Abstract
Main conclusion
Tolerance to heat stress for retention of low-temperature sweetening-resistant phenotype in potato is conferred by insensitivity of acid invertase activity to cold induction.
Heat stress exacerbated cold sweetening (buildup of reducing sugars) of the LTS (low-temperature sweetening)-susceptible potato (Solanum tuberosum L.) cultivars, Ranger Russet and Russet Burbank, and completely abolished the resistance to cold sweetening in the LTS-resistant cultivars/clones, Sage Russet, GemStar Russet, POR06V12-3 and A02138-2. Payette Russet and EGA09702-2, however, demonstrated considerable tolerance to heat stress for retention of their LTS-resistant phenotype. Heat-primed Payette Russet and EGA09702-2 tubers accumulated fourfold more sucrose when subsequently stored at 4 °C, while reducing sugar concentrations also increased marginally but remained low relative to the non-heat-tolerant LTS-resistant clones, resulting in light-colored fries. By contrast, sucrose concentrations in heat-primed tubers of the non-heat-tolerant clones remained unchanged during LTS, but reducing sugars increased fivefold, resulting in darkening of processed fries. Acid invertase activity increased in the LTS-susceptible and non-heat-tolerant LTS-resistant cultivars/clones during cold storage. However, Payette Russet tubers maintained very low invertase activity regardless of heat stress and cold storage treatments, as was the case for Innate® Russet Burbank (W8) tubers, where silenced invertase conferred robust tolerance to heat stress for retention of LTS-resistant phenotype. Importantly, heat-stressed tubers of Payette Russet, EGA09702-2 and Innate® Russet Burbank (W8) demonstrated similar low reducing sugar and high sucrose-accumulating phenotypes when stored at 4 °C. Tolerance to heat stress for retention of LTS-resistant phenotype in Payette Russet and likely its maternal parent, EGA09702-2, is, therefore, conferred by the ability to maintain low invertase activity during cold storage of heat-stressed tubers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fry process quality in potatoes (Solanum tuberosum L.) is largely determined by specific gravity (dry matter and starch content) and reducing sugar content (glucose and fructose; Glc + Fru). Tubers of optimum process quality have low reducing sugars and relatively high specific gravities at harvest and can retain these characteristics for a prolonged period when stored at 9 °C. Storage at lower temperatures (e.g., 4–6 °C) can preserve quality and extend marketability by limiting tuber respiration, fresh weight loss, disease progression, and by prolonging dormancy (Burton 1966, 1978; Schippers 1977; Wiltshire and Cobb 1996); however, process quality (French fries and chips) is often sacrificed by the cold-induced breakdown of starch into reducing sugars (i.e., low-temperature sweetening, LTS) (Isherwood 1976; Sowokinos 2001a; Malone et al. 2006). During the frying process, reducing sugars react with free amino acids in the Maillard reaction, producing dark-colored pigments, off-flavors and acrylamide, a probable human carcinogen (IAREC 1994; Tareke et al. 2002; Mottram et al. 2002; Stadler et al. 2002; Kumar et al. 2004; Stadler and Scholz 2004; Tareke et al. 2000). Therefore, a major goal of breeding programs is to develop varieties that resist low-temperature sweetening (LTS) during cold storage (Novy et al. 2008).
High temperatures during tuber development can alter plant source–sink relationships, disrupt tuber initiation, reduce specific gravity, and increase tuber reducing sugar concentrations and associated physiological disorders, which can greatly compromise yield and overall process quality (Yamaguchi et al. 1964; Krauss and Marshner 1984; Wolf et al. 1991; Midmore 1992; Midmore and Prange 1992; Midmore and Roca 1992; Struik and Ewing 1995; Lafta and Lorenzen 1995; Stevenson et al. 2001; Timlin et al. 2006; Sowokinos 2007; Thompson et al. 2008). High sugar (sucrose, Glc, and Fru) content, sugar end development (Thompson et al. 2008; Thornton et al. 2010), mottling, translucent tissue defect (Zommick et al. 2013) and other sugar-related disorders can either manifest at harvest or develop later during storage as a consequence of in-season temperature stress at key stages of tuber development (Zommick et al. 2014). The threshold tolerance to heat stress for inducing sweetening-related disorders and loss of process quality varies significantly by cultivar.
While potato breeding programs have been successful in developing LTS-resistant varieties (Novy et al. 2008, 2010), recent work has demonstrated that heat stress can attenuate this resistance, resulting in LTS susceptibility (Zommick et al. 2014). The heat-induced loss of process quality due to sweetening is only expressed when tubers are stored at cold temperatures (data reported herein). Heat stress somehow modifies the response to cold, inducing changes in carbohydrate metabolism that compromise quality during low-temperature storage. Understanding the mechanism(s) by which heat stress alters susceptibility to LTS is prerequisite to developing cultivars with more durable and robust tolerance to high temperature for retention of the LTS-resistant phenotype.
In studies reported herein, cultivars/clones with varying degrees of resistance to LTS were challenged with in-season and postharvest heat stress to identify those with tolerance to heat stress for retention of LTS-resistant phenotype. These approaches facilitated classification of eight conventionally bred cultivars/clones and the genetically engineered Innate® Russet Burbank (W8) cultivar (provided by the J.R. Simplot Co., Boise, ID, USA) into one of three categories: ‘LTS-susceptible’, ‘LTS-resistant but non-heat tolerant’, or ‘LTS-resistant and heat-tolerant’. Subsequent evaluation of the LTS phenotypes, invertase/invertase inhibitor activities and starch phosphorylase activities of a subset of these cultivars/clones in response to heat and cold treatments demonstrated that heat stress alters how LTS-resistant but heat-susceptible clones perceive cold to induce invertase activity and accumulation of reducing sugars during storage at low temperature. By contrast, retention of the LTS-resistant phenotype in heat-tolerant cultivars/clones was conferred by the resistance of invertase to cold induction.
Materials and methods
Plant material
Certified seed tubers (G3 from nuclear) of cvs. Ranger Russet, Russet Burbank, GemStar Russet and Sage Russet were acquired directly from commercial seed growers in the fall (Table 1). Seed tubers of LTS-resistant advanced clones (numbered lines) were provided by the Pacific Northwest Variety Development Program and included POR06V12-3, A02138-2, Payette Russet (A02507-2LB) and its maternal parent, EGA09702-2. EGA09702-2 was originally selected from true potato seed provided by Dr. Ewa Zimnoch-Guzowska, Młochów Research Center, Plant Breeding and Acclimatization Institute (IHAR), Młochów, Poland. Seedling tubers from the Polish seed were generated at Aberdeen, ID in 1997, with field selection of EGA09702-2 in Corvallis, OR, USA in 1998 (Novy et al. 2016). Innate® Russet Burbank (W8) seed tubers possessing robust resistance to LTS by virtue of silenced (RNAi) invertase (Clark et al. 2014) were provided by the J.R. Simplot Co. (Boise, ID, USA). All seed tubers were stored at 4 °C (95% RH) until cutting and planting in mid-April for the studies described below.
In-season heat stress studies
To assess the effects of in-season heat stress on subsequent retention of postharvest process quality and LTS, tubers of Ranger Russet (LTS susceptible) and the LTS-resistant clones, POR06V12-3, A02138-2 and Payette Russet were subjected to elevated soil temperatures for 20 and 40 days during both the bulking (80–120 DAP) and maturation (120–160 DAP) stages of tuber development. The five treatments for each cultivar included two durations of heat × two stages of development plus an ambient (non-heat stressed) control (3 replicates). Seed tubers (120–180 g) of each cultivar/clone were hand cut into 50- to 64-g seed pieces, blocked for portion (apical and basal portions assigned to different replicates) and wound healed at 9 °C (95% RH) for 3 days prior to planting.
Seed pieces were planted 20 cm deep and 25 cm apart in a Shano silt loam soil (Lenfesty 1967) at the Washington State University Irrigated Research and Extension Unit, Othello, WA, USA (46.8°N Lat, −119.0°W Long) on April 16, 2014. Plots of each cultivar/clone (4.5-m-long; 18 seed pieces per plot) were arranged in a randomized block design in 61-m rows flanked by guard rows of cv. Ranger Russet. Heat-treated rows were equipped with two 61-m, 1000-W soil heating cables (Redi-Heat, Wrap-On Co., Inc., Bedford Park, IL, USA) installed 20–25 cm apart at seed depth (20 cm after hilling), as described by Zommick et al. (2014). Control (non-heated) rows lacked heat cables. Seed pieces in the heated rows were hand planted (Fig. 1a) and then hilled with a two-row assist feed planter that simultaneously planted and hilled the adjacent guard row. Rows were spaced 86 cm apart.
a Two soil-warming cables (61 m in length) were installed in furrow to increase soil temperature during the bulking and maturation phases of tuber development. Seed pieces were spaced 25.4 cm apart and were 20.3 cm deep after hilling. Hill cross-sectional temperature profiles for control (ambient, 19 °C) (b) and high-temperature (27 °C) rows (c) during tuber bulking were measured on July 23, 2014
Water was provided by a solid-set irrigation system. Soil moisture was monitored by neutron probes and was maintained at a minimum of 65% field capacity throughout the growing season. Irrigation scheduling followed evapotranspiration models for potato production at the WSU Othello Research Unit (WSU AgWeatherNet, http://weather.wsu.edu/awn.php, accessed 23 May, 2016). Pre-plant and in-season fertilizer applications (fertigation through solid set) were adjusted based on soil tests and weekly petiole analyses, respectively. Herbicide and pesticides were applied as needed following standard practices for production of long-season frozen-processing potatoes in the Columbia Basin.
Soil temperature of the heated rows was controlled with rheostats (Redi-Heat™ Thermostat, Phytotronics Inc., Earth City, MO, USA) attached to each heat cable. Soil temperatures were recorded (13 cm depth) hourly from 57 to 165 days after planting (DAP) with Watchdog temperature sensors (Spectrum Technologies Inc., Aurora, IL, USA) installed ca. 10 m in from the beginning and ends of each treatment row. Seasonal temperature profiles for the five treatment rows are shown in Fig. 2. Heated rows averaged 27.0 ± 1.8 °C from 80 to 160 DAP compared with 19.3 ± 2.7 °C for control. The average difference in soil temperature between non-heated control and heated plots was 8.2 ± 2.3 °C from 80 to 160 DAP. A 30.5-cm-long temperature probe (Oakton Instruments, Vernon Hills, IL, USA) was used to map the temperature of hills in cross section (Fig. 1b, c). Hill temperatures were recorded in a grid pattern at 5-cm intervals from the top of the hill to a depth of 23 cm and at 8.9-cm intervals across the 63-cm-wide hills (n = 40) for control and heated rows (Zommick et al. 2014).
Plots were harvested with a single-row harvester on 2 October 2014 (169 DAP). The tubers were washed, counted and individually weighed with a custom-built automated cup-type sorter. Tubers were sorted into the following size classes: <113, 113–170, 170–284, 284–340, 340–397, >397 g, or culls. Total yield is the weight of all tubers including culls. Marketable yield equals total yield minus culls. The 170- to 340-g tubers were used in postharvest studies (see below) to assess the effects of in-season heat stress treatments on LTS and process quality.
Postharvest handling and storage
Tubers of each cultivar and treatment were initially stored at 9 °C (95% RH) for 14 days to wound heal following harvest. The tubers were then blocked for size into three replicates and specific gravity was measured by the weight in air/weight in water method (Gould 1999) (4 tubers per replicate). Prior to placing tubers at 4 °C (95% RH) for the LTS phase of storage, zero-time samples were taken to compare initial French fry process quality (fry color), percent dry matter, and sucrose, glucose (Glc) and fructose (Fru) concentrations. Tubers were then stored at 4 °C (95% RH) with subsequent sampling at 10, 20 and 30 days (LTS phase of storage) to assess the effects of in-season heat treatments on changes in process quality (fry color) and sweetening over time. There were three replicates (4 tubers per replicate) of each treatment (5 in-season heat treatments) for each cultivar at each sampling date (including zero-time).
Tissue sampling and process quality assessment
The procedures for sampling tubers for French fry process quality and carbohydrate analyses are described in Herman et al. (2016). Briefly, at 0, 10, 20 and 30 days of storage (4 °C), tubers (3 replicates of 4 tubers per replicate) were halved along the apical to basal axis. A French fry plank (9.5 mm thick × 2.9 cm wide × length of tuber) was cut from one of the halves and a 1.5-mm-thick tissue slice (periderm intact) was shaved from the cut surface of the other half with a mandoline slicer. Slices from each of the four tubers per replicate were stacked and halved again along the apical to basal axis. Half the tissue was dried at 60 °C for 72 h in a forced-air drying oven and dry matter was determined as percentage fresh weight. The remaining tissue (collectively representing four tubers per sample) was frozen (−80 °C) and lyophilized. The lyophilized samples were ground with mortar and pestle and sieved through a 60-mesh screen (0.246 mm) for analysis of sucrose and reducing sugars (see below) following completion of the 30-day LTS study.
French fry process quality was evaluated at each sampling time as described by Zommick et al. (2014). French fry planks from each of the 12 tubers (see above) per treatment were fried collectively for 3.5 min at 191 °C in soybean oil. Color of the apical and basal ends of each plank was then quantified with a Photovolt reflectance meter (Model 577, Photovolt Instruments Inc., Indianapolis, IN, USA). Basal reflectance data were plotted vs. days at 4 °C to characterize the effects of in-season heat treatments on changes in process color during LTS for each cultivar/clone. The basal reflectance values were also translated into USDA color ratings (≥31 = USDA 0; 25–30 = USDA 1; 20–24 USDA 2; 19–15 USDA 3; and ≤14 USDA 4) (Stark et al. 2016). Photovolt reflectance values less than 20 (USDA 3 or greater) are unacceptable by industry standards (Driskill et al. 2007).
Sucrose and reducing sugar analyses
Sucrose, Glc and Fru were extracted from 250-mg samples of ground-lyophilized tissue by vortexing with 3 mL 60% (v/v) methanol in triethanolamine (TEA) buffer (30 mM, pH 7.0) as described in Herman et al. (2016). The extracts were centrifuged (10,000g, 15 min) and Glc, Fru and sucrose were quantified in the supernatant by methods of Bergmeyer et al. (1974) and Bernt and Bergmeyer (1974) as modified for microplate determination (Knowles et al. 2009). In this assay, the conversion of Glc and Fru to 6-phosphogluconate was coupled to the stoichiometric reduction of NADP (monitored at A 340) via the action of glucose-6-phosphate dehydrogenase, hexokinase and phosphoglucose isomerase. A separate aliquot of extract was incubated with acid invertase to hydrolyze sucrose, which was then quantified as the difference between moles total Glc and moles free Glc (Bergmeyer and Bernt 1974). Sugar concentrations were determined based on standard curves containing equimolar (0.05–1.8 mM) mixtures of Glc, Fru, and sucrose.
Dormancy break
The effects of timing (bulking vs. maturation) and duration (20 vs. 40 days) of in-season heat stress on sprouting of tubers were assessed over an 87- and 165-day postharvest storage period for Ranger Russet and Payette Russet, respectively. Twenty (170- to 340-g) tubers of each cultivar from each of the five in-season heat treatments were stored at 9 °C (95% RH) in the dark directly following harvest. Length of the longest sprout on each tuber was measured approximately every 10 days beginning 30 days after harvest (DAH). Sprout length ± SE is plotted vs. time. Final total sprout fresh weights (g tuber−1) were compared at the end of the storage period.
Postharvest heat stress (PHHS) studies
Additional studies were conducted to screen cultivars and advanced breeding lines for tolerance to heat stress (HS) for retention of LTS-resistant phenotype using the PHHS protocol described by Zommick et al. (2014). All LTS-susceptible and -resistant cultivars/clones (Table 1) except EGA09702-2 were grown full season (up to 7 seasons, 2014 data shown as representative) at the Washington State University Irrigated Research and Extension Unit using standard commercial practices for production of long-season processing russets in the Columbia Basin. Tubers of EGA09702-2 (the maternal parent of Payette Russet) were grown at Aberdeen, ID and provided by R. Novy (USDA-ARS). Following harvest, the tubers were washed, sorted for size (described previously) and initially wound healed for 16 days at 9 °C (95% RH). The 170- to 284-g tubers of each cultivar were blocked for size into four replicates (three tubers per replicate) and subjected to four storage temperature treatments (control, HS, cold stress (CS), HS + CS). Control tuber samples were stored at 9 °C; HS tubers were held at 32 °C for 21 days; CS tubers were held at 9 °C for 21 days and then 4 °C for 32 days to invoke LTS; tubers subjected to the combination treatment were heat primed at 32 °C for 21 days followed by 32 days at 4 °C (HS + CS). Fry plank and tissue samples were taken at the end of each treatment for analysis of process quality, sucrose and reducing sugars as described above for the in-season heat stress study. Acid invertase and starch phosphorylase activities were also compared, along with expression levels of the vacuolar acid invertase and apoplastic and vacuolar invertase inhibitor genes.
Enzyme analysis
Acid invertase activity was determined using methods detailed in Herman et al. (2016). Invertase was extracted from 200 mg of lyophilized tissue (composite of 3 tubers per replicate; 4 replicates) with 1 mL of HEPES buffer (50 mM, pH 7.5) containing 15 mM MgCl2·6 H2O, 2 mM Na2EDTA·2 H2O, 5 mM NaHSO3, 10% (v/v) glycerol, 2% PVPP and 2 µL of protease inhibitor cocktail (2.5 µL mL−1 of leupeptin, chymostatin, pepstatin and antipain, and 20 µL mL−1 of 4-aminobenzamidine and benzamidine) at 4 °C. The homogenate was centrifuged (10,000g, 15 min, 4 °C) and the supernatant was combined with 60% (NH4)SO4 to precipitate the protein. Once pelleted (10,000g, 20 min, 4 °C), the protein was re-solubilized in the original extract buffer (minus PVPP) and stored at −18 °C. Invertase activity was determined colorimetrically by a microplate adaptation of Brummell et al. (2011) as described by Zommick et al. (2013) in the presence (basal activity) and absence (total activity) of endogenous inhibitor. Basal and total activities are expressed as nmol sucrose hydrolyzed mg−1 protein h−1. Soluble protein was quantified (Bradford 1976) using protease-free BSA (Sigma-Aldrich, St. Louis, MO, USA) as a standard.
Activity gel electrophoresis (Steup 1990) was used to evaluate relative changes in activities of the plastidic (L-type; low glycogen affinity) and cytosolic (H-type; high glycogen affinity) isoforms of starch phosphorylase (α-1,4-glucan phosphorylase) in response to PHHS treatments. One hundred milligrams of lyophilized tissue (25 mg combined from each of 4 replicates representing 12 tubers) for each treatment was homogenized with 1 mL of 0.1 M Tris buffer (pH 6.8) containing 10% (w/v) glycerol, 1% NaHSO3, 2 µL of protease inhibitor cocktail (as above), and 1% (w/v) Triton™ X-100 (Sigma-Aldrich, St. Louis, MO, USA). The plastidic and cytosolic isoforms were separated on 8.5% native polyacrylamide gels containing 0.65% (w/v) glycogen (115 µg of protein per lane). Following electrophoresis (180 min, 150 V), gels were washed and subsequently incubated (37 °C for 120 min) in 100 mM MES buffer (pH 6.2) containing 0.5 mg mL−1 of soluble potato starch and 12 mM Glc-1-phosphate. The gels were stained with KI/I to compare starch synthesizing activities of the two isoforms. Data are presented for the plastidic isoform only since activity of the cytosolic isoform was not affected by the PHHS treatments.
RNA extraction and qPCR
Changes in gene expression of acid invertase and its apoplastic (Inh1, AY864820) and vacuolar (Inh2, AY864821β) inhibitors in response to PHHS treatments were compared for Russet Burbank, A02138-2, Payette Russet and Innate® Russet Burbank (W8) tubers. Primers were designed and synthesized as described previously (Zommick et al. 2013, 2014). Forward and reverse primer sequences, annealing temperatures and amplified PCR product lengths for invertase (SGN-U270188), Inh1 (AY864820) and Inh2 (AY864821) are detailed by Zommick et al. (2013, 2014).
Nucleic acids were extracted from tuber tissue (4 replicates of 3 tubers per replicate) as described by Kumar et al. (2015). First-strand cDNA was synthesized from DNase-treated samples (TURBO DNA-freeTM Kit, Ambion Inc., Foster City, CA, USA) using the Verso cDNA Synthesis Kit with anchored oligo-(dT) primer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Real-time quantitative PCR (qPCR) analysis was performed using the LightCycler® 480 High-Resolution Melting Master kit (Roche Diagnostics Corp., GmbH, Germany) on the LightCycler® 480 Real-Time PCR System version 1.5 (Roche Diagnostics Corp., Indianapolis, IN, USA). Reaction mixtures were prepared according to kit specifications and consisted of 50 ng of cDNA, 5 µM forward and reverse primer, 1.5 µM MgCl2 and 10 µL of master mix (2×) to a final volume of 20 µL. The PCR amplification ran for 10 min at 95 °C, followed by 40 cycles of 45 s (95 °C), 45 s (56 °C) and 45 s (72 °C), ending with 10 min at 72 °C for final extension. INV, Inh1 and Inh2 mRNA levels were normalized to EF-1-α (Nicot et al. 2005) using the DDCT method (Livak and Schmittgen 2001). Final qPCR products were visualized on a 1% (w/v) agarose gel containing EtBr.
Data analysis and presentation
Tuber number, weight, yield, specific gravity, dry matter and sprout fresh weight data for each cultivar were subjected to analysis of variance (ANOVA) with single degree-of-freedom contrasts partitioned for main effects (stage of application and duration of in-season heat stress) and their interaction (stage × duration). Means are separated by LSD (P < 0.05). Effects of stage and duration of heat stress on tuber size distribution were similar across cultivars/clones. Therefore, data for yields of the six tuber size classes were averaged across clones, expressed as percent marketable yield, and summarized in polygonal plots to depict the effects of stage and duration on shifts in tuber size distribution. Fry color (basal-end Photovolt reflectance), sucrose and reducing sugar data were plotted ± SE vs. time over the 30-day interval of storage at 4 °C (LTS phase of study).
The effects of PHHS treatments (control, HS, CS, HS + CS) on process quality (basal fry color), sucrose, reducing sugars and invertase (±inhibitor) were analyzed by one-way (for each cultivar) and three-way (cultivar × HS × CS) ANOVA. The three-way ANOVA included planned comparisons between groups of LTS-susceptible and -resistant cultivars/clones. PHHS treatment effects on expression (qPCR) of invertase Inh1 and Inh2 were analyzed separately by cultivar. All data are plotted ±SE with means separated by LSD (P < 0.05).
Results
In-season heat stress studies
Soil-warming cables installed in furrow (Fig. 1a) were used to implement heat stress during the bulking (80–120 DAP) and maturation (120–160 DAP) phases of tuber development. Soil temperatures at 13-cm depth were effectively increased from 20.2 ± 1.9 °C (ambient) to 27.8 ± 1.1 °C during the bulking period (Fig. 2a). Similarly, temperatures during maturation averaged 18.5 ± 3.1 and 27.1 ± 1.7 °C for ambient and heated rows, respectively (Fig. 2b). The temperature treatments were averaged over the 80–160 DAP bulking and maturation periods and are henceforth denoted as 19 and 27 °C for the ambient (control) and heated plots, respectively.
Cross-sectional temperature profiles (heat maps) of the hills were prepared at 98 DAP to assess temperature uniformity of heated vs. control rows. The non-heated (ambient) row averaged 18 °C through most of the hill except the outermost 6.5 cm, which was 2 °C higher (20 °C) (Fig. 1b). Consistent with location of the heat cables, the heated row (Fig. 1c) averaged 30 °C from 16 to 23 cm depth in the central portion of the hill and temperature decreased to 24 °C at the outer periphery of the hill.
Tuber set, size, yield and raw quality
Effects of the elevated soil temperature treatments on yield and tuber quality (specific gravity, dry matter) depended on stage (bulking vs. maturation) and duration of heat and were similar across the four cultivars/clones (see supplementary Table). Data are, therefore, averaged across cultivars/clones and presented in Table 2. The effect of early heat (bulking phase) on tuber number per plant depended on duration. Twenty days of growth at 27 °C during bulking (80–100 days) increased tuber number per plant by 1.3 tubers over control (ambient 19 °C) but 40 days at 27 °C (80–120 DAP) had no effect on tuber number. High soil temperature during maturation decreased tuber number per plant relative to the control (19 °C) and 27 °C bulking treatments, and the effect was greater for 40- vs. 20-day exposure. On average, stage of exposure to high soil temperature (bulking vs. maturation) had a greater effect on tuber number per plant than the duration of exposure and no interaction between stage and duration was apparent.
Elevated soil temperature reduced tuber yields (total and marketable), size (fresh weight), specific gravity and percent dry matter and the extent of reduction depended on stage and duration of heat (Table 2). Averaged over duration, tuber yields fell by 25 and 46% when soil temperature was maintained at 27 °C during the bulking and maturation stages, respectively. Increasing the duration of heat from 20 to 40 days further reduced yields by the same amount for both developmental stages, as evident by the lack of interaction between these treatments. On average, yield losses were 28% higher for the 40- vs. 20-days duration of high soil temperature (averaged over stage).
While 27 °C soil temperature during bulking was less detrimental to yield than during maturation, tuber specific gravity and dry matter were more negatively affected by heat during bulking (Table 2). Under ambient conditions, specific gravity and dry matter averaged 1.088 and 24.2%, respectively. High soil temperature during bulking reduced specific gravity to 1.068 and dry matter to 19.5% (averaged over duration), compared with 1.080 specific gravity and 22.7% dry matter when heat was applied during tuber maturation. However, specific gravity was the only component affected by an interaction between stage and duration. Duration of heat had no effect on specific gravity during the maturation phase, but 40 days at 27 °C during bulking reduced tuber-specific gravity more than 20 days.
The heat-induced decrease in marketable yields and effects on tuber number resulted in a general reduction in average tuber fresh weight for all cultivars/clones in response to elevated soil temperature (Table 2; supplementary Table). Averaged across 20- and 40-days duration, tuber fresh weights were 36 and 28% lower when grown at 27 °C during bulking (146.5 g tuber−1) and maturation (164 g tuber−1), respectively, compared with control (228 g tuber−1). Duration of heat also affected average tuber size during both stages of development. Tubers grown at 27 °C for 20 days averaged 171 g tuber−1 compared with 139.5 g tuber−1 for 40-days duration. These decreases in average tuber size reflect effects of the high soil temperature on tuber size distribution. As percent marketable yield, tuber size distribution shifted away from a relatively high percentage of tubers >284 g to increased percentage of <170-g tubers when grown at 27 °C during bulking and maturation (Fig. 3). The 170–284-g tubers remained relatively constant at ca. 35% of marketable yield regardless of treatment. Moreover, the heat-induced shift in tuber size distribution was greatest during bulking vs. maturation and for the 40- vs. 20-day duration of heat at both stages of development.
Polygonal plots depicting the shift in tuber size distribution induced by a +8 °C increase in soil temperature (19 vs. 27 °C) for 0, 20 or 40 days during the bulking (a) and maturation (b) phases of tuber development (average of 4 cultivars/clones). The yields of <113, 113–170, 170–284, 284–340, 340–397 and >397-g US No. 1 tubers are plotted as percent marketable yield on each axis. Marketable yields (Mkt yld), tuber numbers and average tuber fresh weights are compared in the inset table (letters indicate mean separation by LSD, P < 0.05). Yields with factorial ANOVA results are presented in Table 2
At-harvest process quality and reducing sugar accumulation during LTS
Relative to the other clones, Ranger Russet had the highest reducing sugar concentrations and darkest zero-time (following wound healing at 9 °C) fry color across all in-field heat treatments (Table 3; Fig. 4). While reducing sugar concentrations increased and fry colors darkened over the 30-day interval of cold sweetening for all cultivars/clones, the rates and magnitudes of these changes depended on clone and in-season heat stress treatment. Non-heat-stressed (ambient, 19 °C) Ranger Russet tubers accumulated reducing sugars (i.e., sweetened) at a faster rate than non-heat-treated POR06V12-3, A02138-2 and Payette Russet tubers during storage at 4 °C, reflecting the LTS-resistant phenotypes of the latter three clones (Fig. 4). Relative to tubers grown at ambient temperature, late season heat (27 °C) during maturation for 20 days had no appreciable effect on reducing sugar buildup and fry colors during LTS for all clones except POR06V12-3 where reducing sugar levels were lower and fry colors lighter in tubers from this treatment (Fig. 4; Table 3).
a Changes in process quality (basal end French fry color), b concentrations of reducing sugars (glc + fru) and c sucrose during storage of Ranger Russet, POR06V12-3, A02138-2 and Payette Russet tubers at 4 °C as affected by 20 or 40 days of heat stress (+8 °C soil temperature) during the bulking (80–100 and 80–120 DAP) or maturation (120–140 and 120–160 DAP) phases of tuber development. Data are plotted ± SE. Color shading indicates the USDA color ratings (0–4) of French fries. ANOVA results comparing the effects of soil treatments on fry color, sucrose and reducing sugar concentrations at zero and 30 days of LTS are given in Table 3
Heat stress of Ranger tubers during bulking (20 and 40 days) and maturation (40 days) resulted in unacceptable USDA 3 color fries (basal end) by 10 days and USDA 3–4 color fries by 30 days at 4 °C (Fig. 4). Importantly, A02138-2 tubers from the 40-day, 27 °C bulking treatment accumulated slightly higher reducing sugar concentrations (28.3 mg g−1 dry wt) during 30 days of LTS than the non-heat-stressed (19 °C) tubers of Ranger Russet (26.5 mg g−1 dry wt) (Table 3; Fig. 4). Similarly, POR06V12-3 tubers from the high-temperature 40-day bulking treatment sweetened the most relative to Ranger tubers grown at ambient temperature. In-season heat stress, therefore, abolished or greatly attenuated the LTS-resistant phenotypes of these two cultivars.
In contrast to the other clones, Payette Russet tubers maintained the lowest reducing sugar concentrations and lightest fry colors (USDA 0) regardless of in-season heat treatment when challenged with LTS for 30 days (Fig. 4; Table 3). Conversely, Payette Russet tubers built up higher sucrose concentrations than the other clones by 30 days of LTS for most of the in-season treatments. The lower reducing sugar, lighter fry color and higher sucrose content of Payette Russet tubers following 30-days storage at 4 °C indicate superior tolerance to heat stress for retention of LTS-resistant phenotype when compared with POR06V12-3 and A02138-2 and suggests that this heat tolerance may involve inhibition of cold-induced sucrose inversion, a possibility evaluated further in the PHHS studies described below.
Dormancy break and sprout growth
Stage and duration of heat affected the length of dormancy of Ranger Russet and Payette Russet tubers when stored at 9 °C (95% RH). Non-heat-treated Ranger Russet tubers grown at ambient soil temperature (19 °C) emerged from dormancy (days to 2.5-mm sprouts) approximately 56 DAH compared with 112 days for Payette Russet tubers, characterizing intrinsic differences in dormancy length of these two cultivars (Fig. 5a, b). Heat (27 °C) during maturation of Ranger tubers accelerated emergence from dormancy regardless of duration (20 or 40 days) but the subsequent rate of sprout elongation was greatest for tubers from the 40-days maturation treatment (Fig. 5a). Heat during tuber bulking had little effect on emergence from dormancy of Ranger Russet tubers. By contrast, Payette Russet tubers from the 27 °C, 40-day bulking treatment and 20- and 40-day maturation treatments sprouted considerably earlier than non-heat-stressed (19 °C) tubers (Fig. 5b). However, bulking for 20 days at 27 °C had no effect on dormancy break and subsequent sprout growth of Payette Russet tubers. Total sprout fresh weight per tuber at 65 days (Ranger) and 165 days (Payette) of storage was equivalent for tubers from all treatments except those exposed to 27 °C for 40 day during maturation (Fig. 5c, d). Tubers from this latter treatment produced the greatest final sprout fresh weights in accordance with the earliest dormancy break from these tubers. Moreover, 40 days of heat during maturation also significantly reduced apical dominance in both cultivars, as evidenced by an increase in the number of eyes with sprouts and multiple sprouts within each eye (data not shown).
Effects of duration (20 vs. 40 days) of elevated soil temperature (+8 °C above ambient) during tuber bulking (80–100 and 80–120 DAP) and maturation (120–140 and 120–160 DAP) on emergence from dormancy and sprout growth from Ranger Russet (a, c) and Payette Russet (b, d) tubers stored at 9 °C (95% RH). Each point represents the average of 20 tubers ± SE. Final sprout fresh weights (c, d) were determined at 87 and 165 DAH for Ranger Russet and Payette Russet, respectively. Letters indicate mean separation by LSD (P < 0.05)
PHHS studies
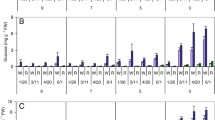
The PHHS protocol (Zommick et al. 2014) was evaluated as a screening tool for heat stress tolerance in LTS-resistant cultivars/clones. The LTS responses of heat-primed (21 days, 32 °C) tubers of Russet Burbank, Ranger Russet (both LTS-susceptible) and the LTS-resistant cultivars/clones, Sage Russet, POR06V12-3, A02138-2, Payette Russet, EGA090702-2, Innate® Russet Burbank W8 (Table 1) and GemStar Russet (data not shown) were compared. As expected, all cultivars/clones produced light (USDA 0, average 42 reflectance units) and uniform fry color (apical to basal end) following an initial 16 days of wound healing (9 °C control, Fig. 6a, b) and extending through the entire 69-day storage period at 9 °C (data not shown). However, consistent with their higher reducing sugar concentrations (Fig. 7a), Russet Burbank, Ranger Russet and Sage Russet tubers produced significantly darker fries than the other clones following harvest (Fig. 6b). Process quality (basal fry color) improved slightly (lightened 16%) for Russet Burbank and Ranger Russet tubers in response to heat priming (21 days at 32 °C) but decreased slightly (darkened 13% but remained USDA 0) for five of the six LTS-resistant cultivars/clones for which fry color data for the heat-primed tubers were measured (Fig. 6b; no data for Sage Russet). Heat priming had no effect on reducing sugar concentrations except in Russet Burbank tubers where reducing sugars declined 62% in response to the heat treatment (Fig. 7a). However, heat priming significantly increased tuber sucrose concentrations in six of eight cultivars/clones (Fig. 7b). Russet Burbank and Ranger Russet tubers accumulated 5.7- and 2.4-fold more reducing sugars during LTS (32 days at 4 °C) than the average of POR06V12-3, A02138-2, Payette Russet, EGA09702-2 and Innate® Russet Burbank (W8) tubers, resulting in significantly darker (USDA 2–3) fries and demonstrating the robust LTS-resistant phenotypes of the latter five clones/cultivars (P < 0.05, Figs. 6a, b, 7a). The reducing sugar concentration of Sage Russet tubers, however, was equal to Ranger Russet tubers following 32 days at 4 °C (Fig. 7a) but fry color remained 17% lighter (USDA 1) (Fig. 6a, b). Importantly, heat priming substantially increased the sensitivity of Russet Burbank, Ranger Russet, Sage Russet, POR06V12-3, A02138-2 (Fig. 7a) and GemStar Russet (data not shown) tubers to cold sweetening. The reducing sugar concentrations of heat-primed tubers increased 1.2- (R. Burbank), 2.9- (Ranger), 1.8- (Sage), 2.3- (POR06V12-3), 8.3- (A02138-2) and 1.9-fold (GemStar Russet) during LTS over the respective non-heat-stressed cold-stored tubers, with associated loss of process color (USDA 3–4 fry color, Fig. 6). Heat stress thus exacerbated LTS and loss of process quality in the LTS-susceptible cultivars, Russet Burbank and Ranger Russet, and extinguished the LTS-resistant phenotypes of Sage Russet, POR06V12-3 and A02138-2 (Figs. 6, 7a). The LTS resistance of these latter three cultivars/clones can thus be classified as heat labile (non-heat tolerant) for retention of LTS resistance (Fig. 6a). By contrast, during LTS, heat-primed Payette Russet, EGA09702-2 and Innate® Russet Burbank (W8) tubers accumulated significantly lower concentrations of reducing sugars (Fig. 7a) but much higher levels of sucrose (Fig. 7b) than all other cultivars/clones. The buildup of sucrose rather than reducing sugars allowed heat-primed tubers of these clones to maintain acceptably light-colored fries (USDA 0–1) during LTS (Fig. 6). Payette Russet, EGA09702-2 and Innate® Russet Burbank (W8) were, therefore, classified as heat tolerant for retention of their LTS-resistant phenotypes. Moreover, consistent with the LTS responses of Payette Russet to in-season heat stress, the PHHS data further suggest that heat tolerance may involve invertase.
a Changes in process quality (color) of French fry planks from LTS-susceptible and resistant cultivars/clones as affected by storage for 32 days at 4 °C (CS) or the combination of HS (21 days at 32 °C) plus CS. Control tubers were stored at 9 °C. Fry planks are oriented with the stem (basal) end down. The four fry planks for each treatment are from different tubers and represent the average color observed from a 12-tuber sample. Numbers on fries depict USDA color ratings for the average basal photovolt reflectance value. b Changes in basal end Photovolt reflectance values (fry color) as affected by the PHHS treatments (n = 12, ±SE). Letters indicate LSD (P < 0.05) for comparison across PHHS treatments and cultivars
Changes in a reducing sugar (Glc + Fru) and b sucrose concentrations of LTS-susceptible and -resistant cultivars/clones as affected by PHHS treatments (see Fig. 6). Each point represents the average of 12 tubers (n = 4, ±SE). Letters indicate LSD (P < 0.05) for comparison of PHHS treatments within a cultivar
Invertase and starch phosphorylase activities
Changes in basal (endogenous inhibitor present) and total (endogenous inhibitor absent) invertase activities in response to the PHHS treatments depended on cultivar/clone. While total invertase activity (Fig. 8b) was higher than basal activity (Fig. 8a), the treatment-induced trends for each cultivar/clone were identical regardless of the presence of endogenous inhibitor. At 9 °C, the LTS-susceptible cultivars (Ranger Russet and Russet Burbank) had 7.9- and 10.6-fold higher basal invertase activities, respectively, than the average of the three LTS-resistant clones (A02138-2, Payette Russet, Innate® Russet Burbank W8). Heat stress alone had no effect on invertase activity. However, activities increased markedly during 32 days of LTS at 4 °C (CS) in Ranger Russet, Russet Burbank and A02138-2 tubers, but not in Payette Russet and Innate® Russet Burbank (W8) tubers, which maintained the lowest activities across all storage temperature treatments. Heat stress prior to CS resulted in lower invertase activity in Ranger Russet tubers relative to CS alone, but increased the invertase activity in A02138-2 tubers. Relative to the control non-stressed tubers (9 °C), basal invertase activities of heat-primed tubers of Ranger, Payette Russet, Russet Burbank and Innate® Russet Burbank (W8) did not increase when subjected to LTS (HS + CS). The lack of induction of invertase by CS or HS + CS in Payette Russet and Innate® Russet Burbank (W8) tubers correlates well with the significant increases in sucrose accumulation induced by these treatments (Fig. 7b).
a Changes in basal (endogenous inhibitor present) and b total (endogenous inhibitor removed) acid invertase activities of LTS-susceptible and -resistant cultivars/clones in response to CS (32 days at 4 °C), HS (21 days at 32 °C) and the combined CS + HS treatment. Data represent the average of 12 tubers (n = 4, ±SE). Letters indicate LSD (P < 0.05) for comparing PHHS treatments within a cultivar/clone
Relative changes in activities of the plastidic and cytosolic isoforms of α-1,4-glucan phosphorylase in response to the PHHS treatments were assessed in Russet Burbank, Payette Russet, and Innate® Russet Burbank (W8) tubers. While treatments had no discernable effect on activities of the cytosolic isoform (data not shown), activities of the plastidic isoform depended on PHHS treatment and cultivar. Regardless of storage treatment, activity was not detected in Innate® Russet Burbank tubers due to gene silencing (Clark et al. 2014) (Fig. 9). By contrast, activity was relatively low but detectable in non-heat-stressed Russet Burbank tubers stored at 9 °C (control) and was not affected by heat stress (21 days at 32 °C) alone. While Russet Burbank tubers subjected to LTS at 4 °C for 32 days (CS) showed a marginal increase in activity, activity was greatly stimulated if tubers were first heat stressed and then subjected to LTS (HS + CS). Heat priming and LTS treatments alone had no effect on plastidic starch phosphorylase activities in Payette Russet tubers, which were barely detectable and lower than the activities observed for Russet Burbank tubers. However, similar to Russet Burbank, activity increased substantially when heat-stressed Payette Russet tubers were subsequently stored at 4 °C, which correlates well with the buildup in sucrose (Fig. 7b) and relatively low invertase activity (Fig. 8) and reducing sugar concentration (Fig. 7a) observed in these tubers in response to the combined HS + CS treatment.
Effects of PHHS treatments (see Fig. 8) on activities of the plastidic isoform of α-1,4-glucan phosphorylase from Russet Burbank, Innate® W8 and Payette Russet tubers. The plastidic isoform was separated on glycogen-containing native gels, which were subsequently incubated with glc-1-phosphate and starch to initiate starch synthesis. Each lane was loaded with a sample extract pooled from four replicates of three tubers per replicate, representing 12 tubers
qPCR analysis of invertase and invertase inhibitors
Changes in the expression of vacuolar acid invertase and apoplastic (Inh1) and vacuolar (Inh2) invertase inhibitor genes in response to LTS (CS) of non-heat-stressed and heat-stressed tubers were compared for Russet Burbank, A02138-2, Innate® Russet Burbank (W8) and Payette Russet tubers. Cold storage and HS prior to CS (HS + CS) treatments had no effect on expression of invertase in Russet Burbank and Innate® (W8) tubers (Fig. 10). However, invertase expression increased significantly (P < 0.05) in heat-primed tubers of A02138-2 in response to LTS (Fig. 10), which concurred with the increased invertase activity (Fig. 8) and higher reducing sugar concentration observed in these tubers (Fig. 7a). Interestingly, CS alone increased invertase expression in Payette Russet tubers; however, this response did not translate to increased invertase activity (Fig. 8), resulting in very low buildup in reducing sugars (Fig. 7a) and no change in sucrose levels (Fig. 7b).
Changes in expression (qPCR) of a acid invertase and b apoplastic (Inh1) and c vacuolar (Inh2) invertase inhibitors in Russet Burbank, A02138-2, Innate® Russet Burbank (W8) and Payette Russet tubers in response to 32 days at 4 °C (CS) or the combination of HS (21 days at 32 °C) plus CS. Control tubers were held at 9 °C. Data ± SE were normalized (DDCT) to expression of EF1-α. Letters indicate LSD (P < 0.05) for comparing PHHS treatments (4 replicates, 3 tubers per replicate) within a cultivar/clone
Expression of apoplastic invertase inhibitor (Inh1) was substantially higher in Innate® W8 tubers than the other clones and increased when heat-primed tubers were subjected to LTS at 4 °C (HS + CS) (P < 0.05; Fig. 10). Similarly, Russet Burbank tubers exposed to the combined (HS + CS) treatments showed a significant (average 3.4-fold) increase in Inh1 expression relative to tubers stored at 9 °C or subjected to CS (32 days at 4 °C) alone (P < 0.05). The combined HS + CS treatment decreased expression of Inh1 in Payette Russet tubers. Inh1 expression in A02138-2 tubers, however, was highest in tubers subjected to CS alone followed by HS + CS and tubers stored at 9 °C.
The expression levels of vacuolar invertase inhibitor (Inh2) were 100-fold lower than invertase and Inh1. Inh2 expression was unaffected by heat and/or cold stress in A02138-2 and Innate® W8 tubers (Fig. 10). In contrast, expression of Inh2 in Russet Burbank tubers was 1.6- and 2.3-fold less in tubers subjected to the CS and HS + CS treatments, respectively, than in tubers stored at 9 °C. Payette tubers subjected to CS and HS + CS also had reduced expression of Inh2 relative to tubers stored at 9 °C.
Discussion
Zommick et al. (2014) showed that increased soil temperatures effectively potentiated cold sweetening in the LTS-susceptible cultivar, Ranger Russet, and the LTS-resistant cultivar and clone, Premier Russet and AO02183-2, respectively. Moreover, tubers of Premier Russet and AO02183-2 varied slightly in their sensitivity to the high-temperature treatments for loss of LTS-resistant phenotype, suggesting inherent differences in tolerance to in-season heat stress for preservation of this trait. Similar to heat stress applied in-season, storage of tubers for 21 days at 32 °C (heat priming) directly following harvest (PHHS) exacerbated LTS in Ranger Russet and abolished the intrinsic resistance of Premier Russet tubers to subsequent cold sweetening (Zommick et al. 2014). The metabolic basis for tolerance to heat stress for retention of cold sweetening resistance is not known. Accordingly, the studies reported herein screened up to nine cultivars/clones with varying degrees of cold sweetening resistance to determine: (1) sensitivity to different durations of high soil temperature applied during bulking and maturation for retention of LTS-resistant phenotype, (2) efficacy of the PHHS treatment protocol for identifying LTS-resistant clones with heat tolerance, and (3) the mechanism of tolerance to heat stress for retention of the cold sweetening-resistant trait.
Tubers produced in 27 °C soil were screened for retention of LTS-resistant phenotype over a 30-days storage period at 4 °C. While ambient soil temperatures in many areas of the Columbia Basin of WA and OR often reach and even exceed 27 °C for short periods during the growing season (US Department of Interior, Bureau of Reclamation, http://www.usbr.gov/pn/agrimet/graphs.html), such temperatures are not sustained for the durations imposed here. The in-season high-heat treatments, therefore, constituted a relatively severe stress for evaluating effects on LTS phenotypes.
While evaluation of the effects of increased soil temperature on tuber yield and specific gravity provided insight into cultivar-dependent tolerance to in-season heat stress, the overall goal was to screen cultivars/clones to potentially identify any with heat tolerance for retention of LTS-resistant phenotype. All cultivars produced acceptably light-colored fries (USDA 0–1) regardless of treatment when stored at 9 °C through wound healing (Table 3) and the entire 44-days storage period (data not shown). However, prior to storage at 4 °C, tubers grown at elevated soil temperatures averaged higher levels of reducing sugars and sucrose and lower specific gravity and dry matter relative to non-heat-stressed tubers, which collectively reflects the inhibition of starch synthesis in tubers during periods of heat stress (Krauss and Marshner 1984; Mohabir and John 1988; Geigenberger et al. 1998; Zommick et al. 2014). Dropping the storage temperature from 9 to 4 °C to invoke LTS-induced declines in process quality coincident with increases in reducing sugars and sucrose in control and heat-stressed tubers and the extent of these changes was clone dependent (Table 3; Fig. 4). Relative to the other cultivars/clones, Payette Russet tubers built up significantly higher levels of sucrose, lower reducing sugars and maintained the lightest (most optimal) process color (USDA 0) regardless of exposure to elevated soil temperature. Payette Russet thus tolerated in-season heat stress for retention of LTS-resistant phenotype and process quality better than the other LTS-resistant clones, POR06V12-3 and A02138-2. These results suggested that heat tolerance may involve invertase and spurred additional screening studies using a PHHS protocol (Zommick et al. 2014) to characterize the metabolic basis of tolerance to heat stress for retention of LTS-resistant phenotype in Payette Russet.
The PHHS studies examined the effects of heat stress on subsequent LTS of nine cultivars/clones with LTS-susceptible and -resistant phenotypes. Heat stress prior to cold storage exacerbated cold-induced sweetening of the LTS-susceptible cultivars Russet Burbank and Ranger Russet, resulting in high reducing sugar concentrations and unacceptable dark-colored fries (Figs. 6, 7). Non-heat-stressed tubers of LTS-resistant Sage Russet, POR06V12-3, A02138-2 and GemStar Russet (data not shown) maintained relatively low reducing sugar concentrations and produced light-colored fries when stored for 32 days at 4 °C. However, heat stress prior to cold storage abolished the LTS resistance of these cultivars/clones, resulting in significant deterioration of process quality. In contrast, heat-stressed tubers of Payette Russet and its maternal parent, EGA09702-2, maintained their LTS-resistant phenotypes, producing light-color fries in response to the HS + CS treatments. This was not the case for Payette’s paternal parent, GemStar Russet, which as described above lost its inherent ability to resist LTS when subjected to heat stress. Therefore, it is likely that Payette Russet inherited its robust tolerance to heat stress from EGA09702-2.
The tolerance of Payette (and likely EGA09702-2) to heat for retention of LTS-resistant phenotype and thus process quality (Fig. 6) appears to be conferred by reduced sensitivity of invertase to cold induction (Fig. 8), resulting in the buildup of sucrose in heat-primed tubers during cold sweetening (Fig. 7). Indeed, heat-primed tubers of Innate® Russet Burbank (W8) tubers in which acid invertase has been silenced (Clark et al. 2014) displayed a sucrose-accumulating/low reducing sugar phenotype similar to Payette Russet tubers during LTS (Fig. 7; Table 4). The total and basal activities of acid invertase in Payette Russet tubers remained low and comparable to the activities observed in Innate® Russet Burbank (W8) tubers regardless of PHHS treatment (Fig. 8; Table 4). However, despite the low invertase activities of these clones, distinct differences in invertase expression levels were observed. Innate® Russet Burbank (W8) tubers, as anticipated, had little detectable expression of acid invertase, consistent with RNAi silencing (Clark et al. 2014). Payette Russet on the other hand readily expressed the vacuolar acid invertase gene which was cold-inducible (Fig. 10), but the increased transcript did not translate to function and invertase activity remained low and unaffected by the PHHS treatments relative to the LTS-susceptible but heat-labile tubers of A02138-2 (Fig. 8). These data indicate inherent differences in the regulation of invertase activity in heat-tolerant vs. non-heat-tolerant clones for retention of LTS-resistant phenotype. The disconnect between invertase gene expression and activity in Payette Russet is not surprising given the many examples of incongruence between expression and enzyme activity. For example, work by Ou et al. (2013) demonstrated weak correlation between transcript levels and acid invertase activities across eight potato genotypes.
The insensitivity of invertase in Payette Russet tubers to PHHS treatments may suggest some form of posttranscriptional regulation affecting invertase protein synthesis/function (Pressey and Shaw 1966; McKenzie et al. 2005; Brummell et al. 2011; Tauzin et al. 2014). Posttranscriptional regulation of invertase activity is complex and was only recently shown to involve an invertase-regulating protein complex that modulates invertase inhibitor activity through phosphorylation of a protein kinase (Lin et al. 2015). Further work should focus on determining the extent to which phosphorylation of sucrose non-fermenting-related protein kinase (Lin et al. 2015) is responsible for maintaining low invertase activity in heat-stressed Payette Russet tubers subjected to cold sweetening conditions. The mechanism by which Payette Russet tubers maintain low activity of invertase in response to heat stress and cold storage is key to the heat tolerance of this cultivar and warrants further investigation.
Heat priming alone had no effect on buildup in reducing sugars in any of the clones; however, consistent with results shown by Zommick et al. (2014), it significantly increased the concentration of sucrose in nearly all clones (Fig. 7; Table 4), demonstrating heat-induced effects on tuber carbohydrate metabolism. The increases in sucrose and/or reducing sugars in response to PHHS treatments reflect increased starch catabolism. Interestingly, heat priming greatly increased the activity of plastidic starch phosphorylase during storage of Russet Burbank and Payette Russet tubers at 4 °C (Fig. 9), which correlated with the substantial increases in sucrose and/or reducing sugars induced by this treatment. By contrast, starch phosphorylase activity was non-detectable in Russet Burbank Innate® (W8) tubers regardless of PHHS treatment. These data demonstrate plasticity in the pathways that mediate starch catabolism in response to heat stress, cold storage and their combination. While starch catabolism can be mediated both hydrolytically (e.g., α- and β-amylases) and phosphorolytically (Smith et al. 2005; Bethke 2013), the phosphorolytic pathway is clearly not necessary, as evidenced by the cold-induced buildup of sucrose in Russet Burbank Innate® (W8) tubers (Figs. 7, 9).
The LTS-resistant clones, Sage Russet, POR06V12-3, A02138-2, GemStar Russet, Payette Russet and EGA09702-2, resist starch degradation during cold storage, which is reflected in their abilities to maintain low reducing sugar and sucrose concentrations and thus light color fries. Differences in susceptibility to LTS have been linked to the regulation of UDP-glucose pyrophosphorylase (Zrenner et al. 1996; Sowokinos 2001b; McKenzie et al. 2005), acid invertase (Pressey and Shaw 1966; Richardson et al. 1990; Zrenner et al. 1996; McKenzie et al. 2013; Zhu et al. 2014), sucrose phosphate synthase (Hill et al. 1996; Reimholz et al. 1997) and β-amylase (Nielsen et al. 1997, Reimholz et al. 1997), key enzymes in carbohydrate metabolism. Clones which exhibit high resistance to LTS potentially have lower activities of one or more of the enzymes that mediate starch and/or sucrose catabolism (McKenzie et al. 2005, 2013). Starch catabolism seemingly increases in response to heat priming, as evidenced by increases in sucrose levels (Fig. 7b). The cold induction of acid invertase (Bethke 2013; Zommick et al. 2014; Herman et al. 2016) then facilitates inversion of sucrose to reducing sugars during subsequent cold storage of LTS-resistant but heat-susceptible clones. The temperature-insensitive and low invertase activities of Payette Russet and Innate® (W8) tubers (Fig. 8) prevent this inversion (Fig. 7b), underscoring the importance of maintaining a low level of acid invertase to confer tolerance to heat stress for retention of the LTS-resistant phenotype. Similarly, RNAi silencing of acid invertase in Ranger Russet and Russet Burbank tubers minimized sugar end development (Zhu et al. 2014), a common defect that manifests in response to heat and other environmental stresses (Thompson et al. 2008).
Increased activity of starch phosphorylase in the leaves of Arabidopsis has been linked to improved tolerance to abiotic stress (Zeeman et al. 2004). Researchers postulated that increases in phosphorolytic starch degradation in leaves provided additional substrate to fuel the oxidative pentose phosphate pathway, which generates reducing equivalents to mitigate stress-induced increases in reactive oxygen species (ROS) (Smirnoff 1993; Mittler 2002; Zeeman et al. 2004). Respiration is a major source of ROS in plants. Blauer et al. (2013) demonstrated with seed tubers that heat priming (21 days at 30 °C) permanently increased tuber respiration rate, which would contribute to increased oxidative stress. Hence, the increased plastidic starch phosphorylase activity in heat-stressed/cold-stored tubers of Russet Burbank and Payette Russet potentially reflect increased need for reducing power to catabolize ROS.
In conclusion, we demonstrate that in-season and/or postharvest heat stress can undermine resistance to LTS in many conventionally bred clones/cultivars. Heat stress exacerbated the LTS susceptibility of Ranger Russet and Russet Burbank and abolished the LTS resistance in Sage Russet, GemStar Russet, POR06V12-3 and A02138-2 by stimulating starch catabolism and increasing the available substrate (sucrose) for inversion to reducing sugars during storage at 4 °C. Heat-stressed tubers of Payette Russet and Russet Burbank Innate® (W8) retained their LTS resistance by maintaining low invertase activity. However, the total sugar (sucrose + Glc + Fru) buildup (i.e., hexose equivalents) in these clones was comparable with that from the LTS-susceptible and -resistant cultivars/clones that had no tolerance to heat stress. Payette Russet’s tolerance to heat stress (and likely EGA09702-2) is conferred by the ability to maintain low invertase activity during cold storage (similar to the silenced activity of RB Innate® W8 tubers). The in-season and PHHS protocols proved effective for screening and identifying genotypes with tolerance to heat stress for retention of LTS-resistant phenotype.
Author contribution statement
All authors contributed equally to conceiving, designing and executing the in-field and postharvest heat stress studies; DJH completed the enzyme, qPCR and dormancy analyses; DJH and LOK completed the sugar and tuber processing fry color analyses. All authors contributed to analyzing and interpreting data, designing graphics, writing and editing the final version of the manuscript.
Abbreviations
- ANOVA:
-
Analysis of variance
- CS:
-
Cold stress
- DAH:
-
Days after harvest
- DAP:
-
Days after planting
- Fru:
-
Fructose
- Glc:
-
Glucose
- HS:
-
Heat stress
- Inh1:
-
Apoplastic invertase inhibitor 1
- Inh2:
-
Vacuolar invertase inhibitor 2 β
- LTS:
-
Low-temperature sweetening
- Mkt:
-
Marketable yield (total yield minus culls)
- PHHS:
-
Postharvest heat stress
- WH:
-
Wound healing
References
Bergmeyer HU, Bernt E (1974) Sucrose. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1176–1179
Bergmeyer HU, Bernt E, Schmidt F, Stork H (1974) Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1196–1201
Bernt E, Bergmeyer HU (1974) d-Fructose. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1304–1307
Bethke PC (2013) Postharvest storage and physiology. In: Navarre R, Pavek MJ (eds) The potato: botany, production, and uses. CABI, Boston, pp 255–271
Blauer JM, Knowles LO, Knowles NR (2013) Evidence that tuber respiration is the pacemaker of physiological aging in seed potatoes (Solanum tuberosum L.). J Plant Growth Regul 32:708–720
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantity of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brummell DA, Chen RKY, Harris JC, Zhang H, Hamiaux C, Kralicek AV, McKenzie MJ (2011) Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J Exp Bot 62:3519–3534
Burton WG (1966) The potato, 2nd edn. Wageningen, Veenman, p 382
Burton WG (1978) Post-harvest behaviour and storage of potatoes. In: Crocker TH (ed) Applied biology III. Academic Press, London, pp 86–228
Clark P, Habig J, Ye J, Collinge S (2014). Petition for determination of nonregulated status for Innate™ potatoes with late blight resistance, low acrylamide potential, reduced black spot, and lowered reducing sugars: Russet Burbank event W8. J.R. Simplot Company Petition JRS01 (USDA Petition 14-093-01p)
Driskill EP, Knowles LO, Knowles NR (2007) Temperature induced changes in potato processing quality during storage are modulated by tuber maturity. Am J Potato Res 84:367–383
Geigenberger P, Geiger M, Stitt M (1998) High-temperature perturbation of starch synthesis is attributed to the inhibition of ADP-Glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. Plant Physiol 117:1307–1316
Gould WA (1999) Potato production, processing, and technology. CTI Publications, Timonium, p 61
Herman DJ, Knowles LO, Knowles NR (2016) Low oxygen storage modulates invertase activity to attenuate cold-induced sweetening and loss of process quality in potato (Solanum tuberosum L.). Postharvest Biol Tech 121:106–117
Hill LM, Reimholz R, Schroder R, Nielson TH, Stitt M (1996) The onset of sucrose accumulation in cold-stored potato tubers is caused by an increased rate of sucrose synthesis and coincides with low levels of hexose-phosphates, an activation of sucrose phosphate synthase and the appearance of a new form of amylase. Plant Cell Environ 19:1223–1237
International Agency for Research on Cancer (1994) Some industrial chemicals: summary of data reported and evaluation. IARC Monographs on the Evaluation of Carcinogenic Risks to Human 60. http://monographs.iarc.fr/ENG/Monographs/vol60/mono60-16.pdf. Accessed 14 April 2016
Isherwood FA (1976) Mechanism of sugar-starch interconversion in Solanum tuberosum. Phytochemistry 15:33–41
Knowles NR, Pavek MJ (2007) WSU potato cultivar yield and postharvest quality evaluations for 2006. Washington State University Special Report
Knowles NR, Pavek MJ (2013) WSU potato cultivar yield and postharvest quality evaluations for 2012. Washington State University Special Report
Knowles NR, Driskill EP, Knowles LO (2009) Sweetening responses of potato tubers of different maturity to conventional and non-conventional storage regimes. Postharvest Biol Tech 52:49–61
Krauss A, Marshner H (1984) Growth rate and sugar metabolism of potato tubers exposed to high temperature. Potato Res 27:297–303
Kumar D, Singh BP, Kumar P (2004) An overview of the factors affecting sugar content of potatoes. Ann Appl Biol 145:247–256
Kumar GNM, Knowles LO, Knowles NR (2015) Zebra chip disease decreases tuber (Solanum tuberosum L.) protein content by attenuating protease inhibitor levels and increasing protease levels. Planta 242:1153–1166
Lafta AM, Lorenzen JH (1995) Effects of high temperature on plant growth and carbohydrate metabolism in potatoes. Plant Physiol 109:637–643
Lenfesty CM (1967) Soil survey: Adams County, Washington. Washington D.C.
Lin Y, Liu T, Liu X, Ou Y, Zhang H, Li M, Sonnewald U, Song B, Xie C (2015) Subtle regulation of potato acid invertase activity by a protein complex of invertase, invertase inhibitor, and SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE. Plant Physiol 168:1807–1819
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCT method. Methods 25:402–408
Love SL, Novy RG, Whitworth J, Corsini DL, Pavek JJ, Mosley AR, Pavek MJ, Knowles NR, Brown CR, James SR, Hare DC, Miller JC (2006) GemStar Russet: a potato variety with high yield, good culinary quality, excellent fresh market appearance, and resistance to common scab. Am J Potato Res 83:171–180
Malone JG, Mittova V, Ratcliffe RG, Kruger NJ (2006) The response of carbohydrate metabolism in potato tubers to low temperature. Plant Cell Physiol 47:1309–1322
McKenzie MJ, Sowokinos JR, Shea IM, Gupta SK, Lindlauf RR, Anderson JAD (2005) Investigations on the role of acid invertase and UDP-glucose pyrophosphorylase in potato clones with varying resistance to cold-induced sweetening. Am J Potato Res 82:231–239
McKenzie MJ, Chen RKY, Harris JC, Ashworth MJ, Brummell DA (2013) Post-translational regulation of acid invertase activity by vacuolar invertase affects resistance to cold-induced sweetening of potato tubers. Plant Cell Environ 36:176–185
Midmore DJ (1992) Potato production in the tropics. In: Harris P (ed) The potato crop: the scientific basis for improvement, 2nd edn. Chapman and Hall, London, pp 728–793
Midmore DJ, Prange RK (1992) Growth responses of two Solanum species to contrasting temperatures and irradiance levels: relation to photosynthesis, dark respiration and chlorophyll fluorescence. Ann Bot 69:13–20
Midmore DJ, Roca J (1992) Influence of production and storage conditions on subsequent growth and tuber yield of potato (Solanum spp.) in the hot tropics. J Agric Sci 119:45–58
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2:405–410
Mohabir G, John P (1988) Effect of temperature on starch synthesis in potato tuber tissue and in amyloplasts. Plant Physiol 88:1222–1228
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419:448–449
Nicot N, Hausman J, Hoffman L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Nielsen TH, Deiting U, Stitt M (1997) α-amylase in potato tubers is induced by storage at low temperature. Plant Physiol 113:503–510
Novy RG, Whitworth JL, Stark JC, Love SL, Corsini DL, Pavek JJ, Vales MI, James SR, Hane DC, Shock CC, Charlton BA, Brown CR, Knowles NR, Pavek MJ, Brandt TL, Olsen N (2008) Premier Russet: a dual-purpose potato cultivar with significant resistance to low temperature sweetening during long-term storage. Am J Potato Res 85:198–209
Novy RG, Whitworth JL, Stark JC, Love SL, Corsini DL, Pavek JJ, Vales IM, James SR, Hane DC, Shock CC, Charlton BA, Brown CR, Knowles NR, Pavek MJ, Brandt TL, Gupta S, Olsen N (2010) Clearwater Russet: a dual-purpose potato cultivar with cold sweetening resistance, high protein content, and a low incidence of external defects and sugar ends. Am J Potato Res 87:458–471
Novy RG, Whitworth JL, Stark JC, Schneider B, Knowles NR, Pavek MJ, Knowles LO, Charlton BA, Sathuvalli V, Yilma S, Brown CR, Thornton M, Brandt TL, Olsen N (2016) Payette Russet: a dual-purpose cultivar with cold-sweetening resistance, low acrylamide formation, and resistance to late blight and potato virus Y. Am J Potato Res (in press)
Ou Y, Song B, Liu X, Lin Y, Zhang H, Li M, Fang H, Liu J (2013) Profiling of StvacINV1 expression in regulation to acid invertase activity and sugar accumulation in potato cold-stored tubers. Potato Res 56:157–165
Pavek MJ, Knowles NR (2016) WSU potato cultivar yield and postharvest quality evaluations for 2015. Washington State University Special Report. (http://potatoes.wsu.edu/wp-content/uploads/2016/01/Potato-Cultivar-Yield-and-Postharvest-Quality-Evaluations-Research-Edition-2015.pdf)
Pavek JJ, Corsini DL, Love SL, Hane DC, Holm DG, Iritani WM, James SR, Martin MW, Mosley AR, Ojala JC, Shager CE, Thronton RE (1992) Ranger Russet: a long russet potato variety for processing and fresh market with improved quality, disease resistance, and yield. Am J Potato Res 69:483–488
Pressey R, Shaw R (1966) Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol 41:1657–1661
Reimholz R, Geiger M, Haake V, Deiting U, Kruase KP, Sonnewald U, Stitt M (1997) Potato plants contain multiple forms of sucrose phosphate synthase, which differ in their tissue distributions, their levels during development, and their responses to low temperature. Plant Cell Environ 20:291–305
Richardson DL, Davies HV, Ross HA, Mackay GR (1990) Invertase activity and its relation to hexose accumulation in potato tubers. J Exp Bot 41:95–99
Schippers PA (1977) The rate of respiration of potato tubers during storage 1. Review of literature. Potato Res 20:173–188
Smirnoff N (1993) The role of active oxygen in the response of plants to water-deficit and desiccation. New Phytol 125:27–58
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Sowokinos JR (2001a) Biochemical and molecular control of cold-induced sweetening in potatoes. Am J Potato Res 78:221–236
Sowokinos JR (2001b) Allele and isozyme patterns of UDP-glucose pyrophosphorylase as a marker for cold-sweetening resistance in potatoes. Am J Potato Res 78:57–64
Sowokinos JR (2007) Internal physiological disorders and nutritional and compositional factors that affect market quality. In: Vreugdenhil D (ed) Potato biology and biotechnology advances and perspectives. Elsevier, Amsterdam, pp 501–523
Stadler RH, Scholz G (2004) Acrylamide: an update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr Rev 62:449–466
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S (2002) Acrylamide from Maillard reaction products. Nature 419:449–450
Stark JC, Novy RG, Whitworth JL et al (2016) Mountain Gem Russet: a potato variety with high early and full season yield potential and excellent fresh market and early processing characteristics. Am J Potato Res 93:158–171
Steup M (1990) Starch degrading enzymes. In: Lea PJ (ed) Methods in plant biochemistry, vol 3. Elsevier, Amsterdam, pp 103–128
Stevenson WR, Loria R, Franc GD, Weingartner DP (2001) Physiological disorders of tubers: internal symptoms. In: Stevenson WR, Loria R, Franc GD, Weingartner DP (eds) Compendium of potato diseases, 2nd edn. APS Press, St. Paul
Struik PC, Ewing EE (1995) Crop Physiology of potato (Solanum tuberosum): responses to photoperiod and temperature relevant to crop modeling. In: Haverkort AJ, MacKerron DKL (eds) Potato ecology and modelling of crop under conditions of limiting growth, current issues in production ecology. Springer, Netherlands, pp 19–40
Tareke E, Rydberg P, Karlsson P, Eriksson P, Törnquist M (2000) Acrylamide: a cooking carcinogen? Chem Res Toxicol 13:517–522
Tareke E, Rydberg P, Karlsson P, Eriksson P, Törnquist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Tauzin AS, Sulzenbacher G, Lafond M, Desseaux V, Reca IB, Perrier J, Bellincampi D, Fourquet P, Leveque C, Giardina T (2014) Functional characterization of a vacuolar invertase from Solanum lycopersicum: post-translational regulation by N-glycosylation and proteinaceous inhibitor. Biochimie 101:39–49
Thompson AL, Love SL, Sowokinos JR, Thornton MK, Shock CC (2008) Review of sugar end disorder (Solanum tuberosum L.). Am J Potato Res 85:375–386
Thornton ML, Buhrig W, Olson N (2010) The relationship between soil temperature and sugar ends in potato. Am J Potato Res 53:289–296
Timlin D, Lutfor RSM, Baker J, Reddy VR, Fleisher D, Quebedeaux B (2006) Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron J 98:1195–1203
Wiltshire JJJ, Cobb AH (1996) A review of the physiology of tuber dormancy. Ann Appl Biol 129:553–569
Wolf S, Marani A, Rudich J (1991) Effect of temperature on carbohydrate metabolism in potato plants. J Exp Bot 65:179–185
Yamaguchi M, Timm H, Spurr AR (1964) Effects of soil temperature on growth and nutrition of potato plants and tuberization, composition, and periderm structure of tubers. Proc Am Soc Hortic Sci 84:412–423
Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dustan H, Haldimann P, Bechtold N, Smoth AM, Smith SM (2004) Plastidial α-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol 135:849–958
Zhu X, Richael C, Chamberlain P, Busse JS, Bussan AJ, Jiang J, Bethke PC (2014) Vacuolar invertase gene silencing in potato (Solanum tuberosum L.) improves process quality by decreasing the frequency of sugar-end defects. PLoS One 9:e93381. doi:10.1371/journal.pone.0093381
Zommick DH, Kumar GNM, Knowles LO, Knowles NR (2013) Translucent tissue defect in potato (Solanum tuberosum L.) tubers is associated with oxidative stress accompanying an accelerated aging phenotype. Planta 238:1125–1145
Zommick DH, Knowles LO, Pavek MJ, Knowles NR (2014) In-season heat stress compromises postharvest quality and low-temperature sweetening resistance in potato (Solanum tuberosum L.). Planta 239:1243–1263
Zrenner R, Schuler K, Sonnewald U (1996) Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198:246–252
Acknowledgements
We gratefully acknowledge financial support from the USDA-ARS, Northwest Potato Research Consortium and the Washington State Potato Commission. We thank the J.R. Simplot Company, Boise, ID for providing Innate® Russet Burbank (W8) seed potatoes and Dr. Richard G. Novy (USDA-ARS, Aberdeen, ID) for providing tubers of EGA09702-2.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herman, D.J., Knowles, L.O. & Knowles, N.R. Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 245, 563–582 (2017). https://doi.org/10.1007/s00425-016-2626-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2626-z