowledge, which has investigated adult-age differences in multi-tasking involving low-level timing in repetitive movement production tasks, was conducted by Krampe et al. (2010). They had young and older adults who perform isochronous tapping of sub- and suprasecond intervals (550 ms, 2100 ms) along with an N-Back working memory task. Under dual-task conditions, young and even more so older adults showed reliably more drift and this was also true for the sub-second (550 ms) condition. Importantly, older but not young adults showed pronounced increases in variability in the slow (2100 ms) target duration condition. Both age groups produced shorter intervals under dual-task compared with single-task conditions in the 2100 ms condition, while only older adults shortened their intervals in the 550 ms condition. This systematic shortening of intervals under dual-task conditions is at odds with the dual-task predictions generated from the pacemaker–accumulator model and pointed towards differences between interval production and duration judgment. Given that the majority of dual-task and age effects were found for the slow tapping tempo (2100 ms), the authors concluded that slow movement timing involved specific contributions of high-level cognitive control processes, which disadvantaged older adults. A potential limitation of this study was that the cognitive task required participants to give vocal responses at relatively constant time intervals (the interstimulus intervals of the N-Back task), while they were tapping. As the authors discuss, this setting lends itself to entrainment of taps and verbal responses, which might have contributed to dual-task interference over and above shared central cognitive processes. In the present study, we use a secondary task that does not require continuous responding during the dual-task phase to assess underlying component processes while avoiding structural limitations in perceptual or output channels. Furthermore, we focus on sub-second target intervals to target the performance range typical of low-level timing.
Outline of the study
Although timing processes have enjoyed considerable attention in multi-tasking research, the evidence concerning multi-tasking and low-level timing is inconclusive. There is clear evidence for dual-task interference between duration judgement tasks and concurrent cognitive tasks, with most studies supporting the interval lengthening predicted by the pacemaker–accumulator model and its attentional gating mechanism. Movement production in multi-task contexts has been studied less frequently and is considered automatic unless tasks involve sequencing, complex movement trajectories, or long (>1 s) target durations. Low-level timing at sub-second intervals appears to defy negative effects of healthy adult-aging and age-differential decrements in timing accuracy due to multi-tasking emerge but at longer target durations. A general limitation of multi-tasking studies on timing is that they use a variety of concurrent cognitive tasks, each of which involves multiple component processes. This situation has made it difficult to identify specific processes underlying multi-task interference in young and older adults’ timing processes.
In this study, we focus on adult-age differences in multi-tasking and we challenge the view that low-level timing is automatic. To this end, we had older and young adults who perform isochronous tapping tasks at below 1 s target durations (300 and 600 ms) under single- and dual-task conditions. For the dual-task condition, we systematically varied the cognitive control demands exerted by the concurrent cognitive task by presenting the same two tasks in non-switch and switch contexts. Our main prediction was that dual-task interference would be pronounced in older compared with young adults and that this effect would be most evident when the concurrent cognitive task required maximal cognitive control (the switch condition). Our second goal was to determine the time-course and locus of dual-task interference during tapping. This approach aimed at the differences between perceptual (duration judgement) and production aspects of movement timing and the differential sensitivities to multi-tasking demands reported in the literature. By analyzing the lengthening/shortening of produced intervals immediately following the presentation of stimuli from the concurrent cognitive task, we evaluated the predictions of the pacemaker–accumulator model and its applicability to repetitive movement production.
Method
Participants
24 young (M = 19.40 years, SD 1.61) and 24 older adults (M = 70.48 years, SD 3.86) recruited from Potsdam schools or drawn from the subject-pool of the Potsdam Center for Cognitive Psychology participated in the experiments. There were 13 women and 11 men in the group of young adults and 12 women and 12 men in the elderly sample. With the exception of one elderly individual, all reported to be at least of average health and they had no known history of neurological diseases or dementia. None of the participants had a background of playing a musical instrument. Older adults (M = 32.79, SD 1.64) performed better at a test of word knowledge (MWT-A, Lehrl, 1977) than young adults (M = 31.63, SD 2.32), t(46) = 2.012, p = 0.050. Higher scores were observed in young (M = 62.25, SD 7.69) compared with older adults (M = 47.33, SD 7.60) for digit symbol substitution (WAIS; Wechsler, 1981), t(46) = 6.75, p < 0.001. Values and age differences are typical for studies comparing young and older adults (Verhaeghen & Salthouse, 1997). Participants received approximately US$7 per session. Informed consent was obtained from all individual participants included in the study.
Apparatus
Participants, seated on height-adjustable chairs, produced interval sequences on electronic drum equipment using standard drumsticks held with their preferred hand. Taps caused deformations of piezo-ceramic sensors in the drum pads (KAT Inc.). An ALESIS DM5 drum-synthesizer with a 48 MHz processor sampled these signals and triggered drum sounds. Signals were transmitted by an MIDI TimePiece II (Mark of the Unicorn) digitizer to the serial port of a Macintosh PowerPC (7100/66) which time-stamped events to the nearest millisecond. Tapping feedback and the cognitive task were presented on a 17″ screen. Drum sounds and pacing signals (500 Hz sine wave, 30 ms duration) were presented through external speakers.
Movement timing tasks
We used the continuation paradigm introduced by Wing and Kristofferson (1973). Participants listened to a pacing beat as long as they wanted, synchronized for another three taps after which the signal was discontinued, and they then continued to produce taps without external pacing for 20 s. We assessed low-level timing for two target durations, 300 and 600 ms. The end of the trial was marked by a computer sound signal. Graphical feedback was given after each trial with columns indicating the length of each produced interval and a reference line denoting the target duration. Outlier intervals exceeding ±50% of the target duration were marked. Mean produced interval durations and standard deviations were also given for each trial. Instructions encouraged accuracy of timing as well as calculation performance on the cognitive tasks. A trial was terminated if participants did not produce a tap for more than 2.5 s.
Cognitive tasks
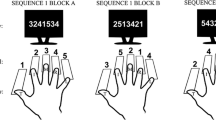
In each trial, participants watched nine successive displays of digits appearing in one of four identical quadrants forming a square in the center of a 17 in. computer monitor (Fig. 1). Digits were in white on a dark background. The quadrant where the first digit appeared varied randomly across trials and consecutive digits appeared in adjacent quadrants in clockwise order. For each trial, the computer determined beforehand nine random positions for stimulus display based on the expected number of taps during 20 s continuation phase (e.g., 9 positions selected from 33 taps in the 600 ms condition). The actual stimulus onsets were then triggered by participants’ taps with presentation starting half a target duration after the pre-specified tap and remaining on the screen for 300 ms. A constraint was that the minimum number of taps between stimuli was four for the 300 ms and two for the 600 ms condition. This method enabled us to analyze timing perturbations in individual intervals as a function of their position relative to the presentation of cognitive task stimuli.
Graphic representation of the cognitive control task. Participants watched nine successive displays of digits appearing in adjacent quadrants in clockwise order. In the add-number condition, participants added up the numbers of digits in the displays. In the add-value condition, participants added up the values of the digits, while in the switch condition, participants switched between the add-value and add-number task by every crossing of the horizontal separation of quadrants
Six different stimulus sets were used: ‘1 1’, ‘1 1 1’, ‘2’, ‘2 2 2’, ‘3’, and ‘3 3’. In the add-number condition, participants focused on the number of digits in a display regardless of the digits’ values (i.e., ‘1 1 1’ = ‘2 2 2’ = 3) and added these numbers up across the nine displays. In the add-value condition, participants added up the values of the displayed digits ignoring their numbers in a given display. Participants performed the two tasks in pairwise alternation in the switch condition applying the add-value procedure to stimuli appearing in the two upper quadrants and the add-number rule to those in the two lower quadrants. Thus, crossing the horizontal separation of quadrants during clockwise stimulus presentation amounted to implementing a task-set reconfiguration, followed by two performances of a certain task followed by another switch, and so on.
To assess cognitive task performance under single-task conditions, we used a reaction-time format. Tasks and their presentation were as described above. In this version, participants pressed the spacebar as soon as they had processed a stimulus, which was then replaced by the next one in the following location. In addition to RT versions of add-number, add-value, and switch tasks, we also assessed simple reaction time. To this end, participants reacted as fast as possible by pressing the spacebar to any stimulus in the sequence appearing at random response-stimulus intervals (range 500–2000 ms). Instructions emphasized speed and accuracy. Immediate feedback was provided about participants’ performance and reaction time.
Procedure
The entire study comprised six sessions with a full run through all conditions for both target durations (300 and 600 ms) taking two sessions. We refer to the first two sessions as Phase 1, the third and fourth sessions as Phase 2, and the last two sessions as Phase 3. Each session took place on a separate day and took 45–60 min. In each session, participants performed five blocks of trials in four conditions: one block of single-task tapping (participants just watched stimuli being displayed while tapping), tapping with add-values (hereafter referred to as add-values), and tapping with add-numbers (hereafter referred to as add-numbers), respectively, and two blocks of tapping with task-set switching. Instructions in movement timing tasks emphasized maintaining a steady tempo while tapping as regularly as possible. For concurrent task conditions, we emphasized accuracy of the cognitive task while maintaining optimal timing performance (equal emphases instruction). To gradually familiarize participants with task challenges, they performed tasks in the order single-tapping, add-numbers, add-values, and switch in Phase 1 with three warm-up trials in each condition. To maximize training gains, we used a progressive testing approach: the number of trials depended on participants’ performance. Trials terminated prematurely, those with mean produced interval durations exceeding ±15% of the target duration, or trials with incorrect results in the cognitive task were immediately repeated up to the minimum number of correct trials for all participants. Participants performed a minimum of six trials per block with a maximum of 30. In Phases 2 and 3, the minimum number of trials was ten with a maximum of 20, and the order of tasks and target tempos was counterbalanced across sessions and participants.
The single-task reaction-time version of the cognitive tasks was administered in Phase 3 (Sessions 5 + 6) of the experiment. In each of the two sessions, participants performed ten blocks of trials including two trials each for simple reaction time, adding numbers, and adding values and four trials of the switch task. Order of tasks was as listed in blocks 1–5 and counterbalanced across participants in blocks 6–10. Again, we applied a progressive testing approach repeating trials to a minimum of four correct trials in blocks 1–5 and eight correct trials in blocks 6–10. Testing was limited to a maximum of ten trials in blocks 1–5 and 20 trials in blocks 6–10.
Results
Pre-processing (errors and detrending) of tapping time-series was done with MATLAB (TheMathWorks, 2007); all other statistical analyses were performed with IBM SPSS statistics 23 for Windows. For analyses of cognitive task performance, we used the minimum number of trials specified for the progressive testing approach, that is, the first six per condition for Phase 1 and the first ten for Phases 2 and 3. We averaged performances in add-number and add-value tasks, and refer to it as non-switch condition in the following. For the analysis of dual-task timing accuracy we only used the trials in which cognitive task performance was correct.Footnote 1 This way, it was guaranteed that participants had directed attention to the cognitive task throughout the trial.
Participants prematurely terminated tapping on 1.20% (SD 2.16) of the trials and these were discarded. To avoid outliers, only trials with mean produced intervals within ±15% of the target duration were included, affecting a mean percentage of 0.12 (SD 0.32) of the trials. Single intervals with durations exceeding ±50% of the produced mean were not used for the calculation of mean and variability. Such instances occurred on 1.27% (SD 1.48) of the trials. These exclusion criteria match the criteria employed in the previous studies (Krampe et al., 2005, 2010).
Cognitive task performance
A mixed-design ANOVA on percentage correct solutions for the concurrent cognitive tasks using target duration (300 vs 600 ms), Phase (1, 2, 3), and task (non-switch, switch) as within- and age group as between-subjects factors showed no effect of target duration on cognitive task performance. We performed pre-planned comparisons for the training effects by comparing Phase 1 to the mean of Phases 2 and 3 [phase contrast (1)], and by comparing Phase 2 to Phase 3 [phase contrast (2)]. Both contrasts related to training were reliable [phase contrast (1) F(1, 46) = 68.32, p < 0.001, ŋ 2 = 0.598; phase contrast (2) F = 37.74, p < 0.001, ŋ 2 = 0.451, M Phase1 = 79.46, SDPhase1 = 8.23, MPhase2 = 85.44, SDPhase2 = 9.12, MPhase3 = 90.08, SDPhase3 = 7.95].
Improvements between Phases 2 and 3 were reliable for the non-switch task in both target duration conditions (300 ms ∆M = 3.33, SD 8.46; 600 ms ∆M = 4.90, SD 9.02), and for the switch task in the 300 ms condition (∆M = 7.40, SD 12.07), but only marginally reliable for the switch task in the 600 ms condition (∆M = 2.91, SD 10.91), F = 4.16, p = 0.047, ŋ 2 = 0.083. However, this effect was similar across age groups. Overall, performance was lower in the switch compared with the non-switch condition, F = 107.70, p < 0.001, ŋ 2 = 0.701 (M switch = 83.77, SDswitch = 10.74, Mnon-switch = 91.74, SDnon-switch = 6.55), and this difference was larger in older adults (∆M = 13.14, SD 6.93) than in young adults (∆M = 4.85, SD 4.90), F = 22.90, p < 0.001, ŋ 2 = 0.332 (Fig. 2). The main effect of age group, F = 21.82, p < 0.001, ŋ 2 = 0.322, generalized to the non-switch condition, where differences between older and young adults (∆M = 4.26, SE 1.74) already reached significance [t(46) = 2.45, p = 0.018]. In general, age effects tended to be larger for 300 ms (∆M = 10.48, SE 2.09) compared with 600 ms (∆M = 6.33, SE 1.89) target durations, F = 5.79, p < 0.05, ŋ 2 = 0.112, while the pattern of age-differential switch costs held for both target durations.
Timing performance
For the analysis of timing performance, we conducted mixed-design ANOVAs with tapping tempo (target intervals 300 vs 600 ms), Phase (1, 2, 3), and task (single-task, non-switch, and switch) as within- and age group as between-subjects factors. For the phase factor, we included pre-planned contrasts by comparing Phase 1 with the mean of Phase 2 and 3 [phase contrast (1)], and by comparing Phase 2 with Phase 3 [phase contrast (2)]. For the task factor, we included pre-planned comparisons between single- and dual-task tapping performance [task contrast (1)], and between non-switch and switch secondary tasks [task contrast (2)].
Before approaching effects on realized intervals and their variability, we determined whether multi-tasking and the cognitive control demands of the concurrent cognitive task impaired participants’ abilities to maintain a steady tempo. To this end, we analyzed drift for each continuation tapping time-series and calculated as the slope of the linear regression of produced duration on interval position in the series. As a first step, we analyzed the absolute drift, that is tempo changes irrespective of sign (speeding up or slowing down within trials). The mixed-factor ANOVA produced a main effect of target duration, F(1, 46) = 229.92, p < 0.001, ŋ 2 = 0.833, due to the fact that absolute drift was stronger for the 600 ms target duration (M = 0.35, SD 0.10) compared with the 300 ms target duration (M = 0.13, SD 0.05).Footnote 2
Analysis of signed drift allowed assessing whether experimental manipulations or differences between age groups induced systematic speed-ups or slowing down within trials. The mixed-factor ANOVA revealed main effects of target duration, F = 6.23, p = 0.016, ŋ 2 = 0.119, a main effect of the task (1) contrast (single vs dual), F = 31.78, p < 0.001, ŋ 2 = 0.409, and a reliable interaction of the two factors, F = 73.04, p < 0.001, ŋ 2 = 0.614. This pattern reflected that in the 300 ms target duration condition, participants showed slowing down within trials in the dual- (M = 0.02 ms/tap, SD 0.11) and speeding up in the single-task conditions (M = −0.02, SD 0.11), t(47) = 3.79, p < 0.001. In contrast, in the 600 ms condition, they changed from slowing down during single-task tapping (M = 0.08, SD 0.26) to speeding up under dual-task conditions (M = −0.15, SD 0.26). This pattern was similar in young and older adults. In addition, we found a reliable change in drift between the second (M = −0.04, SD 0.16) and the last phases (M = −0.004, SD 0.16), F = 5.72, p = 0.021, ŋ 2 = 0.111, which amounted to a larger reduction in older (∆M = −0.07, SD 0.11) compared with young adults (∆M = −0.001, SD 0.11) F(1, 46) = 5.32, p = 0.026, ŋ 2 = 0.104.
Variability and cognitive control demands
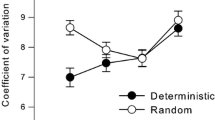
To consider the observed effects on drift, we applied linear detrending to our data and calculated the SD of the residuals (detSD). The initial analyses using detSD showed effects of target duration F(1, 46) = 585.50, p < 0.001, ŋ 2 = 0.927, which reflected the well-documented increase of variability with target duration. This relation has been found to be strictly linear for intervals below 1 s, thereby following Weber’s law (Gibbon, 1977; Krampe et al., 2005; Wing, 1980). We took advantage of this relation by calculating variation coefficients (detSD/M), which effectively wiped all effects related to target duration. Figure 3 shows variation coefficients as a function of cognitive control demands separately for young and older adults.
We conducted a mixed-factor ANOVA on variation coefficients with age group as between and target duration, phase, and task as within-subject factors, with pre-planned comparisons for single versus dual [task contrast (1)], switch versus non-switch [task contrast (2)], Phase 1 versus mean Phase 2 and 3 [phase contrast (1)], and Phase 2 versus Phase 3 [phase contrast (2)]. Both phase contrasts were reliable [phase contrast (1) F(1, 46) = 126.75, p < 0.001, ŋ 2 = 0.734; phase contrast (2) F = 26.58, p < 0.001, ŋ 2 = 0.366], indicating that participants continued to improve across phases. Improvement between Phases 2 and 3 was larger for 600 ms (∆M = −0.20, SD 0.22) compared with the 300 ms (∆M = −0.05, SD 0.28) condition, F = 8.31, p < 0.01, ŋ 2 = 0.153, and this effect was similar for young and older adults. We also found a reliable task contrast (1) by target–duration interaction, F = 4.44, p = 0.041, ŋ 2 = 0.088, reflecting stronger dual-task decrements in the 600 ms (∆M = 0.07, SD 0.30) compared with the 300 ms (∆M = −0.08, SD 0.42) target-duration condition. Again, this effect was similar for the age groups. The key findings relate to age differences in the effects of multi-tasking and task switching, which were independent of tempo and training gains. Figure 3 illustrates that the increase in variability due to multi-tasking demands was higher in older (∆M = 0.07, SD 0.32) compared with young adults (∆M = −0.08, SD 0.17), F(1, 46) = 4.17, p < 0.05, ŋ 2 = 0.083. Likewise, the additional costs due to concurrent performance of a cognitive switch compared with non-switch tasks were higher in older (∆M = 0.13, SD 0.24) compared with young (∆M = −0.08, SD 0.14) adults, F = 13.72, p < 0.001, ŋ 2 = 0.228.
To investigate the dual-task costs for the age groups relative to the single-task performance, we calculated proportional dual-task costs, as performances in (dual minus single)/single × 100, expressed in % costs in dual-task conditions. A mixed-design ANOVA with age group as between and target duration, phase, and task (non-switch, switch) as within-subject factors, with pre-planned comparisons for Phase 1 versus mean Phase 2 and 3 [phase contrast (1)], and Phase 2 versus Phase 3 [phase contrast (2)], showed reliable training effects for the phase (1) contrast, F(1, 46) = 9.84, p < 0.005, ŋ 2 = 0.176, and the phase (2) contrast, F(1, 46) = 4.52, p = 0.039, ŋ 2 = 0.090, and those training effects were similar for the age groups. In general, the proportional dual-task costs were higher in the 600 ms (M = 3.76, SD 9.45) than in the 300 ms condition (M = −0.55, SD 12.54), F(1, 46) = 4.28, p = 0.044, ŋ 2 = 0.085. The proportional dual-task costs for the non-switch tasks were not reliable different from zero. In the switch task, young (M = −2.32, SD 6.72) and older adults (M = 6.33, SD 12.33) showed dual-task costs in different directions F(1, 46) = 10.59, p = 0.002, ŋ 2 = 0.187 (Fig. 4). This finding indicates that young adults’ timing performance improved in switch dual-task conditions, while older adults’ timing performance deteriorated.
Cognitive task performance under single-task conditions: reaction-time version
The goal of administering reaction-time (RT) versions of the cognitive tasks was to provide benchmarks for the processing time demands of the cognitive tasks and related age differences. A mixed-design ANOVA on percentage correct (cognitive task performance) with age group as between-subjects factor and task (non-switch, switch) as within-subjects factors showed that young adults performed better (M = 90.10, SD 8.52) than older adults (M = 83.53, SD 7.83), F(1, 46) = 7.75, p = 0.008, ŋ 2 = 0.144. The comparison of non-switch versus switch tasks showed that performance on non-switch tasks was higher (M = 89.71, SD 8.50) than on switch tasks (M = 83.92, SD 10.59), F = 25.93, p < 0.001, ŋ 2 = 0.360, and this effect was similar for young and older adults.
We performed a mixed-design ANOVA on reaction times in correct trials (Fig. 5) with age group as between and task (simple RT, non-switch, switch) as within factor and pre-planned comparisons of simple reaction time versus cognitive tasks [task contrast (1)], and switch versus non-switch tasks [task contrast (2)]. Simple RT task performance was similar for young and older adults, t(46) = 0.493, p > 0.62. A main effect of the task (1) contrast showed longer RTs for the cognitive tasks compared with single RTs, F(1, 46) = 396.01, p < 0.001, ŋ 2 = 0.896. This effect was present in both age groups, but pronounced in older adults (∆M = 811 ms, SD 282) compared with young adults (∆M = 478 ms, SD 144), F = 26.44, p < 0.001, ŋ 2 = 0.365. A main effect of the task (2) contrast showed that RTs were shorter in the non-switch in comparison with the switch task, F = 326.57, p < 0.001, ŋ 2 = 0.877. Although switch costs were higher in older compared with young adults, the interaction failed significance by a slight margin, F = 3.78, p = 0.058, ŋ 2 = 0.076.
Local effects of concurrent cognitive task demands on timing
This precise timing of visual task stimuli presentation provided an opportunity to investigate local interference with the timing of individual intervals. We analyzed the residuals of the produced intervals after linear detrending to consider drift and overall differences in performance tempos. Figure 6 shows deviations from overall tempo as a function of position relative to the concurrent cognitive task stimulus. The x-axis represents the interval lag, with 0 referring to the interval during which the stimulus was presented. Lags 1–4 refer to the intervals directly succeeding this interval. Based on our analyses of the response times in the self-paced task, we estimated a minimum processing time for young adults of 673 ms (older 963 ms) for concurrent non-switch and 930 ms (older 1288 ms) for concurrent switch tasks. Even if we subtracted the time to produce a motor response into account (bounded by the simple RT task), we could expect at least intervals pos0, lag1, and lag2 (older pos0–lag3) to be affected by concurrent non-switch processing in young adults for the 300 ms target duration and an additional interval in the switch condition. In slower tapping (600 ms target duration), effects should be mostly visible for the pos0 and lag1 intervals during young adults’ concurrent tapping and at higher lags for the switch condition and in older adults. These considerations were based on the assumption, of course, that participants do not adapt to multi-task constraints by postponing concurrent processing.
Deviations from overall tempo (residuals) as a function of position relative to the concurrent cognitive task stimulus separately for age groups and target durations, averaged across phases. Position 0 refers to the interval during which the stimulus was presented and lags 1–4 represent the succeeding intervals
We performed a series of mixed-design ANOVAs separately for the two target durations with age group as between, and task (3) and position as within-subject factors. For the task factor, we used the usual pre-planned comparisons for single versus dual [task contrast (1)] and non-switch versus switch [task contrast (2)]. In separate analyses, we contrasted position 0 (the presentation of the visual stimulus) with positions lag1, lag2, lag3, and lag4, respectively. Naturally, interactions between the task contrasts and position effects were of prime interest, because they signaled local multi-tasking effects. To protect against spurious interactions in multiple comparisons, we adopted an alpha level of p < 0.01 for all analyses.
Target duration 300 ms
As a first step, we compared the residuals in the single-task tapping condition with zero, testing our assumption that this condition provided a valid baseline. This expectation was met, t’s < 1.145, p’s > 0.258. Mixed-factor ANOVAs of the 300 ms condition revealed reliable effects for the comparisons of pos0 with lag1 and lag3 intervals, respectively. For the pos0–lag1 comparison, we obtained a main effect of task contrast (1), F(1, 46) = 11.69, p = 0.001, ŋ 2 = 0.203, a main effect of position, F(1, 46) = 21.04, p < 0.001, ŋ 2 = 0.314, and a task contrast (1) by position interaction, F(1, 46) = 10.32, p = 0.002, ŋ 2 = 0.183. Post hoc t tests showed no differences between single- and dual-task conditions for the pos0 intervals; however, participants reliably shortened the lag1 interval in dual (∆M = −0.72, SD 0.90) t(47) = 5.54, p < 0.001, but not in single-task conditions (∆M = 0.12, SD 1.18). For the pos0–lag3 comparison, we obtained a main effect of task contrast (2), F(1, 46) = 16.33, p < 0.001, ŋ 2 = 0.262, and a task contrast (2) by position interaction, F(1, 46) = 9.81, p = 0.003, ŋ 2 = 0.176. Post hoc t tests showed no differences between non-switch- and switch-task conditions for the pos0 intervals; however, participants reliably shortened the lag1 interval in non-switch (∆M = −0.91, SD 2.00) t(47) = 3.15, p = 0.003, but not in switch conditions (∆M = −0.14, SD 2.13).
Target duration 600 ms
We performed the same analyses as in the 300 ms condition. The comparisons of the residuals in the single-task tapping condition with zero indicated that the single-task tapping provided a valid baseline, because t’s < 1.463, p’s > 0.150. Mixed-factor ANOVAs of the 600 ms condition revealed reliable effects for the comparisons of pos0 with lag1 and lag3 intervals, respectively. For the pos0–lag1 comparison, we obtained main effects of task contrast (1), F(1, 46) = 11.42, p = 0.001, ŋ 2 = 0.199, task contrast (2), F(1, 46) = 26.45, p < 0.001, ŋ 2 = 0.365, a main effect of position, F(1, 46) = 17.17, p < 0.001, ŋ 2 = 0.272, a task contrast (1) by position interaction, F(1, 46) = 10.25, p = 0.002, ŋ 2 = 0.182, and a task contrast (2) by position interaction F(1, 46) = 9.29, p = 0.004, ŋ 2 = 0.168. Post hoc t tests showed no differences between single- and dual-task conditions for the pos0 intervals; however, participants reliably shortened the lag1 interval in dual (∆M = −2.98, SD 5.38) t(47) = 3.830, p < 0.001, but not in single-task conditions (∆M = 0.44, SD 2.64). The non-switch and switch conditions did not differ for the pos0 intervals; however, participants reliably shortened the lag1 interval in the switch condition (∆M = −2.34, SD 5.48) t(47) = 2.962, p = 0.005, and this effect was even more pronounced in the non-switch condition (∆M = −3.61, SD 5.65) t(47) = 4.423, p < 0.001. Regarding age effects, we found a main effect of age group F(1, 46) = 8.72, p = 0.005, ŋ 2 = 0.159, and an age group by position interaction F(1, 46) = 9.58, p = 0.003, ŋ 2 = 0.172. Post hoc t tests showed no difference between pos0 and lag1 for young adults (∆M = −0.54, SD 2.42) and a reliable difference for older adults (∆M = −3.72, SD 4.41) t(23) = 4.128, p < 0.001. For the pos0–lag3 comparison, we obtained a main effect of position F(1, 46) = 10.20, p = 0.003, ŋ 2 = 0.181, which reflected a general difference between residuals at position 0 (M = 0.41, SD 0.90) and residuals at lag 3 (M = −0.35, SD 1.48).
Discussion
Our goals were to investigate adult-age differences in multi-tasking and to test extant theories about low-level timing. Under single-task conditions, low-level timing in older adults in our study was as accurate as in young adults replicating findings from earlier studies (Duchek et al., 1994; Krampe et al., 2005; Salthouse et al., 1979). Under dual-task conditions, both age groups showed similar increases in drift, which were modest in size, however. Our key findings relate to increases in timing variability in the older adult group when timing had to be performed concurrently with a cognitive switch task. Our findings that young adults and (in the non-switch condition) even older adults were able to maintain timing accuracies demonstrate that the critical effects are not the result of multi-tasking as such, but they are driven by cognitive control demands.
Our results also challenge extant timing models in that they demonstrate that low-level timing is far from automatic even if target durations come from the sub-second range. In that respect, they clearly go beyond earlier findings, which were based on a single sub-second interval duration and open to alternative accounts (Krampe et al., 2010). The design of the present study allowed us to track the time-course of dual-task interference, because stimuli were precisely timed and required no direct or continuous responses. We observed a shortening of isochronous tapping intervals directly after stimulus presentation, which is clearly at odds with the predictions derived from the pacemaker–accumulator model (Buhusi & Meck, 2005). In our view, the observed patterns of interval shortening followed by lengthening at longer lags point to multi-task interferences at the level of interval monitoring and error correction. However, our analysis of local multi-tasking effects remains both tentative and exploratory, and future research is required to unravel the underlying mechanisms. Nonetheless, an implication of our findings is that the pacemaker–accumulator models based on duration judgment paradigms cannot directly be applied to timing in movement production.
In conclusion, we argue that adult-age differences in cognitive control (task-coordination) provide the best account for our findings. This conclusion is supported by the fact that young adults actually managed to reduce their timing dual-task costs in the switch relative to the non-switch condition. Older adults may not have had this option in the first place, given that the concurrent cognitive tasks taxed their cognitive control to a larger degree. In the cognitive task in single-task settings, they already showed more costs of task switching in RTs than young adults, replicating earlier findings (Mayr, 2001). An additional explanation to be considered is that low-level timing demands more cognitive control in older compared with young adults to begin with. This is a possible scenario, although both groups performed at comparable levels under single-task conditions. In our view, these two accounts are not mutually exclusive. Future studies should attempt to disambiguate these accounts by individually adjusting concurrent task demands, by applying differential emphasis manipulations, or by trying to assess the cognitive effort involved in young and older adults’ low-level timing through neuroimaging techniques.
Notes
For analysis of timing performance, we only used the trials (of the first ten that were used for the cognitive task) that had correct cognitive task performance. This is a very conservative method in the sense that it does filter out all trials with performance decrements in the cognitive task that may have been caused by the dual-tasking. Cognitive task performance got better over the phases and was higher in young than in older adults, so we filtered out relatively more trials in the first phase and in the older adults and those trials were most likely to show decrements in timing as well. Repeating the same analysis with the incorrect cognitive task performance trials included as well yielded very similar results to our initial analysis. The only difference in results was that in this new analysis two additional significant interactions showed up in the analysis of the detrended SD coefficient, namely task contrast (1) by tempo and age group by phase contrast (2). This finding supports our prediction that we used a rather conservative method for the analysis of our timing data.
The only other significant effect in this analysis was the interaction between the task (1) contrast (single vs dual), target duration, and the phase (2) contrast (changes between the second and the last testing phase), F(1, 46) = 10.91, p = 0.002, ŋ 2 = 0.192. In the 600 ms condition, reduction in drift with practice was larger in the dual (∆M = −0.05, SD 0.14, t(47) = 2.293, p = 0.026) compared with single-task conditions (∆M = 0.06, SD 0.03, t(47) = 2.088, p = 0.042), whereas no practice effects were found for drift in the 300 ms condition.
References
Anderson, J. W., Rueda, A., & Schmitter-Edgecombe, M. (2014). The stability of time estimation in older adults. The International Journal of Aging and Human Development, 78(3), 259–276. doi:10.2190/AG.78.3.c.
Bangert, A. S., & Balota, D. A. (2012). Keep up the pace: Declines in simple repetitive timing differentiate healthy aging from the earliest stages of Alzheimer’s disease. Journal of the International Neuropsychological Society, 18(6), 1052–1063. doi:10.1017/S1355617712000860.
Block, R. A., Hancock, P. A., & Zakay, D. (2010). How cognitive load affects duration judgments: A meta-analytic review. Acta Psychologica, 134(3), 330–343. doi:10.1016/j.actpsy.2010.03.006.
Boisgontier, M. P., Beets, I. A., Duysens, J., Nieuwboer, A., Krampe, R. T., & Swinnen, S. P. (2013). Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neuroscience and Biobehavioral Reviews, 37(8), 1824–1837. doi:10.1016/j.neubiorev.2013.07.014.
Brown, S. W. (2006). Timing and executive function: bidirectional interference between concurrent temporal production and randomization tasks. Memory & Cognition, 34(7), 1464–1471.
Brown, S. W., Collier, S. A., & Night, J. C. (2013). Timing and executive resources: Dual-task interference patterns between temporal production and shifting, updating, and inhibition tasks. Journal of Experimental Psychology: Human Perception and Performance, 39(4), 947–963. doi:10.1037/a0030484.
Buhusi, C. V., & Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience, 6(10), 755–765. doi:10.1038/nrn1764.
Collier, G. L., & Ogden, R. T. (2004). Adding drift to the decomposition of simple isochronous tapping: an extension of the Wing–Kristofferson model. Journal of Experimental Psychology: Human Perception and Performance, 30(5), 853–872. doi:10.1037/0096-1523.30.5.853.
Creelman, C. D. (1962). Human discrimination of auditory duration. Journal of the Acoustical Society of America, 34(5), 582–593.
Drake, C., Jones, M. R., & Baruch, C. (2000). The development of rhythmic attending in auditory sequences: Attunement, referent period, focal attending. Cognition, 77(3), 251–288. doi:10.1016/S0010-0277(00)00106-2.
Droit-Volet, S., Wearden, J. H., & Zelanti, P. S. (2015). Cognitive abilities required in time judgment depending on the temporal tasks used: A comparison of children. Quarterly Journal of Experimental Psychology, 68(11), 2216–2242. doi:10.1080/17470218.2015.1012087.
Duchek, J. M., Balota, D. A., & Ferraro, F. R. (1994). Component analysis of a rhythmic finger tapping task in individuals with senile dementia of the Alzheimer’s type and in individuals with Parkinson’s disease. Neuropsychology, 8, 218–226.
Fisk, J. E., & Sharp, C. A. (2004). Age-related impairment in executive functioning: updating, inhibition, shifting, and access. Journal of Clinical and Experimental Neuropsychology, 26(7), 874–890. doi:10.1080/13803390490510680.
Fortin, C., & Breton, R. (1995). Temporal interval production and processing in working memory. Perception and Psychophysics, 57, 203–215.
Fortin, C., & Masse, N. (2000). Expecting a break in time estimation: Attentional time-sharing without concurrent processing. Journal of Experimental Psychology-Human Perception and Performance, 26(6), 1788–1796. doi:10.1037//0096-1523.26.6.1788.
Gibbon, J. (1977). Scalar expectancy theory and Weber’s law in animal timing. Psychological Review, 84, 279–325.
Greene, L. S., & Williams, H. G. (1993). Age-related differences in timing control of repetitive movement: application of the Wing–Kristofferson model. Research Quarterly for Exercise and Sport, 64(1), 32–38. doi:10.1080/02701367.1993.10608776.
Ivry, R. B., & Keele, S. W. (1989). Timing functions of the cerebellum. Journal of Cognitive Neuroscience, 1(2), 136–152. doi:10.1162/jocn.1989.1.2.136.
Johannsen, L., Li, K. Z. H., Chechlacz, M., Bibi, A., Kourtzi, Z., & Wing, A. M. (2013). Functional neuroimaging of the interference between working memory and the control of periodic ankle movement timing. Neuropsychologia, 51(11), 2142–2153. doi:10.1016/j.neuropsychologia.2013.07.009.
Krampe, R. T., Doumas, M., Lavrysen, A., & Rapp, M. (2010). The costs of taking it slowly: Fast and slow movement timing in older age. Psychology and Aging, 25, 980–990. doi:10.1037/a0020090.
Krampe, R. T., Mayr, U., & Kliegl, R. (2005). Timing, sequencing, and executive control in repetitive movement production. Journal of Experimental Psychology-Human Perception and Performance, 31(3), 379–397. doi:10.1037/0096-1523.31.3.379.
Lehrl, S. (1977). Mehrfach-Wortschatz-Test B (MWT-B) (Multiple-Choice Vocabulary Test B). Erlangen: Straube.
Lewis, P. A., & Miall, R. C. (2003). Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia, 41(12), 1583–1592. doi:10.1016/S0028-3932(03)00118-0.
Maes, P. J., Wanderley, M. M., & Palmer, C. (2015). The role of working memory in the temporal control of discrete and continuous movements. Experimental Brain Research, 233(1), 263–273. doi:10.1007/s00221-014-4108-5.
Mayr, U. (2001). Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging, 16(1), 96–109. doi:10.1037/0882-7974.16.1.96.
Ogden, R. S., Salominaite, E., Jones, L. A., Fisk, J. E., & Montgomery, C. (2011). The role of executive functions in human prospective interval timing. Acta Psychologica, 137(3), 352–358. doi:10.1016/j.actpsy.2011.04.004.
Pütz, P., Ulbrich, P., Churan, J., Fink, M., & Wittmann, M. (2012). Duration discrimination in the context of age, sex, and cognition. Journal of Cognitive Psychology. doi:10.1080/20445911.2012.709230.
Salthouse, T. A., Wright, R., & Ellis, C. L. (1979). Adult age and the rate of an internal clock. Journal of Gerontology, 34, 53–57.
The MathWorks. (2007). MATLAB (Version 7.4). Natick: The MathWorks, Inc.
Treisman, M. (1963). Temporal discrimination and the indifference interval: Implications for a model of the “internal clock”. Psychological Monographs, 77(13), 1–31.
Verhaeghen, P., & Salthouse, T. A. (1997). Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin, 122, 231–249.
Vorberg, D., & Wing, A. M. (1996). Modelling variability and dependence in timing. In H. Heuer & S. W. Keele (Eds.), Handbook of Perception and Action (Vol. 3, pp. 181–261)., Motor Skills London: Academic Press.
Wechsler, D. (1981). The psychometric tradition: Developing the wechsler adult intelligence scale. Contemporary Educational Psychology, 6(2), 82–85. doi:10.1016/0361-476X(81)90035-7.
West, R. L. (1996). An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin, 120(2), 272–292.
Wiener, M., Turkeltaub, P., & Coslett, H. B. (2010). The image of time: A voxel-wise meta-analysis. NeuroImage, 49(2), 1728–1740. doi:10.1016/j.neuroimage.2009.09.064.
Wing, A. M. (1980). The long and the short of timing in response sequences. In G. E. Stelmach & J. Requin (Eds.), Tutorials in Motor Behavior (pp. 469–486). Amsterdam: North-Holland.
Wing, A. M., & Kristofferson, A. B. (1973). Response delays and the timing of discrete motor responses. Perception & Psychophysics, 14, 5–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was conducted at the Center for Cognitive Studies, University of Potsdam, Germany, as part of the project Formal Models of Cognitive Complexity (German Research Foundation Grant INK12). Continued work on this project was supported by BOF grant OT25/05 from the Research Council of KU Leuven and FWO grant (Fonds Wetenschappelijk Onderzoek) G.0379.06 to the author.
Conflict of interest
AM Meijer declares that she has no conflict of interest. R. T. Krampe declares that he has no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in approved by the local ethical committee and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Meijer, AM., Krampe, R.T. Movement timing and cognitive control: adult-age differences in multi-tasking. Psychological Research 82, 203–214 (2018). https://doi.org/10.1007/s00426-017-0876-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-017-0876-4