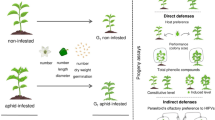
Abstract
Main conclusion
Our results indicate caterpillars and aphids cause similar levels of induced defences and resistance against caterpillars in wild cotton plants. These symmetrical effects are not consistent with patterns predicted by plant defensive signaling crosstalk and call for further work addressing the biochemical mechanisms underpinning these results.
Abstract
Plant-induced responses to attack often mediate interactions between different species of insect herbivores. These effects are predicted to be contingent on the herbivore’s feeding guild, whereby prior feeding by insects should negatively impact subsequent feeding by insects of the same guild (induced resistance) but may positively influence insects of a different guild (induced susceptibility) due to interfering crosstalk between plant biochemical pathways specific to each feeding guild. We compared the effects of prior feeding by leaf-chewing caterpillars (Spodoptera frugiperda) vs. sap-sucking aphids (Aphis gossypii) on induced defences in wild cotton (Gossypium hirsutum) and the consequences of these attacks on subsequently feeding caterpillars (S. frugiperda). To this end, we conducted a greenhouse experiment where cotton plants were either left undamaged or first exposed to caterpillar or aphid feeding, and we subsequently placed caterpillars on the plants to assess their performance. We also collected leaves to assess the induction of chemical defences in response to herbivory. We found that prior feeding by both aphids and caterpillars resulted in reductions in consumed leaf area, caterpillar mass gain, and caterpillar survival compared with control plants. Concomitantly, prior aphid and caterpillar herbivory caused similar increases in phenolic compounds (flavonoids and hydroxycinnamic acids) and defensive terpenoids (hemigossypolone) compared with control plants. Overall, these findings indicate that these insects confer a similar mode and level of induced resistance in wild cotton plants, calling for further work addressing the biochemical mechanisms underpinning these effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attack by multiple herbivore species on shared host plants has been shown to often lead to indirect interactions between the species whereby induced plant responses to one early-arriving attacker affect subsequent attackers (Rodriguez-Saona et al. 2010; Hernández-Cumplido et al. 2016; Abdala-Roberts et al. 2019a). The induced plant responses by which these indirect interactions take place are orchestrated by phytohormonal signalling pathways such as those related to jasmonic acid (JA) and salicylic acid (SA) (Howe and Jander 2008; Erb et al. 2012). A number of studies have shown that the JA pathway is mainly associated with responses against chewing insects and necrotrophic pathogens, whereas the SA pathway is mainly triggered in response to attack by phloem-feeding insect herbivores and biotrophic pathogens (Koornneef and Pieterse 2008; Pieterse et al. 2012), thus providing a framework for understanding specificity in plant-induced responses and the outcomes of plant-mediated herbivore interactions.
Empirical studies documenting the impacts of sequential attacks by herbivores associated with different plant hormonal pathways have shown that JA and SA pathways antagonize or interfere with each other (i.e. so-called crosstalk), with resulting effects on the outcome of these plant-mediated interactions (Koornneef and Pieterse 2008; Rodriguez-Saona et al. 2010; Zhang et al. 2011). This antagonism between plant pathways is predicted to arise, for example, when attackers are of a different guild (i.e. chewing vs. piercing-sucking herbivores), leading to induced susceptibility of the plant to the subsequent attacker, whereas herbivores of the same guild (and thus upregulating the same plant pathway) should lead to induced resistance and thus negative effects on the subsequent feeder (Erb et al. 2012; Thaler et al. 2012; reviewed by Moreira et al. 2018). While evidence from several plant species, mainly crops (e.g. tomato, maize, tobacco and cabbage) has often provided support for these predictions (Erb et al. 2012; Thaler et al. 2012), a recent meta-analysis suggests that antagonism between these defensive-related pathways is not necessarily the norm. In particular, the analysis found that many initial JA-inducing attackers have strong negative effects on both JA- and SA-inducing subsequent attackers, whereas SA-inducing attackers have more variable effects and no significant overall effect on either JA- or SA-triggering herbivores (Moreira et al. 2018). This suggests that JA-mediated defences have more consistent effects on subsequent attackers, whereas SA-mediated effects are much more variable. This highlights the basic need for more work across a variety of plant–insect systems to better understand plant defence upregulation and its consequences for attacking herbivores, as well as further mechanistic work to understand how or when complex (and highly reticulate) hormonal pathways function and interact (Erb et al. 2012).
Variability in predicted crosstalk effects of early herbivory on subsequently attacking herbivores reported thus far could be explained by at least two features. First, most studies have measured either plant-induced responses to initial attackers or effects on subsequent herbivores, whereas work that simultaneously measured both induced defences and herbivory or herbivore performance is less common (e.g. Poelman et al. 2008; Soler et al. 2012; Kroes et al. 2015). As a result, links between the mechanisms of plant induction and the effects on attacker performance are often poorly understood (Viswanathan et al. 2007; Soler et al. 2012). Second, studies mainly focused on the effects of one type or species of initial attacker on another (in a unidirectional manner). To obtain a more comprehensive and realistic evaluation of the indirect interactions among herbivores on shared host plants, studies should include multiple initial attackers and/or subsequent attackers. Recent work is moving in this direction (and under increasing complexity; see Mertens et al. 2021; de Bobadilla et al. 2022), but more work with different insect guilds and across more plant–insect systems is needed.
We tested the effects of prior herbivory on wild cotton (Gossypium hirsutum) by leaf-chewing caterpillars (Spodoptera frugiperda) or piercing-sucking aphids (Aphis gossypii) on the performance of subsequently feeding caterpillars (S. frugiperda). To further understand the physiological changes associated with plant secondary metabolism (i.e. induced defences) underlying plant-mediated effects, we also evaluated the effects of caterpillar and aphid feeding on the induction of phenolic compounds and terpenoid aldehydes, which are known to negatively affect insect herbivory. In particular, we asked the following: (1) Do caterpillar and aphid feeding differentially induce plant defences? (2) Does prior feeding by caterpillars or aphids affect the performance of subsequently attacking caterpillars, and how do such effects compare in direction and strength? (3) Do the induced defence patterns match (and potentially explain) impacts on subsequent caterpillar performance? Given that previous work has shown that the induction of terpenoids and phenolic compounds is associated with the JA pathway (Bi et al. 1997; Dixit et al. 2020) and triggered by feeding by JA-inducing insect herbivores (Rodriguez-Saona et al. 2010; Quijano-Medina et al. 2021), we predicted that caterpillar feeding would induce these compounds to a greater extent than SA-inducing aphids. In turn, following the crosstalk hypothesis, initial feeding by caterpillars should decrease the performance of subsequently attacking caterpillars, whereas initial feeding by aphids should have the opposite effect. Overall, this study sheds light on plant-induced responses underlying plant-mediated herbivore interactions.
Materials and methods
Study system
Gossypium hirsutum (Malvaceae) is a perennial shrub species that is naturally distributed in Central America, Mexico, and the Caribbean Basin (D’Eeckenbrugge and Lacape 2014; Yuan et al. 2021). It is especially abundant on the coast of the Yucatan Peninsula (Mexico), where it is found in the coastal shrubland under conditions of high soil salinity, low and highly seasonal rainfall, and elevated temperatures (Abdala‐Roberts et al. 2019b; Quijano-Medina et al. 2021; Clancy et al. 2023). At these sites, wild cotton is attacked mainly by leaf-chewing insects (e.g. caterpillars and grasshoppers) which cause 25% of leaf area loss on average, and to a lesser extent by phloem feeders (e.g. bugs and aphids) (Abdala-Roberts et al. 2019b). In particular, Spodoptera frugiperda (like other Spodoptera species) is a known herbivore of cultivated cotton. While it is relatively rare in wild cotton populations in Yucatan, a number of studies have shown that Spodoptera spp. induce direct (e.g. terpenoids and phenolic compounds) and indirect (volatile organic compounds and extrafloral nectar) defences in wild and cultivated cotton (Hegde et al. 2011; Zebelo et al. 2017; Arce et al. 2021; Quijano-Medina et al. 2021; Mamin et al. 2023). Similarly, Aphis gossypii can be found at some wild cotton populations (T. Quijano-Medina, pers. observation) and work with cultivated varieties has shown that it induces cotton defences (e.g. volatiles and terpenoid aldehydes) that may decrease the performance of aphid conspecifics (Hegde et al. 2011; Williams et al. 2017). Although there has been work testing for plant-mediated effects between different herbivore species in cultivated cotton (e.g. Rodríguez-Saona et al. 2003; Eisenring et al. 2018), these have not been studied in wild cotton populations. One exception is a recent study by our group testing for the effects of early caterpillar herbivory on subsequent attack by leaf-chewing insects and ants (Abdala-Roberts et al. 2019a).
Plant material
In March 2019, we collected seeds from seven plants (hereafter genotypes) separated by at least 1 m and sourced from a population located near the coastal town of Celestún, Yucatan (Mexico) (21°00ʹ 37.7ʺ N–90°19ʹ 41.9ʺ W). In December 2019, we germinated seeds in Petri dishes at 35 °C and individually transplanted them to 25 × 30 cm low-density polyethylene nursery bags containing a steam-sterilized (100 °C for l h over 3 consecutive days; Azcón and Barea (1997)) mix of sandy soil (collected from the sampling site), native tropical forest substrate, and perlite (1:1:2, by weight). After transplantation, we kept all seedlings in a greenhouse at the Campus de Ciencias Biológicas y Agropecuarias (CCBA) of the University of Yucatan (Mexico, 20°52ʹ 00.6ʺ N 89°37ʹ 29.5ʺ W) for 2.5 months before starting the herbivory experiment and we watered them three times a week with 300 mL tap water.
Herbivory treatments and experimental design
In early March 2020, when plants had 10–12 leaves, we randomly assigned plants of each genotype to one of the following treatments: undamaged control (n = 83 plants), herbivory by the aphid A. gossypii (n = 79 plants), or herbivory by the caterpillar S. frugiperda (n = 80 plants). Cotton genotypes were similarly represented across treatments. We collected aphids from a site nearby the CCBA on Ruellia nudiflora plants and placed them on cotton plants in the greenhouse 2 days before starting the experiment. In the case of S. frugiperda, we obtained eggs from a colony at the Chemical Ecology Lab in ECOSUR (Chiapas, Mexico) and reared caterpillars with a wheat germ-based artificial diet, and 2 days before the experiment we fed them wild cotton leaves. For the aphid herbivory treatment, we placed 20 adult aphids on the apical meristem or new leaves of each plant assigned to this treatment, allocated over a 3 day period (nine aphids the first day, eight the second, and three the last day) to achieve a gradual build-up in aphid density. The day after the third day of infestation, we removed aphids. For the caterpillar herbivory treatment, we placed two third-instar S. frugiperda larvae on the fifth leaf (counting from top to bottom) of each plant on the third day of aphid infestation and then covered the leaf with a mesh bag to prevent the caterpillar from escaping. We kept caterpillars on plants for 24 h and then removed them the same day aphids were removed. Two days after removing herbivores from plants, we collected the first two or three apical leaves (undamaged in the case of plants assigned herbivory) for chemical analyses (see next) for close to two thirds of the plants of each treatment (n = 58 plants for undamaged control, n = 59 for aphid treatment, and n = 65 plants for caterpillar treatment), and conducted a bioassay on remaining plants to test for treatment effects on the performance of subsequent caterpillars (n = 25 plants for undamaged control, n = 20 for aphid treatment, and n = 15 plants for caterpillar treatment).
Leaf chemical analyses
We harvested leaves, immediately frozen them on dry ice, and stored them at − 80 °C until processing (see next). We conducted quantification of terpenoids at the Fundamental and Applied Research in Chemical Ecology (FARCE Lab) and the Neuchâtel Platform of Analytical Chemistry (NPAC) at the University of Neuchâtel (Switzerland), in November 2022. Briefly, we ground frozen leaves under liquid nitrogen, and we extracted terpenoids using 50 mg of frozen leaf powder with 200 μL of a solution of acetonitrile, Milli-Q water, and formic acid (80:18.5:1.5, by vol.). We homogenized samples with three to five glass beads (1.25–1.65 mm diameter) in a mixer mill for 3 min at 30 Hz (TissueLyser II, Qiagen, Hilden, Germany) and ultrasonicated for 5 min. They were then centrifuged for 3 min at 8000 g. We centrifugated the recovered supernatant a second time before transferred it to amber glass vials. We directly analysed samples through ultra-performance liquid chromatography with diode array detection (UPLC-DAD, Ultimate 3000 Dionex, Thermo Fisher Scientific, Waltham, MA, USA). The DAD detector was set at 288 ± 2 nm. 10 μL was injected into an ACQUITY UPLC® BEH C18 column (2.1 × 100 mm, 1.7 μm; Waters). We held the flow rate constant at 0.45 mL min−1, and kept the temperature at 40 °C. The mobile phase solvent A consisted of 0.05% formic acid in Milli-Q water (18 Ω), and the mobile phase solvent B consisted of 0.05% formic acid in acetonitrile (HiPerSolv, VWR Chemicals®, Fontenay-sous-Bois, France). We increased solvent B from 45 to 90% in 8 min, then to 100% in 0.5 min, and held it at 100% for 2.5 min, which was followed by re-equilibration at 45% solvent B for 3.5 min. We identified hemigossypolone, gossypol, and heliocides (grouped together) by their retention time. We quantified terpenoids based on linear regression from six calibration points (5–250 μg/mL) in gossypol equivalents. We expressed concentrations in μg/g tissue on a fresh mass (weight) basis.
We conducted analyses of phenolic compounds at the Misión Biológica de Galicia (MBG-CSIC, Salcedo, Pontevedra, Spain) in August 2020. We extracted these compounds from 20 mg of dry plant tissue (dried at 35ºC for 24 h and macerated to powder) with 1 mL of 70% methanol in an ultrasonic bath for 15 min, followed by centrifugation. For phenolic compound identification, we used ultra-performance liquid chromatography coupled with electrospray ionization (ESI) quadrupole (Thermo Dionex Ultimate 3000 LC) time-of-flight mass spectrometry (UPLC-Q-TOF–MS/MS) (Bruker Compact™). We performed chromatographic separation in a Kinetex™ 2.6 µm C18 82–102 Å and LC column 100 × 4.6 mm column using a binary gradient solvent mode consisting of 0.05% formic acid in water (solvent A) and acetonitrile (solvent B). We used the following gradient: from 10 to 30% solvent B (0–5 min), from 30 to 50% solvent B (5–10 min), from 50 to 100% solvent B (10–12 min), hold 100% solvent B until 14 min, from 100 to 10% solvent B (14–15 min), and hold 10% solvent B until 17 min. The injection volume was 3 µL, we established the flow rate at 0.4 mL/min, and controlled the column temperature at 35 °C. We operated MS analysis in a spectra acquisition range from 50 to 1200 m/z. We used negative (−) ESI modes under the following specific conditions: gas flow 8 L min−1, nebulizer pressure 38 psi, dry gas 7 L min−1, and dry temperature 220 °C. We set capillary and end-plate offsets to 4500 and 500 V, respectively. We performed MS/MS analysis based on the previously determined accurate mass and RT and fragmented using different collision energy ramps to cover a range from 15 to 50 eV. We identified individual compounds based on the data obtained from the standard substances or published literature including RT, λmax, ([M–H] −), and major fragment ions. For phenolic compound quantification, we injected 3 µL of each sample using the same column and conditions mentioned in the previous paragraph, in an UHPLC (Nexera LC-30AD; Shimadzu) equipped with a Nexera SIL-30AC injector and one SPD-M20A UV/VIS photodiode array detector. We recorded chromatograms at 330 nm. We identified two groups of phenolic compounds from the samples, namely flavonoids and hydroxycinnamic acids. We quantified flavonoids as rutin equivalents and hydroxycinnamic acids as ferulic acid equivalents. We achieved the quantification of these compounds by external calibration using calibration curves at 0.25, 0.5, 1, 2, and 5 μg mL−1. We expressed concentrations were expressed in μg/g tissue on a dry mass (weight) basis.
Caterpillar performance bioassay
We initially fed larvae used for this bioassay on artificial diet and then fed them on wild cotton 24 h before the experiment. We starved larvae for 12 h before the bioassay and individually weighed them for two hours before being placed on plants used for the bioassay. For each experimental plant, we placed a single second-instar S. frugiperda larva for 3 days on the third or fourth leaf using a clip cage. We recorded survival every 24 h, measured leaf consumption at 48 h, and weighed surviving larvae at 72 h. We estimated the amount of leaf area consumed by placing a transparent acetate on each leaf to shade the missing area and then overlaying it on a sheet with a 1 mm2 grid. We then counted the area of squares corresponding to missing tissue and expressed the leaf area consumed in mm2. Caterpillar mortality was very low (< 5%) during the first 24 h and therefore did not affect leaf consumption results. Finally, we estimated the effects on caterpillar growth in terms of mass gain as the difference between initial mass and that at 72 h (in mg).
Statistical analyses
We ran general linear mixed models testing for the effects of herbivory treatment (three levels: control, caterpillars, and aphids) on the concentration of terpenoids (hemigossypolone, gossypol, and heliocides in μg/g FW), phenolic compounds (hydroxycinnamic acids and flavonoids, in μg/g DW), caterpillar survival (binary), leaf area consumed (mm2), and caterpillar mass gain (mg). We log-transformed terpenoid and hydroxycinnamic acid concentrations to achieve the normality of residuals, and we analysed survival with a binomial distribution (logit link). Models also included plant genotype (treated as random) to control for genetic variation and/or maternal effects. We report model least-square means and standard errors (back-transformed when applicable) as descriptive statistics. We performed all analyses in R (RStudioTeam 2016), using the rstatix package (Kassambara 2023). When the herbivory treatment was statistically significant, we performed follow-up Tukey’s tests to assess pairwise differences between treatment level means using the emmeans package (Lenth 2020).
Results
Effects of prior herbivory on wild cotton induced defences
We found a significant effect of prior herbivory treatment on the concentration of leaf hemigossypolone (Table 1a). Specifically, aphid and caterpillar herbivory drove significant increases (24% and 15%, respectively) in the concentration of hemigossypolone relative to controls (aphid herbivory: 1457.7 ± 69 μg/g; caterpillar herbivory: 1345.5 ± 64.4 μg/g; control: 1169.7 ± 55.7 μg/g; Fig. 1a). The aphid and caterpillar treatments did not differ significantly (Fig. 1a), indicating that caterpillars and aphids drove a similar magnitude of induction of this compound. In contrast, there was no significant effect of prior herbivory on the concentration of heliocides or gossypol (Table 1a; Fig. 1b, c).
Effects of wild cotton (Gossypium hirsutum) prior herbivory treatment, namely undamaged, caterpillar (Spodoptera frugiperda) or aphid (Aphis gossypii) feeding on the concentration of terpenoid aldehydes (a, b, c) and phenolic compounds (d, e) expressed as μg/g FW or DW, respectively. Bars are model least-squares means and standard errors (n = 58 plants for undamaged control, n = 59 for aphid treatment, and n = 65 plants for caterpillar treatment). Different letters above the bars indicate statistically significant differences (at P < 0.05) between treatments
We also found a significant effect of prior herbivory treatment on the concentration of leaf phenolic compounds (Table 1b). Again, aphid and caterpillar herbivory drove significant increases (77% and 62%, respectively) in the concentration of flavonoids relative to controls (aphid herbivory: 3.92 ± 0.31 μg/g; caterpillar herbivory: 3.59 ± 0.29 μg/g; control: 2.22 ± 0.18 μg/g; Fig. 1d). Likewise, aphid and caterpillar herbivory caused significant increases (91% and 62%, respectively) in hydroxycinnamic acid concentration relative to controls (aphid herbivory: 4.27 ± 0.38; caterpillar herbivory: 3.63 ± 0.33 μg/g; control: 2.23 ± 0.20 μg/g; Fig. 1e). The aphid and caterpillar herbivory treatments did not differ significantly for either flavonoids or hydroxycinnamic acids (Fig. 1d, e).
Effects of prior herbivory on subsequently feeding caterpillars
The prior herbivory treatment had a significant effect on several performance-related variables of subsequently feeding S. frugiperda caterpillars (Table 1c). In particular, we found that S. frugiperda consumed significantly less leaf area on plants previously attacked by aphids and caterpillars (72% and 77%, respectively), compared with undamaged controls (aphid herbivory: 72.8 ± 43 mm2; caterpillar herbivory: 58.4 ± 56.4; control: 257.3 ± 39.6 mm2) (Table 1c; Fig. 2a). Caterpillar leaf consumption did not differ significantly between the prior aphid and caterpillar herbivory treatments (Table 1c; Fig. 2a). In addition, the prior herbivory treatment had a significant effect on caterpillar weight gain (Table 1c), with the mean value being significantly (59%) lower for the aphid treatment relative to controls (Fig. 2b). The prior caterpillar herbivory treatment did not differ significantly from controls but showed a similar trend in reduction (51% lower than controls), and the herbivory treatments did not differ themselves (aphid herbivory: 14.1 ± 5.84 mg; caterpillar herbivory: 16.9 ± 8.24 mg; control: 34.7 ± 4.68 mg) (Fig. 2b). Finally, caterpillar survival was also significantly affected by the prior herbivory treatment (Table 1c), with 88% (21 of 24) of caterpillars surviving on control plants, 70% (14 of 20) on plants subjected to prior aphid herbivory, and 47% (7 of 15) on prior caterpillar herbivory. The binomial model indicated that prior herbivory by caterpillars drove a significant reduction in caterpillar survival (46%) relative to controls (Fig. 2c), whereas prior aphid herbivory did not differ from controls but showed a similar trend in reduction (albeit weaker, 20%) relative to controls (Fig. 2c). Herbivory treatments did not differ themselves (aphid herbivory: 0.7 ± 0.12; caterpillar herbivory: 0.47 ± 0.13; control: 0.88 ± 0.07) (Fig. 2c).
Effects of wild cotton (Gossypium hirsutum) prior herbivory treatment, namely undamaged, caterpillar (Spodoptera frugiperda) or aphid (Aphis gossypii) feeding the performance, estimated as foliar area consumed in mm2 (a), mass gain in mg (b), and survival as back-transformed logit, i.e., odds ratio values (c) of subsequently feeding S. frugiperda caterpillars. Bars are model least-squares means and standard errors (n = 25 plants for undamaged control, n = 20 for the prior aphid treatment, and n = 15 plants for the prior caterpillar treatment). Different letters above bars indicate statistically significant differences (at P < 0.05) between treatments
Discussion
Our findings indicated that prior feeding by aphids and caterpillars similarly induced chemical defences in wild cotton, namely of phenolic compounds and terpenoids. This presumably in turn drove reductions in performance-related traits of subsequently feeding S. frugiperda larvae. That aphids were found to have similar effects on cotton defences and induce resistance as caterpillars was somewhat surprising, especially the fact that the magnitude of the effects was strikingly similar between the two herbivores.
Effects of caterpillar and aphid herbivory on cotton defence induction
We found that hemigossypolone, flavonoids, and hydroxycinnamic acids were significantly induced after prior herbivory in wild cotton, and in most cases, caterpillar and aphid feeding produced a similar level of induction of these compounds. The induction of phenolic compounds has been well studied for plants attacked by chewing insects, i.e., JA-associated (e.g. Stout et al. 1994; Nykänen and Koricheva 2004; Złotek et al. 2019). In the case of cotton, studies have reported induced responses to chewing and phloem-feeding insects, by and large with cultivated varieties (but see Abdala-Roberts et al. 2019a; Quijano-Medina et al. 2021), including work on phenolic compounds and terpenoids (e.g. McAuslane et al. 1997; Opitz et al. 2008; Zebelo et al. 2017; reviewed by Hagenbucher et al. 2013). Whereas work measuring cotton defence induction by chewing insects, mainly caterpillars, has often found increases in JA-associated secondary metabolites (e.g. terpenoids; see Eisenring et al. 2018), studies involving phloem-feeding insects have reported more variable findings. Some have found that they can induce certain JA-associated phenolic compounds (Rodriguez-Saona et al. 2010; for tomato, see Su et al. 2020), including enzymes involved in the synthesis of jasmonates and phenolic compounds, namely LOX and PAL, in the case of A. gossypii (Qin et al. 2005). In addition, Eisenring et al. (2018) found that A. gossypii reduced cotton SA levels and did not induce terpenoids. Other phloem feeders such as mealybugs and whiteflies have been shown to suppress JA-dependent induced responses (Zarate et al. 2007; Zhang et al. 2019), including work with cotton plants (Zhang et al. 2011). A closer look at the metabolic pathways associated with different types of phenolic compounds (relative to terpenoids) can shed light on these findings. Terpenoid production is strongly dependent on JA (Singh and Sharma 2014; Rosenkranz et al. 2021), whereas phenolic compounds comprise different classes associated with seemingly different pathways and, in some cases, their induction patterns could reflect co-dependency on more than one pathway (Appel 1993; Mouradov and Spangenberg 2014). In our case, for example, hydroxycinnamic acid and flavonoid metabolism is related to both the JA and SA pathways (Mouradov and Spangenberg 2014).
Given that A. gossypii is a cotton specialist, we should also consider the possibility that the aphids have evolved a way to manipulate their host plant and that they might benefit from inducing the JA defence pathway. Similar to biotrophic pathogens, piercing-sucking insects commonly cause the upregulation of SA defences (Morkunas et al. 2011), which can lead to callose formation and render leaves less penetrable to insect stylets (Ellinger and Voigt 2014; Li et al. 2018), as shown also in cotton (Tanatsiwa Mbiza et al. 2022). If the aphid indeed controls cotton defence induction, it might circumvent this response by upregulating JA defences to suppress SA defences. For the whitefly Bemisia tabaci, the opposite has been proposed, and it appears to benefit from the fact that it upregulates the SA pathway at the cost of the JA pathway (Zarate et al. 2007; Zhang et al. 2013, 2019). It could be that for A. gossypii the reverse holds true and that it will perform better on already-infested cotton plants. This could be tested by conducting a complete factorial design involving aphid early herbivory effects on subsequently feeding aphids and caterpillars to test whether aphid–caterpillar indirect interactions are reciprocal and symmetrical (or not) in strength.
We note that there were no significant effects of prior herbivory (by either insect) on gossypol or heliocides. Previous work on cultivated cotton has highlighted hemigossypolone and heliocides as being more responsive than gossypol to induction in young leaves (Opitz et al. 2008). Our sampling time point of 2 days after herbivory may have missed the peak accumulation of gossypol and heliocides, as these compounds are derived from hemigossypol and hemigossypolone, respectively, and they accumulate some days after induction by herbivory (McAuslane et al. 1997; Bezemer et al. 2004; Eisenring et al. 2018). Furthermore, the lack of significant induction of gossypol and heliocides could also be attributed to differences in the defence responses between wild and cultivated cotton. In this sense, previous work has found differences in the inducibility of direct defences among cotton-cultivated varieties (Agrawal and Karban 2000). Follow-up work involving measurements of hormones, precursors of end products, and defence-related gene expression levels (Huang et al. 2015; Li et al. 2016) is needed to elucidate the molecular and biochemical mechanisms behind the upregulation of different types of phenolic compounds and terpenoids under herbivory by chewers and phloem feeders on cotton, including wild genotypes.
Effects of prior herbivory on subsequently feeding caterpillars
Mirroring herbivory effects on wild cotton defence induction, the performance of subsequently feeding S. frugiperda decreased when feeding on plants subjected to prior herbivory by aphids or caterpillars. Both types of prior herbivory caused similar reductions in leaf consumption by subsequently feeding caterpillars, whereas only prior aphid feeding significantly reduced mass gain and only prior caterpillar feeding significantly reduced the survival of subsequent caterpillars. Still, reductions in mass gain relative to controls were of similar magnitude in both cases (though more variable for prior caterpillar herbivory) and aphids tended to also reduce survival, suggesting biologically meaningful effects in these cases. The fact that aphid and caterpillar herbivory generally reduced S. frugiperda performance to a similar extent is inconsistent with the crosstalk hypothesis, according to which aphid feeding would be expected to result in induced susceptibility to caterpillars and therefore higher (rather than lower) caterpillar performance due to JA-SA antagonism (Pieterse et al. 2012; Thaler et al. 2012). Whereas previous studies with other plant species such as tomato, cabbage, and milkweed have indeed shown that piercing-sucking insects positively influence the performance of subsequent chewing herbivores (e.g. Rodriguez-Saona et al. 2010; Soler et al. 2012; Ali and Agrawal 2014), work with others have found either no effect or negative effects of piercing-sucking on later-attacking chewing insects (e.g. Soler et al. 2012; Kroes et al. 2016). For cotton, a previous study by Eisenring et al. (2018) reported that A. gossypii prior feeding did not affect the performance of subsequently feeding S. littoralis. However, this study was conducted with cultivated cotton plants and measured responses by another caterpillar species (S. littoralis) that is known as a well-adapted cotton pest in North Africa and the Middle East (Salama et al. 1970; Hosny et al. 1986). This leaves open several questions, such as whether the same types of compounds were induced by aphid feeding, whether they have a role in induced resistance against different Spodoptera species, and whether there are differences between cultivated vs. wild genotypes. Moreover, other intervening factors such as aphid density could result in contrasting effects on subsequent caterpillars as shown in Arabidopsis (Kroes et al. 2015).
Matching patterns of prior aphid and caterpillar herbivory effects on cotton-induced defences and performance of subsequently feeding S. frugiperda suggest that observed increases in phenolic compounds and the terpenoid aldehyde hemigossypolone were responsible for induced resistance to caterpillars. In cultivated cotton, flavonoids and other phenolic compounds have been shown to reduce herbivory by Helicoverpa zea (Bi et al. 1997), H. armigera, S. litura (Dixit et al. 2017), and Heliothis virescens (Hedin et al. 1988). In wild cotton, a recent study by our group also showed a negative correlation between leaf flavonoid levels and herbivory by chewing insects in a field experiment (Abdala‐Roberts et al. 2019a). Induction of terpenoid compounds such as gossypol and heliocides has similarly been associated with reductions in growth and/or feeding of Spodoptera spp. (e.g. McAuslane et al. 1997; Eisenring et al. 2018; Mamin et al. 2023). While we did not find evidence that these two compound types were significantly induced by prior herbivory, hemigossypolone, a product of the same pathway, was induced and this is consistent with these prior findings. It is important to acknowledge that we did not measure JA and SA to directly address crosstalk mechanisms. Further work assessing the biochemical underpinnings of these interaction outcomes (e.g. correlating metabolite concentrations with JA and SA levels or inducing plants with JA and SA artificial elicitors) is needed to better understand why results deviate from predictions by the defensive crosstalk hypothesis.
Final remarks
Research on plant-mediated interactions has focused more on the effects of early feeding by chewing insects compared with that by early sucking-piercing insects (or pathogens) (Moreira et al. 2018). By testing for aphid initial effects, our findings could have potentially important implications for cotton-mediated interactions among insect herbivores. Having said this, our experiment used aphids sourced from a single site and tested for the effects of a caterpillar species that is infrequent in wild cotton populations. To further deepen our understanding of plant-mediated interactions in this species, work including additional insect herbivores, both native to wild cotton and native and non-native pests on cultivated cotton, would be highly valuable, together with comparisons (e.g. in common garden experiments) of responses by and plant-mediated effects with both cultivated and wild cotton to shed insight into the ecology and evolution of cotton induced defences and the effects of domestication and agricultural settings on plant-mediated effects (Gols and Harvey 2023). Field experiments should also consider detailed measurements of plant-induced responses involving multiple types of defence compounds (end products) and their precursors, hormone levels (see above), and leaf nutrient content to better explain the mechanisms behind the effects on herbivore performance. In addition, reciprocal tests of plant-mediated effects are needed to test whether there is an asymmetry in effects (e.g. Ali and Agrawal 2014), as well as a function of the order of arrival and identity of early vs. late herbivores (Poelman et al. 2008; Soler et al. 2012). Such detailed studies are essential for a more robust understanding of the induced responses governing the outcome of plant-mediated interactions between herbivores.
Data availability
The data generated or analysed during this study are not included in this article but can be made available as per request.
Change history
28 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00425-024-04344-y
Abbreviations
- JA:
-
Jasmonic acid
- SA:
-
Salicylic acid
References
Abdala-Roberts L, Pérez Niño B, Moreira X, Parra-Tabla V, Grandi L, Glauser G, Benrey B, Turlings TCJ (2019a) Effects of early-season insect herbivory on subsequent pathogen infection and ant abundance on wild cotton (Gossypium hirsutum). J Ecol 107:1518–1529. https://doi.org/10.1111/1365-2745.13131
Abdala-Roberts L, Quijano-Medina T, Moreira X, Vázquez-González C, Parra-Tabla V, Berny Mier Y, Teran J, Grandi L, Glauser G, Turlings TCJ, Benrey B (2019b) Bottom-up control of geographic variation in insect herbivory on wild cotton (Gossypium hirsutum) by plant defenses and climate. Am J Bot 106:1059–1067. https://doi.org/10.1002/ajb2.1330
Agrawal AA, Karban R (2000) Specificity of constitutive and induced resistance: pigment glands influence mites and caterpillars on cotton plants. Entomol Exp Appl 9:39–49. https://doi.org/10.1046/j.1570-7458.2000.00677.x
Ali JG, Agrawal AA (2014) Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct Ecol 28:1404–1412. https://doi.org/10.1111/1365-2435.12271
Appel HM (1993) Phenolics in ecological interactions: The importance of oxidation. J Chem Ecol 19:1521–1552. https://doi.org/10.1007/BF00984895
Arce CM, Besomi G, Glauser G, Turlings TCJ (2021) Caterpillar-induced volatile emissions in cotton: the relative importance of damage and insect-derived factors. Front Plant Sci 12:709858. https://doi.org/10.3389/fpls.2021.709858
Azcón R, Barea JM (1997) Mycorrhizal dependency of a representative plant species in Mediterranean shrublands (Lavandula spica L.) as a key factor to its use for revegetation strategies in desertification-threatened areas. Appl Soil Ecol 7:83–92. https://doi.org/10.1016/S0929-1393(97)00013-9
Bezemer TM, Wagenaar IR, van Dam NM, van der Putten WH, Wäckers FL (2004) Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol 30:53–67
Bi JL, Murphy JB, Felton GW (1997) Antinutritive and oxidative components as mechanisms of induced resistance in cotton to Helicoverpa zea. J Chem Ecol 23:97–117. https://doi.org/10.1023/B:JOEC.0000006348.62578.fd
Clancy MV, Mamine M, Flückinger G, Quijano-Medina T, Pérez-Niño B, Abdala-Roberts L, Turlings TCJ, Bustos-Segura C (2023) Terpene chemotypes in wild cotton (Gossypium hirsutum) from the Yucatan Peninsula. Phytochemistry 205:113454. https://doi.org/10.1016/j.phytochem.2022.113454
D’Eeckenbrugge G, Lacape JM (2014) Distribution and differentiation of wild, feral, and cultivated populations of perennial upland cotton (Gossypium hirsutum L.) in Mesoamerica and the Caribbean. PloS One 9:e107458. https://doi.org/10.1371/journal.pone.0107458
de Bobadilla MF, Weichen RV, Gort G, Poelman EH (2022) Plasticity in induced resistance to sequential attack by multiple herbivores in Brassica nigra. Oecologia 198:11–20. https://doi.org/10.1007/s00442-021-05043-1
Dixit G, Praveen A, Tripathi T, Yadav VK, Verma PC (2017) Herbivore-responsive cotton phenolics and their impact on insect performance and biochemistry. J Asia-Pac Entomol 20:341–351. https://doi.org/10.1016/j.aspen.2017.02.002
Dixit G, Srivastava A, Rai KM, Dubey RS, Srivastava R, Verma PC (2020) Distinct defensive activity of phenolics and phenylpropanoid pathway genes in different cotton varieties toward chewing pests. Plant Signal Behav 15:e1747689. https://doi.org/10.1080/15592324.2020.1747689
Eisenring M, Glauser G, Meissle M, Romeis J (2018) Differential impact of herbivores from three feeding guilds on systemic secondary metabolite induction, phytohormone levels and plant-mediated herbivore interactions. J Chem Ecol 44:1178–1189. https://doi.org/10.1007/s10886-018-1015-4
Ellinger D, Voigt CA (2014) Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot 114:1349–1358. https://doi.org/10.1093/aob/mcu120
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259. https://doi.org/10.1016/j.tplants.2012.01.003
Gols R, Harvey JA (2023) Integrating chemical plant trait- and ecological-based approaches to better understand differences in insect herbivory between cultivated and natural systems. Agr Ecosyst Environ 356:108643. https://doi.org/10.1016/j.agee.2023.108643
Hagenbucher S, Olson DM, Ruberson JR (2013) Resistance mechanisms against arthropod herbivores in cotton and their interactions with natural enemies. Cr Rev Plant Sci 32:458–482. https://doi.org/10.1080/07352689.2013.809293
Hedin PA, Parrott WL, Jenkins JN, Mulrooney JE, Menn JJ (1988) Elucidating mechanisms of tobacco budworm resistance to allelochemicals by dietary tests with insecticide synergists. Pestic Biochem Physiol 32:55–61. https://doi.org/10.1016/0048-3575(88)90121-6
Hegde M, Oliveira JN, da Costa JG, Bleicher E, Santana AEG, Bruce TJA, Caulfield J, Dewhirst SY, Woodcock CM, Pickett JA, Birkett MA (2011) Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii. J Chem Ecol 37:741–750. https://doi.org/10.1007/s10886-011-9980-x
Hernández-Cumplido J, Glauser G, Benrey B (2016) Cascading effects of early-season herbivory on late-season herbivores and their parasitoids. Ecology 97:1283–1297. https://doi.org/10.1890/15-1293.1
Hosny MM, Topper CP, Moawad GM, El-Saadany GB (1986) Economic damage thresholds of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) on cotton in Egypt. Crop Prot 5:100–104. https://doi.org/10.1016/0261-2194(86)90088-8
Howe AG, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66. https://doi.org/10.1146/annurev.arplant.59.032607.092825
Huang X-Z, Chen J-Y, Xiao H-J, Xiao Y-T, Wu J, Wu J-X, Zhou J-J, Zhang Y-J, Guo Y-Y (2015) Dynamic transcriptome analysis and volatile profiling of Gossypium hirsutum in response to the cotton bollworm Helicoverpa armigera. Sci Rep 5:11867. https://doi.org/10.1038/srep11867
Kassambara A (2023) rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.2. Retrieved from https://rpkgs.datanovia.com/rstatix/
Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146:839–844. https://doi.org/10.1104/pp.107.112029
Kroes A, van Loon JJA, Dicke M (2015) Density-dependent interference of aphids with caterpillar-induced defenses in Arabidopsis: Involvement of phytohormones and transcription factors. Plant Cell Physiol 56:98–106. https://doi.org/10.1093/pcp/pcu150
Kroes A, Stam JM, David A, Boland W, van Loon JJA, Dicke M, Poelman EH (2016) Plant-mediated interactions between two herbivores differentially affect a subsequently arriving third herbivore in populations of wild cabbage. Plant Biol 18:981–991. https://doi.org/10.1111/plb.12490
Lenth R (2020) emmeans: Estimated marginal means, aka least-squares means. Package version 1.8.9. Retrieved from https://github.com/rvlenth/emmeans
Li J, Zhu L, Hull JJ, Liang S, Daniell H, Jin S, Zhang X (2016) Transcriptome analysis reveals a comprehensive insect resistance response mechanism in cotton to infestation by the phloem feeding insect Bemisia tabaci (whitefly). Plant Biotechnol 14:1956–1975. https://doi.org/10.1111/pbi.12554
Li B, Förster C, Robert CAM, Züst T, Hu L, Machado RAR, Berset J-D, Handrick V, Knauer T, Hensel G, Chen W, Kumlehn J, Yang P, Keller B, Gershenzon J, Jander G, Kölner TG, Erb M (2018) Convergent evolution of a metabolic switch between aphid and caterpillar resistance in cereals. Sci Adv 4:eaat6797. https://doi.org/10.1126/sciadv.aat6797
Mamin M, Vallat A, Turlings TCJ (2023) Cotton plants as ideal models for teaching and research on inducible direct plant defenses. Front Ecol Evol 11:1119472. https://doi.org/10.3389/fevo.2023.1119472
McAuslane HJ, Alborn HT, Toth JP (1997) Systemic induction of terpenoid aldehydes in cotton pigment glands by feeding of larval Spodoptera exigua. J Chem Ecol 23:2861–2879. https://doi.org/10.1023/A:1022575313325
Mertens D, Fernández de Bobadilla M, Rusman Q, Bloem J, Douma JC, Poelman EH (2021) Plant defence to sequential attack is adapted to prevalent herbivores. Nat Plants 7:1347–1353. https://doi.org/10.1038/s41477-021-00999-7
Moreira X, Abdala-Roberts L, Castagneyrol B (2018) Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J Ecol 106:2353–2364. https://doi.org/10.1111/1365-2745.12987
Morkunas I, Mai VC, Gabryś B (2011) Phytohormonal signaling in plant responses to aphid feeding. Acta Physiol Plant 33:2057–2073. https://doi.org/10.1007/s11738-011-0751-7
Mouradov A, Spangenberg G (2014) Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front Plant Sci 5:620. https://doi.org/10.3389/fpls.2014.00620
Nykänen H, Koricheva J (2004) Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos 2:247–268. https://doi.org/10.1111/j.0030-1299.2004.12768.x
Opitz S, Kunert G, Gershenzon J (2008) Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J Chem Ecol 34:508–522. https://doi.org/10.1007/s10886-008-9453-z
Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Bi 28:489–521. https://doi.org/10.1146/annurev-cellbio-092910-154055
Poelman EH, Broekgaarden C, van Loon JJA, Dicke M (2008) Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol Ecol 17:3352–3365. https://doi.org/10.1111/j.1365-294X.2008.03838.x
Qin Q, Shi X, Liang P, Gao X (2005) Induction of phenylalanine ammonia-lyase and lipoxygenase in cotton seedlings by mechanical wounding and aphid infestation. Prog Nat Sci 15:419–423. https://doi.org/10.1080/10020070512331342330
Quijano-Medina T, Turlings TCJ, Sosenski P, Grandi L, Cervera JC, Moreira X, Abdala-Roberts L (2021) Effects of soil salinity on the expression of direct and indirect defences in wild cotton Gossypium hirsutum. J Ecol 109:354–3698. https://doi.org/10.1111/1365-2745.13483
Rodriguez-Saona C, Crafts-Brandner SJ, Canas LA (2003) Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J Chem Ecol 29:2539–2550. https://doi.org/10.1023/A:1026314102866
Rodriguez-Saona C, Musser RO, Vogel H, Hum-Musser SM, Thaler JS (2010) Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J Chem Ecol 36:1043–1057. https://doi.org/10.1007/s10886-010-9854-7
Rosenkranz M, Chen Y, Zhu P, Vlot AC (2021) Volatile terpenes – mediators of plant-to-plant communication. Plant J 108:617–631. https://doi.org/10.1111/tpj.15453
RStudioTeam (2016) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA: RStudio, Inc. Retrieved from http://www.rstudio.com/
Salama HS, Dimetry NZ, Salem SA (1970) On the host preference and biology of the cotton leaf worm Spodoptera littoralis. Z Angew Entomol 67:261–266. https://doi.org/10.1111/j.1439-0418.1971.tb02122.x
Singh B, Sharma RA (2014) Plant terpenes: defence responses, phylogenetic analysis, regulation. 3 Biotech 5:129–151
Soler R, Badenes-Pérez FR, Broekgaarden C, Zheng S, David A, Boland W, Dicke M (2012) Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: from insect performance to gene transcription. Funct Ecol 26:156–166. https://doi.org/10.1111/j.1365-2435.2011.01902.x
Stout MJ, Workman J, Duffey SS (1994) Differential induction of tomato foliar proteins by arthropod herbivores. J Chem Ecol 20:2575–2594. https://doi.org/10.1007/BF02036193
Su Q, Yang F, Yao Q, Peng Z, Tong H, Wang S, Wen X, Wu Q, Zhang Y (2020) A non-vector herbivore indirectly increases the transmission of a vector-borne virus by reducing plant chemical defences. Funct Ecol 34:1091–1101. https://doi.org/10.1111/1365-2435.13535
Tanatsiwa Mbiza NI, Hu Z, Zhang H, Zhang Y, Luo X, Wang Y, Wang Y, Liu T, Li J, Wang X, Zhang J, Yu Y (2022) GhCalS5 is involved in cotton response to aphid attack through mediating callose formation. Front Plant Sci 13:892630. https://doi.org/10.3389/fpls.2022.892630
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270. https://doi.org/10.1016/j.tplants.2012.02.010
Viswanathan DV, Lifchits OA, Thaler JS (2007) Consequences of sequential attack for resistance to herbivores when plants have specific induced responses. Oikos 116:1389–1399. https://doi.org/10.1111/j.0030-1299.2007.15882.x
Williams L, Rodriguez-Saon C, Castle SC (2017) Methyl jasmonate induction of cotton: a field test of the ‘ attract and reward ’ strategy of conservation biological control. AoB Plants 9:1–15. https://doi.org/10.1093/aobpla/plx032
Yuan D, Grover CE, Hu G, Pan M, Miller ER, Conover JL, Hunt SP, Udall JA, Wendel JF (2021) Parallel and intertwining threads of domestication in allopolyploid cotton. Adv Sci 8:2003634. https://doi.org/10.1002/advs.202003634
Zarate SI, Louisa A, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143:866–875
Zebelo S, Disi J, Balusu R, Reeves B, Fadamiro H (2017) Spodoptera exigua modulates gossypol biosynthesis in cotton Gossypium hirsutum. J Plant Interact 12:121–127. https://doi.org/10.1080/17429145.2017.1298853
Zhang P, Zhu X, Huang F, Liu Y, Zhang J, Lu Y, Ruan Y (2011) Suppression of jasmonic acid-dependent defense in cotton plant by the mealybug Phenacoccus solenopsis. PLoS One 6:e22378. https://doi.org/10.1371/journal.pone.0022378
Zhang P-J, Xu C-X, Lu Y-B, Zhang J-M, Liu Y-Q, David A, Boland W, Turlings TCJ (2013) Phloem-feeding whiteflies can fool their host plants, but not their parasitoids. Funct Ecol 27:1304–1312. https://doi.org/10.1111/1365-2435.12132
Zhang P-J, Wei J-N, Zhao C, Zhang Y-F, Li C-Y, Liu S-S, Dicke M, Yu X-P, Turlings TCJ (2019) Airborne host-plant manipulation by whiteflies via an inducible blend of plant volatiles. Proc Natl Acad Sci USA 116:7387–7396. https://doi.org/10.1073/pnas.1818599116
Złotek U, Szymanowska U, Jakubczyk A, Sikora M, Świeca M (2019) Effect of arachidonic and jasmonic acid elicitation on the content of phenolic compounds and antioxidant and anti-inflammatory properties of wheatgrass (Triticum aestivum L.). Food Chem 288:256–261. https://doi.org/10.1016/j.foodchem.2019.02.124
Acknowledgements
We thank A. Vallat for help with running samples with the HPLC. In addition, Nicolás Salinas, Emiliano Sosa, Alexander Suárez, Biiniza Pérez, Martha Reyes, and Diego Angulo provided assistance in the field. The authors have no conflicts of interest to declare.
Funding
This study was in part supported by funds from the Swiss Science Foundation awarded to TCJT (315230_185319), two grants from the Spanish National Research Council (COOPA20477 and INCGL20004) to XM and LAR, and a grant from the Regional Government of Galicia (IN607D 2016/001) to XM.
Author information
Authors and Affiliations
Contributions
TQM, LAR, and XM conceived the ideas and designed the methodology; TQM collected the data; TQM and LAR analysed the data; TQM, USR, MM, MC, MF, and CBS performed the chemical analyses; and TQM, LAR, and XM wrote the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work and have nothing to disclose.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quijano-Medina, T., Interian-Aguiñaga, J., Solís-Rodríguez, U. et al. Aphid and caterpillar feeding drive similar patterns of induced defences and resistance to subsequent herbivory in wild cotton. Planta 258, 113 (2023). https://doi.org/10.1007/s00425-023-04266-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04266-1