Abstract
Main conclusion
A terrestrial orchid, Cymbidium sinense appears to utilizes “remedy strategy”, while an epiphytic orchid, C. tracyanum , employs a “precaution strategy” to drought stress based on morphological, physiological and proteomic analysis.
Drought condition influences plant growth and productivity. Although the mechanism by which plants adapt to this abiotic stress has been studied extensively, the water-adaptive strategies of epiphytes grown in water-limited habitats remain undefined. Here, root and leaf anatomies, dynamic changes in physiological and proteomic responses during periods of drought stress and recovery studied in an epiphytic orchid (Cymbidium tracyanum) and a terrestrial orchid (C. sinense) to investigate their strategies for coping with drought. Compared with C. sinense, C. tracyanum showed stronger drought-resistant adaptive characteristics to drought because its leaves had more negative water potential at turgor loss point and roots had higher proportion of velamen radicum thickness. Although both species demonstrated quick recovery of photosynthesis after stress treatment, they differed in physiological and proteomic responses. We detected and functionally characterized 103 differentially expressed proteins in C. sinense and 104 proteins in C. tracyanum. These proteins were mainly involved in carbon and energy metabolism, photosynthesis, and defense responses. The up-regulated expression of plastid fibrillin may have contributed to the marked accumulation of jasmonates only in stressed C. sinense, while ferredoxin-NADP reductase up-regulation was only found in C. tracyanum which possibly related to the stimulation of cyclic electron flow that is linked with photoprotection. These physiological and proteomic performances suggest distinct adaptive strategies to drought stress between C. sinense (remedy strategy) and C. tracyanum (precaution strategy). Our findings may help improve our understanding about the ecological adaptation of epiphytic orchids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epiphytes are an important component of tropical and subtropical flora, and are especially rich within Orchidaceae (Zotz and Bader 2009). Both epiphytic and terrestrial life forms occur in the genus Cymbidium (Orchidaceae), which is distributed in tropical and subtropical Asia, and in northern Australia. Approximately 70% of the members within Cymbidium grow on trees (Zhang et al. 2001; Motomura et al. 2008). Numerous species in Cymbidium have long been cultivated as desirable ornamental plants worldwide. Some of them are now endangered in wild due to the destruction of natural habitats and climate change (Luo et al. 2002; Liu et al. 2009). Therefore, studying the adaptive mechanisms by which epiphytic and terrestrial Cymbidium species adapt to abiotic stress is beneficial for their utilization and conservation, and can improve our understanding of ecological strategy in orchids.
Because of their divergent life forms, closely related species may develop different mechanisms for adapting to their habitats (Zhang et al. 2016). Compared with terrestrial environments, epiphytic sites are usually characterized by restricted capacity to store available water and nutrients, as well as extreme fluctuations in light and temperature (Théry 2001). Among these abiotic factors, water is arguably the most limiting for the growth of vascular epiphytes (Zotz and Hietz 2001). However, the physiological and proteomic mechanisms that epiphytic orchids utilize under drought stress have remained undefined.
Plants optimize their morphology, physiology and metabolic processes at organ and cellular levels to cope with drought stress. The strategies for drought resistance include avoidance and tolerance, with the former being achieved via enhanced water uptake and reduced water loss (Chaves et al. 2002; Price et al. 2002). An adaptive structure for drought avoidance is velamen radicum, which may be important for maintaining the water balance in epiphytic orchids. This unique dead structure occurs on the root surface of most epiphytic orchids, acting as a sponge to absorb water within seconds during and immediately after a rain fall (Zotz and Tyree 1996; Zotz and Winkler 2013). We previously reported that epiphytic orchids have higher values for leaf mass per unit area, leaf thickness, epidermal thickness, saturated water content and the time required to dry saturated leaves to 70% relative water content. This indicated that ability to avoid drought of epiphytes is greater than terrestrial species (Zhang et al. 2015).
The well-known mechanisms for strategies of drought tolerance include the biosynthesis/de-conjugation of abscisic acid (ABA) and its signaling pathways, which lead to stomatal closure. Other tolerance mechanisms are mediated through osmotic adjustments, various secondary messengers, and transcriptional/post-transcriptional regulation that activates various drought-related genes (Reddy et al. 2004; Golldack et al. 2014). However, the entire process is more complex. For example, ABA is not the only phytohormone involved in drought stress response, and much evidence has been found of cross-talk between it and other phytohormones, such as jasmonates (JA) (Wilkinson et al. 2012). Furthermore, plants can be divided into two categories—isohydric or anisohydric—according to their stomatal regulation of water status (Tardieu and Simonneau 1998). Isohydric plants show reduced stomatal conductance when the soil water potential (Ψ soil) decreases and atmospheric conditions are dry, making them able to maintain a relatively constant midday leaf water potential (Ψ leaf) regardless of drought conditions. In contrast, anisohydric species allow midday Ψ leaf to decline as Ψ soil declines. For C. sinense, the transportation rate and stomatal resistance are more drought-sensitive than leaf water content, leaf water potential and chlorophyll content (Pan et al. 1993). This suggests that this species relies upon isohydric regulation of stomata. Photoprotection of photosystem II (PSII) and photosystem I (PSI) is another important process in the drought response. We have previously determined that, under strong irradiance or chilling stress, the activities of PSII and PSI are more sensitive in C. sinense than in C. tracyanum, and that stimulation of cyclic electron flow (CEF) may be a primary photoprotective mechanism in the latter species (Kuang and Zhang 2015; Li and Zhang 2016). Therefore, because of the complexity of mechanisms in drought response, further studies should focus on the physiological-, biochemical- and molecular-based aspects of the divergent life forms displayed among species of Cymbidium.
Although the molecular mechanisms for drought adaptations are now better understood (Yordanov et al. 2000; Shinozaki and Yamaguchi-Shinozaki 2007; Manavalan et al. 2009), investigating those mechanisms in members of Orchidaceae are more challenging because of their large genome size, low transformation efficiency, extended regeneration period, and long life cycle (Hossain et al. 2013). However, with the development of the proteomics technologies that have enabled more holistic studies of a wider range of plant species (Hajheidari et al. 2005; Bonhomme et al. 2009; Fulda et al. 2011), researchers can now examine strategies for drought adaptation in Cymbidium at cell, tissue and organic levels.
In the present study, we investigated the mechanisms and strategies underlying the responses by an epiphytic orchid (C. tracyanum) and a terrestrial orchid (C. sinense) to gradually anabatic drought stress and consequent water recovery by applying physiological and comparative proteomics. By characterizing the adaptive mechanisms of the two species, we may ultimately be able to improve their utilization and conservation.
Materials and methods
Plant materials
We studied two species in Cymbidium to compare differences in the adaptive strategies by terrestrial and epiphytic plants to drought. C. sinense is a typical terrestrial orchid that always occurs on forest floors or in well-drained, shaded thickets in subtropical and tropical forests of southeastern China at elevations of 300–1500 m. In contrast, C. tracyanum is an epiphytic orchid that always grows on tree trunks in the subtropical forests of southwestern China at 1200–2000 m. To minimize the potential effect of developmental differences on our experimental results, we selected 30 mature individuals of fairly uniform size for each species. They were planted in the plastic pots containing bark mixtures, and placed in a greenhouse at the Kunming Institute of Botany, Kunming, China. Their growing conditions included an air temperature of 18–24 °C, relative humidity (RH) of 50–70, and 20% full sunlight. The plants were watered to maintain soil relative water content (RWC) of 65–75% before experimental treatments began. The soil RWC was defined by weighing individual pots, and was calculated as: (initial weight − final weight)/initial weight × 100.
Drought treatments
We investigated the response of C. sinense and C. tracyanum to gradually anabatic drought stress and water recovery. Before treatment, records were made of leaf morphology, root anatomy, gas exchange, chlorophyll fluorescence, midday leaf water content (Ψ MD), concentrations of JA and ABA, activity of antioxidant enzymes catalase (CAT, EC1.11.1.6) and superoxide dismutase (SOD, EC1.15.1.1), and the concentrations of nonstructural carbohydrate in samples from each species. The leaf materials for proteomic analysis were frozen in liquid nitrogen and stored in −80 °C. Half of the plants for each species were arranged in a completely randomized design and watered to maintain soil RWC of 65–75% as the well-watered control treatment, and irrigation was halted for the other half to induce different degrees of drought stress. When the soil RWC values had declined to 40–45, 20–25, 10–15 and 5–10%, the parameters mentioned above were measured and leaf materials were collected from both species for proteomic analysis. After these drought treatments were completed, the soil RWC of those stressed plants was recovered to 60–75% by rewatering. During this phase of recovery, the gas exchange was measured daily until the values for stomatal conductance (g s) and net photosynthesis (A n) returned to the level recorded before drought treatment began. Following this 3-day recovery period, those parameters were again measured and leaf materials were collected.
Examination of root anatomy and leaf morphology
For each species, eight fresh mature roots were collected from individual plants. Transverse sections were examined and photographed with a digital camera mounted on a Leica DM2500 microscope (Leica Microsystems Vertrieb GmbH, Wetzlar, Germany). Photographs at 1.6× magnification were used to measure the ratio of velamen thickness to root semi-diameter with the Image J program, while the photographs at 10× magnification were used to observe the xylem conduits diameter (XCD) and the number of xylem conduits (NXC) in the roots.
Values for RWC and saturated water content (SWC) were calculated according to the method of Ogburn and Edwards (2012). The time needed to dry a saturated leaf to 70% RWC (T 70) was measured as described by Hao et al. (2010). Pressure–volume (P–V) curves were acquired using a WP4C Dewpoint Potentia Meter (Decagon, Pullman, WA, USA), based on the method of Ogburn and Edwards (2012). Water relations parameters derived from P–V curves included osmotic potential at full turgor (Π o), water potential at turgor loss point (Ψ TLP), relative water content at turgor loss point (RWCTLP), volumetric elastic modulus (ε), capacitance before turgor loss (C FT), and capacitance after turgor loss (C TLP) (Tyree and Hammel 1972; Ogburn and Edwards 2012).
Measurements of gas exchange and chlorophyll fluorescence
The Li-6400 open gas exchange system (Li-Cor Inc., Lincoln, NE, USA) was used to determine the A n and g s from six mature leaves of each species. During this monitoring period, the RH was 60% and the air temperature was 22 °C, the CO2 concentration was maintained at 400 µmol mol−1. All measurements were made from 09:00 to 11:30 when CO2 uptake was maximal.
The in vivo chlorophyll fluorescence of PSII and P700 redox state were measured with Dual PAM-100 (Heinz Walz, Effeltrich, Germany) that was connected to a computer with control software. The following parameters were examined: minimum fluorescence (F 0), maximum fluorescence (F m), minimum fluorescence in light-adapted state (\(F_{\text{o}}^{\prime }\)), the maximum quantum yield of PSII after dark adaptation overnight (F v/F m), and the effective quantum yield of PSII [Y(II)] (Kramer et al. 2004). Saturation pulses (10,000 μmol m−2 s−1) were applied for assessing P700 parameters. Maximum photo-oxidizable P700 (P m) was determined by applying a saturation pulse after pre-illumination with far-red light. Afterward the maximum change in P700 in a given light state (\(P_{\text{m}}^{\prime }\)), the photochemical quantum yield of PSI [Y(I)] were calculated (Tikkanen et al. 2014). The value for Y(NA) represented the fraction of overall P700 that could not be oxidized by a saturation pulse in a given state due to a lack of acceptors, was calculated as (P m − \(P_{\text{m}}^{\prime }\))/P m. We estimated cyclic electron flow around PSI (CEF) as the difference in electron flow between PSI and PSII (Miyake et al. 2005).
Analysis of JA and ABA accumulation
After the leaf tissue was ground in fine power under liquid nitrogen, approximately 200 mg of each sample was collected in centrifugal tube. 1 mL of ethyl acetate was spiked with 200 ng of D2-JA and 40 ng of D4-ABA which was used as the internal standards for JA and ABA, was added to each crushed sample, respectively. The samples were then vortexed for 10 min. After centrifugation at 13,000g for 10 min at 4 °C, the supernatants were transferred to fresh 2-mL tubes. Each sample was re-extracted with 0.5 mL of ethyl acetate without internal standard. The supernatants were combined and then evaporated to dryness on a vacuum concentrator (Eppendorf, Hamburg, Germany). Each residue was re-suspended in 0.5 mL of 70% methanol (v/v), vortexed for 10 min, and centrifuged at 13,000g for 10 min at 4 °C to clarify phases. The supernatants were pipetted to glass vials and then analyzed by HPLC–MS/MS (high pressure liquid chromatography–mass spectrometry; LCMS-8040 system, Shimadzu, Kyoto, Japan). Measurements were conducted on a 1200 L LC–MS (Varian, Palo Alto, CA, USA). At a flow rate of 0.1 mL min−1, 15 mL of each sample was injected onto a Pursuit C8 column (3 m, 150 × 2 mm; Varian). A mobile phase comprising solvent A (0.05% formic acid) and solvent B (0.05% formic acid in methanol) was used in a gradient mode for separation.
Analyses of total soluble sugars and starch
Approximately 0.1 g of dried leaves was put into a 10-mL centrifuge tube, and 5 mL of 80% ethanol was added. This mixture was incubated in an 80 °C water bath for 30 min, and then centrifuged at 4000g for 15 min. The concentration of total sugars was determined with a UV–visible spectrophotometer (UV-2500; Shimadzu, Kyoto, Japan) at 620 nm by the anthrone method (Seifter et al. 1949), and was calculated on a dry matter basis (% d.m.). Starch in the residue was released in 2 mL of distilled water for 15 min in a boiling water bath, and the concentration was measured with the UV–visible spectrophotometer 620 nm, using anthrone reagent. It was calculated by multiplying the glucose concentrations by a conversion factor of 0.9 (Li et al. 2008).
Monitoring the activities of antioxidant enzyme activity
Approximately 0.3 g of leaf collected from each sample was homogenized in 5 mL of 50 mM sodium phosphate (pH 7.0) buffer containing 1 mM EDTA, 1 mM dithiothreitol (DTT), 1 mM glutathione, 5 mM MgCl2.6H2O, 1% (w/v) PVP-40, and 20% (v/v) glycerin. The homogenates were centrifuged at 12,000g for 15 min at 4 °C, and the concentration of total soluble protein in the supernatants was measured by the Bradford method (Kruger 1994). Activity of CAT and SOD, activities were determined as the previously described (Nakano and Asada 1981; Jiang and Zhang 2001).
Protein extraction and two-dimensional gel electrophoresis
Protein extraction and two-dimensional electrophoretic (2DE) separation were performed according to a reported method with minor modifications (Yang et al. 2012; Li et al. 2014). Approximately 10 g of the leaf sample was ground in liquid nitrogen. Total soluble protein was extracted with 10% (w/v) TCA and 1% (w/v) DTT. The homogenates were maintained at −20 °C for 4 h, and then centrifuged at 25,000g for 30 min at 4 °C. The resultant pellets were washed with acetone containing 1% (w/v) DTT at −20 °C for 1 h, and then centrifuged. The final pellets were vacuum-dried and then dissolved in 8 M urea, 20 mM DTT, 4% (w/v) CHAPS, and 2% (w/v) ampholyte (pH 3–10). Samples in the ampholyte were vortexed thoroughly for 1 h at room temperature and then centrifuged at 25,000g for 20 min at 20 °C. Those supernatants were collected for 2DE experiment, and each experiment was repeated three times.
In-gel digestion, MALDI-TOF/TOF analysis and database search
Protein spots that showed significant changes in expression in parallel with changes in water status were excised manually from colloidal CBB-stained 2-DE gels. After the proteins were digested with trypsin mass spectrometry analyses were conducted using a MALDI-TOF/TOF mass spectrometer 4800-plus Proteomics Analyzer (Applied Biosystems, Farmington, MA, USA). The primary and secondary MS data were transferred to Excel files and used as inputs to search against an NCBI non-redundant database. This search was restricted to viridiplantae (green plants) using the MASCOT search engine.
Statistical analysis
Our statistical analyses were performed with SPSS 16.0. All data were subjected to analysis of variance (ANOVA), and Tukey’s multiple comparison tests was used at the level of α = 0.05 level to determine whether significant differences existed between treatments.
Results
Differences in water-related traits between C. sinense and C. tracyanum
The cross-sections of roots from C. sinense and C. tracyanum were anatomically similar, with showing a multilayer velamen radicum, epidermis, cortex, and pericycle (Fig. 1). However, C. tracyanum had higher ratios of velamen radicum thickness to root semi-diameter and xylem conduit diameter (Supplement Information Table S1).
Compared with C. sinense, the Ψ TLP value for C. tracyanum was more negative, its RWCTLP was lower, and its T 70 was longer (Table S1). These findings suggested that leaf water was better conserved in C. tracyanum than in C. sinense.
Physiological responses of C. sinense and C. tracyanum to drought
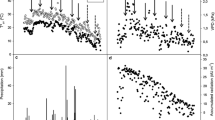
Under well-watered control conditions, the values were significantly higher for A n (p < 0.01) and g s (p < 0.05) in C. tracyanum than in C. sinense. However, the levels of both parameters were decreased for both species when the drought stress was intensified. Under both stress and recovery conditions, values for ΨMD did not change significantly (Fig. 2a). Nevertheless, A n remained significantly higher (p < 0.05) in C. tracyanum than in C. sinense after the soil RWC dropped to 10–15% (Fig. 2b, c). In parallel with the decline in g s, ABA concentration in both species increased gradually as soil RWC decreased. Although the ABA concentration was higher in C. tracyanum than in C. sinense under control conditions, it was lower when the soil RWC dropped to 10–15% (p < 0.01) (Fig. 2e). JA concentration did not significantly change in C. tracyanum during drought treatment, but dramatically increased in C. sinense when soil RWC dropped to 10–15% (Fig. 2d). After the plants were re-watered for 3 days, the values for A n, g s, ABA and JA concentration recovered quickly within 3 days.
Changes in midday leaf water potential (Ψ MD) (a); stomatal conductance (g s) (b); net photosynthesis (A n) (c); concentrations of jasmonic acids (JA) (d); and abscisic acid (ABA) (e) in Cymbidium sinense and C. tracyanum plants during drought-stress treatment. Each vertical bar represents mean ± SE for five measurements from individual plants. Different letters above bars indicate significant differences in each parameter between treatments (p < 0.05, based on ANOVA, followed by Tukey’s post hoc tests for comparison)
During both the drought period and 3 days of recovery, neither F v/F m nor P m changed significantly (Fig. 3a). However, CEFmax was more strongly stimulated by drought in C. tracyanum than in C. sinense during treatment (Fig. 3b). The value for Y(NA)233 significantly increased in C. sinense when soil RWC dropped to 5–10% at a photon flux density of 233 mol m−2 s−1. In contrast, no remarkable change was observed in C. tracyanum. Under both stress and recovery conditions, Y(NA)233 values were higher in the former than the latter (Fig. 3c).
Changes in maximum quantum yield of photosystem II after dark adaption (F v/F m) and maximum photo-oxidizable P700 (P m) (a), maximum ratio of cyclic electron flow around PSI (CEFmax) (b) and PSI acceptor side limitation at 233 µmol photons m−2 s−1 [Y(NA)233] (c) for Cymbidium sinense and C. tracyanum plants during drought-stress treatment. Each vertical bar represents mean ± SE for four measurements from individual plants. Different letters above bars indicate significant differences in each parameter between treatments (p < 0.05, based on ANOVA, followed by Tukey’s post hoc tests for comparison). Statistical differences (p values) between the two species at each treatment were determined with independent-sample t tests. (ns p > 0.05; *p < 0.05; **p < 0.01, ***p < 0.001)
Before the drought treatments began, sugar concentration was higher in C. tracyanum than in C. sinense (p < 0.05), starch concentration was not significantly different between species before treatment, but decreased dramatically in C. sinense when soil RWC dropped to 10–15%, and did not recover after 3 days of re-watering (Fig. 4a, b). Finally, under both stress and recovery conditions, the activity did not significantly altered for either CAT (Fig. 4c) or SOD (Fig. 4d).
Changes in starch concentration (a), sugar concentration (b), activities of antioxidant CAT (c) and SOD (d) for Cymbidium sinense and C. tracyanum during period of drought-stress. Each vertical bar represents mean ± SE for four measurements from individual plants. Different letters above bars indicate significant differences in each parameter between treatments (p < 0.05, based on ANOVA, followed by Tukey’s post hoc tests for comparison). Statistical differences (p values) between the two species at each treatment were determined with independent-sample t tests. (ns p > 0.05; *p < 0.05; **p < 0.01, ***p < 0.001)
Leaf protein profiles for C. sinense and C. tracyanum
The leaf proteome was analyzed under different soil water status, i.e., control (65–75% RWC) drought stress (40–45, 20–25, 10–15, or 5–10%), and 3-day water recovery (60–75%). Those experimental conditions led to significant variations in the protein yields for both species. Out of the 410–460 protein spots detected 132 (C. sinense) and 139 (C. tracyanum) significantly changed in response to stress (p < 0.05). When compared with the samples from the control plants, all of these proteins showed increased abundance (>1.5) or decreased abundance (<−1.5) under stress and recovery conditions. Among them 103 proteins from C. sinense and 104 proteins from C. tracyanum were identified by MALDI-TOF/TOF and corresponded to eight putative classes of biological functions (Fig. 5). For C. sinense, the proteins related to carbon and energy metabolism (28.2%), defense responses (16.5%) and photosynthesis (13.6%) accounted for 58.3% of the total proteins; whereas for C. tracyanum, proteins related to carbon and energy metabolism (29.8%), photosynthesis (17.4%) and antioxidation (14.4%) accounted for 61.6% of all proteins. Other functions included protein kinase and phosphatase, cell structure and division, protein synthesis, degradation and refolding and transcriptional and signal factor (Fig. 6). The results of our Venn diagram analysis proved that more proteins were up-regulated rather than down-regulated as drought conditions were intensified (Fig. 7).
Dynamic changes in protein spot abundance in Cymbidium sinense and C. tracyanum during drought-stress treatment. Plant samples were collected at different soil relative water content (65–75, 40–45, 20–25, 10–15, 5–10, 60–75%) and 1 mg of total protein was extracted and loaded into gels BR-20-stained 2-D gels of total protein. Enlarged windows from panel a showing spot changes in the representative gels from samples collected during drought-stress treatment
The expression levels of several proteins involved in photosynthesis were altered with water status. The expression of oxygen-evolving enhancer protein (Table 1; spot T66, spot T67, spot T69) in C. tracyanum was up-regulated at soil RWC of 40–45, 20–25 and 10–15%, but was down-regulated for C. sinense (Table 1; spot S68, spot S94) when soil RWC dropped down to 40–45 and 10–15%, and the recovery. In C. tracyanum, the FtsH-like protein Pftf (Table 1; spot T17) was down-regulated as soil RWC decreased to 5–10%, but was recovered as the stress alleviated. The level of ferredoxin-NADP reductase (FNRs) was up-regulated during the stress period and recovery phase (Table 1; spot T55). However, the expression level of cytochrome b6-f complex iron–sulfur subunit (Table 1; spot S99; spot T100) fluctuated in both species.
In the group of protein carbon and energy metabolism, the expression level of Rubisco activase was up-regulated under drought as well as recovery (Table 1; spot T41; spot T42, spot T49) in C. tracyanum, while for C. sinense, some Rubisco activase (Table 1; spot S33, spot S29, S35) was down-regulated during drought treatment and even recovery condition. Expression of phosphoglycolate phosphatase (Table 1; spot T70) was up-regulated in C. tracyanum, and the expression of fructose-bisphosphate aldolase (Table 1; spot S42) was significantly up-regulated (7.7- to 13.1-fold) in drought stress in C. sinense.
In the group of antioxidation, the expression level of CAT (Table 1; spot S19) was up-regulated at the beginning of the drought treatments, and down-regulated during recovery phase in C. sinense. The expression level of SOD (Table 1; spot T97, spot T101) was continuously up-regulated during the drought treatment period in C. tracyanum. Expression level of APX of C. tracyanum (Table 1; spot T77, spot T85) was up-regulated during the drought period. For C. sinense, the expression of chloroplast l-ascorbate peroxidase isoform (Table 1; spot S53) was up-regulated during water recovery.
The expression level of plastid fibrillin (Table 1, spot S84), a protein related to defense response, was up-regulated only in C. sinense when soil RWC dropped to 10–45%.
Discussion
Water-adaptive traits of C. sinense and C. tracyanum
When compared with C. sinense, C. tracyanum showed stronger adaptive characteristics of drought resistance. This is consistent with our earlier conclusion that epiphytes are more drought-resistant than terrestrial species in Cymbidium (Zhang et al. 2015). The velamen radicum has been studied extensively in Orchidaceae (Porembski and Barthlott 1988). Although it is more common in epiphytic orchids, it occurs in both epiphytic and terrestrial species of Cymbidium (Yukawa and Stern 2002). The most important role of velamen radicum appears to be the absorption of water and nutrients, but it also reduces water loss (Zotz and Winkler 2013; Benzing et al. 1982). Here, the epiphytic C. tracyanum had a higher radio of velamen thickness to root thickness, along with larger-diameter xylem conduits than those of the terrestrial C. sinense. This further indicated that C. tracyanum has a greater capacity to conserve water and avoid the negative effects of drought (Chaves et al. 2002).
The loss of cell turgor has an important impact on cellular structural integrity, metabolism and whole-plant performance (Brodribb et al. 2003; McDowell 2011). Thus, researchers use the parameter of leaf water potential at turgor loss to assess physiological drought tolerance. Plants with low Ψ TLP tend to maintain more normal stomatal conductance, hydraulic conductance, photosynthetic gas exchange, and growth under drought stress (Blackman et al. 2010; McDowell 2011). We also noted that the Ψ TLP of C. tracyanum was more negative than C. sinense, indicating that the former has greater capacity for drought tolerance.
Drought-induced stomatal closure in C. sinense and C. tracyanum
Values for g s gradually decline in both species along with the stability of Ψ MD. Anisohydric tree species tend to occupy more drought-prone habitats when compared with isohydric species, and xylem in the former is more resistant to negative water potential (McDowell et al. 2008; Klein 2014). Thus, stomatal regulation in our two tested species appears to be isohydric.
Although the two species utilize similar mechanisms for controlling stomatal activity, they differ somewhat in their regulatory processes. Under well-watered condition, gs was significantly higher in C. tracyanum than in C. sinense, but no statistically significant difference in g s was observed between species during the drought period. As a controlling factor of stomatal closure and positive response to drought stress (Mittler and Blumwald 2015), ABA concentrations increased faster and reached a higher level in C. sinense than in C. tracyanum, possibly showing that the stomatal control is more flexible in the latter. In contrast, amount of ABA in C. sinense was perhaps remedied by improving the level of signals.
The phytohormone JA has also been proposed as an important signal for stomatal closure considering its accumulation and positive regulatory role in stomatal closure under drought stress (Suhita et al. 2004). Here, JA concentration in C. sinense was dramatically increased and was higher than in C. tracyanum when soil RWC dropped to 10–15%. This phenomenon might support the above-mentioned “remedy strategy”. In Arabidopsis, plant fibrillin (FIB1-2) initiates the chloroplast stress-related biosynthesis of JA (Youssef et al. 2010). For C. sinense, the level of plastid fibrillin (Table 1; spot S84) was up-regulated when soil RWC dropped to 10–45%, possibly contributing to the rise in the JA concentration.
Photoprotection of C. sinense and C. tracyanum
During the drought-treatment period, the leaf water status in C. sinense and C. tracyanum was stabilized because of the rapid closure of stomata. However, such quick activity can limit CO2 uptake and the utilization of absorbed light energy. Redundant energy will lead to an over-accumulation of electrons in photosynthetic electron transfer chain, which can then result in photoinhibition. However, we detected no significant change in the values for F v/F m in dark-adapted leaves and in P m for either species under drought stress, thereby indicating that the activities of PSII and PSI were not influenced by photoinhibition. While some proteins were found to be involved in linear electron flow (LEF) from water to NADP+ via PSII and PSI in series and cyclic electron flow (CEF) around PSI, our proteomics analysis showed that these proteins had either a positive or negative response to drought stress. When we compared the changes in proteins between species, we found that C. tracyanum had greater capacity for photoprotection, resulting in little risk of photoinhibition for PSII, effectively balance between CEF and LEF and increasing photorespiration.
Within the photosynthetic machinery, PSII is particularly sensitive to abiotic stresses, and its photoinhibition is determined by the balance between the rate of photodamage and repair (Nishiyama et al. 2006). Both oxygen-evolving enhancer protein and two other molecules (33 and 17 kDa) form an oxygen-evolving complex (James et al. 1989) that is associated with PSII photodamage (Ohnishi et al. 2005). For C. sinense, the expression level of oxygen-evolving enhancer protein (Table 1; spot S68, spot S94) was down-regulated when soil RWC dropped down to either 40–45 or 10–15%. This also occurred during the recovery. However, the expression of oxygen-evolving enhancer protein (Table 1; spot T66, spot T67, spot T69) in C. tracyanum was up-regulated at soil RWC of 40–45, 20–25 and 10–15%. Repairing PSII photodamage requires several steps: degradation of damaged D1 protein; de novo synthesis of D1 protein; and installation of the newly synthesized D1 protein into PSII (Takahashi and Murata 2008). In the chloroplasts of Arabidopsis, FtsH2 and FtsH5 participate in the repair of photodamaged PSII by digesting and removing damaged D1 protein (Bailey et al. 2002; Sakamoto et al. 2002). The FtsH proteases are involved in the primary cleavage of the D1 protein under moderate heat stress (Yoshioka et al. 2006). We found that, in C. tracyanum, the FtsH-like protein Pftf (Table 1; spot T17) was down-regulated as soil RWC decreased to 5–10%, but was recovered as the stress abated. This indicated that there was little risk of PSII photoinhibition in C. tracyanum under more severe drought conditions.
Cyclic electron flow is an important mechanism for protecting PSI and PSII against the effects of drought stress (Lehtimäki et al. 2010; Huang et al. 2013). Here, CEF was more strongly stimulated in C. tracyanum than in C. sinense. Furthermore, the level of ferredoxin-NADP reductase (FNRs) was up-regulated during the stress period and recover phase, but only for C. tracyanum (Table 1; spot T55). The physiological role of FNRs is catalyze the final step of photosynthetic electron transport, namely, the transfer of electron from the iron–sulfur protein ferredoxin (Fd) reduced by PSI to NADP+ (Shin and Arnon 1965). A super-complex mediation CEF including FNRs, has been separated in Chlamydomonas reinhardtii, which regulates the energy balance of PSII and PSI, and switches the mode of photosynthetic electron flow that is controlled by a photoacclimation mechanism called state transition to main cellular ATP homeostasis (Iwai et al. 2010). During the state transition from state 1 to state 2 (in which most of the excitation energy is used by PSI photochemistry, and CEF around PSI prevails over LEF) (Finazzi et al. 2002), cytochrome b6-f complex migrates from the appressed region in the thylakoid membranes, where PSII resides, to the non-appressed region, where PSI resides (Vallon et al. 1991). Here, we determined that fluctuations in the expression of cytochrome b6-f complex iron–sulfur subunit (Table 1; spot S99; spot T100) in both species were possibly related to the state transition, and therefore, to the balance between CEF and LEF.
Ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) has dual functions: CO2 fixation and oxygenase reactions. However, because Rubisco has no binding sites for CO2 and O2 at the same time, so the competing reactions between the two depend upon their concentrations (Spreitzer and Salvucci 2002). Under drought stress, the reduced intercellular CO2 concentration in a leaf that results from stomatal closure may lead to the increased oxygenation of RuBP by Rubisco and photorespiration. In C. tracyanum, expression of Rubisco activase was up-regulated under drought as well as recovery (Table 1; spot T41; spot T42, spot T49). Meanwhile, phosphoglycolate phosphatase (Table 1; spot T70) involved in the process of photorespiration, was also up-regulated. This demonstrated that photorespiration in C. tracyanum can be up-regulated by water stress. Photorespiration can help avoid inhibition of the synthesis of D1 protein, which is important for the repair of photodamaged PSII (Takahashi et al. 2007). In contrast, for C. sinense, some Rubisco activase (Table 1; spot S33, spot S35) was down-regulated during drought treatment, and even some proteins (Table 1; spot S29) were down-regulated when the stress relieved. These findings indicated that C. tracyanum can alleviate photodamage by increasing its photorespiration, thereby enhancing its adaptability to drought conditions.
Changes in the carbon balance and antioxidant activity during water stress
Sugars play a central role in plant metabolism, because they are a source of carbon and energy in cells (Pinheiro et al. 2001). However, their role in drought tolerance is debatable. For example, Ramel et al. (2009) found that the pre-stress sugar concentration is correlated with subsequent stress tolerance. In contrast, Chaves and Oliveira (2004) suggested that leaf concentrations of soluble sugars are not consistently altered in plants under drought conditions. We also did not detect any marked change in levels of soluble sugars in our stressed plants, although those concentrations were higher in C. sinense than in C. tracyanum. Starch is required as a buffer during periods of abiotic stress (Kozlowski and Pallardy 2002). In Arabidopsis, starch appears to be a key factor in coordinating the drought response, photosynthesis, ABA accumulations, reactive oxygen species (ROS) activation, and transcription of several amylases and sucrose synthases, and it is also possibly associated with transcription of amylase and catalase genes (Pinheiro et al. 2001). We determined that the starch concentration in C. sinense decreased dramatically when soil RWC dropped to 10–15%, but did not recover when the stress was alleviated. No significant changes in starch concentration were found in C. tracyanum. The expression of fructose-bisphosphate aldolase (Table 1; spot S42) was significantly up-regulated (7.7- to 13.1-fold) in drought-stressed plants of C. sinense, which possibly contributed to the decrease in starch levels there. These alterations in sugar and starch concentrations, combined with elevated levels of proteins associated with carbon metabolism, might demonstrate that the greater capacity to balance the carbon source–sink helps to improve the drought tolerance in C. tracyanum.
When ROS production is induced by various abiotic stresses, it can disrupt normal metabolism of plants by damaging DNA, and inhibiting the functions of proteins, chlorophyll and membranes (Alscher et al. 1997). In response to severe drought-related oxidative stress, plants trigger complex antioxidant enzymes, including SOD and CAT (Gill and Tuteja 2010). We found that the activities of both were higher in C. sinense than in C. tracyanum throughout the entire treatment period. The level of CAT in C. sinense (Table 1; spot S19) was up-regulated at the beginning of the drought treatment and down-regulated during recovery phase. For C. tracyanum, the level of SOD (Table 1; spot T97, spot T101) was continuously up-regulated through drought treatment.
The ascorbate–glutathione (ASC–GSH) cycle also has a key role in removing H2O2, which is catalyzed by a set of four enzymes: ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), glutathione-dependent dehydroascorbate reductase (DHAR), and glutathione reductase (GR). In contrast with CAT, APX, as a reductant in the first step of the ASC–GSH cycle, can efficiently remove low concentrations of H2O2 (Noctor and Foyer 1998). For C. sinense, the expression of chloroplast l-ascorbate peroxidase isoform (Table 1; spot S53) was up-regulated during water recovery. Expression level of APX of C. tracyanum (Table 1; spot T77, spot T85) was up-regulated during the drought period. Exogenous JA is effective in protecting plants against drought-induced oxidative damage because it can enhance the activity of antioxidant enzymes (Riemann et al. 2015). This is supported by the increase in JA levels that was found in C. sinense. Therefore, although the activity of antioxidant enzymes differed somewhat between two species, C. sinense could remedy its relatively weak capacity to prevent ROS generation under drought conditions by improving its ability to eliminate ROS.
Conclusion
We investigated the adaptive mechanisms of C. sinense and C. tracyanum to drought stress based on their morphology, physiology and proteomics. Whereas C. sinense appears to employ “remedy strategy”, C. tracyanum utilizes a “precaution strategy”. We have modeled these contrasting strategies for adaptations, as presented in Fig. 8. The unique water-related traits associated with their root anatomy and leaf physiology mean that C. tracyanum is more drought-tolerant when compared with C. sinense. In C. tracyanum, the stimulation of CEF and enhancement of photorespiration improved its photoprotection under water stress. These plants also demonstrated greater capacity to maintain carbon balance and the responded more effectively to ABA. Although the photosystem of C. sinense was more sensitive to drought stress, increase of phytohormones concentration and antioxidant activity helped this species survive under our stress treatment. All of these findings explained the distinct water-adaptation strategies of epiphytic and terrestrial orchids, and may contribute to our understanding about the ecological adaptations of epiphytic orchids.
Author contribution statement
J-WL, S-BZ and X-YH conceived and designed research; J-WL and X-DC conducted experiments; J-WL, LM and X-YH analyzed data; J-WL and S-BZ wrote the manuscript; all authors read and approved the manuscript.
Abbreviations
- A n :
-
Net photosynthesis
- g s :
-
Stomatal conductance
- Ψ MD :
-
Midday leaf water content
- F v/F m :
-
Maximum quantum yield of PSII after dark adaptation overnight
- P m :
-
Maximum photo-oxidizable P700
- CEF:
-
Cyclic electron flow
- JA:
-
Jasmonates
- ABA:
-
Abscisic acid
References
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233
Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C, Mann NH (2002) A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the photosystem II repair cycle in vivo. J Biol Chem 277:2006–2011
Benzing D, Ott D, Friedman W (1982) Roots of Sobralia macrantha (Orchidaceae): structure and function of the velamen-exodermis complex. Am J Bot 69:608–614
Blackman CJ, Brodribb TJ, Jordan GJ (2010) Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol 188:1113–1123
Bonhomme L, Monclus R, Vincent D, Carpin S, Lomenech AM, Plomion C, Brignolas F, Morabito D (2009) Leaf proteome analysis of eight Populus × euramericana genotypes: genetic variation in drought response and in water-use efficiency involves photosynthesis-related proteins. Proteomics 9:4121–4142
Brodribb T, Holbrook N, Edwards E, Gutierrez M (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26:443–450
Chaves M, Oliveira M (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field. photosynthesis and growth. Ann Bot 89:907–916
Finazzi G, Rappaport F, Furia A, Fleischmann M, Rochaix JD, Zito F, Forti G (2002) Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep 3:280–285
Fulda S, Mikkat S, Stegmann H, Horn R (2011) Physiology and proteomics of drought stress acclimation in sunflower (Helianthus annuus L.). Plant Biol 13:632–642
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:151
Hajheidari M, Abdollahian-Noghabi M, Askari H, Heidari M, Sadeghian SY, Ober ES, Hosseini Salekdeh G (2005) Proteome analysis of sugar beet leaves under drought stress. Proteomics 5:950–960
Hao GY, Sack L, Wang AY, Cao KF, Goldstein G (2010) Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Funct Ecol 24:731–740
Hossain MM, Kant R, Van PT, Winarto B, Zeng S, Teixeira da Silva JA (2013) The application of biotechnology to orchids. Crit Rev Plant Sci 32:69–139
Huang W, Fu P-L, Jiang YJ, Zhang JL, Zhang SB, Hu H, Cao KF (2013) Differences in the responses of photosystem I and photosystem II of three tree species Cleistanthus sumatranus, Celtis philippensis and Pistacia weinmannifolia exposed to a prolonged drought in a tropical limestone forest. Tree Physiol 33:e220
Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464:1210–1213
James HE, Bartling D, Musgrove J, Kirwin P, Herrmann R, Robinson C (1989) Transport of proteins into chloroplasts. Import and maturation of precursors to the 33-, 23-, and 16-kDa proteins of the photosynthetic oxygen-evolving complex. J Biol Chem 264:19573–19576
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Klein T (2014) The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol 28:1313–1320
Kozlowski T, Pallardy S (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218
Kruger NJ (1994) The Bradford method for protein quantitation. Methods Mol Biol 32:9–15
Kuang M, Zhang SB (2015) Physiological response to high light in Cymbidium tracyanum and C.sinense. Plant Divers Resour 37:55–62
Lehtimäki N, Lintala M, Allahverdiyeva Y, Aro EM, Mulo P (2010) Drought stress-induced upregulation of components involved in ferredoxin-dependent cyclic electron transfer. J Plant Physiol 167:1018–1022
Li JW, Zhang SB (2016) Differences in the responses of photosystems I and II in Cymbidium sinense and C. tracyanum to long-term chilling stress. Front Plant Sci 6:1097
Li MH, Xiao WF, Shi P, Wang SG, Zhong YD, Liu XL, Wang XD, Cai XH, Shi ZM (2008) Nitrogen and carbon source-sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ 31:1377–1387
Li X, Yang Y, Sun X, Lin H, Chen J, Ren J, Hu X, Yang Y (2014) Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS One 9:e107605
Liu Z, Chen L, Liu K, Li L, Zhang Y, Huang L (2009) Climate warming brings about extinction tendency in wild population of Cymbidium sinense. Acta Ecol Sin 29:3443–3455
Luo Y, Jia J, Wang C (2002) A general review of the conservation status of Chinese orchids. Chin Biodivers 11:70–77
Manavalan LP, Guttikonda SK, Tran LSP, Nguyen HT (2009) Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol 50:1260–1276
McDowell NG (2011) Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol 155:1051–1059
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Mittler R, Blumwald E (2015) The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27:64–70
Miyake C, Horiguchi S, Makino A, Shinzaki Y, Yamamoto H, Tomizawa K (2005) Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chl fluorescence in tobacco leaves. Plant Cell Physiol 46:1819–1830
Motomura H, Yukawa T, Ueno O, Kagawa A (2008) The occurrence of crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. J Plant Res 121:163–177
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim et Biophys Acta-Bioenerg 1757:742–749
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Ogburn R, Edwards EJ (2012) Quantifying succulence: a rapid, physiologically meaningful metric of plant water storage. Plant Cell Environ 35:1533–1542
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44:8494–8499
Pan R, Zheng X, Wen Z (1993) Change of water physiology of Cymbidium sinense during soil drought period. Acta Bot Yunnanica 16:379–384
Pinheiro C, Chaves MM, Ricardo CP (2001) Alterations in carbon and nitrogen metabolism induced by water deficit in the stems and leaves of Lupinus albus L. J Exp Bot 52:1063–1070
Porembski S, Barthlott W (1988) Velamen radicum micromorphology and classification of Orchidaceae. Nord J Bot 8:117–137
Price AH, Cairns JE, Horton P, Jones HG, Griffiths H (2002) Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot 53:989–1004
Ramel F, Sulmon C, Gouesbet G, Couée I (2009) Natural variation reveals relationships between pre-stress carbohydrate nutritional status and subsequent responses to xenobiotic and oxidative stress in Arabidopsis thaliana. Ann Bot 104:1323–1337
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Riemann M, Dhakarey R, Hazman M, Miro B, Kohli A (2015) Exploring jasmonates in the hormonal network of drought and salinity responses. Front Plant Sci 6:1077
Sakamoto W, Tamura T, Hanba-Tomita Y, Murata M (2002) The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells 7:769–780
Seifter S, Dayton S, Novic B, Muntwyler E (1949) The estimation of glycogen with the anthrone reagent. Biochemistry 25:191–220
Shin M, Arnon DI (1965) Enzymic mechanisms of pyridine nucleotide reduction in chloroplasts. J Biol Chem 240:1405–1411
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53:449–475
Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate-and abscisic acid-induced stomatal closure. Plant Physiol 134:1536–1545
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Takahashi S, Bauwe H, Badger M (2007) Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol 144:487–494
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49:419–432
Théry M (2001) Forest light and its influence on habitat selection. In: Linsenmair KE, Davis AJ, Fiala B, Speight MR (eds) Tropical forest canopies: ecology and management. Springer, Dordrecht, pp 251–261
Tikkanen M, Mekala NR, Aro E-M (2014) Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim et Biophys Acta -Bioenerg 1837:210–215
Tyree M, Hammel H (1972) The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J Exp Bot 23:267–282
Vallon O, Bulte L, Dainese P, Olive J, Bassi R, Wollman F-A (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci 88:8262–8266
Wilkinson S, Kudoyarova GR, Veselov DS, Arkhipova TN, Davies WJ (2012) Plant hormone interactions: innovative targets for crop breeding and management. J Exp Bot 63:3499–3509
Yang Y, Chen J, Liu Q, Ben CC, Todd CD, Shi J, Yang Y, Hu X (2012) Comparative proteomic analysis of the thermotolerant plant Portulaca oleracea acclimation to combined high temperature and humidity stress. J Proteome Res 11:3605–3623
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38:171–186
Yoshioka M, Uchida S, Mori H, Komayama K, Ohira S, Morita N, Nakanishi T, Yamamoto Y (2006) Quality control of photosystem II cleavage of reaction center D1 protein in spinach thylakoids by FtsH protease under moderate heat stress. J Biol Chem 281:21660–21669
Youssef A, Laizet Yh, Block MA, Maréchal E, Alcaraz JP, Larson TR, Pontier D, Gaffé J, Kuntz M (2010) Plant lipid-associated fibrillin proteins condition jasmonate production under photosynthetic stress. Plant J 61:436–445
Yukawa T, Stern WL (2002) Comparative vegetative anatomy and systematics of Cymbidium (Cymbidieae: Orchidaceae). Bot J Linn Soc 138:383–419
Zhang M, Sun C, Hao G, Ye X, Liang C, Zhu G (2001) A preliminary analysis of phylogenetic relationships in Cymbidium (Orchidaceae) based on nrITS sequence data. Acta Bot Sin 44:588–592
Zhang SB, Dai Y, Hao GY, Li JW, Fu XW, Zhang JL (2015) Differentiation of water-related traits in terrestrial and epiphytic Cymbidium species. Front Plant Sci 6:260
Zhang W, Hu H, Zhang SB (2016) Divergent adaptive strategies by two co-occurring epiphytic orchids to water stress: escape or avoidance? Front Plant Sci 7:588
Zotz G, Bader M (2009) Epiphytic plants in a changing world: global change effects on vascular and non-vascular epiphytes. In: Canovas FM, Luttge U, Matyssek R (eds) Progress in botany. Springer, Dordrecht, pp 147–170
Zotz G, Hietz P (2001) The physiological ecology of vascular epiphytes: current knowledge, open questions. J Exp Bot 52:2067–2078
Zotz G, Tyree MT (1996) Water stress in the epiphytic orchid, Dimerandra emarginata (G. Meyer) Hoehne. Oecologia 107:151–159
Zotz G, Winkler U (2013) Aerial roots of epiphytic orchids: the velamen radicum and its role in water and nutrient uptake. Oecologia 171:733–741
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (31370362, 31670342), and National Key Project of the Ministry of Science and Technology of China (2015BAD10B03).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, JW., Chen, XD., Hu, XY. et al. Comparative physiological and proteomic analyses reveal different adaptive strategies by Cymbidium sinense and C. tracyanum to drought. Planta 247, 69–97 (2018). https://doi.org/10.1007/s00425-017-2768-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2768-7