Abstract
Main conclusion
Maize produces an array of herbivore-induced terpene volatiles that attract parasitoids to infested plants and a suite of pathogen-induced non-volatile terpenoids with antimicrobial activity to defend against pests.
Plants rely on complex blends of constitutive and dynamically produced specialized metabolites to mediate beneficial ecological interactions and protect against biotic attack. One such class of metabolites are terpenoids, a large and structurally diverse class of molecules shown to play significant defensive and developmental roles in numerous plant species. Despite this, terpenoids have only recently been recognized as significant contributors to pest resistance in maize (Zea mays), a globally important agricultural crop. The current review details recent advances in our understanding of biochemical structures, pathways and functional roles of maize terpenoids. Dependent upon the lines examined, maize can harbor more than 30 terpene synthases, underlying the inherent diversity of maize terpene defense systems. Part of this defensive arsenal is the inducible production of volatile bouquets that include monoterpenes, homoterpenes and sesquiterpenes, which often function in indirect defense by enabling the attraction of parasitoids and predators. More recently discovered are a subset of sesquiterpene and diterpene hydrocarbon olefins modified by cytochrome P450s to produce non-volatile end-products such kauralexins, zealexins, dolabralexins and β-costic acid. These non-volatile terpenoid phytoalexins often provide effective defense against both microbial and insect pests via direct antimicrobial and anti-feedant activity. The diversity and promiscuity of maize terpene synthases, coupled with a variety of secondary modifications, results in elaborate defensive layers whose identities, regulation and precise functions are continuing to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The United States produced 370 million metric tons of maize (Zea mays) in 2017. Furthermore, China, Brazil, Ethiopia, and the European Union collectively produced an additional 436 million, totaling more than 807 million metric tons in the top 5 maize-producing regions of the world. Despite a global reliance on maize, significant economic losses continue to impact production because of natural enemy damage, resulting in 6–19% yield reductions from insects and other herbivores (Oerke 2006) and an additional 10% due to pathogens (Mueller et al. 2016). In an effort to minimize and overcome these losses, numerous research efforts over the past six decades have focused on the elucidation of endogenous direct chemical defenses in maize such as benzoxazinoids, maysin, and a range of terpenoids (Meihls et al. 2012). As the production and function of benzoxazinoids and maysin have been extensively characterized and described in maize (Wouters et al. 2016; Casas et al. 2016), the current review will focus primarily on volatile terpenoids and non-volatile terpenoid defenses.
Terpenoids (also called isoprenoids) are a large and structurally diverse group of compounds that originate from conjugation of the five-carbon compound dimethylallyl diphosphate (DMAPP) and its isomer isopentenyl diphosphate (IPP). Prenyl transferases catalyze the condensation of DMAPP with multiple IPP units in a head–tail orientation to form prenyl diphosphates of variable chain lengths. Fusion of two, three, or four C5-units yields geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15) and geranylgeranyl diphosphate (GGPP, C20), respectively (Tholl 2015). Prenyl diphosphates are then used to form monoterpenes (C10), diterpenes (C20), triterpenes (C30), tetraterpenes (C40) and polyterpenes (> C40). Subsequent rearrangements of these molecules via terpene synthases (TPSs) and cytochrome P450s, the primary enzymes involved in terpenoid diversification, result in thousands of different isoprenoids. The products of these reactions perform a variety of functions, including sterols and quinones that are important for normal cellular function and plant hormones such as brassinosteroids, gibberellins, strigolactones, abscisic acid and isoprenoid cytokinins that have developmental (Tarkowska and Strnad 2018) as well as possible plant resistance functions (Balmer et al. 2013). However, many additional terpenoids not directly known to act as endogenous signals form a vital part of the maize chemical defense arsenal against biotic agents, predominantly acting as antimicrobial/anti-insect compounds or as volatile signals attracting parasitoids or predatory insects to infested plants. Increasingly, initial products of maize TPSs are being annotated; biosynthetic pathways further confirmed in vivo and biological functions experimentally investigated. Despite recent advances, the expansive genetic diversity present between maize inbred lines ensures that many bioactive TPS products and derivatives remain to be identified. The challenge to improve maize resistance traits is matched by the opportunity to continue uncovering novel anti-microbial and anti-insect terpenoid pathways that combine to optimize stress resilience.
Biosynthesis of terpenoid volatiles
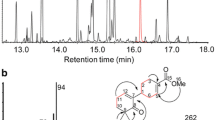
For decades, maize has served as an important monocot model for studying terpenoid metabolism, especially in the context of volatile terpenes (Kollner et al. 2004a; Degenhardt 2009; Chen et al. 2011). While often at low levels, blends of terpene volatiles have been detected from all maize tissues examined and known volatile products of maize TPSs can be seen in Fig. 1. However, the composition and quantity of the volatile blend produced is dependent on the maize cultivar, developmental stage, and organ, as well as, abiotic and biotic stress exposure (Gouinguene et al. 2001; Gouinguene and Turlings 2002; Block et al. 2017; Kollner et al. 2004a; Block et al. 2018; Becker et al. 2014). The cultivar-specific variations occur in both the amount of volatiles produced in response to biotic stress and in the composition of the blend, including differences in the ratios between monoterpenes and sesquiterpenes (Gouinguene et al. 2001). These cultivar-specific variations are likely due to the high degree of genetic variability retained by maize during domestication coupled with the rapid evolution and promiscuity of terpene synthase gene families.
Terpenoid structural diversity is largely a consequence of the diverse carbon backbones formed by TPSs, the enzymes that convert the respective prenyl diphosphate substrates into monoterpenes, sesquiterpenes and diterpenes (Davis and Croteau 2000). The majority of these enzymes are class I TPSs that initiate electrophilic reactions by cleaving the diphosphate moiety, yielding a carbocation intermediate (Liang et al. 2018; Christianson 2006). The carbocation can undergo a series of structural rearrangements including cyclization, hydride shifts and methyl migrations prior to either direct deprotonation, resulting in terpene hydrocarbon formation, or the capture of a water molecule leading to the addition of a hydroxyl group and the production of terpene alcohols (Liang et al. 2018; Christianson 2006, 2008). Maize TPS17 (GRMZM2G010356), however, can generate dually hydroxylated products directly from FPP via an initial addition of water followed by protonation of the internal carbon-6,7-double bond, cyclization and further addition of water to form eudesmane-2,11-diol and two closely related structural isomers. Given the unusual activity, TPS17 has been assigned the name eudesmandiol synthase (EDS) (Liang et al. 2018). Class II TPSs, on the other hand, initiate reactions by the protonation of the double bond at the opposite side of diphosphate moiety. This mechanism is characteristic of several diterpene synthases, for example, those synthesizing copalyl diphosphate that serves as a substrate for other diterpene synthases, such as kaurene synthases discussed below (Tholl 2015; Bensen et al. 1995; Harris et al. 2005). For catalysis, TPSs are dependent on a divalent metal ion cofactor, such as Mg2+. Thus, all TPS genes have a highly conserved Asp-rich region, the DDxxD motif, which is involved in the binding of a divalent metal cofactor (Bohlmann et al. 1998).
Like most plant species, maize has a mid-sized family of approximately 30 TPS genes (Chen et al. 2011; Ding et al. 2017). Extensive research efforts have led to the genetic, biochemical, and ecological characterization of about half of these maize genes. A single TPS commonly produces multiple products, and can catalyze reactions with different prenyl diphosphate substrates. However, some of the predicted TPS genes may be inactive in given inbreds, as has been suggested for TPS3 (GRMZM2G064406) (Richter et al. 2016), and others appear to be functionally redundant. The direct products of many TPSs have high vapor pressure at room temperature which allows them to be released into the environment as volatiles. Maize TPS1 (GRMZM2G049538) catalyzes the formation of the acyclic monoterpene volatiles linalool and geraniol from GPP, and the volatile sesquiterpenes (E)-β-farnesene, (E,E)-farnesol, and (E)-nerolidol from FPP (Schnee et al. 2002). TPS2 (GRMZM2G046615) can produce the monoterpene, sesquiterpene and diterpene alcohols, linalool, (E)-nerolidol, and (E,E)-geranyllinalool, respectively (Richter et al. 2016). The two closely related enzymes TPS4 (GRMZM2G117319) and TPS5 (GRMZM2G074309) both form the same complex mixture of sesquiterpenes including 7-epi-sesquithujene, sesquithujene, (Z)-α-bergamotene, (E)-α-bergamotene, sesquisabinene B, sesquisabinene A, (E)-β-farnesene, (S)-β-bisabolene, β-curcumene, and γ-curcumene from the FPP precursor, but the proportion of these products vary due to four amino acid substitutions in the catalytic sites, resulting in stereoselectivity between the two enzymes (Kollner et al. 2004b). Furthermore, functionality differences between TPS4 and TPS5 result in variable volatile terpene composition between maize varieties.
Another pair of functionally redundant enzymes, TPS6/11(GRMZM2G127087) catalyzes the formation of the acyclic monoterpenes, β-myrcene and linalool, along with minor amounts of the cyclic compounds limonene, α-thujene, sabinene, and α-terpinolene in the presence of GPP. However, in the presence of FPP, TPS6 and TPS11 produce the monocyclic sesquiterpene, β-bisabolene, and the uncommon bicyclic olefin, β-macrocarpene likely involved in zealexin biosynthesis as discussed below (Kollner et al. 2008b). TPS7 (AC217050.4_FG007) produces a blend of sesquiterpenoids of which τ-cadinol predominates (Ren et al. 2016). TPS8 (GRMZM2G038153) produces three bicyclic olefins, α-copaene, (E)-β-caryophyllene, and the macrocyclic sesquiterpene germacrene D (Fontana et al. 2011). TPS10 (GRMZM2G179092) catalyzes the formation of predominantly (E)-β-farnesene, (E)-α-bergamotene, but also several other minor sesquiterpene hydrocarbons, α-copaene, (E)-β-caryophyllene, sesquisabinene A, germacrene D, zingiberene, α-muurolene, β-bisabolene, δ-cadinene, and sesquiphellandrene (Schnee et al. 2006). Although most maize TPS can utilize FPP, TPS26 (GRMZM2G030583) has specificity for the GPP substrate and is exclusively a monoterpene synthase responsible for the formation of α-terpineol, limonene, γ-terpinene, β-myrcene, terpinolene, and 4-terpineol (Lin et al. 2008). Most of these products have been identified in the volatile profiles of aboveground maize tissues responding to biotic stress (Turlings et al. 1990; Kollner et al. 2004a; Schnee et al. 2006; Gouinguene et al. 2001; Becker et al. 2014). However, volatile emission is not limited to the atmosphere, TPS23 (GRMZM2G127336) is expressed in both maize leaves and roots, and is responsible for the formation and emission of (E)-β-caryophyllene in the rhizosphere following root herbivory (Kollner et al. 2008a).
Other terpene volatiles are formed through further product modifications by oxidation, dehydrogenation, acylation, and other cytochrome P450-mediated reactions. For example, (E)-nerolidol and (E,E)-geranyllinalool produced by TPS2 are subsequently converted into (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT) by oxidative degradation which is catalyzed by the P450 monooxygenases, CYP92C5 (GRMZM2G102079) and CYP92C6 (GRMZM2G139467), respectively (Richter et al. 2016). Both DMNT and TMTT are volatile homoterpenes that are also synthesized in response to biotic stress and are frequently found in the headspace of damaged maize tissues (Turlings et al. 1990). However, chemical modifications and the addition of multiple functional groups to volatile TPS products dramatically increases boiling points and results in the formation of non-volatile specialized metabolites that accumulate near the site of synthesis. For example, α- and β-selinene volatiles produced by TPS21 (GRMZM2G011151) can predominate in multiple ecological contexts yet are commonly converted into the corresponding oxygenated derivatives, α/β-costols and α/β-costic acids which display dramatically lower volatility (Ding et al. 2017).
Biosynthesis of terpenoid phytoalexins
Maize produces a range of non-volatile terpenoids that are elicited in response to biotic attack. Currently, our knowledge of the diversity, biosynthesis and activity of these maize defense compounds remains a rapidly expanding area of research. Several families of maize non-volatile terpenoid phytoalexins occur as mixes of related compounds (Fig. 2). Kauralexins, for example, are labdane-related diterpenoids that are produced in a manner similar to that of the phytohormone gibberellic acid. The maize kauralexin A series metabolites identified to date are: ent-kauran-17-oic acid, ent-kauran-17,19-dioic acid, and ent-kaur-19-al-17-oic acid, termed kauralexins A1 through A3, respectively. The maize kauralexin B series includes ent-kaur-15-en-17-oic acid, ent-kaur-15-en-17,19-dioic acid, and ent-kaur-15-en-19-al-17-oic acid, termed kauralexins B1–B3, respectively (Schmelz et al. 2011). Kauralexins are produced by the bi-cyclization of GGPP into ent-copalyl diphosphate by the ent-copalyl diphosphate synthase, Anther ear 2 (An2, GRMZM2G044481) (Vaughan et al. 2015; Harris et al. 2005). Ent-copalyl diphosphate is then converted into ent-kaurene by ent-kaurene synthases. Three genes in maize have demonstrated in vitro ent-kaurene synthase activity, TPS1, KSL5 (GRMZM2G093526) and KSL3 (GRMZM2G093603), with KSL3 being solely responsible for the production of gibberellic acid (Fu et al. 2016). The kauralexin A series metabolites are likely derived from ent-kaurene, while the kauralexin B series are likely from its isomer ent-isokaurene produced as a minor product of these genes (Fu et al. 2016). Based on comparisons between the synthesis of gibberellins and labdane-related diterpenoids in rice, it has been suggested that cytochrome P450s and short chain alcohol dehydrogenases may be responsible for the additional modifications required to produce the bioactive kauralexins, although the specific enzymes required remain unknown (Schmelz et al. 2014).
Maize has three other ent-kaurene synthase-like genes KSL1 (GRMZM2G391312), KSL2 (Zm00001d041082) and KSL4 (GRMZM2G016922). It was recently shown that KSL4 converts ent-copalyl diphosphate into dolabradiene rather than ent-kaurene (Mafu et al. 2018). Dolabradiene is then converted into 15,16-epoxydolabrene (epoxydolabrene) and epoxydolabranol via sequential C-16 epoxidation and C-3 hydroxylation by the cytochrome P450, CYP71Z16 (GRMZM2G067591). Epoxydolabranol can be further modified to form trihydroxydolabrene, though the enzyme(s) responsible for this predominant end-product has(have) yet to be identified (Mafu et al. 2018). Collectively, these labdane-related diterpenoids are termed dolabralexins. Interestingly, the maize An2 mutant, which is deficient in kauralexins, is also deficient in dolabralexins, indicating that An2 provides ent-copalyl diphosphate for both families of labdane-related diterpenoid phytoalexins (Mafu et al. 2018; Vaughan et al. 2015).
Zealexins are non-volatile sesquiterpenoid phytoalexins produced from the bicyclic olefin β-macrocarpene. The duplicated terpene synthases TPS6 and TPS11 both produce β-macrocarpene from FPP and are predicted to be responsible for zealexin production, though this has yet to be confirmed genetically (Kollner et al. 2008b; Huffaker et al. 2011). The C15 methyl group of β-macrocarpene is oxidized to a carboxylic acid by the cytochrome P450 mono-oxygenases CYP71Z16 and CYP71Z18 (Zm00001d014134) to form Zealexin A1 (Mao et al. 2016; Mafu et al. 2018). Zealexin A1 can be further modified by hydroxylation at the C1 or C8 positions to form Zealexin A2 and Zealexin A3, respectively (Huffaker et al. 2011). The C8 hydroxyl of Zealexin A3 can be further modified, likely by additional hydroxylation to transform the C8 alcohol into a germinal diol that spontaneously dehydrates into a ketone, forming Zealexin A4 (Christensen et al. 2017). Zealexin B1, has a C15 carboxylic acid similarly to Zealexin A1 but also has a C1–C6 double bond, resulting in a conjugated 1,1′,3′-triene system (Huffaker et al. 2011). The genes required for these modifications remain under investigation.
Many genes involved in producing kauralexins, dolabralexins and zealexins have been identified through in vitro activity assays targeting strongly elicited transcript accumulation. While providing functional information, in vitro assays alone have the potential to lead to unexpected findings due to enzyme promiscuity and the level of endogenous relevance of the selected combinatorial pairings. For instance, CYP71Z18 and CYP71Z16 are tandemly duplicated genes both shown to produce Zealexin A1 from β-macrocarpene (Mao et al. 2016; Mafu et al. 2018) and similarly both enzymes can produce epoxydolabranol from dolabradiene (Mafu et al. 2018). Collectively, loss of function mutants in combinations of these genes will be required to determine relative contributions to the proposed pathways and processes. As evidence also exists for as yet unidentified zealexins and dolabralexins (Huffaker et al. 2011; Mafu et al. 2018) considerable work remains to elucidate the biosynthesis, regulation and function of terpenoid phytoalexins in maize.
Functions of terpenoid defense compounds
As the biosynthesis pathways of maize terpenoid defenses are being elucidated, progress is also being made on determining the functions of these compounds against different biotic stresses. One of the most prominent defensive functions of maize terpenoids is to directly impact the growth and reproduction of maize pests. Many terpenoid compounds are elicited in response to various biotic stresses, but not all are effective in direct defense. For example, production of the diterpenoid epoxydolabranol is strongly elicited in maize roots during infection with the fungal pathogens Fusarium verticillioides and Fusarium graminearum and was demonstrated to have strong antimicrobial activity against both pathogens in vitro (Mafu et al. 2018). Conversely, eudesmane-2,11-diol is detectible in maize roots following F. verticillioides infection, but does not display direct antifungal activity against this pathogen (Liang et al. 2018).
Zealexins are elicited in response to infection with diverse fungal pathogens including Cochliobolus heterostrophus, F. graminearum, Rhizopus microsporus, Colletotrichum sublineolum and Aspergillus flavus, but only weakly in response to Colletotrichum graminicola (Huffaker et al. 2011; Christensen et al. 2017). Among these pathogens, zealexins demonstrated strong in vitro anti-fungal activity against R. microsporus, A. flavus, and F. graminearum. In vivo evidence for the antimicrobial activity of zealexins was observed by partial virus-induced gene silencing of the putative maize zealexin biosynthesis genes tps6 and tps11, which led to increased susceptibility to the biotrophic fungal pathogen Ustilago maydis (van der Linde et al. 2011). Interestingly, some zealexins have demonstrated variable antimicrobial activity against different fungal pathogens. For instance, zealexin A1 inhibits the growth of A. flavus, F. graminearum and R. microsporus, while zealexins A3 and A4 inhibit A. flavus and F. graminearum but have no activity against R. microsporus (Huffaker et al. 2011; Christensen et al. 2017, 2018). Furthermore, zealexin A2 has not displayed significant inhibitory activity against any fungus tested to date (Huffaker et al. 2011). Surprisingly, zealexin A4 promotes the growth of C. graminicola in in vitro assays suggesting that the lack of zealexin elicitation in maize in response to this pathogen may be beneficial to the plant (Christensen et al. 2017).
Kauralexins are elicited in maize stems in response to infection with F. graminearum, R. microsporus, C. heterostrophus, and C. graminicola (Schmelz et al. 2011; Christensen et al. 2018). Kauralexin A3 and kauralexin B3 display in vitro antifungal activity against R. microsporus and C. graminicola (Schmelz et al. 2011) and a mixture of kauralexin A2 and kauralexin B2 has in vitro antifungal activity against F. graminearum and F. verticillioides (Christensen et al. 2018). Interestingly, the kauralexin-deficient An2 mutant has increased susceptibility to C. heterostrophus and F. verticillioides but not C. graminicola or F. graminearum (Christensen et al. 2018), suggesting fungal-dependent specificity. Moreover, near isogenic lines (NILs) lacking functional copies of TPS21 responsible for maize β-costic acid production displayed increased susceptibility to multiple Fusarium species compared to functional wild-type NILs (Ding et al. 2017). Collectively, these results highlight the need for continued efforts to identify isogenic maize lines deficient in the production of specific terpenoids to understand the biological significance of individual compounds in particular pathosystems.
Terpenoids that display anti-fungal activity may also have defensive functions against insect pests. For instance, the biotic stress-induced β-costic acid inhibited the growth of F. verticillioides, F. graminearum, R. microsporus, Aspergillus parasiticus, and C. heterostrophus in antimicrobial assays (Ding et al. 2017). Similarly when applied to healthy maize root tissues, β-costic acid negatively impacted the growth of Diabrotica balteata (Banded cucumber beetle) but not the specialist Diabrotica virgifera virgifera (Western corn rootworm) (Ding et al. 2017). Kauralexins are induced in maize stems in response to infestation with the stem borer Ostrinia nubilalis (European corn borer) and kauralexin A3 and kauralexin B3 displayed in vitro feeding deterrence activity against O. nubilalis in choice assays (Schmelz et al. 2011). Modest accumulation of zealexins are also observed in response to O. nubilalis herbivory in maize stems suggesting that they too could have anti-insect properties (Huffaker et al. 2011). In addition to kauralexins and zealexins being induced by stem caterpillar feeding, secondary fungal infections in the humid feeding tunnels are likely to promote kauralexin and zealexin production. Other maize terpenoids likely play a role in direct defense against insect pests. For instance, a loss of function mutation in the single TPS2/3 gene in maize inbred W22 led to a reduction in linalool, lavandulyl, and menthadiene. These compounds are induced by Rhopalosiphum maidis (corn leaf aphid) feeding and have a negative impact on R. maidis reproduction (Tzin et al. 2015).
Many terpene volatiles play a role in indirect defense against insect pests by attracting parasitoids or predators to infested plants. Turlings et al. (1990) demonstrated that maize seedlings released large amounts of volatiles when infested with Spodoptera exigua (beet armyworm). These volatiles include linalool, DMNT, indole, (E)-α-bergamotene, (E)-β-farnesene, (E)-nerolidol, and TMTT, the blend of which is attractive to the S. exigua parasitoid Cotesia marginiventris (Turlings et al. 1991). Subsequent studies have shown that terpenoids attract other parasitoids. For instance, linalool attracts naive Campoletis chlorideae, a larval parasitoid of Mythmna separate (Northern armyworm) (Yan and Wang 2006) and (E)-β-caryophyllene attracts naïve Cotesia sesamiae, a larval parasitoid of the stem borer Chilo partellus (Spotted stalk borer) (Tamiru et al. 2017). Below-ground tissues of maize can also emit terpene volatiles to attract parasitoids or predators to their prey. Feeding by the larvae of D. virgifera virgifera on the roots of maize induces the release of (E)-β-caryophyllene, attracting entomopathogenic nematodes that infest the attacking larvae (Rasmann et al. 2005; Degenhardt et al. 2009). The D. virgifera virgifera larvae themselves also use (E)-β-caryophyllene emission to help locate suitable host plants (Robert et al. 2012).
The function of several terpene volatiles have been assessed by the expression of terpene synthases in alternate systems. For instance, expression of the maize TPS10 in Arabidopsis (Arabidopsis thaliana) leads to the production of (E)-β-farnesene and (E)-α-bergamotene, rendering Arabidopsis attractive to experienced C. marginiventris (Schnee et al. 2006). Maize TPS8, when expressed in Arabidopsis, also increased its attractiveness to experienced C. marginiventris and the combined expression of TPS10, TPS8 and TPS5 was more attractive than the expression of these genes individually (Fontana et al. 2011). The collective expression of these genes, however, does not increase the attractiveness of Arabidopsis to naïve C. marginiventris, indicating that use of these volatiles for host location is a learned response. These studies suggest that optimum chemical defenses often consist of a suite of complementary defense compounds that act in concert to provide protection.
The promiscuous nature and variety of terpene synthases available, coupled with the wide range of modifications possible in terpenoid families, help to facilitate effective chemical defenses against a broad range of biological threats. While this review exclusively details terpenoid defense compounds in maize, other plant species utilize these specialized metabolites, including volatile terpenes and non-volatile phytoalexins (Chen et al. 2011). Some of the terpenoids produced are found through-out the plant kingdom and likely play similar roles in diverse species, while others are species specific. For example Oryza sativa (rice) produces a series of labdane-related diterpenoids similar to the kauralexins of maize, including momilactones, oryzalexins and phytocassanes (see review, Schmelz et al. 2014).
Chemicals used in defense can be less effective against specialized pests that acquire mechanisms of tolerance or detoxification. Plants counter this by modifying the compounds to make them more effective or more resistant to detoxification. Our increased knowledge of terpenoid function and biosynthesis will contribute to programs that breed crop varieties with enhanced terpenoid concentrations for optimal defense against specific pests. Furthermore, the engineering or movement of enzymes between different plant species has the potential to enable plants to produce compounds effective against, but not usually encountered by, their major pests. Such combinatorial chemistry from across the plant kingdom could even lead to novel modifications and the discovery of new anti-microbial or insecticidal compounds. Such combinatorial chemistry across the plant kingdom could potentially reduce the need for external pesticide application and facilitate a higher-yielding agro-economic industry.
Author contribution statement
AB conceived the review and wrote the sections on terpenoid phytoalexins and functions of terpenoid defense compounds. MV wrote the section of biosynthesis of terpenoid volatiles. SC wrote the introduction and made Fig. 2 with the assistance of ES. MV made Fig. 1 with the assistance of AB, and ES wrote the abstract. All authors edited the manuscript.
References
Balmer D, de Papajewski DV, Planchamp C, Glauser G, Mauch-Mani B (2013) Induced resistance in maize is based on organ-specific defence responses. Plant J 74(2):213–225. https://doi.org/10.1111/tpj.12114
Becker EM, Herrfurth C, Irmisch S, Kollner TG, Feussner I, Karlovsky P, Splivallo R (2014) Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J Agric Food Chem 62(22):5226–5236. https://doi.org/10.1021/jf500560f
Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP (1995) Cloning and characterization of the maize An1 gene. Plant Cell 7(1):75–84. https://doi.org/10.1105/tpc.7.1.75
Block A, Vaughan MM, Christensen SA, Alborn HT, Tumlinson JH (2017) Elevated carbon dioxide reduces emission of herbivore-induced volatiles in Zea mays. Plant Cell Environ 40(9):1725–1734. https://doi.org/10.1111/pce.12976
Block AK, Hunter CT, Rering C, Christensen SA, Meagher RL (2018) Contrasting insect attraction and herbivore-induced plant volatile production in maize. Planta 248(1):105–116. https://doi.org/10.1007/s00425-018-2886-x
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95(8):4126–4133. https://doi.org/10.1073/pnas.95.8.4126
Casas MI, Falcone-Ferreyra ML, Jiang N, Mejia-Guerra MK, Rodriguez E, Wilson T, Engelmeier J, Casati P, Grotewold E (2016) Identification and characterization of maize salmon silks genes involved in insecticidal maysin biosynthesis. Plant Cell 28(6):1297–1309. https://doi.org/10.1105/tpc.16.00003
Chen F, Tholl D, Bohlmann J, Pichersky E (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J 66(1):212–229. https://doi.org/10.1111/j.1365-313X.2011.04520.x
Christensen SA, Huffaker A, Sims J, Hunter CT, Block A, Vaughan MM, Willett D, Romero M, Mylroie JE, Williams WP, Schmelz EA (2017) Fungal and herbivore elicitation of the novel maize sesquiterpenoid, zealexin A4, is attenuated by elevated CO2. Planta. https://doi.org/10.1007/s00425-017-2830-5
Christensen SA, Sims J, Vaughan M, Hunter C, Block A, Willett D, Alborn HT, Huffaker A, Schmelz EA (2018) Commercial hybrids and mutant genotypes reveal complex protective roles for inducible terpenoid defenses. J Exp Bot. https://doi.org/10.1093/jxb/erx495
Christianson DW (2006) Structural biology and chemistry of the terpenoid cyclases. Chem Rev 106(8):3412–3442. https://doi.org/10.1021/cr050286w
Christianson DW (2008) Unearthing the roots of the terpenome. Curr Opin Chem Biol 12(2):141–150. https://doi.org/10.1016/j.cbpa.2007.12.008
Davis EM, Croteau R (2000) Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes. Top Curr Chem 209:53–95
Degenhardt J (2009) Indirect defense responses to herbivory in grasses. Plant Physiol 149(1):96–102. https://doi.org/10.1104/pp.108.128975
Degenhardt J, Hiltpold I, Kollner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106(32):13213–13218. https://doi.org/10.1073/pnas.0906365106
Ding YZ, Huffaker A, Kollner TG, Weckwerth P, Robert CAM, Spencer JL, Lipka AE, Schmelz EA (2017) Selinene volatiles are essential precursors for maize defense promoting fungal pathogen resistance. Plant Physiol 175(3):1455–1468. https://doi.org/10.1104/pp.17.00879
Fontana A, Held M, Fantaye CA, Turlings TC, Degenhardt J, Gershenzon J (2011) Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson). J Chem Ecol 37(6):582–591. https://doi.org/10.1007/s10886-011-9967-7
Fu J, Ren F, Lu X, Mao H, Xu M, Degenhardt J, Peters RJ, Wang Q (2016) A tandem array of ent-kaurene synthases in maize with roles in gibberellin and more specialized metabolism. Plant Physiol 170(2):742–751. https://doi.org/10.1104/pp.15.01727
Gouinguene SP, Turlings TC (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129(3):1296–1307. https://doi.org/10.1104/pp.001941
Gouinguene S, Degen T, Turlings TCJ (2001) Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11(1):9–16. https://doi.org/10.1007/Pl00001832
Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, Allard S, Kathiresan A, Ouellet T, Peters RJ (2005) The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol 59(6):881–894. https://doi.org/10.1007/s11103-005-1674-8
Huffaker A, Kaplan F, Vaughan MM, Dafoe NJ, Ni XZ, Rocca JR, Alborn HT, Teal PEA, Schmelz EA (2011) Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol 156(4):2082–2097. https://doi.org/10.1104/pp.111.179457
Kollner TG, Schnee C, Gershenzon J, Degenhardt J (2004a) The sesquiterpene hydrocarbons of maize (Zea mays) form five groups with distinct developmental and organ-specific distribution. Phytochemistry 65(13):1895–1902. https://doi.org/10.1016/j.phytochem.2004.05.021
Kollner TG, Schnee C, Gershenzon J, Degenhardt J (2004b) The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell 16(5):1115–1131. https://doi.org/10.1105/tpc.019877
Kollner TG, Held M, Lenk C, Hiltpold I, Turlings TC, Gershenzon J, Degenhardt J (2008a) A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20(2):482–494. https://doi.org/10.1105/tpc.107.051672
Kollner TG, Schnee C, Li S, Svatos A, Schneider B, Gershenzon J, Degenhardt J (2008b) Protonation of a neutral (S)-beta-bisabolene intermediate is involved in (S)-beta-macrocarpene formation by the maize sesquiterpene synthases TPS6 and TPS11. J Biol Chem 283(30):20779–20788. https://doi.org/10.1074/jbc.M802682200
Liang J, Liu J, Brown R, Jia M, Zhou K, Peters RJ, Wang Q (2018) Direct production of dihydroxylated sesquiterpenoids by a maize terpene synthase. Plant J. https://doi.org/10.1111/tpj.13901
Lin CF, Shen BZ, Xu ZN, Koellner TG, Degenhardt J, Dooner HK (2008) Characterization of the monoterpene synthase gene tps26, the ortholog of a gene induced by insect herbivory in maize. Plant Physiol 146(3):940–951. https://doi.org/10.1104/pp.107.109553
Mafu S, Ding Y, Murphy KM, Yaacoobi O, Addison JB, Wang Q, Shen Z, Briggs SP, Bohlmann J, Castro-Falcon G, Hughes CC, Betsiashvili M, Huffaker A, Schmelz EA, Zerbe P (2018) Discovery, biosynthesis and stress-related accumulation of dolabradiene-derived defenses in maize. Plant Physiol 176(4):2677–2690. https://doi.org/10.1104/pp.17.01351
Mao HJ, Liu J, Ren F, Peters RJ, Wang Q (2016) Characterization of CYP71Z18 indicates a role in maize zealexin biosynthesis. Phytochemistry 121:4–10. https://doi.org/10.1016/j.phytochem.2015.10.003
Meihls LN, Kaur H, Jander G (2012) Natural variation in maize defense against insect herbivores. Cold Spring Harb Symp Quant Biol 77:269–283. https://doi.org/10.1101/sqb.2012.77.014662
Mueller DS, Wise KA, Sisson AJ, Allen TW, Bergstrom GC, Bosley DB, Bradley CA, Broders KD, Byamukama E, Chilvers MI, Collins A, Faske TR, Friskop AJ, Heiniger RW, Hollier CA, Hooker DC, Isakeit T, Jackson-Ziems TA, Jardine DJ, Kinzer K, Koenning SR, Malvick DK, McMullen M, Meyer RF, Paul PA, Robertson AE, Roth GW, Smith DL, Tande CA, Tenuta AU, Vincelli P, Warner F (2016) Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Prog 17:211–222. https://doi.org/10.1094/PHP-RS-16-0030
Oerke EC (2006) Crop losses to pests. J Agric Sci 144:31–43. https://doi.org/10.1017/S0021859605005708
Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TC (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434(7034):732–737. https://doi.org/10.1038/nature03451
Ren F, Mao H, Liang J, Liu J, Shu K, Wang Q (2016) Functional characterization of ZmTPS7 reveals a maize tau-cadinol synthase involved in stress response. Planta 244(5):1065–1074. https://doi.org/10.1007/s00425-016-2570-y
Richter A, Schaff C, Zhang Z, Lipka AE, Tian F, Kollner TG, Schnee C, Preiss S, Irmisch S, Jander G, Boland W, Gershenzon J, Buckler ES, Degenhardt J (2016) Characterization of biosynthetic pathways for the production of the volatile homoterpenes DMNT and TMTT in Zea mays. Plant Cell 28(10):2651–2665. https://doi.org/10.1105/tpc.15.00919
Robert CA, Erb M, Duployer M, Zwahlen C, Doyen GR, Turlings TC (2012) Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol 194(4):1061–1069. https://doi.org/10.1111/j.1469-8137.2012.04127.x
Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni XZ, Rocca JR, Alborn HT, Teal PE (2011) Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci USA 108(13):5455–5460. https://doi.org/10.1073/pnas.1014714108
Schmelz EA, Huffaker A, Sims JW, Christensen SA, Lu X, Okada K, Peters RJ (2014) Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J 79(4):659–678. https://doi.org/10.1111/tpj.12436
Schnee C, Kollner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E, E)-farnesol after herbivore damage. Plant Physiol 130(4):2049–2060. https://doi.org/10.1104/pp.008326
Schnee C, Kollner TG, Held M, Turlings TC, Gershenzon J, Degenhardt J (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103(4):1129–1134. https://doi.org/10.1073/pnas.0508027103
Tamiru A, Bruce TJA, Richter A, Woodcock CM, Midega CAO, Degenhardt J, Kelemu S, Pickett JA, Khan ZR (2017) A maize landrace that emits defense volatiles in response to herbivore eggs possesses a strongly inducible terpene synthase gene. Ecol Evol 7(8):2835–2845. https://doi.org/10.1002/ece3.2893
Tarkowska D, Strnad M (2018) Isoprenoid-derived plant signaling molecules: biosynthesis and biological importance. Planta 247(5):1051–1066. https://doi.org/10.1007/s00425-018-2878-x
Tholl D (2015) Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biot 148:63–106. https://doi.org/10.1007/10_2014_295
Turlings TC, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250(4985):1251–1253. https://doi.org/10.1126/science.250.4985.1251
Turlings TC, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE (1991) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol 17(11):2235–2251. https://doi.org/10.1007/BF00988004
Tzin V, Fernandez-Pozo N, Richter A, Schmelz EA, Schoettner M, Schafer M, Ahern KR, Meihls LN, Kaur H, Huffaker A, Mori N, Degenhardt J, Mueller LA, Jander G (2015) Dynamic maize responses to aphid feeding are revealed by a time series of transcriptomic and metabolomic assays. Plant Physiol 169(3):1727–1743. https://doi.org/10.1104/pp.15.01039
van der Linde K, Kastner C, Kumlehn J, Kahmann R, Doehlemann G (2011) Systemic virus-induced gene silencing allows functional characterization of maize genes during biotrophic interaction with Ustilago maydis. New Phytol 189(2):471–483. https://doi.org/10.1111/j.1469-8137.2010.03474.x
Vaughan MM, Christensen S, Schmelz EA, Huffaker A, Mcauslane HJ, Alborn HT, Romero M, Allen LH, Teal PEA (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ 38(11):2195–2207. https://doi.org/10.1111/pce.12482
Wouters FC, Blanchette B, Gershenzon J, Vassao DG (2016) Plant defense and herbivore counter-defense: benzoxazinoids and insect herbivores. Phytochem Rev 15(6):1127–1151. https://doi.org/10.1007/s11101-016-9481-1
Yan ZG, Wang CZ (2006) Identification of Mythmna separata-induced maize volatile synomones that attract the parasitoid Campoletis chlorideae. J Appl Entomol 130(4):213–219. https://doi.org/10.1111/j.1439-0418.2006.01055.x
Acknowledgements
The use of trade name, commercial product or corporation in this publication is for the information and convenience of the reader and does not imply an official recommendation, endorsement or approval by the U.S. Department of Agriculture or the Agricultural Research Service for any product or service to the exclusion of others that may be suitable. USDA is an equal opportunity provide and employer. This work was funded by United States Department of Agriculture-Agricultural Research Service projects 6036-11210-001-00D and 5010-42000-048-00-D and by United States Department of Agriculture-National Institute of Food and Agriculture Grant 2018-51181-28419.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Block, A.K., Vaughan, M.M., Schmelz, E.A. et al. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 249, 21–30 (2019). https://doi.org/10.1007/s00425-018-2999-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-2999-2