Abstract
Main conclusion
In this study, we show that ectopic expression of either HtNHX1 or HtNHX2, from Helianthus tuberosus plant (located at vacuolar and endosome membranes, respectively), in rice plants could enhance its tolerance to aluminum (Al3+) stress and soil acidity.
Abstract
Plant sodium (potassium)/proton (Na+(K+)/H+ antiporters of the NHX family have been extensively characterized as they are related to the enhancement of salt tolerance. However, no previous study has reported NHX transporter functions in plant tolerance to Al3+ toxicity. In this study, we demonstrate their role as a component of the Al3+ stress tolerance mechanism. We show that the ectopic expression of either HtNHX1 or HtNHX2 , from Helianthus tuberosus plant, in rice (located at vacuole and endosome, respectively) could also enhance rice tolerance to Al3+ stress and soil acidity. Expression of either HtNHX1 or HtNHX2 reduced the inhibitory effect of Al3+ on the rice root elongation rate; both genes were reported to be equally effective in improvement of stress conditions. Expression of HtNHX1 enhanced Al3+-trigged-secretion of citrate acids, rhizosphere acidification, and also reduced K+ efflux from root tissues. In contrast, expression of HtNHX2 prevented Al3+-trigged-decrease of H+ influx into root tissues. Al3+-induced damage of the cell wall extensibility at the root tips was impaired by either HtNHX1 or HtNHX2. Co-expression of HtNHX1 and HtNHX2 further improved rice growth, particularly under the Al3+ stress conditions. The results demonstrate that HtNHX1 and HtNHX2 improved rice tolerance to Al3+ via different mechanisms by altering the K+ and H+ fluxes and the cell wall structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World-wide acidic soils account for nearly 30% of current potential arable lands where soluble Al3+ is the major obstacle for crop production (Kochian et al. 2015). The phytotoxic effects of Al3+ include reducing cell wall extensibility (Ma et al. 2004), damaging plasma membrane (Panda et al. 2009; Ma et al. 2014), triggering oxidation of mitochondria and inhibiting uptake of water and nutrients (Kichigina et al. 2017). The primary phytotoxic effect of Al3+ toxicity is confined at the root apex, where Al3+ inhibits cell division and elongation within hours at micromolar concentrations (Ryan and Kochian 1993; Jones and Kochian 1995). To cope with this toxicity, plants have developed multiple strategies for detoxifying Al3+. Sequestration of Al3+ into the vacuole by ABC transporters and aquaporins are the components of the internal detoxification mechanism (Zhang et al. 2001; Yang et al. 2008; Huang et al. 2009, 2012). During binding of Al3+ to the extracellular matrix, secretion of organic acids including citrate, oxalate and malate by the roots to chelate Al3+ at the root surface is the dominant strategy for external detoxification (Ma et al. 2001; Kochian et al. 2004; Zheng et al. 2005).
Massive K+ uptake and sequestration from the vacuole are essential for cell expansion and, therefore, root elongation. K+ uptake at the plasma membrane is mediated by K+ channels transporting K+ down the electro-chemical gradient and/or by HAK/KT/KUP H+/K+ co-transporters facilitating transmembrane H+-gradient to transport K+ against the electro-chemical gradient (Li et al. 2018). Given that the pH in the lumen of the vacuole is about 2 units lower than the pH in the cytosol (Martinière et al. 2013; Bassil and Blumwald 2014; Zhu et al. 2018), the potential of the vacuole is about 30 mV which is relatively positive compared to the cytosol (Sze and Chanroj 2018), compartmentation of K+ into the vacuole requires the involvement of H+-gradient driven antiporters, specifically those from cation proton antiporter (CPA) family (Blumwald and Poole 1985; Apse et al. 1999; Zhang and Blumwald 2001; Bassil and Blumwald 2014). CPAs are Na+(K+)/H+ antiporters that are divided into two subfamilies (CPA1 and CPA2, respectively). The former includes intracellularly located NHXs and plasma membrane-located SOS antiporters. The members of CPA2 (also named as CHXs; Chanroj et al. 2012) are commonly located at the plasma membrane and endomembrane systems, but not at the vacuole, while intracellular located type-I NHXs and type-II NHXs transporters are locates at vacuolar membranes and endosome/trans Golgi/prevacuolar system, respectively (Bassil et al. 2011a, b; Barragán et al. 2012; Chanroj et al. 2012; Andrés et al. 2014; McCubbin et al. 2014). Many studies showed that type-I NHXs are the major players in sequestering K+(Na+) from the vacuole. The functions of type-II NHXs and intracellular located CHXs have been characterized very less and are generally assumed as being essential for regulating luminal ion and pH homeostasis of endomembrane system (Bassil et al. 2012). However, to the best of our knowledge, no previous study has implied the role of NHX transporters in plant tolerance to Al3+ and/or acidic stresses.
Initially, the vacuolar located NHXs (type-I NHXs) were regarded as Na+/H+ antiporters, which may enhance plant salt tolerance by sequestering Na+ in the vacuole (Blumwald and Poole 1985; Apse et al. 1999; Gaxiola et al. 1999; Bassil and Blumwald 2014). However, in vitro ion selectivity assay and physiological studies have demonstrated that they operate as Na+/K+ non-selective transporters (Jiang et al. 2010; Leidi et al. 2010; Bassil et al. 2011b; Barragán et al. 2012; Andrés et al. 2014; Reguera et al. 2014). The type-I NHXs transport K+ mainly in the absence of excessive Na+ (Bassil et al. 2011b). In Arabidopsis, double mutation of AtNHX1 and AtNHX2 reduced the K+ content in the vacuole and impaired turgor generated cell expansion (Barragán et al. 2012). Given that the vacuole occupies about 90% volume of a mature cell, it is easy to understand the critical function of type-I NHXs in the cell volume regulation. Interestingly, mutation of endosome associated type-II NHXs resulted in salt-sensitive phenotypes and impaired turgor-dependent plant growth. Arabidopsis AtNHX5 and AtNHX6 are located at early endosome/trans Golgi network/prevacuolar membranes (TNG/EE/PVC); knock out of both genes resulted in a compromised plant growth associated with decrease of cell size and number, and extreme sensitivity to high salts (Bassil et al. 2011a). Also, increasing bodies of evidences suggest that the luminal pH of endomembrane systems which is associated with the activity of CPAs is critical for delivery or trafficking of materials (including proteins, lipids and the substances for cell walls synthesized in ER and Golgi) in plant cells (Bassil et al. 2012).
Rice (Oryza sativa) is a major food crop and shows a relatively strong tolerance to Al3+ but a sensitivity to salinity in comparison to other food crops (Ma et al. 2002; Munns and Tester 2008). Previously, we have shown that a pair of NHXs, HtNHX1 and HtNHX2, cloned from a salt tolerant cultivar of Jerusalem artichoke (Helianthus tuberosus L.), functioned in improving rice salt tolerance (Zeng et al. 2018). In addition, HtNHX2 improved tolerance to limited supply of nutrients, particularly K+ (Zeng et al. 2018). We also showed that HtNHX1 was located at the tonoplast, while HtNHX2 (which is identical to HtNHX1 except a patch of 114 amino acids missing in the middle of the transporter) was associated with endomembrane systems (Zeng et al. 2018). In this study, we compared the functions of this pair of genes in rice tolerance to the stresses of Al3+ and acidic soil. We show that both transporters could enhance the stress resistance with different mechanisms.
Materials and methods
Plant materials and growth condition
The wild type of rice (Oryza sativa L. ssp. Japonica cv. Nipponbare) and four transgenic lines expressing either HtNHX1 or HtNHX2 cloned from Helianthus tuberosus (cv. Nanyu No. 1) were the same as those generated and used by Zeng et al. (2018). The double expression lines of HtNHX1 and HtNHX2 were developed by a cross between the homozygous HtNHX1 line and HtNHX2 line (designated HtNHX1/HtNHX2) and verified by genotyping along with detecting the simultaneous expression of both genes.
The seeds were sterilized with 30% (v/v) sodium hypochlorite solution for 30 min, rinsed with deionized water, and cultured in a 28 °C culture chamber for 3 d. The germinated seeds were transferred to a 0.5 mM CaCl2 (pH 4.5) solution for 3 days. For further growth, seedlings of the same size were selected and transferred to the international rice research institute (IRRI) nutrient solution on day 7. The nutrient concentration in the nutrient solution is: 1.25 mM NH4NO3, 0.3 mM KH2PO4, 0.35 mM K2SO4, 1 mM CaCl2, 1 mM MgSO4, 0.5 mM Na2SiO3, 20 μM NaFeEDTA, 20 μM H3BO3, 9 μM MnCl2, 0.32 μM CuSO4, 0.77 μM ZnSO4, and 0.38 μM Na2MoO4, pH 5.5. All plants were grown in a greenhouse with a photoperiod of 16 h of light (30 °C)/8 h of darkness (22 °C) and relative humidity of 60–70%.
In soil cultivation experiments, we carried out pot experiments using one acidic red soil at pH 4.5 (soil-A) from Jiangxi Province, China, and one near neutral paddy soil at pH 6.5 (soil-B) from Jiangsu Province, China. The ten-day-old seedlings of WT and the transgenic lines were grown in soil for 30 days, then the biomass of root and shoot was weighted.
Quantifying the root relative growth rate
The three-day-old seedlings were transferred to a 0.5 mM CaCl2 (pH 4.5) solution containing 0, 50 or 100 μM AlCl3 for further growth. The root length was measured daily during the treatment for the total three days. The relative growth rate of the root = (root length measured after the treatment − initial root length). Ten replicates were measured for each WT and transgenic lines.
Measurement of net K+- and H+-flux rate and direction of root elongation zone using non-invasive microelectrode ion flux estimation (MIFE)
Net fluxes of H+ and K+ were measured 45 μm away from root elongation zone (180–300 μm from the root cap) using the non-invasive MIFE® system (University of Tasmania, Hobart, Australia) as described by Newman (2001). The details pertinent to microelectrode fabrication, conditioning, and calibration were described in previous publications (Bose et al. 2010; Jayakannan et al. 2013). Prior to the MIFE measurement, the roots were immobilized and conditioned in a petri dish containing 30 ml IRRI solution (1 mM K+ or 0.1 mM K+, pH 4.5) for 30 min. After that, the Petri dish was placed on the microscope stage of the MIFE system. Ion fluxes were measured under control conditions for 5 min before treatment application. Treatment solutions (the IRRI solution mentioned above with 100 μM AlCl3, pH 4.5) were then added to the petri dishes and thoroughly mixed. The net ion flux was recorded by MIFE Chart software and calculated by the MIFEFLUX program. For each treatment, at least 10 replicates were measured.
Visualization of rhizosphere pH changes
Phytagel (0.4%, w/v) containing 0.05% (w/v) bromocresol purple was used to indicate rhizosphere acidification via color changes (Pacheco-Villalobos et al. 2016). The relatively uniform four-day-old rice seedlings of the wild type and the transgenic lines expressing HtNHX1 or HtNHX2 were selected and transferred to dishes filled with the phytagel. Roots were imbedded into a dish filled with 25 ml gel containing pH indicator, 0.5 mM CaCl2 (pH 6) and without or with 100 µM AlCl3. The roots and gel were photographed before and after 6 h and 12 h treatment using a digital camera. The rhizosphere acidified area of each line was obtained by the semi quantified size of yellow areas using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Four replicates were measured for each WT and transgenic lines.
Collection and measurement of root exudates
The wild type and the transgenic lines were first grown in IRRI solution as described by Yang et al. (2014) for 2 weeks. Then, the roots were washed six times with sterile water and the seedlings were transferred to a sterile 0.5 mM CaCl2 (pH 4.5) solution overnight. Root exudates were collected 24 h after exposing without or with 100 μM AlCl3. After removing the seedlings, the solution was placed in a vacuum freeze dryer (LGJ-18S, Beijing Songyuanhuaxing Biotechnology Co., Ltd, Beijing, China) and lyophilized for 3–5 days until the solution was completely evaporated. The freeze-dried sample was then dissolved in 1 ml of double distilled water. The sample was passed through a 0.45 μm aqueous phase filter and transferred to a 1.5 ml brown liquid bottle. An ultra-high-performance-liquid-chromatography (UPLC) (ACQUITY UPLC system; Waters, Milford, MA, USA) was used to determine the organic acid content of the solution according to the method described by Violeta et al. (2010).
Freeze–thawing experiment for quantifying the mechanical changes in the root apex
The Al-stress trigged mechanical changes in the root apex which has been described as a freeze disruption coefficient which was determined according to Wu et al. (2014), with slight modifications. Three-day-old seedlings of both wild type and transgenic lines were bathed in a 0.5 mM CaCl2 (pH 4.5) solution containing 0 or 100 μM AlCl3 for 24 h. The root tip (0–5 mm) was first cut and embedded in a 5% low temperature melting agar. The slices of 80 μm thickness were transversely sectioned 3 mm from the apexes by a vibratome (Leica VT1200 S, Germany). The sections were placed on glass slides with 50% glycerol, the coverslips were covered and sealed with the neutral balsam. These slices were photographed as “section before treating” (SBT). After placing at − 20 °C overnight, thawed samples were photographed again as “section after treating” (SAT). The cross-sectional areas were analyzed using ImageJ and the freeze-disrupt coefficient (FDC) was calculated according to Wu et al. (2014).
Quantification of aluminum content in root tip
Four-day-old seedlings of the wild type and the transgenic lines were treated with 0.5 mM CaCl2 (pH 4.5) solution containing 0 or 100 μM AlCl3 for 24 h. For extraction of the total Al3+ content from 1 cm of the root tips, four tips were pooled in a tube containing 1 ml of 2 M HCl for two days. For quantifying the total Al3+ in the sap, a pool of 10 tips was quickly frozen at − 80 °C, the samples were centrifugated at 14,000×g for 10 min after being completely thawed. For quantifying the total Al3+ content in cell walls, the collection methods were performed as described by Ma et al. (2004). The extracted soluble Al3+ was measured by inductive coupled plasma (ICP)-emission spectrometer (Agilent Technologies, 700 series ICP-OES).
Statistical analysis
Data were analyzed by ONE WAY ANOVA using the SPSS 10 program (SPSS Inc., Chicago, IL, USA) and represented as mean ± standard error (SE). P ≤ 0.05 is considered as significant difference between the treatments.
Results
Expression of HtNHX1 and HtNHX2 improved rice root growth under Al3+ stress
Four transgenic lines namely NHX1-1, NHX1-2 and NHX2-1, NHX2-2, representing the phenotypes of rice (Nipponbare cultivar) expressing either HtNHX1 or HtNHX2,, were used to examine the function of these two genes in rice Al3+ tolerance. The growth rate of NHX1 and NHX2 lines was nearly the same as that in the wild type (WT, Nipponbare) at no external Al3+ treatment (Fig. 1a, b), which was consistent with our previous data (Zeng et al. 2018). The presence of 50 µM Al3+ in the culture solution impaired root growth rate to a relatively larger extent in WT than in all the transgenic lines, however, expression of either NHX1 or NHX2 did not significantly relieve the impairment of root growth (Fig. 1e). Increasing Al3+ to 100 µM in the solution suppressed root growth rate remarkably, while the growth rate of NHX1- or NHX2-expressing lines was similar and much less inhibited than that of the WT (Fig. 1c, d, f), indicating the functions of both HtNHX1 and HtNHX2 in improving rice tolerance to Al3+ stress.
Ectopic expression of HtNHX1 or HtNHX2 in rice alleviated root growth inhibition during aluminum stress conditions. The seedlings of WT and the transgenic lines of rice were transferred to a 0.5 mM CaCl2 (pH 4.5) solution containing 0 (a, b), 50 (e) or 100 μM AlCl3 (c, d, f) for 3 days. The total root length of each line was measured before and after the treatment. The relative root elongation rate of each line was calculated (see “Materials and methods”) and shown in e and f, respectively. The values in e and f represent mean ± SE (n = 10). Means with ** represent significant differences (P < 0.01 by Tukey’s test). WT wild type (Nipponbare), NHX1-1, NHX1-2, NHX2-1 and NHX2-1, the transgenic lines of ectopic expression of HtNHX1 and HtNHX2, same as the lines used by Zeng et al. (2018). Bar = 5 cm
HtNHX expression alters the rate and direction of net K+ and H+ fluxes in the elongation zone of Al3+ stress roots
As both HtNHX1 and HtNHX2 could enhance K+ uptake and retention in rice roots under salt stress conditions (Zeng et al. 2018), we examined whether this function could still be maintained under Al3+ stress. We measured net K+ flux from the root elongation zone by non-invasive MIFE technique. Under low pH stress (pH 4.5), net K+ efflux was measured from the root cells (Fig. 2a). Addition of 100 µM Al3+ to the bulk solution gradually reduced net K+ efflux in all the roots, showing responses similar to that of Arabidopsis roots to the acidic stress (Bose et al. 2010). In the NHX1-expressing line, the reduction of K+ efflux was much faster than that in the WT and the net K+-flux turned from efflux to influx in 25–30 min after exposure to Al3+. In contrast, NHX2-expressing line was much less sensitive to Al3+ treatment (Fig. 2a). At low K+ (0.1 mM) solution, addition of Al3+ inhibited net K+-flux of roots, but there was much less difference among WT and transgenic lines (Fig. 2b). Net H+ flux across the root surface was also measured concurrently with potassium (Fig. 2c, d). In the solution containing 1 mM K+, net H+-uptake was only slightly reduced in NHX2-expressing lines, while it reduced and turned into net H+-efflux in NHX1 lines and WT after exposure to Al3+ for 20–30 min. At the same time, Al3+ inhibition of H+-influx was much lesser and similar among WT and transgenic lines when they were recorded in 0.1 mM K+ solution (Fig. 2d). These data imply that the effect of HtNHX1 and HtNHX2 on K+ and H+ transport on the plasma membrane is determined by external K+ level.
Ectopic expression of HtNHX1 or HtNHX2 in rice altered the aluminum effect on net K+- and H+-flux rate and direction of root elongation zone. The seedlings were first grown in normal IRRI solution containing 1 mM K+ (a, c) or 0.1 mM K+ (b, d) for 6 days. Transient K+ and H+ fluxes measured at the root elongation zone exposed to 100 μM AlCl3 at pH 4.5 in 1 mM (a, c) or 0.1 mM K+ (b, d) solution, respectively. The values represent mean ± SE (n = 10). The WT and the transgenic lines were the same as those shown in Fig. 1
Expression of HtNHX1, but not HtNHX2, enhanced rhizosphere acidification and root citrate secretion under Al3+ stress
Since short exposure to Al3+ had different impacts on net H+-flux rate and direction between HtNHX1 and HtNHX2 lines, we further examined the pH change in the rhizosphere of these rice lines after prolonged Al3+ treatment by in situ visualization with pH indicator bromocresol purple. The medium color turns from red to yellow, indicating the acidification (Fig. 3). The pH at the rhizosphere showed only slight acidification when no Al3+ was added in the medium (Fig. 3a, d). Remarkably, the acidification surrounding the roots of NHX1-expressing lines was intensified 6 h after exposure to Al3+ and was further enhanced during 12 h exposure (Fig. 3b, e). In contrast, pH surrounding the roots of both WT and NHX2-expressing lines showed much less change and a similar degree of acidification (Fig. 3b, e). The difference in rhizosphere acidification was semi quantified by measuring the size of yellow area and plotted as relative to WT (Fig. 3c, f).
Ectopic expression of either HtNHX1 or HtNHX2 in rice altered the aluminum-stress-induced rhizosphere acidification. Al3+ induces acidification of the medium caused by the secretion of organic acids from the seminal roots. The four-day-old seedlings of WT and the transgenic lines were transferred to a dish filled with 25 ml pH indicator gel in 0.5 mM CaCl2 solution (pH 6) containing 0 (a, d) or 100 (b, e) µM AlCl3. The roots and gel were photographed a before and b after Al3+ treatment for 6 h and another set of the treatment d before and e after Al3+ treatment for 12 h using a digital camera. c, f are the rhizosphere acidified areas relative to WT. The values represent mean ± SE (n = 4). Means with different letters represent significant differences (P < 0.05 by Tukey’s test). WT wild type (Nipponbare); 1-1, 1-2, 2-1, 2-2, the transgenic lines expressing HtNHX1-1, HtNHX1-2, HtNHX2-1, HtNHX2-2, the same as those used in Figs. 1, 2
Citric acid is the most abundant organic acid secreted by rice roots (Ma et al. 2002, 2014). In the absence of Al3+ treatment, the secretion rate of all these rice lines was similar (Fig. 4). Addition of 100 µM Al3+ to the bath medium for 24 h had little influenced on the secretion of citric acid in both WT and NHX2-expressing lines. However, the presence of Al3+ significantly increased the citrate secretion in the NHX1-expressing lines (Fig. 4). We also examined the concentration of malate and oxalate in the exudates, but both were present in extremely low levels.
Ectopic expression of HtNHX1, but not HtNHX2 in rice altered the aluminum-stress-induced citrate secretion of roots. The two-week-old seedlings were treated with sterile 0.5 mM CaCl2 (pH 4.5) solution containing 0 or 100 μM AlCl3 for 24 h and the root exudates were collected during the Al3+ treatment. The citric acid content was determined by UPLC (see “Materials and methods”). The values represent mean ± SE (n = 6). Means with different letters represent significant differences (P < 0.05 by Tukey’s test). The WT and the transgenic lines were the same as those used in Figs. 1, 2
Expression of HtNHX1 and HtNHX2 alters the cell wall plasticity of the rice root apex
The extracellular pH regulates the cell wall flexibility and low pH at the cell wall compartment favors the enzyme activity to increase the cell wall extensibility (Hager 2003). To examine whether the amelioration of Al3+ inhibitory effects on root growth in lines expressing HtNHX1 and HtNHX2 originated from the changes in the cell wall plasticity, we analyzed the resistance of Al3+ treated root apical cell wall to freeze–thawing disruption, as described by Wu et al. (2014). The freeze–thawing treatment itself had little effect on the integrity of root epidermis (Fig. 5a). In comparison to non-Al3+ presence, Al3+ treatment caused the shrinking of epidermis and outer cortex cells of the root apex, and the shrinking was further enhanced after freeze–thawing treatment, particularly in the roots of WT (Fig. 5b). For quantifying this change, the FDC, the value correlated positively with the extent of the root damage (Wu et al. 2014), was calculated. The freeze–thawing treatment disruption of the cell wall integration of the root apex was slight and no significant difference was observed between non-Al3+ stressed WT and transgenic lines (Fig. 5a, c). The Al3+ treatment increased the FDC of WT roots by 4.3-fold, while this increase of FDC in the NHX1- and NHX2-expressing lines was only about two-fold and 0.5-fold, respectively (Fig. 5b, c). Expression of HtNHX2 remarkably prevented the Al3+-induced damage of root apical cell walls. Apparently, the HtNHX2 enhancement of root apical cell wall flexibility was independent of the extracellular acidification (Fig. 3).
Ectopic expression of HtNHX1, particularly HtNHX2, in rice reserved the aluminum-stress-induced plasticity of root apex cells. The change in the sections of the root tips in a the absence or b presence of 100 µM AlCl3 was observed before (SBT) and after (SAT) the treatment. c The freeze-disrupt coefficient was calculated as described in “Materials and methods”. The values in C represent mean ± SE (n = 10). Means with different letters represent significant differences (P < 0.05 by Tukey’s test)
Preventing cytosolic Al3+ accumulation by binding it to the extracellular matrix or secretion of organic acid as a chelator is also an important strategy of plant Al3+ tolerance (Miyasaka et al. 1991; Delhaize et al. 1993; Pellet et al. 1995; Ma et al. 1997, 2014). Therefore, we measured and quantified total Al3+ accumulation at the root tips of these transgenic lines and WT after exposure of 50 or 100 µM Al3+ for 24 h. Al3+ content in the NHX1-expressing line was slightly higher than that in WT and NHX2-expressing line (Fig. 6a). Both the intracellular and extracellular distribution of Al3+ was also assayed after treatment with 100 µM Al3+. In comparison to WT and NHX2-expressing lines, the NHX1 line had a lower Al3+ content at the cell matrix, but significantly higher Al3+ content in the sap of root tips (Fig. 6b, c).
Ectopic expression of HtNHX1, but notHtNHX2 in rice altered the aluminum content in cell wall and entire root apex. The four-day-old seedlings were cultured for 24 h in 0.5 mM CaCl2 (pH 4.5) nutrient solution containing 0, 50, 100 μM AlCl3 , respectively. Then the root tips (0–10 mm) were excised for Al3+ determination. Total Al3+ content in root tips with 0, 50, 100 μM AlCl3 (a ) and Al3+ content in the cell wall of root tips (b ) and in the cell sap of root tips (c ) treated with 100 μM AlCl3. The values represent mean ± SE (n = 5). Means with different letters represent significant differences (P < 0.05 by Tukey’s test)
Expression of HtNHX1 and HtNHX2 enhanced rice tolerance to acid soil
In addition to the response of the transgenic lines expressing HtNHX1 and HtNHX2 to Al3+ stress, we also examined the effects of expressing them on rice growth in acidic soil. The WT and transgenic lines were grown in an acidic red soil with pH 4 and an adequate amount of nitrogen (N), phosphorus (P) and K+ fertilizers was applied. A control experiment was conducted by planting the rice plants in a near neutral paddy soil (pH at 6.5).
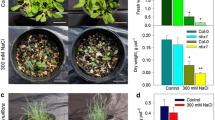
There was no significant difference in the growth and biomass accumulation between WT and the transgenic lines grown in the neutral soil (Fig. 7a, c), while both HtNHX1- and HtNHX2-expressing lines showed much less inhibition to biomass accumulation in the acidic soil (Fig. 7b, d). This result confirmed that NHX genes function in improving rice tolerance to acidic soil environment.
Ectopic expression of either HtNHX1 or HtNHX2 improved rice tolerance to soil acidity. The ten-day-old seedlings of WT and the transgenic lines were grown a in soils-A (pH 6.5) and b soil-B (pH 4.5) for 30 days. The rulers in a and b are the same (50 cm). Bar = 20 cm (a, b). The biomass of root and shoot was weighted in c soil-A and d soil-B. The values represent mean ± SE (n = 8). Means with different letters represent significant differences (P < 0.05 by Tukey’s test)
We assumed that the different responses of the rice expressing HtNHX1 and HtNHX2 to Al3+ stress or acidic soil may be linked to their different subcellular locations (Zeng et al. 2018) and joint expressions may further improve rice tolerance to the stress. Expressing tonoplast located HtNHX1 could drive more cytosolic K+ into the vacuole in exchange of H+ for cytosol K+ and H+ homeostasis, which may reduce extracellular pH, thus making the cell wall more flexible under Al3+ stress. Expressing endosome located HtNHX2 could enhance luminal pH homeostasis of the endomembrane system, resulting in stimulation of secretion and delivery of the membrane and cell wall material to the cell surface and apoplast, thus increasing Al3+ stress tolerance. To examine this hypothesis, we reciprocally crossed the single gene expressing lines and generated four lines of HtNHX1 and HtNHX2 co-expressing rice plants. Notably, unlike their function in acidic soil, single expression of either HtNHX1 or HtNHX2 did not significantly increase rice biomass accumulation at 200 µM Al3+ in culture solution (Fig. 8). This inconsistency between solution and soil culture may be caused due to the difference in time of the stress and the level of Al3+ in the nutrition solution (Fig. 8) and in the soil (Fig. 7), in addition to other unknown soil factors. The duration of the Al3+ stress in the culture solution was only two weeks, while the rice was grown in the acid soil for 30 days. The stress effect on plant growth (particularly shoot biomass accumulation) gradually enhanced as the time extended that is why no significant difference between the single expression of either HtNHX1 or HtNHX2 was observed in short time. In addition, the concentration of Al3+ was 200 mM in the solution culture (Fig. 8), which could not be directly compared with the acidity of the soil (Fig. 7). Nevertheless, single expression of either HtNHX1 or HtNHX2 could improve the root growth at relatively lower levels of Al3+ (50–100 mM) (Fig. 1). The co-overexpressing lines showed significant improvement of rice growth at both non- and 200 µM Al3+ treatment. Their co-expression increased total biomass by 39 ± 2.8% when grown in solution without Al3+ presence (Fig. 8a, c, e). High Al3+ treatment reduced the biomass of WT and the single gene expressing lines, while the biomass of two gene co-expression lines was increased by 51.6 ± 3.9% in comparison to that of WT and single gene expression lines (Fig. 8b, d, e). This result further indicated that HtNHX1 and HtNHX2 have different functions in enhancing the plant tolerance to Al3+ stress or acidic environment.
Ectopic double expression of HtNHX1 and HtNHX2 improved rice growth and particularly aluminum stress tolerance. The seven-day-old seedlings of WT and single or double expression of HtNHX1 and HtNHX2 genes were supplied with IRRI nutrient solution (pH 4.5) containing 0 μM AlCl3 (a, c) or 200 μM AlCl3 (b, d) for 2 weeks. The value of the fresh weight of single gene expression lines and double expression lines relative to the WT fresh weight (e). Bar = 10 cm. The values represent mean ± SE (n = 8). Means with different letters represent significant differences (P < 0.05 by Tukey’s test). WT, NHX1-1, NHX1-2, NHX2-1 and NHX2-2 were the same rice lines as those used in previous figures, 1♀ × 2♂1, 1♀ × 2♂2, 2♀ × 1♂1, and 2♀ × 1♂2 were the transgenic lines with double expression of both HtNHX1 and HtNHX2 genes which were generated by screening of hybridization of single gene expression lines
Discussion
Plant NHX-type cation/H+ antiporters expressed in the membranes of vacuole and vesicles have been shown to mediate Na+(K+)/H+ exchange for salinity tolerance and K+ homoeostasis (Brett et al. 2005; Rodriguez-Rosales et al. 2009; Chanroj et al. 2012). Even though NHX transporters are involved in maintaining the cellular pH homeostasis (Sze and Chanroj 2018), it is not known if these transporters contribute to plant tolerance to Al3+ stress and to low pH soil. We have shown that ectopic expression of Helianthus tuberosus HtNHX1 or HtNHX2 improved rice tolerance to salinity (Zeng et al. 2018). Also, expression of HtNHX2, but not HtNHX1, improves rice growth under K+-limited, salt stress or general nutrient deficienct conditions (Zeng et al. 2018). The possible explanation for this functional difference may be a difference in the subcellular localization, e.g. the fact that HtNHX2 is located at the endomembrane while HtNHX1 is confined to the tonoplast (Zeng et al. 2018).
In this study, we demonstrated that ectopic expression of HtNHX1 or HtNHX2 improved the rice tolerance to Al3+ stress and acidic soil as indicated by alleviating the root growth inhibition (Fig. 1), diminishing the plasticity injury of root apex cells (Fig. 5), and increasing both root and shoot growth (Figs. 7, 8). However, it is notable these two genes show different mechanisms in altering K+ and H+ transport as well as cell wall structure.
Expressing HtNHX1 alleviated Al3+ stress in rice (Fig. 1), which is at least in part due to its enhanced K+ uptake of rice and/or retention in the roots under normal K+ supply levels (Fig. 2a). Given that the vacuole occupies about 90% volume of a mature plant cell, the enhanced Na+(K+)/H+ transporter activity in the tonoplast has been shown to change cytosolic K+ in both tomato and Arabidopsis (Leidi et al. 2010; Barragán et al. 2012). The activity of HtNHX1 unavoidably changes the cytosolic K+ and H+ homeostasis, and thus in turn alters the activities of the respective transporters in the plasma membrane. The activities of both plasma membrane H+-ATPase and K+ transporters are modified by external pH and the presence of Al3+ (Ahn et al. 2002; Wherrett et al. 2005). Low pH in the culture solution can stimulate H+ influx into the root tissue (Shabala et al. 1997) and depolarize membrane potential, thus activating outward rectifying K+ channels (Babourina et al. 2001). Non- invasive MIFE measurements, Bose et al. (2010) showed that Al3+ quickly inhibited low pH-induced H+ influx in the distal elongation zone, concomitantly reducing low pH-induced K+ efflux or even favoring K+ influx into the root tissue in Arabidopsis. We also detected the low pH-induced K+ efflux and H+ influx currents recorded at the elongation zone of rice root tips (Fig. 2, the initial current). Adding Al3+ to the bath medium impaired the net K+ efflux and net H+ influx from all these roots (Fig. 2) showing nearly the same effect as in Arabidopsis roots (Bose et al. 2010). Notably, only HtNHX1 expression could prevent Al3+ -induced root K+ efflux when K+ supply was at the normal level (1 mM), but not at 0.1 mM. It is likely that the activity of HtNHX1 at the tonoplast (Zeng et al. 2018) drives more cytosolic K+ into the vacuole in exchange of H+, and the changed K+ and H+ homeostasis in the cytosol in turn induced change in the K+ channel as well as in the H+-ATPase activity in the plasma membrane (Figs. 2a, 3b, e). K+ channels are able to mediate K+ uptake at low external K+ conditions at extreme membrane hyperpolarization conditions (Hirsch et al. 1998; Hampton et al. 2004). However, the recording bath of low pH (the leak of H+ into the cell) in this study prevented extreme hyperpolarization of the membrane potential, and thus the inward K+ channels were not likely to be activated when the bath K+ concentration was as low as 0.1 mM. Therefore, the HtNHX1 contribution to reduceAl3+-induced K+ efflux was much less significant at low external K+ conditions (Fig. 2b). The HtNHX1 transgenic lines were coupled with quick K+ uptake but not with increased H+ efflux at the plasma membrane (Fig. 2a, c). Nevertheless, the rhizosphere pH of HtNHX1 rice was much lower after prolonged Al3+ exposure (Fig. 3b, e).
Another important feature of HtNHX1 enhancing rice tolerance to Al3+ stress is the stimulation of root secretion of citric acid (Fig. 4). Secretion of organic acids such as citrate, oxalate and malate for chelating Al3+ in the exterior has long been recognized as a major mechanism of Al3+ detoxification (Ma et al. 2001; Kochian et al. 2004). Some plant species secrete predominantly one type of organic acid, such as oxalate by buckwheat, citrate by rice and bean (Ma et al. 1997; Ma 2000; Liu et al. 2018). We detected only citrate in the rice exudations (Fig. 4), which is consistent with a previous report (Ma et al. 2002).
HtNHX2-located endomembrane systems take a small fraction of the cell volume; therefore, the activity of HtNHX2 would change K+ and H+ homeostasis of cytosol much less than the HtNHX1. As expected, the K+ flux in HtNHX2-expressing lines was similar to that in WT (Fig. 2a). It is intriguing that external Al3+ only inhibited H+ influx slightly in the root of HtNHX2 lines in comparison to the rapid inhibition in WT and HtNHX1 lines when K+ supply was sufficient (Fig. 2c), which may relate to HtNHX2 change of the luminal pH and ion-homeostasis of the endomembrane system, and this change affects endosome secretion, thus causing H+-ATPase delivery to plasma membrane (Sze and Chanroj 2018).
The initial phase of Al3+ toxicity is associated with accumulation of Al3+ in the root apex, which binds mostly to the negatively charged pectin of the cell wall and reduces cell wall extensibility (Tabuchi and Matsumoto 2001; Ma et al. 2004; Jones et al. 2006; Yang et al. 2008). We found that the value of FDC that can be used as a proxy for the cell wall extensibility or deformation was small and nearly the same for WT and the transgenic lines under control conditions (Fig. 5a, c). This indicates that the freeze–thaw treatment did not significantly change cell wall structure. However, the transgenic lines showed much more tolerance to freeze–thaw disruption after Al3+ treatment (Fig. 5b, c). After examining the total amount of Al3+ accumulated at the root apex and its apoplastic or intracellular distribution (Fig. 6), it was obvious that enhancement of cell wall extensibility by these two genes was not due to the less Al3+ accumulation in the apoplast. Yet the increase of root tip Al3+ content by HtNHX1 expression is difficult to be explained, and may need to be further characterized in future. The enhancement of the cell wall extensibility by HtNHX1 may be explained by the acid growth theory (Rayle and Cleland 1970), HtNHX1 expression could reduce extracellular pH, making cell wall more flexible. However, the similar phenomenon of HtNHX2 on root FDC is not fulfilled with this theory (Fig. 5). It is easy to understand HtNHX1 enhanced cell wall flexibility, since this gene enhanced extracellular acidification after Al3+ treatment (Fig. 3b, e).
The improved mechanical resistance in HtNHX2 overexpressing plants most likely occurred via a different mechanism. HtNHX2 at early endosome/trans Golgi network/prevacuolar membranes certainly contributes little to cytosolic K+ and H+ homeostasis, but accumulated evidences show that the effect of endosome located NHXs on luminal pH of endomembrane system is critical for endosome trafficking (Pardo et al. 2006; Rodriguez-Rosales et al. 2009; Bassil et al. 2012; Reguera et al. 2014). The cell expansion requires the recruitment of membranes and cell wall. In Arabidopsis, mutation of AtNHX5 and AtNHX6 impaired the endosome secretion pathway (Bassil et al. 2011a). In contrast, HtNHX2 expression in rice likely enhanced endosome secretion and, therefore, more membrane and cell wall would be delivered to cell surface and apoplast.
Impairing nutrients uptake is a major consequence of Al3+ toxicity. In our previous studies, we have noticed that HtNHX2 not only enhanced N, P and K+ uptake, but also improved their translocation from root to shoot at low K+ or limited nutrient supplies, thus largely increasing rice yield in comparison to WT and HtNHX1 lines (Zeng et al. 2018). These results indirectly suggest that HtNHX2 may enhance the delivery of transporter proteins to plasma membrane under Al3+ stress conditions, and thus benefit nutrient uptake. Moreover, phosphorus (P) is an important counter-ion of Al3+ toxicity. Al and P can form insoluble complexes like AlPO4 in cell wall, cytosol and vacuoles (Zheng et al. 2005; Magalhaes et al. 2018). The elevated P content in HtNHX2-expressing rice also likely contributes to its Al3+ tolerance.
In conclusion, both HtNHX1 and HtNHX2 play a significant role in improving rice tolerance to Al3+ and acidic soil by different means. Due to their different subcellular locations, they show a non-redundant function in improving the plant tolerance to salinity (Zeng et al. 2018) and acidic stress (Fig. 7). Their co-expression resulted in additive capacity to stimulate rice growth and tolerate the stresses (Fig. 8). We suggest that such a biotechnological strategy to combine the use of type-I and type-II NHX transporters may be adapted to enhance crop tolerance to other abiotic stresses.
Author contribution statement
LY and GX conceived and designed the experiments; WL, JD, HF and QW conducted the experiments; LY, GX, and WL analyzed the data and wrote the manuscript, SS interpreted the data and revised the manuscript.
Abbreviations
- MIFE:
-
Microelectrode Ion Flux Estimation
- FDC:
-
Freeze-disrupt coefficient
References
Ahn SJ, Sivaguru M, Chung GC, Rengel Z, Matsumoto H (2002) Aluminium-induced growth inhibition is associated with impaired efflux and influx of H+ across the plasma membrane in root apices of squash (Cucurbita pepo). J Exp Bot 53(376):1959–1966. https://doi.org/10.1093/jxb/erf049
Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, Pardo JM (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci USA 111(17):E1806–E1814. https://doi.org/10.1073/pnas.1320421111
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285(5431):1256–1258. https://doi.org/10.1126/science.285.5431.1256
Babourina O, Hawkins B, Lew RR, Newman I, Shabala S (2001) K+ transport by Arabidopsis root hairs at low pH. Aust J Plant Physiol 28(7):635–641. https://doi.org/10.1071/PP01018
Barragán V, Leidi EO, Andres Z, Rubio L, De Luca A, Fernandez JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24(3):1127–1142. https://doi.org/10.1105/tpc.111.095273
Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Biotechnol 22:1–6. https://doi.org/10.1016/j.pbi.2014.08.002
Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011a) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23(1):224–239. https://doi.org/10.1105/tpc.110.079426
Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011b) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23(9):3482–3497. https://doi.org/10.1105/tpc.111.089581
Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63(16):5727–5740. https://doi.org/10.1093/jxb/ers250
Blumwald E, Poole RJ (1985) Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol 78(1):163–167. https://doi.org/10.1104/pp.78.1.163
Bose J, Babourina O, Shabala S, Rengel Z (2010) Aluminium-induced ion transport in Arabidopsis: the relationship between Al tolerance and root ion flux. J Exp Bot 61(11):3163–3175. https://doi.org/10.1093/jxb/erq143
Brett CL, Donowitz M, Rao R (2005) Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288(2):C223–C239. https://doi.org/10.1152/ajpcell.00360.2004
Chanroj S, Wang G, Venema K, Zhang MW, Delwiche CF, Sze H (2012) Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front Plant Sci 3:25. https://doi.org/10.3389/fpls.2012.00025
Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) (II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol 103(3):695–702. https://doi.org/10.1104/pp.103.3.695
Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96(4):1480–1485. https://doi.org/10.1073/pnas.96.4.1480
Hager A (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116(6):483–505. https://doi.org/10.1007/s10265-003-0110-x
Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA, Pritchard J, White PJ (2004) Cesium toxicity in Arabidopsis. Plant Physiol 136(3):3824–3837. https://doi.org/10.1104/pp.104.046672
Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280(5365):918–921. https://doi.org/10.1126/science.280.5365.918
Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF (2009) A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 21(2):655–667. https://doi.org/10.1105/tpc.108.064543
Huang CF, Yamaji N, Chen Z, Ma JF (2012) A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J 69(5):857–867. https://doi.org/10.1111/j.1365-313X.2011.04837.x
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot 64(8):2255–2268. https://doi.org/10.1093/jxb/ert085
Jiang X, Leidi EO, Pardo JM (2010) How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav 5(7):792–795. https://doi.org/10.4161/psb.5.7.11767
Jones DL, Kochian LV (1995) Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell 7(11):1913–1922. https://doi.org/10.1105/tpc.7.11.1913
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29(7):1309–1318. https://doi.org/10.1111/j.1365-3040.2006.01509.x
Kichigina NE, Puhalsky JV, Shaposhnikov AI, Azarova TS, Makarova NM, Loskutov SI, Safronova VI, Tikhonovich IA, Vishnyakova MA, Semenova EV, Kosareva IA, Belimov AA (2017) Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L. Physiol Mol Biol Plants 23(4):851–863. https://doi.org/10.1007/s12298-017-0469-0
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493. https://doi.org/10.1146/annurev.arplant.55.031903.141655
Kochian LV, Pineros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66:571–598. https://doi.org/10.1146/annurev-arplant-043014-114822
Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Fernandez JA, Bressan RA, Hasegawa PM, Quintero FJ, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61(3):495–506. https://doi.org/10.1111/j.1365-313X.2009.04073.x
Li W, Xu G, Alli A, Yu L (2018) Plant HAK/KUP/KT K+ transporters: function and regulation. Semin Cell Dev Biol 74:133–141. https://doi.org/10.1016/j.semcdb.2017.07.009
Liu MY, Lou HQ, Chen WW, Pineros MA, Xu JM, Fan W, Kochian LV, Zheng SJ, Yang JL (2018) Two citrate transporters coordinately regulate citrate secretion from rice bean root tip under aluminum stress. Plant Cell Environ 41(4):809–822. https://doi.org/10.1111/pce.13150
Ma JF (2000) Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol 41(4):383–390. https://doi.org/10.1093/pcp/41.4.383
Ma JF, Zheng SJ, Matsumoto H, Hiradate S (1997) Detoxifying aluminium with buckwheat. Nature 390(6660):569–570. https://doi.org/10.1038/37518
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6(6):273–278. https://doi.org/10.1016/S1360-1385(01)01961-6
Ma JF, Shen R, Zhao Z, Wissuwa M, Takeuchi Y, Ebitani T, Yano M (2002) Response of rice to Al stress and identification of quantitative trait Loci for Al tolerance. Plant Cell Physiol 43(6):652–659. https://doi.org/10.1093/pcp/pcf081
Ma JF, Shen R, Nagao S, Tanimoto E (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45(5):583–589. https://doi.org/10.1093/pcp/pch060
Ma JF, Chen ZC, Shen RF (2014) Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381(1–2):1–12. https://doi.org/10.1007/s11104-014-2073-1
Magalhaes JV, Pineros MA, Maciel LS, Kochian LV (2018) Emerging pleiotropic mechanisms underlying aluminum resistance and phosphorus acquisition on acidic soils. Front Plant Sci 9:1420. https://doi.org/10.3389/fpls.2018.01420
Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N (2013) In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 25(10):4028–4043. https://doi.org/10.1105/tpc.113.116897
McCubbin T, Bassil E, Zhang S, Blumwald E (2014) Vacuolar Na+/H+ NHX-type antiporters are required for cellular K+ homeostasis, microtubule organization and directional root growth. Plants 3(3):409–426. https://doi.org/10.3390/plants3030409
Miyasaka SC, Buta JG, Howell RK, Foy CD (1991) Mechanism of aluminum tolerance in snapbeans. Root exudation of citric acid. Plant Physiol 96(3):737–743. https://doi.org/10.1104/pp.96.3.737
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24(1):1–14. https://doi.org/10.1046/j.1365-3040.2001.00661.x
Pacheco-Villalobos D, Diaz-Moreno SM, van der Schuren A, Tamaki T, Kang YH, Gujas B, Novak O, Jaspert N, Li Z, Wolf S, Oecking C, Ljung K, Bulone V, Hardtke CS (2016) The effects of high steady state auxin levels on root cell elongation in Brachypodium. Plant Cell 28(5):1009–1024. https://doi.org/10.1105/tpc.15.01057
Panda SK, Baluska F, Matsumoto H (2009) Aluminum stress signaling in plants. Plant Signal Behav 4(7):592–597. https://doi.org/10.4161/psb.4.7.8903
Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57(5):1181–1199. https://doi.org/10.1093/jxb/erj114
Paroutis P, Touret N, Grinstein S (2005) The pH of the secretory pathway: measurement, determinants, and regulation. Physiology 19(4):207–215. https://doi.org/10.1152/physiol.00005.2004
Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 196(4):788–795. https://doi.org/10.1007/bf01106775
Rayle DL, Cleland R (1970) Enhancement of wall loosening and elongation by acid solutions. Plant Physiol 46(2):250–253. https://doi.org/10.1104/pp.46.2.250
Reguera M, Bassil E, Blumwald E (2014) Intracellular NHX-type cation/H+ antiporters in plants. Mol Plant 7(2):261–263. https://doi.org/10.1093/mp/sst091
Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M, Cagnac O, Venema K (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4(4):265–276. https://doi.org/10.4161/psb.4.4.7919
Ryan PR, Kochian LV (1993) Interaction between aluminum toxicity and calcium uptake at the root apex in near-isogenic lines of wheat (Triticum aestivum L.) differing in aluminum tolerance. Plant Physiol 102(3):975–982. https://doi.org/10.1104/pp.102.3.975
Ryan PR, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52(1):527–560. https://doi.org/10.1146/annurev.arplant.52.1.527
Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113(1):111–118. https://doi.org/10.1104/pp.113.1.111
Shabala S, Bose J, Fuglsang AT, Pottosin I (2016) On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. J Exp Bot 67(4):1015–1031. https://doi.org/10.1093/jxb/erv465
Sze H, Chanroj S (2018) Plant endomembrane dynamics: studies of K+/H+ antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol 177(3):875–895. https://doi.org/10.1104/pp.18.00142
Tabuchi A, Matsumoto H (2001) Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plant 112(3):353–358. https://doi.org/10.1034/j.1399-3054.2001.1120308.x
Violeta N, Ion T, Mira Elena I (2010) HPLC organic acid analysis in different citrus juices under reversed phase conditions. Not Bot Hort Agrobot Cluj 38(1):44–48. https://doi.org/10.15835/nbha3814569
Wherrett T, Shabala S, Pottosin I (2005) Different properties of SV channels in root vacuoles from near isogenic Al-tolerant and Al-sensitive wheat cultivars. FEBS Lett 579(30):6890–6894. https://doi.org/10.1016/j.febslet.2005.11.038
Wu D, Shen H, Yokawa K, Baluska F (2014) Alleviation of aluminium-induced cell rigidity by overexpression of OsPIN2 in rice roots. J Exp Bot 65(18):5305–5315. https://doi.org/10.1093/jxb/eru292
Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146(2):602–611. https://doi.org/10.1104/pp.107.111989
Yang T, Zhang S, Hu Y, Wu F, Hu Q, Chen G, Cai J, Wu T, Moran N, Yu L, Xu G (2014) The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol 166(2):945–959. https://doi.org/10.1104/pp.114.246520
Zeng Y, Li Q, Wang H, Zhang J, Du J, Feng H, Blumwald E, Yu L, Xu G (2018) Two NHX-type transporters from Helianthus tuberosus improve the tolerance of rice to salinity and nutrient deficiency stress. Plant Biotechnol J. https://doi.org/10.1111/pbi.12773
Zhang H-X, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19(8):765–768. https://doi.org/10.1038/90824
Zhang W-H, Ryan PR, Tyerman SD (2001) Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol 125(3):1459–1472. https://doi.org/10.1104/pp.125.3.1459
Zheng SJ, Yang JL, He YF, Yu XH, Zhang L, You JF, Shen RF, Matsumoto H (2005) Immobilization of aluminum with phosphorus in roots is associated with high aluminum resistance in buckwheat. Plant Physiol 138(1):297–303. https://doi.org/10.1104/pp.105.059667
Zhu X, Pan T, Zhang X, Fan L, Quintero FJ, Zhao H, Su X, Li X, Villalta I, Mendoza I, Shen J, Jiang L, Pardo JM, Qiu QS (2018) K+ efflux antiporters 4, 5, and 6 mediate pH and K+ homeostasis in endomembrane compartments. Plant Physiol 178(4):1657–1678. https://doi.org/10.1104/pp.18.01053
Acknowledgements
This work was supported by the National R&D Program for Transgenic Crops (2016ZX08009003), the National Natural Science Foundation (31272226) and the National Natural Science Foundation (31361140357).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to be declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Du, J., Feng, H. et al. Function of NHX-type transporters in improving rice tolerance to aluminum stress and soil acidity. Planta 251, 71 (2020). https://doi.org/10.1007/s00425-020-03361-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03361-x