Abstract
Main conclusion
The THSG biosynthetic pathway in F. multiflora was characterized, and enzymatic activities responsible for the resveratrol synthesis, hydroxylation, and glycosylation reactions involved in THSG biosynthesis were confirmed in vitro.
The biosynthetic origin of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranoside (THSG) and the enzymes involved in THSG biosynthesis in Fallopia multiflora were studied using stable isotope labeling and biocatalytic methods. UPLC-MS-based analyses were used to unravel the isotopologue composition of the biosynthetic intermediates and products, as well as to detect the products of the enzyme assay experiments. In this study, 13C-labeled l-phenylalanine (l-PHE), sodium pyruvate (SP), and sodium bicarbonate (SB) were used as putative precursors in the feeding experiment. Labeling of polydatin (PD) and THSG using [13C9]L-PHE and [13C1]l-PHE confirmed that the p-coumaric moiety of PD and THSG was derived from PHE. The results of the feeding experiments with [13C] SB and [2, 3-13C2] SP suggested that PD and THSG were derivatives of resveratrol that were synthesized by glycosylation and hydroxylation. We developed methods using total crude protein extracts (soluble and microsomal) for comprehensive and simultaneous analysis of resveratrol synthase, glycosyltransferase, and hydroxylase activities in various tissue types of wild F. multiflora and callus cultures. The activity of each tested enzyme was confirmed in one or more tissue types or cell cultures in vitro. The results of the enzyme activity experiments and the distributions of PD and THSG were used to determine the main site and pathway of THSG biosynthesis in F. multiflora.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fallopia multiflora (F. multiflora, also known as Polygonum multiflorum, common name Chinese Knotweed) is a popular traditional Chinese medicine and an ingredient in numerous prescriptions. Stilbenes are the main characteristic components of F. multiflora. Lin’s review (Lin et al. 2015) mentioned a total of 21 stilbenes and stilbene derivatives in F. multiflora, including a variety of 2,3,5,4′-tetrahydroxystilbene glucosides, resveratrol, polydatin and polygonumosides. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside (THSG, structure shown in Fig. 1), the major bioactive and best-studied stilbene glycoside in F. multiflora, was first isolated and identified in 1976 (Li-Shuang et al. 2006). In vitro and in vivo pharmacological studies have demonstrated that THSG contributes to the antioxidant activity of F. multiflora (Han et al. 2009; Lv et al. 2007). Stilbenes are a small group of phenylpropanoids characterized by a 1,2-diphenylethylene backbone. Resveratrol (3,5,4′-trihydroxystilbene) (Fig. 1), a phytoalexin, possesses valuable biological activities and is the best-researched stilbene (Cullen et al. 2007; Olas et al. 2002; Yang et al. 2008). Resveratrol is formed via the well-characterized phenylalanine/polymalonate biosynthetic pathway. The final step in resveratrol biosynthesis involves the condensation of one molecule of p-coumaroyl-CoA and three molecules of malonyl-CoA in a reaction catalyzed by resveratrol synthase (RS) (Lanz et al. 1990).
The pathway and enzymes involved in resveratrol biosynthesis have been well characterized, and much work has been performed with the goal of producing resveratrol in microbes and plants by metabolic engineering (Delaunois et al. 2009; Jeong et al. 2016). The feasibility of microbial resveratrol production was demonstrated by translating some or all of the genes in the resveratrol biosynthetic pathway into Escherichia coli and Saccharomyces cerevisiae (Lim et al. 2011; Shin et al. 2011). Resveratrol can also be produced by non-stilbene plants by translating the resveratrol synthase gene into these plants (Ma et al. 2009).
Resveratrol biosynthesis and resveratrol synthase are relatively well understood, but little research has been performed on the modification of resveratrol to synthesize stilbene derivatives.
Human and bacterial CYP450s were shown on an analytical scale to be capable of regioselective hydroxylation of resveratrol (Furuya and Kino 2014; Le et al. 2017), but there are no reports regarding functional identification of the CYP450s responsible for resveratrol hydroxylation in plants. With regard to glycosylation, preliminary studies with resveratrol glycosyltransferase have been performed using crude extracts obtained from Gamay Freaux grape cell suspension cultures (Krasnow and Murphy 2004). In addition, a study described the biochemical purification, molecular cloning and functional characterization of a novel bi-functional glucosyltransferase from Vitis labrusca cv. Concord that produces glucosides of stilbenes and glucose esters of hydroxycinnamic acids in vitro (Hall and De Luca 2007).
The biosynthetic pathway of tetrahydroxystilbene glucosides, especially THSG, has attracted our interest, and our group has performed significant research aimed at characterizing the THSG biosynthetic pathway in F. multiflora. In a previous study, we isolated stilbene synthase gene FmPKS, a RS, from F. multiflora (Sheng et al. 2010). We constructed suppression subtractive hybridization (SSH) libraries to allow us to identify genes involved in THSG biosynthesis (Zhao et al. 2014b). To obtain more detailed genetic information, we performed de novo transcriptome assembly and digital gene expression (DGE) profiling of F. multiflora using the Illumina RNA-seq system (Zhao et al. 2014a). We also rapidly established stable suspension cultures of F. multiflora cells in Murashige and Skoog medium as a research model (Shao et al. 2012; Xia et al. 2016). Despite significant research effort, the biosynthetic pathway of THSG in F. multiflora is still not well understood.
The A-rings (Fig. 1) of hydroxystilbenes such as dihydroxystilbene (pinosylvin), trihydroxystilbene (resveratrol) and tetrahydroxystilbene (piceatannol) share the same structure, which is synthesized by linear tetraketide cyclization via intramolecular aldol condensation (Austin and Noel 2003). The different structures of the B-rings of hydroxystilbenes were attributed to accepting different cinnamic acid derivatives as substrates. In F. multiflora, THSG has a distinct A-ring structure with three hydroxyl groups on a benzene ring. Based on the information described above, we propose two hypothetical THSG biosynthetic pathways: (1) THSG is synthesized by hydroxylation of resveratrol in the 2C position to form the corresponding tetrahydroxystilbene, which is then C-glucosylated with a sugar donor; (2) THSG is synthesized by synthesizing a different linear tetraketide or using a cyclization mechanism different from that of resveratrol biosynthesis.
In this study, we developed a method for rapid quantitative analysis of resveratrol, PD and THSG in different parts of F. multiflora and cell culture (callus) by ultra-performance liquid chromatography/quadrupole time-of flight mass spectrometry (UPLC/Q-TOF–MS). We performed feeding experiments on F. multiflora suspension cultures using 13C-labeled precursors to investigate the biosynthetic pathway of THSG. We also characterized the RS, glycosyltransferase (GT), and hydroxylase activities involved in THSG biosynthesis in various tissue types of wild F. multiflora and callus cultures.
Experimental
Reagents and chemicals
Methanol and acetonitrile for the UPLC analysis were obtained from Merck Company (Darmstadt, Germany). Acetic acid for the UPLC analysis was purchased from CNW Technologies GmbH (Germany). Leucine-enkephalin (Sigma, USA) was used as the lock mass. Distilled water was purchased from Watson’s Food & Beverage Co., Ltd. (Guangzhou, China). Absolute ethyl alcohol and ethyl acetate (analytical grade) were purchased from Kermel (Tianjin, China). Precursors [1-13C]-l-phenylalanine (99 atom % 13C), [13C9]-l-phenylalanine (97–99 atom % 13C), [13C]-sodium bicarbonate and [2, 3-13C2]-sodium pyruvate (both 99 atom % 13C) were purchased from Cambridge Isotope Laboratories (Cambridge, MA, USA). THSG, resveratrol, PD and piceatannol were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Uridine 5′-diphosphoglucose disodium salt (UDPG), β-nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt (NADPH), malonyl-CoA, and [13C3]-malonyl-CoA were purchased from Sigma-Aldrich (St. Louis, MO, USA). p-Coumaroyl-CoA was purchased from MicroCombiChem e.K. (Germany).
Plant samples and cell cultures
Fallopia multiflora parts were collected from plants grown at Yaowang Mountain at the Guangzhou University of Chinese Medicine. Callus and suspension cultures were established according to the method of Xia et al. (2016).
Feeding experiments with the 13C-labeled precursors
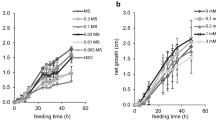
Different amounts of PHE, SP, and SB (with or without a 13C label) were dissolved in distilled water. Preliminary experiments were performed to optimize the conditions under which the precursors were added based on biomass and THSG accumulation. The final concentrations of PHE, SP, and SB in the medium were 60, 1000, and 100 mg L−1, respectively, and the feeding times were 4, 10 and 10 days, respectively (Xia et al. 2016).
To identify the intermediates and final products of the isotope-labeled compounds used in the feeding experiments, the suspension culture cells were harvested 16 days after transportation to new Murashige and Skoog medium. After separating the cells and medium using a sieve and removing the water with filter paper, the fresh cells were dried at 55 °C for 12 h and ground into a fine powder, which was extracted with 60% EtOH. The EtOH extraction was conducted at room temperature for 12 h. The extracts from each experiment were next evaporated to dryness in vacuo, with the resulting residues individually partitioned between ethyl acetate and water. Three biological replicates were performed in each feeding experiment, and the values were averaged to minimize systematic errors. All experimental comparisons were made with the unlabeled treatment control.
Preparation of protein extracts for enzyme activity analyses
All work was done at 4 °C. Fresh tissue samples and callus cultures of F. multiflora were ground to a fine powder in liquid nitrogen in a chilled mortar and pestle. Three times the sample volume of Plant Total Protein Lysis Buffer (Sangon Biotech, Shanghai, China) containing strong protease inhibitors was added to each sample, after which the sample was homogenized to produce concentrated slurry. The slurries were incubated on ice for 3–4 h (with 30 s of vibration per hour) and centrifuged at 1200 rpm for 30 min at 4 °C. The supernatant was applied to a PD-10 desalting column (GE Healthcare, Uppsala, Sweden) equilibrated with ultra-pure water to remove low-molecular-weight compounds. The protein concentration of the crude extract was determined using a Bradford Protein Assay Kit (Sangon Biotech) with BSA as the standard.
Enzyme activity assays
For the RS assay, 100 μL of crude extract was mixed with 150 μM malonyl-CoA, 280 μM p-coumaroyl CoA, and 130 μL 100 mM (pH = 7.6) potassium phosphate buffer (PPB) to reach a final reaction volume of 250 μL. The reaction mixture was held at 30 °C for 1 h. For the hydroxylase assay, 100 μL of the crude extract was mixed with 20 μM of the substrate (resveratrol and PD), 20 mM NADPH, and 50 mM Tris–HCl (pH 7.5) to reach a final reaction volume of 250 μL. The reaction mixture was held at 30 °C for 3 h. The optimum conditions for the RS (Ma et al. 2009) and hydroxylase (Uesugi et al. 2017) assays were determined according to previous studies. The optimum conditions for the GT assay were determined in preliminary experiments using the crude extract of F. multiflora roots (data not shown). For the GT assay, 50 μL of the crude extract was mixed with the acceptor substrate (resveratrol and piceatannol), UDPG, MgCl2, and 50 mM Tris–HCl (pH 7.5) to reach a final reaction volume of 200 μL. The reaction mixture was incubated at 30 °C for 4 h. We also conducted combined enzyme reaction assays, in which two or three enzymatic reactions were allowed to proceed in a tube with their substrates and the crude extract of F. multiflora roots. All reactions were terminated by the addition of 250 μL ethyl acetate. The products and substrates were extracted four times with 250 μL ethyl acetate, after which the combined organic phases were dried using nitrogen. The dried samples were dissolved in 100 μL MeOH prior to UPLC-MS analysis. All determinations were run independently in triplicate.
UPLC/Q-TOF–MS analysis
All wild F. multiflora samples and cell culture samples were collected and ground in liquid nitrogen to a fine powder, which was homogenized in MeOH. The samples were extracted for 12 h at room temperature and centrifuged at 8000 rpm for 10 min.
The prepared samples were analyzed by an Acquity U-HPLC system (Waters Co., Waters, MA, USA) coupled to a Micro-mass Q-Tof micro Mass Spectrometer (Waters Co.,). U-HPLC separation was achieved with a binary solvent delivery system, an auto-sampler, and an Acquity U-HPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm, Waters Co.,) at a flow rate of 0.3 mL/min and at room temperature. The mobile phase consisted of (A) water (including 0.04% acetic acid) and (B) acetonitrile. A linear gradient elution was run as follows: 0 min, 5% B; 5 min, 10% B; 7 min, 20% B; 10 min, 35% B; 12 min, 60% B; 15 min, 75% B; 19 min, 85% B; and 21 min, 100% B.
MS analysis was performed on a Micromass Q-Tof micro Mass Spectrometer (Waters Co.,) equipped with an electrospray ionization (ESI) source operating in negative ion mode (ESI−). The ESI source conditions were as follows: full scan data acquisition was performed from m/z 100 to 600; capillary voltage, 3000 V; cone voltage, 25 V; source temperature, 100 °C; and desolvation temperature, 350 °C. Nitrogen and argon were used as the cone and collision gases, respectively. The cone and desolvation gas flow rates were 50 and 500 L/h, respectively. Leucine-enkephalin was used as the lock mass ([M-H]− m/z 554.2615).
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). Statistical evaluation was performed by one-way analysis of variance (ANOVA) and Tukey’s honest significant difference (HSD) test. The confidence level was > 95% (p < 0.05). The results of the experiments are expressed as mean ± SD.
Results and discussion
Distribution of PD and THSG in various tissues and cultured F. multiflora cells
In the process of chromatographic analysis, PD and THSG are difficult to separate completely because of their similar structures and polarities. In our previous HPLC analysis, resveratrol was undetectable, but in other reports, resveratrol was detected in the roots of F. multiflora (Lin et al. 2015).
In this study, we developed a rapid, convenient, and reliable analytical method to investigate the distributions of PD, resveratrol and THSG in wild plant tissues and cultured F. multiflora cells using a UPLC/Q-TOF–MS system. THSG, PD and resveratrol were identified by analyzing their chromatographic characteristics and comparing their high resolution mass spectrometry (HRMS) profiles with those of standard substances. The base peak intensity (BPI) chromatograms showed that the two compounds had insufficient baseline separation, but they were well separated in the extracted ion chromatogram (EIC) (Online Resource 1), so the quantification was automatically integrated using the extracted ion chromatogram (EIC). The amount of each product was determined using its integrated peak area and the predetermined calibration curve for each substrate.
THSG accumulated to high concentrations within the vines (1504.091 ± 81.672 μg/g fresh weight) and roots (1730.719 ± 37.807 µg/g fresh weight), whereas it accumulated to very low concentrations within the stem tips, stems, leaves and callus (Table 1). The THSG contents in these six samples were basically consistent with the results from our previous HPLC analysis, in which THSG accumulated to high concentrations within the vines and roots (42.751 ± 3.323 and 24.125 ± 3.291 mg/g dry weight, respectively), to very low concentrations within the callus (0.022 ± 0.008 mg/g dry weight), and was not detected in the leaves or stems (Xia et al. 2016).
PD was detected in the leaves, vines, roots, and callus at very low concentrations (Table 1). Resveratrol was not detected in wild F. multiflora or cultured cells.
The aglycone of THSG, 2,3,5,4′-tetrahydroxystilbene, has been speculated to be an intermediate in THSG biosynthesis, but it has not been identified and was not available commercially at the time of this study. We did not attempt to produce 2,3,5,4′-tetrahydroxystilbene in our laboratory. 2,3,5,4′-Tetrahydroxystilbene (m/z = 243.0658) was not detected in F. multiflora tissue samples or callus.
Feeding experiments
To compare the biosynthetic pathways of the primary trihydroxystilbene glucoside (PD) and tetrahydroxystilbene glucoside (THSG) in F. multiflora, we performed stable isotopic tracing experiments together with a high throughput analytical method based on UPLC-MS to track labeled PD and THSG.
In this study, 13C-labeled l-PHE, SP, and SB were used as putative precursors in the feeding experiment to utilize the general phenylpropanoid pathway (Fig. 1), in which PHE is converted to p-coumaroyl-CoA, whereas SP and SB are converted to malonyl-CoA (Jez et al. 2000a). In our previous feeding experiments with unlabeled precursors and F. multiflora suspension cultures, PHE, SP and SB did not increase the culture biomass or THSG yield, which was inconsistent with speculation that THSG synthesis might involve phenylpropanoid pathways (Xia et al. 2016). Thus, further studies were carried out here to determine whether PHE, SP and SB are precursors in PD and THSG biosynthesis, as well as to study the biosynthetic pathways of PD and THSG in F. multiflora suspension cultures. In the biosynthetic pathway of resveratrol, the p-coumaric moiety was derived from routing PHE through cinnamic acid (CA), p-coumaric acid, and p-coumaroyl-CoA.
To investigate the origin of the p-coumaric moieties of PD and THSG, [13C9] L-PHE was applied to F. multiflora suspension cultures, followed by detection of PD and THSG by UPLC/Q-TOF–MS. As expected, [13C9] PD (m/z 398.1557, [M-H + 9]−) and [13C9] THSG (m/z 414.1494, [M-H + 9]−) were detected, which unambiguously confirmed that all nine carbon atoms of l-PHE were incorporated into PD and THSG (Fig. 2). The 13C9 signal was also observed in the MS spectra of CA and p-coumaric acid, which was consistent with the involvement of PHE in the phenylpropanoid pathway (data not shown). These findings confirm that PHE is a precursor of PD and THSG in F. multiflora. In addition, these results show that, like the p-coumaric moiety of resveratrol, those of PD and THSG are derived from PHE.
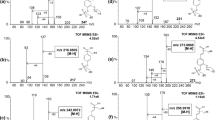
13C labeling patterns of PD and THSG from the experiments with [U-13C]l-PHE and [13C1] l-PHE. A The mass spectra of unlabeled and [13C9]THSG (m/z 414.1494, [M-H + 9]−) obtained from F. multiflora suspension cultures fed with [U-13C9]l-PHE; B the mass spectra of unlabeled and [13C1]THSG (m/z 406.1218, [M-H + 1]−) obtained from F. multiflora suspension cultures fed with [13C1]l-PHE; a The mass spectra of unlabeled and [13C9]PD (m/z 398.1557, [M-H + 9]−) fed with [U-13C9]l-PHE; b The mass spectra of unlabeled and [13C1]PD (m/z 390.1310, [M-H + 9]−) obtained from F. multiflora suspension cultures fed with [13C1]l-PHE; C and c the controls fed with unlabeled l-PHE
The incorporation of all carbon atoms of l-PHE into PD and THSG also indicated that the carbon atom of CO2 formed during stilbene synthase (STS)-catalyzed condensation of p-coumaroyl-CoA and malonyl-CoA was derived from malonyl-CoA, but not from p-coumaroyl-CoA. To confirm the origin of the CO2 produced during this reaction, additional feeding experiments using [13C1] l-PHE with the 13C label at C-1 (using the numbering scheme shown in Fig. 1) were performed. [13C1]PD and [13C1] THSG were detected, which was consistent with the results of the [13C9] L-PHE feeding experiments. These findings confirm that the carbon atom of the CO2 produced during STS-catalyzed condensation of p-coumaroyl-CoA and malonyl-CoA in F. multiflora is derived from malonyl-CoA.
SP and SB are involved in malonyl-CoA biosynthesis. In addition to L-PHE, SP and SB were also shown to be putative precursors of PD and THSG (Fig. 1). To investigate the biosynthetic process of the A-rings of PD and THSG, [13C] SB and [2, 3-13C2] SP were separately applied to F. multiflora suspension cultures.
The high resolution mass spectra of PD and THSG exhibited distinct 13C-labeled signals, which indicated incorporation of SP into PD and THSG. Malonyl-CoA is derived from acetyl-CoA and bicarbonate in a reaction catalyzed by acetyl-CoA carboxylase. Pyruvate undergoes oxidative decarboxylation, in which it loses its carboxyl group (as CO2) to form acetyl-CoA (Jez et al. 2000b).
The analysis described above showed that [2,3-13C2] SP feeding resulted in production of [2,3-13C2] malonyl-CoA (using the numbering scheme shown in Fig. 1). In addition, the 13C2 units of malonyl-CoA were incorporated into the linear tetraketide intermediate, which led to [M + 2], [M + 4], and [M + 6] incorporation patterns after three condensations with malonyl-CoA. The incorporation patterns of the 13C signal in PD and THSG were determined by the intramolecular cyclization patterns of the linear tetraketide intermediate.
Five new ions ([M-H + 1]−, [M-H + 2]−, [M-H + 3]−, [M-H + 4]−, and [M-H + 5]−) indicating 13C-labeled PD and THSG appeared with the same retention time as [12C]-PD and [12C]-THSG in the high resolution mass spectra (Fig. 3) when [13C2]-SP was administered to F. multiflora suspension cultures. These results are consistent with intramolecular C2 → C7 aldol condensation catalyzed by STS by cyclizing the tetraketide intermediate. The STS C2 → C7 reaction requires a thioesterase-like hydrolysis step to cleave the C1 thioester linkage to the STS enzyme, as well as decarboxylative elimination of the resulting C1 carboxylate (Austin et al. 2004). Therefore, when the 13C2 unit was incorporated into the C1 and C2 positions of the linear tetraketide intermediate, [M-H + 1]−, [M-H + 3]−, and [M-H + 5]−appeared because of C1 elimination; otherwise, the [M-H + 2]−and [M-H + 4]−ions appeared in the high resolution mass spectra of PD and THSG. The incorporation patterns of PD (a resveratrol glucoside) in the [2,3-13C2] SP feeding experiments confirmed the resveratrol biosynthetic pathway determined from previous research. Although not fully conclusive, THSG shares the same incorporation patterns of the 13C label with PD, suggesting that THSG biosynthesis is achieved through resveratrol hydroxylation and glycosylation. As expected, [13C] SB was not incorporated into PD and THSG, in accordance with the results of the PHE feeding experiments, which showed that the CO2 produced during biosynthesis of PD and THSG was derived from the carbon atom of malonyl-CoA generated from SB (Fig. 1).
13C labeling patterns of PD and THSG from the experiments with [2, 3-13C2]SP. a 13C labeling patterns of THSG ([M-H + 1]−, [M-H + 2]−, [M-H + 3]−, [M-H + 4]−, and [M-H + 5]−); c 13C labeling patterns of PD([M-H + 1]−, [M-H + 2]−, [M-H + 3]−, [M-H + 4]−, and [M-H + 5]−); b, d the controls fed with unlabeled SP
The results of the [13C] SB and [2,3-13C2] SP feeding experiments indicate that PD and THSG are derivatives of resveratrol, while the CO2 produced during the biosynthesis of PD and THSG is derived from condensation of p-coumaroyl-CoA and malonyl-CoA.
Enzyme assays
Based on the 13C-labeled precursor feeding experiments described above, we concluded that PD and THSG were likely biosynthesized via hydroxylation and glycosylation of resveratrol, and that resveratrol was synthesized via the phenylalanine/polymalonate biosynthetic pathway. Therefore, we characterized the enzymatic activities responsible for resveratrol synthesis and the hydroxylation and glycosylation reactions involved in PD and THSG biosynthesis using crude enzyme extracts, with the goal of understanding how PD and THSG are synthesized in F. multiflora. Crude protein was extracted from various tissue samples from wild F. multiflora and callus cultures (Online Resource 2), after which the protein samples were desalted twice to remove most of the contaminating small molecules.
To confirm the capacity of the enzymes responsible for resveratrol synthesis in F. multiflora cells, p-coumaroyl-CoA and malonyl-CoA were tested as substrates for biocatalysis using crude enzyme extracts from the stem tips, stems, leaves, vines, roots and callus of F. multiflora. The expected product, resveratrol, was synthesized only by the vine crude enzyme extract, which produced 0.105 ± 0.003 nmol resveratrol in the 250-μL reaction system. Therefore, RS activity in F. multiflora is confined to the vines. To further assess RS activity, [13C3]-malonyl-CoA and p-coumaroyl-CoA were incubated with crude protein extracts from six types of tissue. Labeled resveratrol (Fig. 4a; [M-H + 5]−, 232.0882) was detected only in the mixture with the vine extracts, and the labeled pattern was consistent with the incorporation patterns of 13C in PD and THSG in the [2, 3-13C2] SP feeding experiments. This biocatalysis study confirmed the activity of RS in F. multiflora vine cells and the involvement of intramolecular C2 → C7 aldol condensation with elimination of the resulting C1 during biosynthesis of PD and THSG.
Glycosyltransferase-catalyzed attachment of sugar moieties to the hydroxystilbene backbone in F. multiflora cells was investigated by biocatalytic methods in vitro using crude enzyme extracts. Trihydroxystilbene (resveratrol) and tetrahydroxystilbene (piceatannol) were used as aglycons, while UDPG was used as the donor. Moderate concentrations of the aglycons and donor (40 μM resveratrol and piceatannol, 500 μM UDPG) were used in the assessment of GT activity in different parts of F. multiflora and callus.
Four of the sample types (stem tips, stems, leaves, and callus) showed positive results, which consisted of new low peaks at m/z = 389.1244 [M-H]−or statistically significant accumulation of PD, whereas the protein extract from the roots failed to produce a significant change in PD content. The low concentrations of PD produced by the extracts from the stem tips, stems, leaves, callus, and vines (Table 2) demonstrated the weakness of GT activity with resveratrol as the glycosyl acceptor in vitro. In addition, we used a series of substrate concentrations in the biocatalytic experiments with extracts from vines to investigate the influence of the substrate concentration on GT activity. However, no PD was detected when the resveratrol and UDPG concentrations were lower than 10 and 200 μM, respectively. In the other tests with extracts from vines, the PD contents of the control samples and those incubated with various concentrations of resveratrol (20 to 100 μM) and UDPG (200 to 1200 μM) showed no significant differences.
Based on these results, we conclude that the stem tips, stems, leaves, vines, and callus of F. multiflora have GT activity. In the production of PD from protein extracts of these tissues in vitro, resveratrol serves as the acceptor substrate, whereas UDPG serves as the donor substrate. The concentration of each substrate did not significantly affect PD production in vitro.
We also investigated the GT activity of the crude protein extracts with tetrahydroxystilbene piceatannol as the acceptor substrate. Although piceatannol and the aglycon of THSG are both tetrahydroxystilbenes, their hydroxyl group positions differ (Fig. 1). Unfortunately, the aglycon of THSG, 2,3,5,4′-tetrahydroxystilbene, has not been identified and cannot be obtained commercially or produced by our laboratory. Thus, we used piceatannol as a substrate to test the GT activity responsible for tetrahydroxystilbene glycosylation. The new peak at m/z = 405.1197 ([M-H]−) with a retention time at 1.96 min appeared to be an isomer of THSG, which was consistent with the addition of a glycosyl group to piceatannol (Online Resource 5). Thus, this new peak was speculated to be the glycosylated product of a reaction catalyzed by a GT from F. multiflora with piceatannol as the substrate. The crude protein extracts from the stems and callus did not show the ability to glycosylate piceatannol, but the extracts from the stem tips, leaves, vines, and roots showed the ability to glycosylate piceatannol with varying effectiveness (Table 2).
The yield of the glycosylation reaction using piceatannol as its substrate was calculated using THSG as the reference substance (Table 2). The tetrahydroxystilbene (piceatannol) GT activity of the roots and vines was much greater than their trihydroxystilbene (resveratrol) GT activity. Similar to the results of the assessment of GT activity using resveratrol as the substrate, the extracts of the stem tips and leaves showed weak GT activity with piceatannol as a glycosyl acceptor in vitro.
The effects of the substrate concentration on the piceatannol GT activity of the crude enzyme extract from the roots are shown in Online Resource 3 and Online Resource 4. The optimal concentration of UDPG was 1.5 mM when the concentration of piceatannol was 40 μM. The optimal concentration of piceatannol was 100 μM when the concentration of UDPG was 500 μM.
The hydroxylase activity involved in THSG biosynthesis was investigated using resveratrol and PD as substrates by incubating them with crude enzyme extracts in the presence of NADPH. When resveratrol was the substrate, only incubation with the extract from the vines led to a new peak at m/z = 243.0658 ([M-H]−), which was consistent with the addition of a hydroxyl group. This peak was absent in the spectrum of a control sample that did not contain any substrate and had a retention time (2.35 min) different from that of piceatannol (Online Resource 5). The LC–MS results indicated that this peak was an isomer of piceatannol. Therefore, this new peak was speculated to be the hydroxylated product of the reaction catalyzed by resveratrol hydroxylase from the vines of F. multiflora.
In contrast, when PD was used as the substrate, we found much greater hydroxylase activity in comparison with that achieved with resveratrol as the substrate. PD possesses a glycosyl group at position 3, whereas THSG possesses a glycosyl group at position 2. The new peak at m/z = 405.1197 [M-H]− with a retention time at 2.59 min appeared to be an isomer of THSG, which was consistent with the addition of a hydroxyl group to PD. Therefore, this new peak was speculated to be the hydroxylated product of a reaction catalyzed by a hydroxylase from F. multiflora with PD as the substrate. PD hydroxylase activity was found in the stem tips, stems, vines, roots, and callus (Table 2).
We also used piceatannol and THSG as reference substances to calculate the yield of the hydroxylation reactions using resveratrol and PD as substrates (Table 2). The high yield of the tetrahydroxystilbene glucoside when the substrates were incubated with the extracts from the vines and roots suggests that hydroxylase activity was markedly increased when PD was the substrate.
The results of the biocatalysis study demonstrate the complexity of the THSG biosynthetic pathway in various F. multiflora tissue types and callus. The in vitro biocatalysis reactions confirm that RS, GT and hydroxylase reactions occur in F. multiflora cells. The high tetrahydroxystilbene GT activity and PD hydroxylase activity in the vines and roots were consistent with the high accumulation of their reaction products in these tissues. All enzyme assays were conducted in vitro, so the results of these experiments might not be representative of the actual reaction pathways in vivo.
Combinatorial biocatalysis
The enzyme reactions responsible for resveratrol synthesis, hydroxylation, and glycosylation in the THSG biosynthesis pathway in F. multiflora cells and callus are shown in Table 2. However, these results were not sufficient to allow us to draw conclusions regarding the biosynthetic pathway of THSG in different tissues and cell cultures of F. multiflora. Therefore, we conducted experiments in which we incubated the crude enzyme extracts with substrates of more than one reaction step in the biosynthetic pathway of THSG.
When NADPH was incubated with the crude enzyme extracts from the vines, p-coumaroyl-CoA, and malonyl-CoA, a tetrahydroxystilbene product was produced, which was consistent with the results of incubating NADPH and resveratrol (Table 3). Moreover, when non-labeled malonyl-CoA was replaced by 13C-labeled malonyl-CoA, 13C-labeled resveratrol and hydroxyresveratrol were generated (Fig. 4b, c). These results confirm the RS activity of F. multiflora vines and indicate that resveratrol synthesis and hydroxylation can be achieved in a single incubation mixture by two sequential steps.
When we added UDPG and MgCl2 to the incubated mixture of the crude enzyme extract from the vines, p-coumaroyl-CoA and malonyl-CoA, only resveratrol was produced (Table 3). Incubation of the crude extract from the vines with 13C-labeled malonyl-CoA generated 13C-labeled resveratrol. These results confirm the RS activity of the vines of F. multiflora. The lack of PD production was attributed to the low concentration of resveratrol synthesized from p-coumaroyl-CoA and malonyl-CoA, which indicated that resveratrol synthesis and glycosylation could not be achieved in a single incubation by two sequential steps.
The products produced when we used all of the substrates (p-coumaroyl-CoA, 13C-labeled malonyl-CoA, NADPH and UDPG) required for each enzymatic reaction in the THSG biosynthetic pathway in a single incubation are shown in Table 3. No labeled THSG was produced, which suggested that the three enzymatic reactions in the THSG biosynthetic pathway could not be achieved in a single incubation in vitro.
The products produced when resveratrol, NADPH, UDPG, and MgCl2 were co-incubated with extracts from five tissues and callus of F. multiflora are shown in Table 4. PD formation was observed when the extracts from the stems, leaves, vines and callus were incubated, which was consistent with the results of the resveratrol GT activity assay. However, PD was detected in the mixture incubated with the extract from the roots, but not in that incubated with the extract from the stem tips, which was inconsistent with the results of the resveratrol GT activity assay. Tetrahydroxystilbene formation was observed when the extracts from the vines and roots were incubated, which was consistent with the results of the resveratrol hydroxylase activity assay.
The results of the combinatorial biocatalysis experiments indicate that only the hydroxylation reactions in the THSG biosynthetic pathway can proceed following the resveratrol synthesis reaction in a single incubation in vitro. No THSG was produced, which indicated that these three reactions cannot proceed sequentially in vitro in a single incubation.
Conclusion
This study characterized the THSG biosynthetic pathway in F. multiflora. We conclude that THSG is synthesized via glycosylation and hydroxylation of resveratrol in F. multiflora. The enzymatic activities responsible for the resveratrol synthesis, hydroxylation, and glycosylation reactions involved in THSG biosynthesis were confirmed in vitro. The findings of this study provide information regarding the manner in which resveratrol is modified by enzymes in F. multiflora.
Author contribution statement
Shujin Zhao designed and supervised the study; Wanxia Xia performed the experiments; Wen Rui, Wei Zhao, Shujing Sheng and Lei Lei helped in the experiment; Wanxia Xia and Wen Rui analyzed the data, Wanxia Xia and Shujin Zhao prepared the manuscript. All authors read and approved the final manuscript.
Abbreviations
- THSG:
-
2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside
- l-PHE:
-
l-Phenylalanine
- SP:
-
Sodium pyruvate
- SB:
-
Sodium bicarbonate
- PD:
-
Polydatin
- RS:
-
Resveratrol synthase
- GT:
-
Glycosyltransferase
- STS:
-
Stilbene synthase
- UDPG:
-
Uridine 5′-diphosphoglucose disodium salt
- NADPH:
-
β-Micotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt
- CA:
-
Cinnamic acid
References
Austin MB, Noel A (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20:79–110
Austin MB, Bowman ME, Ferrer JL, Schroder J, Noel JP (2004) An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem Biol 11:1179–1194
Cullen JP, Morrow D, Jin Y, von Offenberg Sweeney N, Sitzmann JV, Cahill PA, Redmond EM (2007) Resveratrol inhibits expression and binding activity of the monocyte chemotactic protein-1 receptor, CCR2, on THP-1 monocytes. Atherosclerosis 195:e125–e133
Delaunois B, Cordelier S, Conreux A, Clément C, Jeandet P (2009) Molecular engineering of resveratrol in plants. Plant Biotechnol J 7:2–12
Furuya T, Kino K (2014) Regioselective synthesis of piceatannol from resveratrol: catalysis by two-component flavin-dependent monooxygenase HpaBC in whole cells. Tetrahedron Lett 55:2853–2855
Hall D, De Luca V (2007) Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J 49:579–591
Han L, Wu B, Pan G, Wang Y, Song X, Gao X (2009) UPLC-PDA analysis for simultaneous quantification of four active compounds in crude and processed rhizome of Polygonum multiflorum Thunb. Chromatographia 70:657–659
Jeong Y, An CH, Woo SG, Park JH, Lee K, Lee S, Rim Y, Jeong HJ, Ryu YB, Kim CY (2016) Enhanced production of resveratrol derivatives in tobacco plants by improving the metabolic flux of intermediates in the phenylpropanoid pathway. Plant Mol Biol 92:117–129
Jez JM, Austin MB, Ferrer JL, Bowman ME, Schroder J, Noel JP (2000a) Structural control of polyketide formation in plant-specific polyketide synthases. Chem Biol 7:919–930
Jez JM, Ferrer JL, Bowman ME, Dixon RA, Noel JP (2000b) Dissection of malonyl-coenzyme A decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry 39:890–902
Krasnow MN, Murphy TM (2004) Polyphenol glucosylating activity in cell suspensions of grape (Vitis vinifera). J Agr Food Chem 52:3467–3472
Lanz T, Der GS, Der JS (1990) Differential regulation of genes for resveratrol synthase in cell cultures of Arachis hypogaea L. Planta 181(2):169–175
Le T, Jang H, Nguyen HTH, Doan TTM, Lee G, Park KD, Ahn T, Joung YH, Kang H, Yun C (2017) Highly regioselective hydroxylation of polydatin, a resveratrol glucoside, for one-step synthesis of astringin, a piceatannol glucoside, by P450 BM3. Enzyme Microb Tech 97:34–42
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MAG (2011) High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microb 77:3451–3460
Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, Qu C, Ni J (2015) Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol 159:158–183
Li-Shuang LV, Gu XH, Ho CT, Tang J (2006) Stilbene glycosides from the roots of Polygonum multiflorum Thunb and their in vitro antioxidant activities. J Food Lipids 13:131–144
Lv L, Gu X, Tang J, Ho C (2007) Antioxidant activity of stilbene glycoside from Polygonum multiflorum Thunb in vivo. Food Chem 104:1678–1681
Ma L, Pang X, Shen H, Pu G, Wang H, Lei C, Wang H, Li G, Liu B, Ye H (2009) A novel type III polyketide synthase encoded by a three-intron gene from Polygonum cuspidatum. Planta 229:457–469
Olas B, Wachowicz B, Saluk-Juszczak J, Zieliński T (2002) Effect of resveratrol, a natural polyphenolic compound, on platelet activation induced by endotoxin or thrombin. Thromb Res 107:141–145
Shao L, Zhao S, Cui T, Liu Z, Zhao W (2012) 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glycoside biosynthesis by suspension cells cultures of Polygonum multiflorum Thunb and production enhancement by methyl jasmonate and salicylic acid. Molecules 17:2240–2247
Sheng S, Liu Z, Zhao W, Shao L, Zhao S (2010) Molecular analysis of a type III polyketide synthase gene in Fallopia multiflora. BIOLOGIA 65:939–946
Shin S, Han NS, Park Y, Kim M, Seo J (2011) Production of resveratrol from p-coumaric acid in recombinant Saccharomyces cerevisiae expressing 4-coumarate:coenzyme A ligase and stilbene synthase genes. Enzyme Microb Tech 48:48–53
Uesugi D, Hamada H, Shimoda K, Kubota N, Ozaki SI, Nagatani N (2017) Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci Biotechnol Biochem 81:226–230
Xia W, Lei L, Zhao W, Feng Y, Zhao S (2016) Quantitative analysis of 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glycoside in wild Polygonum multiflorum and suspension cell cultures fed different precursors and elicitors. Phytochem Lett 15:180–185
Yang Y, Paik JH, Cho D, Cho J, Kim C (2008) Resveratrol induces the suppression of tumor-derived CD4+ CD25+ regulatory T cells. Int Immunopharmacol 8:542–547
Zhao W, Sheng S, Liu Z, Di L, Zhu K, Li X, Zhao S, Yao Y (2014a) Isolation of biosynthesis related transcripts of 2,3,5,4′-tetrahydroxy stilbene-2-O-β-d-glucoside from Fallopia multiflora by suppression subtractive hybridization. Acta Soc Bot Pol 83:147–157
Zhao W, Xia W, Li J, Sheng S, Lei L, Zhao S (2014b) Transcriptome profiling and digital gene expression analysis of Fallopia multiflora to discover putative genes involved in the biosynthesis of 2,3,5,4′-tetrahydroxy stilbene-2-O-β-d-glucoside. Gene 547:126–135
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2017_2797_MOESM1_ESM.tif
Online Resource 1 Extracted ion chromatogram of PD (A), THSG (B), piceatannol (C) and resveratrol (D) Mass spectra under ESI (–) mode of PD (a), THSG (b), piceatannol (c) and resveratrol (d) (TIFF 2220 kb)
425_2017_2797_MOESM2_ESM.tif
Online Resource 2 Total protein concentration of the crude extracts from wild F. multiflora tissues and callus (TIFF 17684 kb)
425_2017_2797_MOESM3_ESM.tif
Online Resource 3 Effects of the UDPG concentration on the piceatannol glycosyltransferase activity of the crude enzyme extract from the roots. The concentration of piceatannol was 40 μM (TIFF 80567 kb)
425_2017_2797_MOESM4_ESM.tif
Online Resource 4 Effects of the piceatannol concentration on the piceatannol glycosyltransferase activity of the crude enzyme extract from the roots. The concentration of UDPG was 0.5 mM (TIFF 78003 kb)
Rights and permissions
About this article
Cite this article
Xia, W., Rui, W., Zhao, W. et al. Stable isotope labeling and 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucopyranoside biosynthetic pathway characterization in Fallopia multiflora . Planta 247, 613–623 (2018). https://doi.org/10.1007/s00425-017-2797-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2797-2