Abstract
Main conclusion
Rare red currants colors caused by low anthocyanin content in the pink and a lack of anthocyanins in the white cultivar correlated with low ANS gene expression, enzyme activity, and increased sugar/acid ratios.
Changes in the contents of polyphenols, sugars, and organic acids in berries of the three differently colored Ribes rubrum L. cultivars (‘Jonkheer van Tets’, ‘Pink Champagne’, and ‘Zitavia’) were determined by LC–MS and HPLC at 4 sampling times during the last month of fruit ripening. The activities of the main flavonoid enzymes, chalcone synthase/chalcone isomerase (CHS/CHI), flavanone 3-hydroxylase (FHT), and dihydroflavonol 4-reductase (DFR), and the expression of anthocyanidin synthase (ANS) were additionally measured. Despite many attempts, activities of flavonol synthase and glycosyltransferase did not show reliable results, the reason of which they could not be demonstrated in this study. The pink fruited cultivar ‘Pink Champagne’ showed generally lower enzyme activity than the red cultivar ‘Jonkheer van Tets’. The white cultivar ‘Zitavia’ showed very low CHS/CHI activity and ANS expression and no FHT and DFR activities were detected. The DFR of R. rubrum L. clearly preferred dihydromyricetin as substrate although no 3′,4′,5′-hydroxylated anthocyanins were present. The anthocyanin content of the red cultivar slightly increased during the last three weeks of ripening and reached a maximum of 890 mg kg−1 FW. Contrary to this, the pink cultivar showed low accumulation of anthocyanins; however, in the last three weeks of ripening, their content increased from 14 to 105 mg kg−1 FW. Simultaneously, the content of polyphenols slightly decreased in all 3 cultivars, while the sugar/acid ratio increased. The white cultivar had no anthocyanins, but the sugar/acid ratios were the highest. In the white and pink cultivars, reduction/lack of anthocyanins was mainly compensated by increased relative concentrations of hydroxycinnamic acids and flavonols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Ribes genus consists of many different species and is important in the world production of berry fruits. Currants are highly appreciated for their sour taste, high nutritional value, and also because of the well-known health-promoting properties of their polyphenols: anthocyanins, hydroxycinnamic acids, flavonols, and flavanols (Veberic et al. 2015; Mikulic-Petkovsek et al. 2015, 2016). Anthocyanins, the main pigments responsible for fruit color of black currant (Ribes nigrum L.), are mainly found in the epidermis and in the tissue directly under the skin (Milivojevic et al. 2012). Although, in some red currant (R. rubrum L.) cultivars, anthocyanins can also be found in the pulp to some extent, similarly to few grape cultivars (He et al. 2010), bilberry (Jaakola et al. 2002), or some other berries. In intensely colored berries of black currant, high levels of anthocyanins are typically present, while smaller amounts are accumulated in red currant (Gavrilova et al. 2011; Mattila et al. 2016). In addition, pink and acyanic colored white currants which are color variants of R. rubrum L. could be found, while green currant (R. nigrum L.) additionally lacks anthocyanins (Määttä et al. 2001, 2003). This indicates that the biosynthetic routes of anthocyanins vary between different varieties and also cultivars (Mattila et al. 2016). Due to differently pigmented phenotypes, regulation of anthocyanin biosynthesis has been studied across a number of plants: bilberry (Jaakola et al. 2002; Primetta et al. 2015), grapevine (Castellarin and Gaspero 2007), pear (Yang et al. 2015), cherry (Wei et al. 2015), kiwifruit (Halbwirth et al. 2009), pomegranate (Ben-Simhon et al. 2015; Zhao et al. 2015), etc.
Anthocyanins and other phenolic compounds are synthesized via the phenylpropanoid/flavonoid pathway, which is driven by the structural genes encoding the enzymes that directly participate in the formation of pigments and other flavonoids, and the regulatory genes that control the transcription of structural genes. Differences in the pattern of anthocyanin accumulation and fruit color intensity are, therefore, attributed to variations in the expression of these two types of genes (Zhao et al. 2015).
Biosynthesis starts from phenylalanine to produce phenylpropanoids, which are channeled into the flavonoid pathway by chalcone synthase (CHS), which is one of the key enzymes initializing the pathway. Further steps are catalyzed by chalcone isomerase (CHI), flavanone 3-hydroxylase (FHT), and dihydroflavonol 4-reductase (DFR), which converts dihydroflavonols into leucoanthocyanidins. DFR is a key enzyme in anthocyanin biosynthesis and also affects the biosynthesis of other flavonoids. DFR competes with flavonol synthase for dihydroflavonolos as a common substrate and thus interferes with flavonol production (Davies et al. 2003; Wang et al. 2013). Finally, anthocyanidin synthase (ANS) leads to the synthesis of anthocyanidin pigments, while different glycosyltransferases and other transferases determine the substitution pattern of phenylpropanoid and flavonoid end-products (Tanaka et al. 2008). Although flavonoid metabolism is generally influenced by genetic predisposition, environmental and developmental factors also play an important role in its regulation (Jaakola 2013; Yang et al. 2013; Mikulic-Petkovsek et al. 2015).
The polyphenol composition of black, red, and white currants is generally well investigated (during ripening and among different varieties/cultivars) (Gavrilova et al. 2011; Mikulic-Petkovsek et al. 2015; Mattila et al. 2016). However, to the best of our knowledge, pink colored currants have not previously been studied. Furthermore, flavonoid enzyme activities have also not been investigated in currant berries; only the expression of the ANS gene has previously been studied in R. nigrum L. cv. ‘Broad’ (Li et al. 2015). Polyphenol metabolism in fruits has become an interesting object of study in terms of the regulation and organization of the formation of different flavonoid classes in a well-defined time schedule.
The aim of the present study was to analyze, for the first time, the activities of the main flavonoid enzymes (CHS/CHI, FHT, and DFR) and ANS gene expression through fruit ripening in differently colored R. rubrum L. cultivars. The last few weeks of fruit ripening, as the essential period for fruit quality (firmness, flavor, color, etc.), particularly draw our attention, but visible changes were negligible. Since pink colored cultivar in our study accumulated anthocyanins only in the skin, and according to reports which indicated that anthocyanin biosynthesis key enzymes were expressed predominantly in the skin of grape berries (Grimplet et al. 2007; Mu et al. 2014), we decided to study berry skins only. In addition, berry color parameters, the composition and content of primary metabolites, anthocyanins, and other polyphenolics were determined.
Materials and methods
Plant material
Ribes rubrum L. berries of red (‘Jonkheer van Tets’), pink (‘Pink Champagne’), and white (‘Zitavia’) color cultivars were hand-harvested weekly at the experimental station of the Agricultural Institute of Slovenia in Brdo pri Lukovici (46°10′N, 14°41′E). During the last period of fruit ripening, four samplings were performed (each contained approx. 500 g of fruits, collected from different bushes of each cultivar). The first sampling date was on the 8th (S1), the second on the 15th (S2), the third on the 22nd (S3), and the fourth on the 29th (S4) of June 2015. Color parameters were measured after each harvest. The berry skin was separated from the pulp, shock-frozen in liquid nitrogen, and stored at −80 °C until enzyme analysis. For primary and secondary metabolites analysis, whole berries were stored at −20 °C.
Chemicals
The following standards were used for the determination of sugars and organic acids: fructose, glucose, and sucrose; citric and malic acid from Fluka Chemie (Buchs, Switzerland); and shikimic and fumaric from Sigma-Aldrich Chemie (Steinheim, Germany). The following standards were used for the quantification of phenolic compounds: cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and quercetin-3-O-rutinoside from Sigma-Aldrich Chemie; caffeic, (+)-catechin from Roth (Karlsruhe, Germany), (−)-epicatechin, quercetin-3-O-rhamnoside, quercetin-3-O-glucoside, p-coumaric acid, procyanidin B1, and kaempferol-3-O-glucoside from Fluka Chemie; myricetin-3-rhamnoside from Apin Chemicals (Abingdon, UK). Methanol for the extraction of phenolics was acquired from Sigma-Aldrich Chemie. The chemicals for the mobile phases were HPLC–MS grade acetonitrile and formic acid from Fluka Chemie. Water for the mobile phase was double distilled and purified with the Milli-Q system (Millipore, Bedford, MA, USA). For the total phenolics, Folin-Ciocalteu phenol reagent (Fluka Chemie), sodium carbonate (Merck, Darmstadt, Germany), gallic acid (Sigma-Aldrich Chemie), and ethanol (Sigma-Aldrich Chemie) were used. (2-14C)-Malonyl-coenzyme A was obtained from Amersham International (Freiburg, Germany). (14C)-Labeled flavonoids naringenin, dihydrokaempferol (DHK), dihydromyricetin (DHM), and dihydroquercetin (DHQ) were prepared as described previously (Fischer et al. 2003).
Fruit color measurements
Fruit color was evaluated using a portable colorimeter (Konica Minolta, Tokyo, Japan) on 30 fruits for each sampling date and individual cultivar. The data were expressed in lightness (L*), chroma (C*), and hue angle (h°) values, calculated as tan−1 (b*/a*) in degrees from 0° to 360°. As C* increases, color becomes more intense. The L* value corresponds to a dark-bright scale and represents the relative lightness with a range from 0 to 100 (0 = black, 100 = white) (McGuire 1992).
Extraction and determination of sugars and organic acids
Primary metabolites (sugars and organic acids) were analyzed in the whole berry fruit. For the extraction of primary metabolites, 1.4 g of fruit was homogenized with 7 ml of double distilled water and left for 30 min at room temperature with continuous stirring. After the extraction, the homogenate was centrifuged and the supernatant was filtered into a vial. Sugars and organic acids were measured using a Thermo Finnigan Surveyor HPLC system (Thermo Scientific, Waltham, MA, USA). Further analysis was performed as reported by Zorenc et al. (2016), where detailed method conditions are also reported. For each currant cultivar and sampling date, five replicates were prepared and analyzed. Contents of individual and total sugars and organic acids were expressed in mg g−1 of FW.
Extraction and determination of individual phenolic compounds using HPLC–DAD–MSn analysis
One gram of berry skin was extracted with 4 ml methanol containing 3% (v/v) formic acid in a cooled ultrasonic bath for 1 h and then the extract was centrifuged and filtered into a vial. Phenolic compounds were analyzed on a Thermo Finnigan Accela HPLC system (Thermo Scientific) and identified using a mass spectrometer LCQ Deca XP MAX (Thermo Scientific) with ESI operating in negative (all phenolic groups except anthocyanins) and positive (anthocyanins) ion mode according to Mikulic-Petkovsek et al. (2015). For individual, as well as total phenolic content, five replicates were carried for each cultivar and sampling date. For compounds lacking standards, quantification was carried out using similar compounds as standards. Contents of phenolic compounds were expressed in mg kg−1 of FW.
Determination of total phenolic content (TPC)
The extraction of total phenolics was made according to the same protocol as for individual phenolic compounds. TPC of extracts was assessed by the Folin–Ciocalteu phenol reagent method (Singleton et al. 1999) and was expressed as gallic acid equivalents (GAE) in mg kg−1 of FW.
Extraction and assay of enzymes
Shock-frozen currant skin was ground to powder with liquid nitrogen. A total of 0.20 g fine skin powder, 0.20 g quartz sand, 0.20 g Polyclar AT, and 3 ml extraction buffer (described by Thill et al. 2012b) was homogenized in a mortar. The homogenate was centrifuged for 10 min at 4 °C and 13,000g. To remove low molecular compounds, 400 μl of supernatant were passed through a gel chromatography column (Sephadex G25 medium). The protein solution eluted in the excluded volume of the column (crude extract) was used for enzyme assays.
Enzyme assays were performed as described previously (Slatnar et al. 2012) using the assay conditions optimized for currant skin (Suppl. Table S1). The assays were incubated for 15 min at 30 °C. Values on each sampling date represent an average of three independent biological replicates for each treatment. All assays were run in duplicate at least. To determine the specific enzymatic activity, a modified Lowry method for protein determination (Sandermann and Strominger 1972) with BSA as a standard was used. Activities of CHS/CHI, FHT, and DFR were calculated and expressed as nkat g−1 protein.
Gene expression studies
Expression of ANS was analyzed by qPCR using a StepOnePlus system and the SYBRW Green PCR Master Mix (Applied Biosystems, Darmstadt, Germany) according to the supplier’s instruction. The analysis was carried out in triplicates, and the ANS expression was normalized against actin as control gene. Primers for ANS (RibANS_f: ATGGTGACAGTATCAGAGGCGGC; RibANS_r: TCATTTAGGTAGGAGTTCATCTTGGG) and actin (RibAct_f: TGTTCCCTGGTATT-GCTGAC, RibAct_r: CTGGAAGGTGCTAAGGGATG) were designed on the base of the sequences published in the NCBI database (LN736332, LN736331, and LN736321). Differences between the cycle threshold (Ct) of the target gene and the Actin gene were used to obtain relative transcript levels of the target gene, and calculated as 2 exp-(Ct target − Ct actin).
Statistical analysis
Results were evaluated with the Statgraphics Centurion XV.II program (Statpoint Technologies Inc., Warrenton, VA, USA). Data were tested for any differences among sampling dates within each cultivar and among cultivars (in average data calculated from all harvest dates) using the one-way analysis of variance. The differences among ripening stages were tested using the Duncan’s test and among cultivars using the HSD test with the significance level of 0.05.
Results
Fruit color and primary metabolites content
The fruit color and content of total sugars, organic acids, and sugar/organic acid ratio of the three currant cultivars are presented in Table 1. Significant differences in all color parameters were observed during fruit ripening in all cultivars. The highest color (L*, C*, h°) values were measured at the first sampling date and decreased afterwards in all cultivars, meaning ripening resulted in darker, less intense and redder (‘Jonkheer van Tets’), pinker (‘Pink Champagne’), or more light yellow (‘Zitavia’) berry skin color.
Fructose and glucose were the prevalent sugars in all three currant cultivars (sucrose was detected only in traces), while citric acid was the prevalent organic acid in berries, together with smaller amounts of malic, shikimic, and fumaric acids (data not shown). The lowest content of total sugars was measured in the fruits of all cultivars on the first harvest date (Table 1). Conversely, total organic acid contents were the highest at the beginning of ripening in ‘Pink Champagne’ and ‘Zitavia’. The highest content of total sugars was measured on the last harvest date in ‘Pink Champagne’ and ‘Zitavia’ and on to the third sampling date in ‘Jonkheer van Tets’. White currant ‘Zitavia’ had the highest sugar/organic acid ratio, while red currant ‘Jonkheer van Tets’ was characterized by the lowest.
Phenolic compound composition
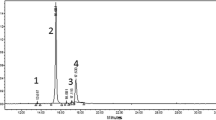
The detailed phenolic compositions and the relative levels of individual phenolics of the red, pink, and white fruiting currant cultivars are presented in Suppl. Tables S2 and S3, while total amounts of the four polyphenol classes (anthocyanins, hydroxycinnamic acids, flavanols, flavonols) and TPC detected in berry skins are presented in Table 2. Figure 1 additionally visualizes the differences in the spectrum of phenolic compounds present in the 3 cultivars.
The highest total anthocyanins content was detected in the red cultivar (Table 2). The pink cultivar contained a 13.1-fold lower content than the previous, while anthocyanins were not detected in the white cultivar. The same four cyanidin glycosides (glucosylrutinoside, sambubioside, xylosylrutinoside, and rutinoside) were found in the red and pink cultivars, but cyanidin-3-O-glucosylrutinoside and cyanidin-3-O-sambubioside were present only in traces (Suppl. Table S2 and S3). Total anthocyanin contents increased during fruit ripening, with the highest increase, of 642%, in the pink cultivar, while only 25% increase was detected in the red cultivar, which was characterized by generally high total anthocyanins in all ripening stages.
The concentration and spectrum of hydroxycinnamic acid derivatives significantly varied in the red and pink cultivars during fruit ripening. The pink and white cultivars showed more than 6-fold higher hydroxycinnamic acids concentration than the red cultivar (Table 2). The latter accumulated only p-coumaric acid derivatives (Suppl. Table S3) and the content decreased during fruit ripening. Contrary to the red cultivar, the two other cultivars accumulated caffeic acid derivatives in addition to p-coumaric acid derivatives, while the total content remained unchanged in the white cultivar and even slightly increased in the pink cultivar during ripening. Relative hydroxycinnamic acid concentration showed strong variation among the three cultivars (Fig. 1), and was higher in the pink and the white cultivars in comparison to the red (23 and 31 times, respectively).
The red cultivar accumulated the highest total flavanols (catechin, epicatechin, and procyanidin dimers), reaching up to 1.8- and 2.8-fold higher levels than the pink and the white cultivars, respectively (Table 2). Total flavanols were highest in the red and white cultivars at the first sampling date and decreased thereafter, while the pink cultivar showed a maximum at the second sampling. In the white and pink cultivars, the relative flavanol concentrations were increased by a factor of 1.8 and 2.0, respectively (Fig. 1). Interestingly, catechin was predominant in the pink and white cultivars, whereas in the red cultivar, catechin, epicatechin, and procyanidin dimers 1 and 2 were almost equally distributed.
Total flavonol glycosides (quercetin, myricetin, and kaempferol glycosides) showed no variation among the 3 cultivars, although the pink and white cultivars contained 8–14% higher contents than the red cultivar (Table 2). Sixteen differently glycosylated flavonols were detected, most of them present in all 3 cultivars (Suppl. Tables S2 and S3), with the exception of kaempferol-3-O-galactoside, which was detected only in the pink cultivar, and quercetin-rhamnosyl hexoside 3 and quercetin-rhamnosyl hexoside pentoside, which were found only in the berry skin of the red cultivar. However, the majority of the flavonols were found to be present in minor concentrations and quercetin dirhamnosyl hexoside 1 and quercetin-3-O-rutinoside were predominant in all 3 cultivars. Whereas the total flavonol content decreased during fruit ripening in the red cultivar, it remained quite stable in the two other cultivars. Relative flavonol concentrations, however, varied markedly between the three cultivars and were higher by a factor of 4 in the pink and by a factor of 5 in the white compared to the red cultivar.
The red cultivar showed the highest TPC, which was clearly due to the high anthocyanin content (Fig. 1 and Table 2). The pink and white cultivars showed significantly lower contents (1.6- and 2.2-fold lower, respectively) and only half of the contents were due to the presence of the main flavonoid classes or hydroxycinnamic acids. In all 3 cultivars, TPC slightly decreased during fruit ripening.
Activities of main flavonoid enzymes and ANS gene expression in currant skin
All enzyme assays had to be developed de novo, due to the absence of protocols for the determination of flavonoid enzymes in the literature. Various methods for enzyme preparations were tested (data not shown) and that by Thill et al. (2012b) provided the best results. The three key enzymes in the pathway leading to anthocyanin formation, CHS/CHI, FHT, and DFR, were included in the study. Since ANS activity cannot be tested from tissues so far, we also included ANS gene expression studies. DFR was tested with DHK, DHM, and DHQ as substrates. DFR from Ribes did not convert DHK and clearly preferred DHM over DHQ (tenfold lower conversion rates compared to DHM, on the average). Despite many attempts, activities of flavonol synthase and glycosyltransferase did not show reliable results, the reason of which they could not be demonstrated in this study.
Changes in the tested specific enzyme activities (CHS/CHI, FHT, and DFR) and ANS expression in the berry skin of red, pink, and white fruiting cultivars during ripening are presented in Fig. 2. In the red cultivar, the flavonoid pathway was highly induced, as indicated by generally high activities of all tested enzymes (Fig. 2a–c) and an ANS/actin ratio above 1 (Fig. 2d). The white cultivar was characterized by low CHS/CHI activity, very low ANS/actin ratio, and a lack of FHT and DFR activity. The ANS/actin ratio in the pink cultivar was below 1, but higher by a factor of 200 than that of the white cultivar. CHS/CHI activities were comparable to those detected with enzyme preparations of the white cultivar, but FHT and DFR activities were clearly present, although they were generally lower than in preparations from berry skins of the red cultivar.
Changes in specific enzyme activity (nkat g−1 protein) of CHS/CHI (a), FHT (b), DFR (c), and ANS/actin expression ratio (d) of red (‘Jonkheer van Tets’), pink (‘Pink Champagne’) and white (‘Zitavia’) currant cultivars in different ripening stages (S1–S4). Different letters (a–c) above the lines denote significant differences among ripening stages (S1–S4) within the cultivar (Duncan’s test P < 0.05)
During fruit ripening, similar patterns were observed in the red cultivar for CHS/CHI and DFR activity, with a maximum at the third stage, whereas FHT activity remained constantly high during the last three weeks of fruit ripening (Fig. 2a–c). No significant change in CHS/CHI activity was observed in the pink and white cultivars during the sampling period. In the pink cultivar FHT showed a high activity and DFR a much lower maximum at the third sampling date, which correlated with the highest ANS/actin expression ratio (Fig. 2d).
Discussion
Ripening is a complex physiological process in fruit crops. Changes in flavonoid patterns are driven by changing physiological requirements, switching from protection against pathogens and herbivores to a timely attraction of seed dispersers. Fruit color is an important evolutionary trait and one of the few important ripening indicators for currants contributing to berry quality and subsequent market value. We, therefore, studied red, pink and white fruiting currant cultivars for changes in their color, different metabolites (primary and secondary), and selected enzyme activity in fruits.
All color parameters in our study decreased during fruit ripening, as reported earlier (Mikulic-Petkovsek et al. 2015). During ripening, all cultivars developed their specific color—red for cv. ‘Jonkheer van Tets’, translucent pink for ‘Pink Champagne’ and pale yellow for ‘Zitavia’ (Suppl. Fig. S1). Fruit color is mostly affected by anthocyanins, which in our study increased with ripening and improved fruit color, while the actual hue also depends on the presence of co-pigments and vacuolar pH (Dixon et al. 2013). We also measured juice pH (data not shown). However, during the fruit ripening of each cultivar, pH values in our study did not show obvious patterns, and therefore, no connection with total anthocyanins was observed.
Sugars and other carbohydrates are common regulators for the expression of genes encoding proteins involved in various processes, including anthocyanin biosynthesis (Solfanelli et al. 2006; Zheng et al. 2009; Shi et al. 2014). In our study, the content of total sugars increased significantly (32–70%) with advanced maturity and could thus influence anthocyanin accumulation. Conversely, total organic acids generally decreased somewhat, contributing to increased sugar/organic acid ratios during ripening. Interestingly, the sugar/organic acid ratio showed a negative correlation with the anthocyanin content of the three tested cultivars, but it remains open whether this is a general phenomenon in currants, since the number of cultivars tested was not high enough.
Some white cultivars showed complete lack of anthocyanins (Määttä et al. 2001, 2003; Mikulic-Petkovsek et al. 2015; Mattila et al. 2016), while a few others had detectable amounts (Pantelidis et al. 2007; Lugasi et al. 2011). No anthocyanins could be detected in our white cv. ‘Zitavia’. Our data suggest that the flavonoid pathway in the white cultivar generally has low performance. The high absolute and relative content of total hydroxycinnamic acids in the white compared to the red cultivar confirms the presence of an early bottleneck in the flavonoid pathway. The higher relative flavonol content of berries of the white cultivar, however, indicates that at some stage, some of the intermediates not processed by the anthocyanin route are redirected towards flavonol formation. Since we could not detect FHT and DFR activity and the flavonol concentration did not further increase in the last three weeks of fruit ripening, we assume that the enzymes upstream DFR should be active in earlier fruit developmental stages. It has been shown for several strawberry varieties that flavonoid metabolism occurs following different gene and enzyme expression patterns in a two-stage process, resulting in the biosynthesis of flavonoid classes at distinct stages during early and late fruit development (Halbwirth et al. 2006; Carbone et al. 2009). The lack of anthocyanin formation in the white cultivar is probably due to the absence of DFR and ANS activity in the last three weeks of fruit ripening, in which the formation of anthocyanins is usually initiated to provide fruit pigmentation as a ripening indicator for animal seed dispersal. The absence of DFR and/or FHT activities in white cultivars of otherwise colored fruits has previously been reported for other species, such as ripe white bilberry (Jaakola et al. 2002; Primetta et al. 2015), strawberry (Thill et al. 2012a), or kiwi fruit (Halbwirth et al. 2009).
The pink cultivar in our study was characterized by a less active flavonoid pathway than with the red cultivar, which results in generally lower concentrations of TPC and particularly of total anthocyanins. The pink coloration is primarily caused by quantitative effects, since the anthocyanin composition showed moderate change and relative increase of cyanidin-3-O-glucosylrutinoside and cyanidin-3-O-rutinoside at the expense of cyanidin-3-O-xylosylrutinoside. The bottleneck in CHS/CHI (Fig. 2a) is correlated with the 23-fold increase of relative hydroxycinnamic acid concentrations. Interestingly, the white and pink cultivars also showed qualitative changes and accumulated up to 16% of glycosylated caffeic acid derivatives, in addition to glycosylated coumaric acids (Suppl. Table S3). We assume that hydroxylation in position 3 of coumaric acid occurs as a result of the tailback of hydroxycinnamic acid intermediates and could be part of a detoxification process. Hydroxylation is often the first step of increasing solubility and enabling storage of phenolic compounds in glycosylated and, frequently, acylated forms in the vacuoles to avoid damage of cell structures (Zhao and Dixon 2010). The occurrence of caffeic acid derivatives in the white cultivar also indicates that the hydroxylation step is favored in the competition of coumaric acid converting enzymes in an increasing pool of hydroxycinnamic acids.
A second important bottleneck in the pink compared to the red cultivar is caused by low DFR activity. This is demonstrated by the relatively high flavonol concentrations in comparison with anthocyanins and flavanols, indicating that the flux is primarily directed towards flavonol biosynthesis, particularly in earlier fruit developmental stages. In the investigated time period, there was a relatively high increase of anthocyanins, which correlates well with a peak of FHT activity and, to a lesser extent, DFR at the third sampling date. In addition to the low DFR activity, the observed substrate specificity could have a strong impact on anthocyanin formation. No delphinidin-based anthocyanins were detected, although DHM was the preferred substrate for DFR, and myricetin derivatives were present to a very low extent (Suppl. Table S3). We assume that the absence of F3′5′H is a key difference between red currants accumulating cyanidin glycosides and black currants accumulating delphinidin glycosides in the late stages of fruit development (Mikulic-Petkovsek et al. 2015). In the absence of 3′,4′,5′-hydroxylated dihydroflavonol precursor DHM, 3′,4′-hydroxylated dihydroflavonol DHQ will probably also be converted but lower contents of anthocyanins in red than in black currants can be expected, due to the low substrate specificity for DHQ. Although myricetin glycosides were present to a very low extent in all 3 cultivars, F3′5′H must have been active at some stage, although we assume that F3′5′H was expressed only at earlier developmental stages and not when anthocyanidins are mainly formed.
Intensely (mostly black) colored berries usually accumulate higher TCP levels than red or white ones (Lugasi et al. 2011; Mikulic-Petkovsek et al. 2015). In our study, the red cultivar contained the highest TPC, mostly due to high anthocyanin content (Fig. 1) and the white cultivar the lowest, probably due to lower or lack of activity of main flavonoid enzymes. These results obtained by the Folin–Ciocalteu method correlated positively with the sum of the concentrations of individual polyphenol classes obtained by HPLC–MS. Contrary to this, calculated sum of polyphenol classes detected by HPLC–MS in tested white and pink cultivars was only half of the amount measured with the Folin-Ciocalteu method.
The present study provides insight into the regulation of the flavonoid metabolism in differently pigmented currant cultivars. Genotype profoundly affected all tested enzymes activities and ANS gene expression, which determined different polyphenol composition and differences in accumulation patterns of all phenolic groups, especially anthocyanins.
Author contributions statement
DK, RV, MMP, and HH designed the study; SM, OSH, MMP, and ZZ performed research; ZZ and HH wrote the manuscript.
Abbreviations
- ANS:
-
Anthocyanidin synthase
- CHS/CHI:
-
Chalcone synthase/chalcone isomerase
- DFR:
-
Dihydroflavonol 4-reductase
- DHK:
-
Dihydrokaempferol
- DHM:
-
Dihydromyricetin
- DHQ:
-
Dihydroquercetin
- FHT:
-
Flavanone 3-hydroxylase
- TPC:
-
Total phenolic content
References
Ben-Simhon Z, Judeinstein S, Trainin T, Harel-Beja R, Bar-Ya’akov I, Borochov-Neori H, Holland D (2015) A “white” anthocyanin-less pomegranate (Punica granatum L.) caused by an insertion in the coding region of the leucoanthocyanidin dioxygenase (LDOX;ANS) gene. PLoS One 10(11):e0142777. doi:10.1371/journal.pone.0142777
Carbone F, Preuss A, De vos RCH, D’amico E, Perrotta G, Bovy AG, Martens S, Rosati C (2009) Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant Cell Environ 32:1117–1131
Castellarin SD, Gaspero GD (2007) Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol 7:46. doi:10.1186/1471-2229-7-46
Davies KM, Schwinn KE, Deroles SC, Manson DG, Lewis DH, Bloor SJ, Bradley JM (2003) Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 131:259–268
Dixon RA, Liu C, Jun JH (2013) Metabolic engineering of anthocyanins and condensed tannins in plants. Curr Opin Biotechnol 24:329–335
Fischer TC, Halbwirth H, Meisel B, Stich K, Forkmann G (2003) Molecular cloning, substrate specificity of the functionally expressed dihydroflavanol 4-reductases from Malus domestica and Pyrus comunis cultivars and the consequences for flavonoid metabolism. Arch Biochem Biophys 412:223–230
Gavrilova V, Kajdžanoska M, Gjamovski V, Stefova M (2011) Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J Agric Food Chem 59:4009–4018
Grimplet J, Deluc LG, Tillet RL, Wheatley MD, Schlauch KA, Cramer GR, Cushman JC (2007) Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genom 187:1–23
Halbwirth H, Puhl I, Haas U, Jezik K, Treutter D, Stich K (2006) Two-phase flavonoid formation in developing strawberry (Fragaria × ananassa) fruit. J Agric Food Chem 54:1479–1485
Halbwirth H, Waldner I, Miosic S, Ibanez M, Costa G, Stich K (2009) Measuring flavonoid enzyme activities in tissues of fruit species. J Agric Food Chem 57:4983–4987
He JJ, Liu YX, Pan QH, Cui XY, Duan CQ (2010) Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) grape berries. Molecules 15:1141–1153. doi:10.3390/molecules15031141
Jaakola L (2013) New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci 18:477–483
Jaakola L, Määttä K, Pirttila AM, Törrönen R, Kärenlampi S, Hohtola A (2002) Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol 130:729–739
Li XG, Wang J, Yu ZY (2015) Cloning of an anthocyanidin synthase gene homolog from blackcurrant (Ribes nigrum L.) and its expression at different fruit stages. Genet Mol Res 14:2726–2734
Lugasi J, Hóvári J, Kádár G, Dénes F (2011) Phenolics in raspberry, blackberry, and currant cultivars grown in Hungary. Acta Aliment 40:52–64
Määttä K, Kamal-Eldin A, Törrönen R (2001) Phenolic compounds in berries of black, red, green, and white currants (Ribes sp.). Antioxid Redox Sign 3:981–993
Määttä KR, Kamal-Eldin A, Törrönen AR (2003) High-performance liquid chromatography (HPLC) analysis of phenolic compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: Ribes species. J Agric Food Chem 51:6736–6744
Mattila PH, Hellström J, Karhu S, Pihlava JH, Veteläinen M (2016) High variability in flavonoid contents and composition between different North-European currant (Ribes spp.) varieties. Food Chem 204:14–20
McGuire RG (1992) Reporting of objective color measurements. HortScience 27:1254–1255
Mikulic-Petkovsek M, Rescic J, Schmitzer V, Stampar F, Slatnar A, Koron D, Veberic R (2015) Changes in fruit quality parameters of four Ribes species during ripening. Food Chem 173:363–374
Mikulic-Petkovsek M, Koron D, Veberic R (2016) Quality parameters of currant berries from three different cluster positions. Sci Hort 210:188–196
Milivojevic J, Slatnar A, Mikulic-Petkovsek M, Stampar F, Nikolic M, Veberic R (2012) The influence of early yield on the accumulation of major taste and health related compounds in black and red currant cultivars (Ribes spp.). J Agric Food Chem 60:2682–2691
Mu L, He JJ, Pan QH, He F, Duan CQ (2014) Tissue-specific accumulation of flavonoids in grape berries is related to transcriptional expression of VvF3′H and VvF3′5′H. S Afr J Enol Vitic 35(1):68–81
Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis G (2007) Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem 102:777–783
Primetta AK, Karppinen K, Riihinen KR, Jaakola L (2015) Metabolic and molecular analyses of white mutant Vaccinium berries show down-regulation of MYBPA1-type R2R3 MYB regulatory factor. Planta 242:631–643. doi:10.1007/s00425-015-2363-8
Sandermann H, Strominger JL (1972) Purification and properties of C55-isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem 247:5123–5131
Shi L, Cao S, Shao J, Chen W, Zheng Y, Jiang Y, Yang Z (2014) Relationship between sucrose metabolism and anthocyanin biosynthesis during ripening in Chinese Bayberry fruit. J Agric Food Chem 62(43):10522–10528
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Slatnar A, Mikulic Petkovsek M, Halbwirth H, Stampar F, Stich K, Veberic R (2012) Polyphenol metabolism of developing apple skin of a scab resistant and a susceptible apple cultivar. Trees 26:109–119
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749. doi:10.1111/j.1365-313X.2008.03447
Thill J, Miosic S, Mikulic-Petkovsek M, Slatnar A, Paltram R, Pober S, Gotame T, Veberic R, Halbwirth H, Stich H (2012a) Polyphenol metabolism in different cultivars and developmental stages of strawberry (Fragaria) fruits. Book of abstracts, 2nd Symposium on Horticulture in Europe, Angers
Thill J, Regos I, Farag MA, Ahmad AF, Kusek J, Castro A, Schlangen K, Carbonero CH, Gadjev IZ, Smith LMY, Halbwirth H, Treutter D, Stich K (2012b) Polyphenol metabolism provides a screening tool for beneficial effects of Onobrychis viciifolia (sainfoin). Phytochemistry 82:67–80
Veberic R, Slatnar A, Bizjak J, Stampar F, Mikulic-Petkovsek M (2015) Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci Technol 60:509–517
Wang H, Fan W, Li H, Yang J, Huang J, Zhang P (2013) Functional characterization of dihydroflavonol-4-reductase in anthocyanin biosynthesis of purple sweet potato underlies the direct evidence of anthocyanins function against abiotic stresses. PLoS One 8(11):e78484. doi:10.1371/journal.pone.0078484
Wei H, Chen X, Zong X, Shu H, Gao D, Liu Q (2015) Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the red and yellow fruits of sweet cherry (Prunus avium L.). PLoS One 10(3):e0121164. doi:10.1371/journal.pone.0121164
Yang B, Zheng J, Laaksonen O, Tahvonen R, Kallio H (2013) Effects of latitude and weather conditions on composition and contents of phenolic compounds in green, red, and white currants (Ribes spp.). J Agric Food Chem 61:3517–3532
Yang Y, Yao G, Yue W, Zhang S, Wu J (2015) Transcriptome profiling reveals differential gene expression in proanthocyanidin biosynthesis associated with red/green skin color mutant of pear (Pyrus communis L.). Front. Plant Sci 6:795. doi:10.3389/fpls.2015.00795
Zhao J, Dixon RA (2010) The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 15(2):72–80. doi:10.1016/j.tplants.2009.11.006
Zhao X, Yuan Z, Feng L, Fang Y (2015) Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J Plant Res 128:687–696
Zheng Y, Tian L, Liu H, Pan O, Zhan J, Huang W (2009) Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul 58:251–260
Zorenc Z, Veberic R, Stampar F, Koron D, Mikulic-Petkovsek M (2016) White versus blue: does the wild ‘albino’ bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem 211:876–882
Acknowledgements
The research is part of program Horticulture No. P4-0013-0481 funded by the Slovenian Research Agency (ARRS). Olly S. Hutabarat gratefully acknowledges Hasanuddin University (Agricultural Engineering Department, Makassar, South Sulawesi, Indonesia), the Ministry of Education and Culture of the Republic of Indonesia (DIKTI), and Austrian Agency for International Cooperation in Education and Research (OeAD-GmbH) for enabling the performance of the PhD studies abroad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special topic: Polyphenols II: biosynthesis and function in plants and ecosystems. Guest editor: Stefan Martens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zorenc, Z., Veberic, R., Koron, D. et al. Polyphenol metabolism in differently colored cultivars of red currant (Ribes rubrum L.) through fruit ripening. Planta 246, 217–226 (2017). https://doi.org/10.1007/s00425-017-2670-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2670-3