Abstract
Recent studies have suggested that the threat of pain may redirect attention towards specific features of the pain stimulus via attentional control settings. For instance, it has been shown that anticipating pain results in attentional prioritization of the location where pain is expected. In contemporary theories on attention and pain, it has been argued that pain control motivation—e.g., attempting to avoid pain—is capable of enhancing these effects. The present study investigated if the threat of pain prioritizes attention towards somatosensory input over other sensory information, and if pursuing a pain control goal augments this effect. In a Temporal Order Judgment experiment, 41 participants were presented with visuo-tactile stimulus pairs and asked to judge which stimulus they had perceived first. Half of all trials were associated with the threat of acute pain, while the other half was not. Furthermore, half of our sample was encouraged to avoid the administration of pain by means of a specified behavioral response, whereas the other half was not. In line with our hypotheses, we found the threat of pain to prioritize attention towards the somatosensory modality, i.e., participants tended to perceive the tactile stimulus as occurring earlier in time than the visual stimulus. Interestingly, in-depth analyses suggested that this effect was predominantly carried by participants who were engaged in pain control efforts. These findings support the idea that pain goals exert top–down attentional control prioritizing pain-relevant sensory information. Clinical relevance and future directions are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an evolutionary tactic, pain may at first glance seem at odds with its purpose. Yet, while it is in itself an unpleasant and unwelcome experience, the ability to feel pain is vital to the survival of later-stage organisms such as humans (Chapman, Tuckett, & Song, 2008). Pain alerts us of the possibility of impending physical harm, prompting us to shift our attention to effectively address the threat in question (Eccleston & Crombez, 1999).
While this attentional demand is most evident in a bottom–up sense, i.e., when the sudden onset of pain disrupts ongoing goal-directed behavior, it is also possible that one’s goals are a priori directed at pain control (Van Damme, Legrain, Vogt, & Crombez, 2010). When faced with contexts that signal the likelihood of hurt, it is adaptive to devote attention to pain-related signals pre-emptively (Öhman et al., 1979). As such strategy permits us to deal with physical threat swiftly and efficiently, it is in effect a way of attempting to gain control over the threat. This is an example of a top–down, goal-driven mode of attentional selection (Legrain et al., 2009).
Goals, such as attempting to prevent or control imminent pain, have a way of adjusting our attentional control settings—a mental set of stimulus features that allows us to efficiently identify and act upon goal-relevant information (Folk & Remington, 2008). When pain becomes the focal point of one’s motivation, it can thus be expected that stimuli sharing perceptual features with the anticipated pain will be selected by attention more easily (the so-called attentional set hypothesis) (Legrain et al., 2009). Prevalent stimulus features of pain include its location and the somatosensory modality. The former feature, i.e., the spatial characteristics of pain, has been the focus of recent research. In favor of the attentional set perspective, these experiments have demonstrated that the anticipation of pain directs one’s attention towards the location where this pain is expected to occur (Durnez & Van Damme, 2015; Vanden Bulcke, Van Damme, Durnez, & Crombez, 2013, Vanden Bulcke, Crombez, Spence, & Van Damme, 2014).
The attentional set hypothesis further predicts that somatosensory stimuli, sharing their modality with pain, will be selected by attention more easily than stimuli from other sensory modalities. Evidence in support of this assertion is scarce yet. A number of cross-modal cueing studies have shown that attention can be selectively directed to the specific modalities using visual cues (Spence, Bentley, Phillips, McGlone, & Jones, 2002; Van Damme, Crombez, Eccleston, & Goubert, 2004). For instance, there is evidence that cueing the word ‘pain’ or ‘tone’ can prioritize attention to the somatosensory and auditory modality, respectively. Also informative in this regard may be the study by Van Damme, Gallace, Spence, Crombez, and Moseley (2009). Using an unspeeded temporal order judgment (TOJ) task, they found that the presentation of an image of physical threat (such as a knife) in front of one hand shortly before a pair of either tactile or auditory stimuli resulted in quicker awareness of tactile stimuli at the “threatened” hand than at the other hand. This prioritization effect was not found for auditory stimuli presented close to the threatened hand. In other words, threat only seemed to prioritize somatosensory information at the threat location, implying a modality-specific effect. However, the experiment did not place auditory and somatosensory information in direct competition for attention. Hence, it does not support any inference with regard to the prioritization of a specific modality, but rather suggests that the prioritization of threatened locations may depend on the modality of the input that requires processing.
In an attempt to address this shortcoming, Jia, Shi, Zang and Müller (2013) conducted a series of bimodal TOJ experiments, in which participants judged the order of audio-tactile stimulus pairs. Results showed that the prior presentation of affectively salient pictures—at a location independent of the audio-tactile stimuli—was capable of shifting attention towards the somatosensory modality, resulting in the quicker perception of tactile stimuli compared to concomitant auditory stimuli. Notably, this effect was only found when stimuli from different modalities were also separated in space. Prioritization effects were found for both positive (e.g., an erotic couple) and negative (e.g., a spider) high-arousal imagery. When disentangling the effects of physically threatening contexts with regard to the locus of threat, prioritization of somatosensory stimuli only occurred when the visual cue represented a near-body threat (e.g., a snake), and not when it depicted remote threat (e.g., a car accident). A limitation of the aforementioned studies (Jia et al., 2013; Van Damme et al., 2009) is that only visual threat cues were used. Effects of the actual anticipation of pain thus remain open to investigation.
As is often the case with laboratory pain studies, the design of the vast majority of aforementioned studies left little or no room for the natural urge to avoid, escape or minimize the pain itself. Thus, these studies seldom allow any conclusion to be made about the presumed role of active pain control goals in attentional prioritization of a threatened location. Few studies on pain-related attention include a pain control option. One experiment demonstrated that attempts to avoid a painful stimulus are capable of prioritizing attention to visual cues predicting pain stimulation (Notebaert et al., 2011). However, this study does not permit any conclusions with regard to prioritization of pain-related stimulus features, specifically. More recently, in a tactile change detection task, researchers observed how attentional prioritization of a threatened location was more pronounced when participants were encouraged to avoid administration of pain by means of a specified behavioral response (Durnez & Van Damme, 2015). This study provides some evidence that pain control goals may activate the location feature of pain in the attentional control settings. However, as only tactile stimuli were used in this study, it cannot tell us anything about the hypothesized prioritization of the somatosensory modality over other modalities.
The present study has two main objectives. First, we examine whether the threat of acute pain prioritizes attention towards somatosensory input, at the cost of input from other modalities. In this study, specifically, a comparison will be made with visual information. In accordance with the attentional set hypothesis, we predict that stimuli more perceptually similar to pain, i.e., somatosensory input, will be processed more swiftly by attention (hypothesis 1). Second, we investigate the significance of pain control motivation by encouraging half of our sample to actively try to avoid administering of pain stimuli. Extending the notion that goal pursuit shapes our attentional control settings (Folk & Remington, 2008), we propose that explicit activation of pain control goals will enhance attentional prioritization of the somatosensory modality (hypothesis 2). To test these ideas, we designed a TOJ study featuring stimuli from two distinct sensory modalities. Participants were required to judge the order in which pairs of stimuli were presented to both hands: one visual stimulus and one somatosensory stimulus. These stimuli were always presented on a different hand (visual left and somatosensory right or vice versa). Stimulus locations were counterbalanced over trials. Half of all trials were made threatening through a simple pain conditioning procedure. To achieve this, we used auditory cues (high frequency vs. low frequency) that indicated either the possibility of receiving a painful stimulus on both hands or the certainty that no such stimulus would follow. Additionally, we divided participants into a pain control group and a comparison group. The former group was actively encouraged to attempt to avoid pain by quickly pressing down on a foot pedal as soon as they heard the pain-indicative cue, whereas the latter group was simply asked to press the foot pedal as an additional timed reaction task. In reality, both groups were given an equal amount of painful stimuli. We expected somatosensory stimuli to be prioritized over visual stimuli when the threat of impending pain was present. In addition, we predicted this prioritization effect to be significantly stronger when participants actively pursued a pain control goal, as this goal-directed behavior would significantly reinforce attentional control settings.
Methods
Participants
Forty-two students of Ghent University (17 male and 25 female; M age 22.76 SDage 7.27) participated in this study, either to earn required course credits or in exchange for a small financial compensation. Three of them were left-handed. All participants had normal or corrected-to-normal vision and normal hearing. The study protocol was approved by the Ethics Committee of the Faculty of Psychology and Educational Sciences of Ghent University. The experiment took approximately 1 h and 10 min. Informed consent was obtained by all individual participants included in the study.
Apparatus and stimulus material
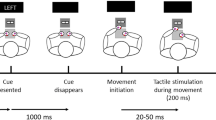
The experiment was conducted in a darkened, sound-isolated room. Participants sat on a chair in front of a desk, with their hands palm-down on marked positions (see Fig. 1). The tactile stimuli used in the experiment were vibrations, presented by means of two resonant-type tactors (C-2 TACTOR, Engineering Acoustics, Inc.) consisting of a housing of 3.05 cm diameter and .79 cm high, with a skin contactor of .76 cm diameter. Their function was controlled and amplified through a custom-built device. The tactors were attached directly to the skin in the center of the back of either hand using double-sided tape rings. The frequency of tactile stimulation was 200 Hz. The stimulus duration was set to 20 ms. Visual stimuli were presented by means of two green light-emitting diodes (LEDs). These LEDs were placed directly on top of the tactors. During the experiment, they were illuminated for a duration of 20 ms, causing them to be perceived by participants as briefly flashing green light. An additional, centrally placed red LED served as a fixation point throughout the different trials of the experiment. Painful stimuli were generated electrically through means of constant current stimulators (Digitimer DS5, 2000). They were delivered via 2 lubricated Fukuda standard Ag/AgCl electrodes (1 cm diameter), placed in close proximity to the tactors and the superficial branch of the radial nerve. These sinusoid electrocutaneous stimuli had a frequency of 200 Hz and a duration of 200 ms. As such, this stimulation can be considered as phasic pain. Throughout the experiment, painful stimulation always occurred on both hands simultaneously. Amplitudes for both the tactile and electrocutaneous stimulation were set using adaptive procedures, as described in the procedure section. Auditory cues were administered using a set of headphones (Sennheiser HD 202 II). These cues consisted of either a high tone (1000 Hz) or a low tone (250 Hz) and had a duration of 1000 ms. As part of the goal manipulation, participants were asked to press a foot pedal at specific moments in a portion of the trials. This foot pedal (Bespeco NT-13 sustain pedal) was attached to the floor at a distance that was comfortable for each participant, so that they could easily and quickly press down on it with their dominant foot. The pedal was connected to a Cedrus response box (RB-530 model) to optimize response time registration.
TOJ paradigm
The task was programmed in the programming language C using the Tscope 5 library package, an upgraded version of the original Tscope. It was presented on a laptop (Dell latitude E5520). Participants were instructed to keep their hands on the marked positions, and keep their gaze fixed on the fixation LED. Visual illustration of a typical trial course is provided in Fig. 2.
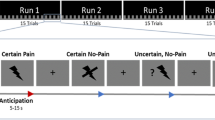
Overview of possible trial courses. Fixation cross presentation is followed by an auditory cue. After a blank interval, one of two possibilities occurs. If the auditory cue indicated threat, there is a chance of 1 out of 11 that a painful stimulus would follow (pain trial). If not, then a TOJ trial is presented, in which two stimuli are presented, separated by an interstimulus interval (stimulus onset asynchrony, or SOA). Participants responded by stating which they perceived first, the light or the vibration. In case of a pain trial, they were asked to report that as well
The experiment was divided into 5 blocks of 84 trials each, resulting in a total of 420 trials. Electrocutaneous stimulation was presented at least once in the first ten trials of each block to maintain contingency perception. Each trial began with an illumination of the fixation LED for 1000 ms. Next, a 1000 ms auditory cue was presented, indicating whether or not electrocutaneous stimulation could follow (within-subjects variable of THREAT). One tone frequency predicted the possible advent of such stimulation (threat trial), while the other signaled that this would not be the case (neutral trial). The frequency of the threatening tone (high vs. low) was counterbalanced. The tone was followed by an interval of 500 ms. One out of eleven threat trials included actual electrocutaneous stimulation. Participants were not informed of this proportion. In this case, no other stimuli were presented. In the remaining 10 threat trials, as well as in all neutral trials (10 in number), the auditory cue was instead followed by the administration of the TOJ stimuli. In each trial, the stimulation on one hand was visual (LED light), while the other hand received a somatosensory stimulus (vibration). In half of the trials, the somatosensory stimulus was presented on the left side and the visual stimulus on the right side. In the other half, the opposite was true. The stimuli were separated in time by 1 of 10 possible stimulus onset asynchronies (SOAs; −200, −90, −55, −30, −10, +10, +30, +55, +90 or +200 ms; see also (Spence, Baddeley, Zampini, James, & Shore, 2003). In TOJ experiments, it is customary to code SOAs so that negative values indicate that the test stimulus was presented first. In this study, we regard somatosensory stimuli as test stimuli, while visual stimuli are treated as reference stimuli. In the remainder of the manuscript, negative SOAs thus refer to trials in which the somatosensory stimulus preceded the visual stimulus. Every SOA occurred an equal number of times during the course of the experiment (8 per block, in which the modality location was counterbalanced).
Participants were asked to verbally report on which hand they noticed the stimulation first. They did this by either saying “light” or “vibration” aloud. When a painful stimulus replaced a TOJ trial, participants were asked to report this by saying “shock”. They had up to 5 s to respond before their response was coded as a blank. All responses were coded by the experimenter using a keyboard.
Procedure
Participants were given a brief description of the experiment and asked to fill in an informed consent form. They then completed a custom-made pre-test questionnaire, which is described in the self-report measures section below. Tactors, electrodes and LEDs were then attached to the locations described above (Fig. 1). Because it has been shown that somatosensory sensitivity can vary depending on which location of the body is stimulated (Weinstein, 1968), we first obtained appropriate tactile and electrocutaneous stimulation amplitudes for each hand. Our aim was to ensure that participants perceived somatosensory stimulation of equal intensity on both hands hand, so as not to privilege either side.
Determining intensity of tactile stimuli
Our custom-made adaptive procedure (see also: Durnez & Van Damme, 2015; Vanden Bulcke et al., 2013, 2014) based on the double random staircase procedure, was designed as follows. Participants were first given a reference stimulus at 50 % of the maximum capacity (p = .425 wt) on the left hand. One second after that, a tactile stimulus was administered to the right hand. The amplitude of this second stimulus was taken from one of two staircases, which were alternated randomly for an equal number of times in total. The initial value for the first staircase was a random integer between 45 and 49, while it was a random integer between 51 and 55, for the second. This way we ensured that participants would encounter both a stimulus that was higher in actual amplitude, and one that was lower in amplitude. After each pair of stimuli, participants were asked whether they perceived the second stimulus as “a lot stronger”, “stronger”, “equally strong”, “weaker” or “a lot weaker”. Their response determined the next value in the staircase (5 units down, 1 unit down, no change, 1 unit up or 5 units up, respectively). This was repeated for 16 times. The continuous coupling of reference stimuli and to-be-rated stimuli was intended to ensure participants could adequately compare both sensations, making sure there was no gradual shift in memory of how the stimulus was perceived. It also served to prevent divergent sensitization effects on both hands. An average was made of all amplitude values which participants had reported to perceive equally strong (supplementary Table 1). This value was used in the main experiment.
Determining intensity of electrocutaneous stimuli
In the following preparatory phase, we determined amplitudes for the electrocutaneous stimulation. We did this by obtaining an appropriate value for the left hand and then finding a matching value for the right hand, both times using a double random staircase procedure of 14 steps. In the first part of this procedure, starting values for both staircases were chosen randomly between 1 and 20 (respectively, .1 and 2.0 mA). A series of 15 stimuli was administered on the left hand. Participants were asked to rate each of these on an 11-point scale (0 = “no pain”, 10 = “unbearable pain”). Reponses determined the next value in the corresponding staircase: a rating over 7 meant 1 unit down, a rating of 7 meant no change, and a rating under 7 meant 1 unit up. We took the average of all values to which participants gave a pain intensity rating of 7. This way we obtained the pain intensity for the left hand (supplementary Table 1). This was then used as a reference stimulus during the second part of the procedure. In this part, a stimulus on the right hand followed the reference stimulus on the left hand. Its functioning was similar to the former procedure for determining tactile intensities—and because of the same reasons. Participants were asked once more whether they perceived the second, right hand stimulus as “a lot stronger”, “stronger”, “equally strong”, “weaker” or “a lot weaker”. Their response pattern over the next fourteen trials determined the pain intensity for the right hand, which was calculated as the average of all right hand intensities which participants had judged to be “equally strong” to the reference stimulus on the left hand.
We proceeded by introducing the TOJ paradigm to the participants and explained the nature of the task. We presented them with 20 practice TOJ trials with two additional pain trials intermixed. We only proceeded when participants scored 70 % accuracy on the trials with the largest SOA (±200 ms). Next, we informed participants about the meaning of the auditory cues. Dependent on which group they were placed in (between-subjects variable of GROUP), participants received additional instructions with regard to the use of the foot pedal. In the pain control group (21 participants), participants were instructed that they could significantly reduce the chance of receiving painful stimuli throughout the experiment, by pressing down on the pedal as soon as they heard the threat-signaling cue. In reality, the timing and occurrence of painful stimuli were predetermined, ensuring that participants in the pain control group received an equal amount of pain stimuli as those in the comparison group. This implies that our goal manipulation depended on subjective control, rather than actual control. In this comparison group (21 participants), participants were also instructed to press down on the pedal upon hearing the threat-signaling cue. These participants, however, were told this served to obtain additional measures of attention and concentration. No instructions related to pain control were given whatsoever. Five TOJ blocks were then presented, as described above.
Self-report measures
Prior to the experiment, participants filled in a custom-made questionnaire on pre-existing pain-related conditions and episodes. All ratings (e.g., “To what degree were you unable to conduct daily activities during the past 6 months because of your pain?”) were indicated on a 11-point Likert scale. In addition, each experimental block was followed by a quick questionnaire on effort (“To what extent did you put effort into the task?”), concentration (“How well could you concentrate on the task?”), attention (“How much attention did you pay to the somatosensory/visual stimuli?”; “How much attention did you pay to the electrocutaneous stimuli?”), fear related to either cue (“To what extent did you fear that a high/low tone would be followed by an electrocutaneous stimulus?”), pain expectancy related to either cue (“To what extent did you expect an electrocutaneous stimulus to follow the high/low tone?”), pain perception (“How painful did you find the electrocutaneous stimulus?”), anxiety (“How anxious did you feel during this block?”) and fatigue (“How tiresome did you find this block?”). Participants in the pain control group were also asked to what degree they attempted to avoid the occurrence of painful stimuli. This question was not posed to the comparison group, so as not to evoke the illusion of underlying control mechanisms. All questions were answered on an appropriately anchored 11-point Likert scale. Answers were averaged over blocks per participants, prior to analysis. Finally, upon completion of all experimental blocks, participants completed the Pain Catastrophizing Scale (PCS; Sullivan, Bishop & Pivik, 1995; Van Damme, Crombez, Bijttebier, Goubert, & Van Houdenhove, 2002).
Statistical analyses
Participants not reaching a mean accuracy of 70 % on trials with the largest SOAs (±200) were excluded from further analyses (Spence et al., 2003). We then analyzed performance on the TOJ-task by fitting these data to functions based on an independent channels model, as described in Alcalá-Quintana and García-Pérez (2013). Using these fits, we obtained Point of Subjective Simultaneity (PSS) measures for each condition. These measures represent the (fictitious) SOA at which observers can be expected to give either response (“vibration” or “light”) with equal probability. Consequently, a shift in this point teaches us about the relative speed with which the competing information is processed. Participants with PSS values greater than the largest SOA were removed from the dataset (see also Spence, Shore, & Klein, 2001). The final PSS values were analyzed using a generalized linear mixed-effects model with a Gaussian link function, as implemented in the R package ‘lme4’ (Bates, Maechler, Bolker, & Walker, 2014). The statistical modeling procedure was as follows.
First, all relevant factors and their interactions were entered in the model as fixed factors. These included THREAT (threat trials vs. neutral trials) and GROUP (pain control group vs. comparison group). By default, a random effect was added introducing adjustments to the intercept conditional on each subject separately. This accounts for by-subject baseline differences. Next, we determined whether the addition of a random slope for the within-subject THREAT variable, conditional on each subject, was necessary. This random effect statistically represents the possibility that the effect of THREAT is different for different subjects. If this random effect increased the model’s goodness of fit, we included it in the final model. In a second step, we sought out the most parsimonious model that fit the data by restricting the full model systematically, starting with higher-order terms. All model comparisons were made using likelihood-ratio tests. In a third and final step, we inspected the ANOVA table of the final model and tested specific hypotheses about possible main effects or interactions (see De Ruddere, Goubert, Stevens, Williams, & Crombez 2013; Durnez & Van Damme, 2015; Verbruggen & Aron, 2010, for a similar approach). As we were interested in the interaction between THREAT and GROUP, type III sum of squares were calculated.
In order to further investigate the nature of the interaction effect, when present, 4 additional (planned) orthogonal contrasts were calculated. These analyses examined the effect of threat in both the comparison group and the pain control group, independently. Similarly, we investigated the separate effect of pain control attempts in neutral trials and threat trials. These contrast analyses were corrected for multiple testing according to the corrections of Holm-Bonferroni (Holm, 1979).
As discussed in the introduction, we expected the threat of pain to prioritize attention towards somatosensory input (hypothesis 1: THREAT main effect). Additionally, we expected that the strength of such threat-induced attentional bias would be increased in the pain control group, relative to the comparison group (hypothesis 2: THREAT x GROUP interaction effect).
Results
Self-report data and manipulation check
Participants assessed their own health as ‘very good’, on average. Twenty-three participants had experienced some form of pain during the past 6 months (M = 19.37 days, SD = 26.76 days). This pain had an average intensity rating of 5.09 (SD = 1.20) and an average disability rating of 3,565 (SD 2.79). One of these participants reported to have suffered from his pain complaint for more than 90 days (intensity rating = 5, disability rating = 4). We found no evidence that including this participant significantly distorted the data. Eight participants reported feeling pain at the moment of testing, on a Likert scale ranging from “no pain” to “worst possible pain”. Their average pain intensity ratings were low (M = 2.13, SD = 1.25).
To verify the effect of the threat manipulation, we applied an ANOVA with the factors CUE (threatening vs. neutral) and GROUP (comparison vs. pain control) on fear and pain expectancy ratings. With regard to fear ratings, we found main effects of both the CUE and GROUP variable (resp. χ 2 = 85.10, p < .001 and χ 2 = 6.27, p = .01). These indicated that participants felt more fearful upon hearing the threat cue (M = 4.97, SD = 2.15) compared to the neutral cue (M = 1.03, SD = 1.69) (d = 2.04, 95 % CI = 1.47–2.62), indicating that the threat manipulation was successful. Interestingly, we found that fear ratings were overall higher in the pain control group (M = 3.57, SD = 3.04) than in the comparison group (M = 3.04, SD = 2.40) (d = .39, 95 % CI = −.08 to .86). The interaction was not significant. A comparable pattern was found with respect to pain expectancy ratings, showing a significant main effect of CUE (χ 2 = 51.66, p < .001) and a now marginally significant main effect of GROUP (χ 2 = 3.01, p = .08). Similarly, hearing the threatening cue led to more pain expectancy (M = 4.23, SD = 2.44) compared to hearing the neutral cue (M = .92, SD = 1.51) (d = 1.63, 95 % CI = 1.09–2.17). Additionally, pain expectancy ratings were slightly higher for participants in the pain control group (M = 2.99, SD = 2.84) than for those in the comparison group (M = 2.20, SD = 2.36) (d = .32, 95 % CI = .15–.79).
The remainder of the self-report measures is summarized in Table 1. Notably, participants in the pain control group reported paying more attention to the electrocutaneous stimulus (M = 6.63, SD = 1.58) than those in the comparison condition (M = 4.99, SD = 1.58) (t 36 = 2.41, p = .02, d = .78, 95 % CI = .08–1.49). Finally, participants in the pain control group reported moderate attempts to control the painful stimulus (M = 4.27, SD = 2.60).
TOJ data
We eliminated 3 participants (1 in the comparison group, 2 in the pain control group) whose accuracy on trials with the largest SOA (±200 ms) fell under the cut-off level of 70 %. Of the remaining participants, one showed a PSS value outside of the SOA range (−210.84 ms), prompting this participant’s exclusion. This left us with 20 participants in the comparison group, and 18 participants in the pain control group.
Upon closer inspection of the PSS table, we noticed one value standing out remarkably. This value (PSS = −184.34) was identified as an outlier by a Grubbs test (G = 4.75, U = .70, p < .001). While data restriction in TOJ experiments typically ends after the application of the aforementioned exclusion criteria, we chose to bar this participant from the analyses. The distribution of the remaining PSS values is shown per condition in Fig. 3 and Table 2.
We compared individual Point of Subjective Simultaneity (PSS) measurements across conditions. For every participant in both the comparison group and the pain control group, we calculated the Point of Subjective Simultaneity (PSS) (smaller, hollow circles). Mean PSS values are indicated as well (larger, solid circles). More positive values means somatosensory information is processed quicker relative to visual information. The illustrated pattern shows that threat prioritizes somatosensory information, particularly in the pain control group
Analysis of PSS measures
The best fitting statistical model included all fixed factors and interactions, and a random subject-based intercept. No other random effects were necessary (see Table 3). The interaction effect was marginally significant (χ 2 = 3.51, p = .06). We chose not to restrict the model any further. This model’s intercept was strongly significant, indicating that tactile information was generally perceived quicker than visual information, regardless of our experimental manipulations. This result has been found before on several occasions (e.g., Spence et al. 2001, 2003). In addition, we found a significant main effect of the THREAT variable (χ 2 = 22.01, p < .001), indicating higher PSS values when threat was presented. In our coding scheme, higher PSS values indicate that somatosensory input was processed relatively quicker than visual input following a threatening cue. We also found a main effect of GROUP (χ 2 = 4.62, p = .03), showing generally higher PSS values in the pain control group compared to the comparison group. The interaction suggested that the effect of THREAT was stronger in the pain control group than it was in the comparison group, although this was only a tentative result with borderline significance (see Table 4).
In order to further dissect this near-significant interaction, 4 additional contrasts were calculated (α = .05/4 = .0125). The effect of threat was not significant in the comparison group (χ 2 = 3.61, p = .06). In the pain control group, however, threat significantly increased PSS measures (χ 2 = 16.21, p < .001)—and thus, facilitated the detection of somatosensory input compared to visual input. In addition, we found no evidence that PSS values for neutral trials differed between groups (χ 2 = 1.37, p = .24). In contrast, threat trials yielded significantly higher PSS measurements in the pain control group, compared to the comparison group (χ 2 = 9.48, p < .01). This suggests that pain control attempts prioritize somatosensory information over other input, but only when critical threat is presented.
Discussion
The aim of the present study was twofold. On the one hand, we set out to investigate if the anticipation of pain led participants to prioritize all somatosensory input over input from other modalities—in this case visual information. We thus directly compared processing speed for stimuli in both these modalities. Our predictions were derived from the attentional set hypothesis, which proposes that stimuli sharing features with a motivationally salient target stimulus will be selected by attention more readily. We thus expected all somatosensory input to be prioritized, as this input shares its modality with the anticipated pain (hypothesis 1). On the other hand, we were interested in verifying whether motivational factors—in this case the goal to control pain—have the capability to enhance this somatosensory prioritization (hypothesis 2) (Van Damme et al., 2010). We predicted such an increase in attention, as we estimated that inducing a pain control goal would increase the salience of the goal-relevant stimulus (the anticipated pain), thus further strengthening the prioritization of pain-related features through these participants’ attentional set.
The results largely substantiated our hypotheses, showing larger (and positive) PSS values in the threat condition compared to the neutral condition. Given our coding scheme, a positive PSS means that visual information needs to be presented earlier than somatosensory information in order for both stimuli to be perceived as simultaneously occurring. In other words, positive PSS values show that somatosensory input is processed more quickly than visual input—the so-called prior-entry effect (Spence & Parise, 2010; Titchener, 1908). In turn, the finding that PSS values are on average larger in threat trials compared to neutral trials signifies that this difference in processing speed is enlarged through the anticipation of pain. This confirms our first hypothesis, which predicted that the threat of pain would prioritize attention towards the somatosensory modality at the cost of competing information in other modalities.
With respect to the second hypothesis, the pattern of results appears to be a little more nuanced. We predicted that the addition of a pain control option would further enhance the prioritization of somatosensory information following the anticipation of pain. Indeed, analyses uncovered that the threat-induced shift of PSS values towards the somatosensory modality was more pronounced in this pain control group than in the comparison group. Still, this effect narrowly failed to reach the applied significance level, making it harder to draw strong inferences. A noteworthy finding in this regard, though, is the fact that the main effects of both our threat manipulation and motivation manipulation were largely carried by the magnitude of PSS values generated by threat trials in the pain control group. It thus appears as though the threat manipulation was significantly more powerful when participants were concurrently occupied with pain control attempts. Given that the effect of our threat manipulation was borderline significant in the comparison group, it is not entirely clear whether pain control attempts were essential in producing the main effect of threat. In that sense, it may prove useful to conduct a replication study, aimed towards a more decisive separation of the effects of motivation on the one hand, and the anticipation of pain on the other.
In this regard, it may be worthwhile noting that we found both increased fear ratings and increased pain expectancy ratings, for threat trials, when comparing the pain control group to the comparison group—even though pain perception itself was stable across groups. It is possible that these differences contributed to the higher PSS values for pain controllers facing threat, when compared to threat trials in the comparison group. An earlier meta-analysis reported that anxiety is significantly related to attentional prioritization of threat-related information, albeit with a medium effect size (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). However, a meta-analytic study that investigated attentional bias to pain-related information, specifically, failed to confirm that individual differences—such as pain-related fear or catastrophizing—significantly affected the magnitude of such biases (Crombez, Van Ryckeghem, Eccleston, & Van Damme, 2013). This avenue of interpretation is, therefore, not wholly unquestionable, and warrants further investigation. Note that the fear and pain expectancy ratings in our study were mainly included as a threat manipulation check. Adding these ratings to our analyses would force us beyond the (statistical) scope of this study.
Several additional points of discussion present themselves. First, it is important to note that while participants in the pain control group were encouraged to attempt to avoid the painful stimulus, they never exerted actual control during trials. This type of goal induction has been implemented in several earlier studies, showing that such a manipulation is in fact capable of altering attentional prioritization (Durnez & Van Damme, 2015; Notebaert et al., 2011). Even when participants deemed their pain control attempts fruitless after a period of time, this does not necessarily diminish the effect of our manipulation. In fact, the frustration of goal pursuit can serve to activate the pertinent goal even more (Moskowitz, 2002).
Second, stimuli in the present experiment were always spatially separated. We made sure not to cue one specific location by always presenting electrocutaneous stimulation on both sites simultaneously, in the case of a pain trial. Additionally, stimulus modalities (i.e., visual or somatosensory) and stimulus locations (i.e., left or right hand) were fully counterbalanced, so that there could never be any confound between these features—thus safeguarding the validity of our results. However, in the study conducted by Jia et al. (2013), the spatial separation of stimuli appeared to be a prerequisite in the search for the emotional modulation of TOJs. When stimuli were presented at the same location no effects were found. The authors explained this through the idea of cross-modal integration, citing that multisensory stimuli stemming from the same location are more prone to be processed as a unitary object, rather than as multiple events in multiple modalities (Welch & Warren, 1980; Spence et al., 2003; Stein & Stanford, 2008). It might then be worthwhile to replicate the present study, limiting both visual, somatosensory and electrocutaneous stimuli to one fixed location instead. Assuming that the anticipation of pain—and more importantly, attempts to control said pain—indeed primarily impacts attention through modification of the attentional set, such a design would place modal prioritization effects in an interesting direct competition with the unity effect (Welch & Warren, 1980). If somatosensory prioritization would still be evident, and the unity effect thus overcome, an even more robust case would be made for the capability of pain to significantly impact attention.
Third, another obvious difference between the present study and its predecessor studies (Jia et al., 2013; Van Damme et al., 2009) can be found in the stimulus material used. Whereas our experiment juxtaposed somatosensory stimuli against visual information, these latter studies used audio-tactile stimulus pairs. This discrepancy holds no implications for the soundness of our current hypotheses and results. More so, it is likely that visual information is more functionally relevant in the context of pain. That said, it would be interesting to replicate the experiment with audio-tactile stimulus material, as such replication could provide us with additional support for the attentional set perspective.
Fourth, it should be noted that the painful stimulation used in our experiment was of a phasic nature. It has been shown that phasic and tonic pain can impact attentional processes differently (Sinke, Schmidt, Forkmann, & Bingel, 2015). As such, it would be ill-advised to generalize the results of the present study to instances of tonic pain.
Finally, it is important to note the potential implications of this line of research. Experimental studies on pain-related attention, and particularly those that investigate psychometric effects of pain control motivation, serve to provide a scientific substrate and basis of credibility for contemporary models of chronic pain. Individuals suffering from this condition are characterized by unrelenting pain symptoms for which often no clear-cut medical explanation can be found. Current theoretical accounts, such as the misdirected problem solving model (Eccleston & Crombez, 2007), propose that when patients strongly adhere to a biomedical framework of their pain problem, problem solving is often directed at gaining control over a—largely uncontrollable—pain problem. In failing to do so, worry is magnified, which further motivates such “misdirected problem solving”, resulting in more disability and distress. Along with the amplification of the worry process, attention to pain and pain-related information may increase considerably—often referred to as a hypervigilant state (Crombez, Van Damme, & Eccleston, 2005). The precise manifestation of hypervigilance in chronic pain patients, particularly in terms of body location and sensory modality, is still under debate (Rollman, 2009; Van Damme et al., 2009). The present study can be construed as an attempt to recreate a rudimentary state of increased vigilance to pain by inducing—intrinsically dysfunctional—pursuit of the goal to control pain. Its results at the very least suggest that such a context comes with heightened and generalized attention to pain-specific features, which may in turn influence pain perception. The reported findings thus—preliminarily—suggest that interventions, designed to directed patients’ focus away from pain control and towards acceptance (e.g., Acceptance and Commitment Therapy: Hayes, Luoma, Bond, Masuda, & Lillis, 2006), are a step in the right direction.
References
Alcalá-Quintana, R., & García-Pérez, M. A. (2013). Fitting model-based psychometric functions to simultaneity and temporal-order judgment data: MATLAB and R routines. Behavior Research Methods, 45, 972–998. doi:10.3758/s13428-013-0325-2.
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van Ijzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. doi:10.1037/0033-2909.133.1.1.
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2014). lme4: Linear mixed-effects models using S4 classes. R package version 1.1-6. R. http://cran.r-project.org/package=lme4
Chapman, C. R., Tuckett, R. P., & Song, C. W. (2008). Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. Journal of Pain, 9(2), 122–145. doi:10.1016/j.jpain.2007.09.006.
Crombez, G., Van Damme, S., & Eccleston, C. (2005). Hypervigilance to pain: An experimental and clinical analysis. Pain, 116(1–2), 4–7. doi:10.1016/j.pain.2005.03.035.
Crombez, G., Van Ryckeghem, D. M. L., Eccleston, C., & Van Damme, S. (2013). Attentional bias to pain-related information: A meta-analysis. Pain, 154(4), 497–510. doi:10.1016/j.pain.2012.11.013.
De Ruddere, L., Goubert, L., Stevens, M., de C Williams, A. C., & Crombez, G. (2013). Discounting pain in the absence of medical evidence is explained by negative evaluation of the patient. Pain, 154(5), 669–676. doi:10.1016/j.pain.2012.12.018.
Durnez, W., & Van Damme, S. (2015). Trying to fix a painful problem: The impact of pain control attempts on the attentional prioritization of a threatened body location. The Journal of Pain, 16(2), 135–143.
Eccleston, C., & Crombez, G. (1999). Pain demands attention. Psychological Bulletin, 125(3), 356–366.
Eccleston, C., & Crombez, G. (2007). Worry and chronic pain: A misdirected problem solving model. Pain, 132(3), 233–236. doi:10.1016/j.pain.2007.09.014.
Folk, C. L., & Remington, R. W. (2008). Bottom-up priming of top-down attentional control settings. Visual Cognition, 16(2–3), 215–231. doi:10.1080/13506280701458804.
Hayes, S. C., Luoma, J. B., Bond, F. W., Masuda, A., & Lillis, J. (2006). Acceptance and commitment therapy: Model, processes and outcomes, 44, 1–25. doi:10.1016/j.brat.2005.06.006
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70.
Jia, L., Shi, Z., Zang, X., & Müller, H. J. (2013). Concurrent emotional pictures modulate temporal order judgments of spatially separated audio-tactile stimuli. Brain Research, 1537, 156–163. doi:10.1016/j.brainres.2013.09.008.
Legrain, V., Van Damme, S., Eccleston, C., Davis, K. D., Seminowicz, D. A., & Crombez, G. (2009). A neurocognitive model of attention to pain: Behavioral and neuroimaging evidence. Pain, 144(3), 230–232. doi:10.1016/j.pain.2009.03.020.
Moskowitz, G. B. (2002). Preconscious effects of temporary goals on attention. Journal of Experimental Social Psychology, 38, 397–404. doi:10.1016/S0022-1031(02)00001-X.
Notebaert, L., Crombez, G., Vogt, J., De Houwer, J., Van Damme, S., & Theeuwes, J. (2011). Attempts to control pain prioritize attention towards signals of pain: An experimental study. Pain, 152(5), 1068–1073. doi:10.1016/j.pain.2011.01.020.
Öhman, A. (1979). The orienting response, attention and learning: an information-processing perspective. In Kimmel H.D., Van Olst E.H. & Orlebeke J.F. (Eds.) The orienting reflex in human (pp. 55–80). Hillsdale: Erlbaum.
Rollman, G. B. (2009). Perspectives on hypervigilance. Pain, 141(3), 183–184. doi:10.1016/j.pain.2008.12.030.
Sinke, C., Schmidt, K., Forkmann, K., & Bingel, U. (2015). Phasic and tonic pain differentially impact the interruptive function of pain. PLoS One, 10(2), 1–13. doi:10.1371/journal.pone.0118363.
Spence, C., Baddeley, R., Zampini, M., James, R., & Shore, D. I. (2003). Multisensory temporal order judgments: When two locations are better than one. Perception and Psychophysics, 65(2), 318–328. doi:10.3758/BF03194803.
Spence, C., Bentley, D. E., Phillips, N., McGlone, F. P., & Jones, A. K. (2002). Selective attention to pain: A psychophysical investigation. Experimental Brain Research, 145(3), 395–402. doi:10.1007/s00221-002-1133-6.
Spence, C., & Parise, C. (2010). Prior-entry: A review. Consciousness and Cognition, 19(1), 364–379. doi:10.1016/j.concog.2009.12.001.
Spence, C., Shore, D. I., & Klein, R. M. (2001). Multisensory prior entry. Journal of Experimental Psychology: General, 130(4), 799–832.
Stein, B. E., & Stanford, T. R. (2008). Multisensory integration: current issues from the perspective of a single neuron. Nature Reviews Neuroscience, 9, 255–266.
Sullivan, M. J. L., Bishop, S., & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7, 524–535.
Titchener, E. B. (1908). Lectures on the elementary psychology of feeling and attention. New York: Macmillan.
Van Damme, S., Crombez, G., Bijttebier, P., Goubert, L., & Van Houdenhove, B. (2002). A confirmatory factor analysis of the Pain Catastrophizing Scale: Invariant factor structure across clinical and non-clinical populations. Pain, 96, 319–324. Accessed Feb 7, 2014 from http://www.sciencedirect.com/science/article/pii/S0304395901004638
Van Damme, S., Crombez, G., Eccleston, C., & Goubert, L. (2004). Impaired disengagement from threatening cues of impending pain in a cross modal cueing paradigm. European Journal of Pain, 8(3), 227–236. doi:10.1016/j.ejpain.2003.08.005.
Van Damme, S., Crombez, G., Wiech, K., Legrain, V., Peters, M. L., & Eccleston, C. (2009a). Why become more general when we can be more specific? Comment on Hollins et al. “Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis” [Pain 2009; 141:215–221], and on Rollman. Pain, 144(3), 342–343. doi:10.1016/j.pain.2009.04.035. (author reply 343–4).
Van Damme, S., Gallace, A., Spence, C., Crombez, G., & Moseley, G. L. (2009b). Does the sight of physical threat induce a tactile processing bias? Modality-specific attentional facilitation induced by viewing threatening pictures. Brain Research, 1253, 100–106. doi:10.1016/j.brainres.2008.11.072.
Van Damme, S., Legrain, V., Vogt, J., & Crombez, G. (2010). Keeping pain in mind: A motivational account of attention to pain. Neuroscience and Biobehavioral Reviews, 34(2), 204–213. doi:10.1016/j.neubiorev.2009.01.005.
Vanden Bulcke, C., Crombez, G., Spence, C., & Van Damme, S. (2014). Are the spatial features of bodily threat limited to the exact location where pain is expected? Acta Psychologica, 153, 113–119. doi:10.1016/j.actpsy.2014.09.014.
Vanden Bulcke, C., Van Damme, S., Durnez, W., & Crombez, G. (2013). The anticipation of pain at a specific location of the body prioritizes tactile stimuli at that location. Pain, 154(8), 1464–1468. doi:10.1016/j.pain.2013.05.009.
Verbruggen, F., & Aron, A. R. (2010). Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proceedings of the National Academy of Sciences, 107(31), 13966–13971. doi:10.1073/pnas.1001957107.
Weinstein, S. (1968). Intensive and extensive aspects of tactile sensitivity as a function of body part, sex and laterality. In Kenshalo D. R. (Ed.), The Skin Senses (pp. 195–200). Springfield: Thomas.
Welch, R. B., & Warren, D. H. (1980). Immediate perceptual response to intersensory discrepancy. Psychological Bulletin, 88(3), 638–667. doi:10.1037/0033-2909.88.3.638.
Acknowledgments
This study was supported by a research Grant (BOF11/STA/004) by the Special Research Foundation of Ghent University (BOF) awarded to S. Van Damme. The authors wish to thank Anand Ramamoorthy for carefully proofreading the manuscript, and Iege Bassez for valuable help with the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest that may arise as a result of this research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Durnez, W., Van Damme, S. Let it be? Pain control attempts critically amplify attention to somatosensory input. Psychological Research 81, 309–320 (2017). https://doi.org/10.1007/s00426-015-0712-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-015-0712-7