Abstract
Main conclusion
The present study provides evidence of a unique function of RGG1 in providing salinity stress tolerance in transgenic rice without affecting yield. It also provides a good example for signal transduction from the external environment to inside for enhanced agricultural production that withstands the extreme climatic conditions and ensures food security.
The role of heterotrimeric G-proteins functioning as signalling molecules has not been studied as extensively in plants as in animals. Recently, their importance in plant stress signalling has been emerging. In this study, the function of rice G-protein γ subunit (RGG1) in the promotion of salinity tolerance in rice (Oryza sativa L. cv. IR64) was investigated. The overexpression of RGG1 driven by the CaMV35S promoter in transgenic rice conferred high salinity tolerance even in the presence of 200 mM NaCl. Transcript levels of antioxidative genes, i.e., CAT, APX, and GR, and their enzyme activities increased in salinity-stressed transgenic rice plants suggesting a better antioxidant system to cope the oxidative-damages caused by salinity stress. The RGG1-induced signalling events that conferred tolerance to salinity was mediated by increased gene expression of the enzymes that scavenged reactive oxygen species. In salinity-stressed RGG1 transgenic lines, the transcript levels of RGG2, RGB, RGA, DEP1, and GS3 also increased in addition to RGG1. These observations suggest that most likely the stoichiometry of the G-protein complex was not disturbed under stress. Agronomic parameters, endogenous sugar content (glucose and fructose) and hormones (GA3, zeatin and IAA) were also higher in the transgenic plants compared with the wild-type plants. A BiFC assay confirmed the interaction of RGG1 with different stress-responsive proteins which play active roles in signalling and prevention of aggregation of proteins under stress-induced perturbation. The present study will help in understanding the G-protein-mediated stress tolerance in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth and development is severely affected by abiotic stresses which ultimately results in heavy economic losses and food crisis. Salinity is a major abiotic stress that negatively affects the productivity of plants (Mahajan et al. 2006; Tuteja et al. 2013). Rice is the staple food for billions of Asians, which is severely affected by salinity stress (Singh et al. 2012). High salt percentage in soil enhances the production of reactive oxygen species (ROS) (Tuteja et al. 2013). Various genetic engineering advances have been employed to boost salt tolerance in a range of plants including rice (Sanan-Mishra et al. 2005; Misra et al. 2007; Gill et al. 2013; Tuteja et al. 2013).
G-protein (guanine nucleotide-binding proteins) signalling pathway is an evolutionarily conserved extracellular signal transduction which comprises the Gα subunit of 39–52 kDa, the Gβ subunit of 34–36 kDa and the Gγ subunit of 7–10 kDa (Gilman 1987; Tuteja and Sopory 2008). The pathway transduces signals from the outside environment to inside possibly through regulators (Tuteja 2009). G-proteins are engaged in a diverse range of vital biological pathways including extracellular signal transduction, hormone regulation, light perception, ion channel regulation, cell proliferation, multiple developmental processes, defence responses, cell wall modification and its regulation, and innate immunity (Jones 2002; Jones and Assmann 2004; Perfus-Barbeoch et al. 2004; Trusov et al. 2007, 2009; Zhang et al. 2008; Dupre et al. 2009; Klopffleisch et al. 2011; Trusov and Botella 2012; Urano et al. 2013; Cheng et al. 2015; Maruta et al. 2015). Subunits of G-protein have been reported in humans, Arabidopsis, wild oat, pea, rice, lupin soybean, tobacco, tomato spinach and lotus (Merlet et al. 1999; Assmann 2002; Jones and Assmann 2004; Misra et al. 2007; Yadav et al. 2012). Under stress, the G-proteins in peas have been shown to be regulated (Misra et al. 2007; Bhardwaj et al. 2011). Furthermore, Arabidopsis G-proteins are multifunctional units, interacting and playing significant roles in the development and environmental stress combat (Klopffleisch et al. 2011).
While multiple subunits of G-proteins have been reported in animals, the Arabidopsis genome has been reported to have only one gene for the Gα (GPA1) subunit and Gβ (AGB) subunit and three genes for Gγ (AGG1, AGG2 and AGG3) subunits (Perfus-Barbeoch et al. 2004; Thung et al. 2012; Trusov et al. 2012). Rice genome contains only one canonical Gα (RGA), one Gβ (RGB) and five Gγ subunits; in addition to RGG1 and RGG2 three other RGG3 subunits namely GRAIN SIZE 3 (GS3), DENSE AND ERECT PANICLE1 (DEP1) and G-protein gamma subunit type C number 2 of rice (OsGGC2) have been identified (Trusov et al. 2012). There are three different types of gamma subunits in plants: type “A” (i.e. the animal looking classic one), type “B” (lacking the isoprenylation motif (Subramaniam et al. 2016) and type “C” (with the long tail) (Botella 2012). Gγ provides functional selectivity to the heterotrimer and is essential for its proper targeting to the plasmalemma (Trusov et al. 2007; Choudhury et al. 2011; Thung et al. 2012). Studies have shown that AGB1 (Arabidopsis thaliana GB1) is necessary for survival during salt stress (Colaneri et al. 2014; Yu and Assmann 2015) and is also involved in ER stress tolerance (Cho et al. 2015). In A. thaliana, AGG1 is involved in auxin signalling with brassinosteroid (Domagalska et al. 2007) and overexpression of AGG3 in Camelina sativa resulting yield increase and heavy metal stress tolerance (Alvarez et al. 2015). Recently, it was reported that Arabidopsis Gα (GPA1) also functions in mediating ethylene-induced stomatal closure via H2O2 production (Ge et al. 2015). Three extra-large G-proteins XLG1, XLG2 and XLG3 in Arabidopsis are found to be acting as signal molecules for tuning plant G protein responses (Chakravorty et al. 2015).
Our previous work has reported that the expression of two isoforms of Gγ, i.e. RGG1 and RGG2, was increased 10- to 20-fold by NaCl stress (Yadav et al. 2012). In the present study, RGG1 overexpressing transgenic rice plants (Oryza sativa L., cv. IR64) were developed which shows enhanced tolerance to salinity stress with reduced oxidative damage. We have also identified some interacting partners of RGG1 protein using yeast two-hybrid screening assay. These partners probably play role in signalling, cell cycle control and prevention of aggregation of proteins under stress-induced perturbations.
Materials and methods
Isolation of RNA and quantitative real-time PCR
25-day-old rice seedlings (O. sativa cv. IR64; seeds obtained from ICAR-National Rice Research Institute, Cuttack, India) were stressed by keeping in 200 mM NaCl, 200 mM KCl and 10 µM ABA, and heat stress was given at 45 °C temperature separately under controlled parameters. Leaf samples were harvested at various time intervals (1, 2, 6, 12 and 24 h) and used for RNA isolation and qRT-PCR as described earlier (Tuteja et al. 2013). For qRT-PCR, the RGG1 gene-specific primers (forward 5′-GCGCTTTCTCGAGGAACTTGAAG-3′ and reverse 5′-CTTGCCAGTCTTGGGACAGATGGTTTG–3′) were used. The expression was normalised using α-tubulin gene-specific primers (forward 5′-GGTGGAGGTGATGATGCTTT-3′ and reverse 5′–ACCACGGGCAAAGTTGTTAG–3′) and fold change was calculated using \( 2^{{ - \Delta \Delta C_{\text{t}} }} \) method from three independent experiments (Livak and Schmittgen 2001). The RGG1 transgenic plants were used to check the transcript levels of different subunits such as RGG2 RGB and RGA. The qRT-PCR experiment was performed using gene-specific primers such as (forward 5′-CAAGAAGCTCGAGCAAGAGG-3′ and reverse 5′-CGGACCTTCAAACCATCTGT-3′) for RGG2 (forward 5′-GTCCTGTGGGATGTTACTACTG-3′ and reverse 5′-GCATCACATGAACCCGAAAC-3′) for RGB and (forward 5′-CTGGGAAACAGGAGGTTGAA-3′ and reverse 5′-GGTCTAGGGTCGTAGTTCTGTA-3′) for RGA.
Cloning of the gene RGG1 and its Agrobacterium-mediated transformation in IR64 rice
The coding region of RGG1 gene (282 bp) from rice was PCR-amplified using forward (5′-GGTACCATGCAGGCCGGAGGAGGAGGGGA-3′) and reverse primers (5′-GAATTCTCACAAAAACCAGCATTTGCATCTG-3′) and the amplified fragment was sequenced (GenBank accession number: GU111573.1). The CDS RGG1 (282 bp) was cloned in plant transformation vector pCAMBIA1302 to generate the plasmids pCAMBIA1302-RGG1. The IR64 rice was transformed with this construct using the Agrobacterium tumefaciens (LBA4404) transformation method (Sahoo and Tuteja 2012).
Segregation analysis of the T1 transgenic lines
The T1 seeds of transgenic IR64 plants were allowed to germinate on MS medium containing hygromycin (50 mg l−1). Segregation analysis was carried out by the progenies that were resistant to hygromycin.
Polymerase chain reaction (PCR), Southern- and western-blot analysis
The incorporation of the gene RGG1 in the transgenic plants was checked by PCR and Southern-blot analysis using the method described previously (Sahoo and Tuteja 2012). The crude plant extract from WT and the overexpressing lines was done using the process described earlier (Hurkman and Tanaka 1986). Equal amount of crude protein was denatured and separated using SDS-PAGE and electroblotted onto polyvinylidene fluoride (PVDF) membrane and then probed with mouse polyclonal antibodies (1:1000 dilution) raised against full length RGG1 protein. Crude extract from WT plant was used as negative control. Western-blot analysis using anti-RGG1 (1:1000 dilution) primary and alkaline phosphatase-conjugated anti-mouse IgG (Sigma) secondary antibodies (1:10000 dilution) was performed to check the production of the protein by the transgenic lines. The blot was developed as per manufacturer’s protocol (Sigma).
Germination and leaf disc assay
The leaf disc assay and chlorophyll content were measured by employing the process described (Sanan-Mishra et al. 2005). The germination of seeds was checked for salinity tolerance.
Analysis of transgenic, WT and vector control (VC) plants
45-day-old seedlings of transgenic, WT and VC plants were allowed to grow in 200 mM NaCl kept in a tank. The changes in phenotypic characters were recorded at an interval of 45 days.
Measurement of photosynthetic activities, agronomic attributes, and Na+ and K+ content
The different photosynthetic parameters were measured according to previously depicted method (Tuteja et al. 2015). The endogenous ions’ (phosphorous, potassium and sodium) contents were measured as described (Tuteja et al. 2013). The T2 RGG1 transgenic plants were analysed as described above.
Isolation of RNA and real-time PCR of antioxidant genes
25-day-old rice (O. sativa cv. IR64) plants were kept in H2O and 200 mM NaCl for 24 h. Leaf samples of WT, vector control (VC) and transgenic lines were collected for RNA isolation and qRT-PCR was achieved as described earlier (Tuteja et al. 2013). Three major antioxidant genes such as CATa, APX1 and GR2 were chosen for this qRT-PCR experiment and in all these cases gene-specific primers were used. The expression was normalised to α–tubulin (forward 5′-GGTGGAGGTGATGATGCTTT-3′ and reverse 5′-ACCACGGGCAAAGTTGTTAG-3′) and calculated using the \( 2^{{ - \Delta \Delta C_{\text{t}} }} \) method from three sovereign experiments (Livak and Schmittgen 2001). List of primers used in this experiment has been provided in (Supplementary Table S1).
Biochemical assays of RGG1 transgenic plants
The biochemical analyses, like lipid peroxidation, catalase a (CATa), ascorbate peroxidase one (APX1), glutathione reductase two (GR2), proline and hydrogen peroxide, were carried out using 25-day-old WT and transgenic rice seedlings exposed for 24 h to salt stress. The electrolytic leakage and relative water content (RWC) was measured as previously described (Garg et al. 2012).
Measurement of soluble sugar and endogenous hormones (GA3, zeatin and IAA)
Leaves from T2 transgenic and WT plants, grown for 24 h on 200 mM NaCl, were used for estimation of glucose and sucrose following the method of Karkacier et al. (2003). The T2 RGG1 rice (L1–L5) along with wild-type (WT) plants were grown under 200 mM NaCl. After 24 h, the plant samples were collected for endogenous hormone assays (Chen et al. 1996).
Yeast two-hybrid and bi-molecular fluorescence complementation (BiFC) assays
The interacting partners of RGG1 were isolated using the technique described earlier (Gill et al. 2013). Open reading frames (ORFs) were amplified using the primer pair (Supplementary Table S2) and were initially cloned in pJET1.2 and then finally subcloned into pSY728 and pSY738 vectors. The vectors pSY728 and pSY738 were used to express proteins of interest fused at their C-termini to fragments of YFP, using gene-specific primers and apt restriction enzymes (Supplementary Table S2). This gives rise to translational fusion between the coding sequences and BiFC probes (Knip et al. 2013). The constructs were co-bombarded into onion epidermal pieces on agar plates containing Murashige and Skoog salts (Sigma-Aldrich) using a biolistic PDS-1000/He system (Bio-Rad). After incubation for 24–30 h at 30 °C, the expression of YFP fluorescence was observed by confocal-laser microscopy. Following excitation with a 476.5–493.5 nm band-path filter, along with a triple band filter set (Zeiss Iberia, Madrid, Spain; filter set 40) the YFP appears as bright green (Cell observer SD, Apotome 2, LSM 710, Zeiss) (Bracha-Drori et al. 2004; Gu et al. 2014).
Quantitative real-time PCR (qRT-PCR) of RGA, RGB, RGG1 and RGG2
25-day-old rice (O. sativa cv. IR64) plants were kept in H2O and 200 mM NaCl for 24 h. Leaf samples of WT, VC and transgenic lines were collected for the isolation of RNA and qRT-PCR as described earlier (Tuteja et al. 2013). The expression was normalised to α-tubulin (forward 5′-GGTGGAGGTGATGATGCTTT-3′ and reverse 5′-ACCACGGGCAAAGTTGTTAG-3′) and calculated using the \( 2^{{ - \Delta \Delta C_{\text{t}} }} \) method from three repeats (Livak and Schmittgen 2001). List of primers used in this experiment has been provided in (Supplementary Table S3).
Statistical analysis
The data were analysed statistically as mentioned earlier (Tuteja et al. 2013).
Results
Relative expression of RGG1 gene in IR64 rice wild-type (WT) plants under different abiotic stresses
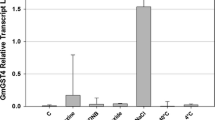
RGG1 transcript level was up-regulated up to threefold during 1–24 h of heat stress (45 °C) treatment (Fig. 1a). Similarly, ABA treatment increased RGG1 expression by up to fourfold (Fig. 1b). The relative expression of RGG1 in WT plants was less when treated with KCl (Fig. 1c). The transcript abundance of RGG1 was also up-regulated up to 14-fold during 1–24 h of salt (200 mM NaCl) treatment (Fig. 1d). It seems to be a strong response towards NaCl exposure. The expression of RGG1 was only threefold after treatment with an equal concentration, i.e. 200 mM of KCl suggesting that the high level of expression in response to NaCl was due to the stress created by sodium ions (Fig. 1d; 24 h). These results demonstrate that RGG1 is highly up-regulated due to salt (NaCl) treatment in rice.
Polymerase chain reaction, Southern- and Western-blot analysis of RGG1 transgenic rice plants
The T–DNA construct of the RGG1 gene was used for developing T1 transgenic rice plants (Fig. 2a). The original T–DNA map of pCAMBIA-1302 binary vector has been provided in (Supplementary Fig. S3). Phenotypically, the transgenic rice plants were significantly taller (L1–L5) than the WT (Fig. 2b). 35S forward and the gene-specific reverse primers were used in PCR to confirm the successful integration of the transgene (RGG1). The expected size band of 480 bp was observed (Fig. 2c). Western-blot analysis revealed that the protein was expressed to almost similar levels in all the transgenic lines from L1 to L5 (Fig. 2d). Southern-blot analysis confirmed the integration and the copy number of the transgene. The results show that a single copy transcript is present in the transgenic lines (L1-L5) driven by the constitutive 35S promoter and in the WT (native gene) (Fig. 2e). The incorporation of the RGG1 gene was established by PCR in T2 plants and the band of expected size (480 bp) was observed (Fig. 2f).
Analysis of RGG1 over expressing transgenic T1 IR64 rice plants. a The OsRGG1 gene cloned in pCAMBIA1302 vector at HindIII site. The directionality of the insert depicted was confirmed by sequencing. b Transgenic plants (L1–L5) along with WT. c PCR analysis of the RGG1 over expressing transgenic (T1) lines along with wild type (WT), positive control (PC) and negative control (NC) shows the amplification of the 480 bp fragment. d Western-blot analysis showing the production of RGG1 protein (~11 kDa). e Southern-blot analysis showing the integration and copy number of the RGG1 gene. f PCR analysis of the RGG1 over expressing transgenic (T2) lines along with WT, positive control (PC) and negative control (NC) showing the amplification of a 480 bp fragment
Response of T1 transgenic plants under salinity stress condition
The effect of saline stress during germination of seeds was studied by growing WT and T1 transgenic seeds on MS plates supplemented with 200 mM NaCl (Murashige and Skoog 1962). The seeds of transgenic lines were able to germinate and continue growth even in the presence of 200 mM NaCl saline stress (Fig. 3a). Leaf disc assays demonstrated that the reduction of the amount of chlorophyll under the influence of salt stress was lower after 72 h in the RGG1 T1 transgenic lines compared to WT plants (Fig. 3b). As a control, the 0 h NaCl treatment showed no sign of bleaching (data not shown). The data of the chlorophyll content supported the findings of the leaf disc assay results under both, 100 and 200 mM, NaCl saline stress (Fig. 3c). Senescence changes in the leaves of WT plants after 3 days of salt (200 mM NaCl) treatment were observed (Fig. 3d); however, after 45 days of treatment with salt, the RGG1 plants (L1–L3) matured and produced viable seeds (Fig. 3e), while the WT plants expired. The transgenic lines L4 and L5 demonstrated results similar to the L1- L3 plants (Supplementary Fig. S1).
Analysis of RGG1 over expressing transgenic T2 IR64 rice plants. a Germination test of T2 seeds on solid MS medium with 200 mM NaCl. b Leaf disc senescence assay under 100 and 200 mM NaCl. c Chlorophyll content (mg/g FW) in T2 RGG1 transgenic lines after salt stress. d Salt tolerance response of T2 RGG1 transgenic plants (L1, L2 and L3) and WT in 0 day of 200 mM NaCl stress. e Salt tolerance of same set of mature plants after 45 days of NaCl stress
Segregation ratio and agronomic performance of RGG1 T1 transgenic plants under stress
The survival rates of seedlings of T1 transgenic (after 3 days of treatment in 200 mM NaCl saline stress) were contrasted with the WT seedlings (without stress) and VC (empty vector control). The difference was not significant in this case (Table 1). The results showed that the segregation ratio of seeds was ~3:1 in the presence of hygromycin (Table 1). Under the influence of saline stress, the RGG1 IR64 plants evidenced superior growth and development when compared with the WT and VC (Table 2). Other parameters of growth of the RGG1 IR64 plants were found to be better than WT and VC plants (Table 3).
Photosynthetic characteristics and endogenous ion contents of RGG1 T1 transgenic plants under stress
The photosynthetic characteristics of transgenic as well as WT and VC plants were measured in the matured stage (harvesting time) after one week of induction of salt (200 mM NaCl) stress. The photosynthetic rate declined by 33% in WT and 35% in VC as compared with RGG1 transgenic lines. The stomatal conductance, net photosynthetic rate and intracellular CO2 were also higher in transgenic lines in comparison with the WT and VC plants (Table 2). In the presence of NaCl (200 mM), the WT and VC plants accumulated excess sodium whereas the transgenic lines had reduced amounts of sodium in their leaves. Salt-treated T1 transgenic lines showed higher accumulation of phosphorus and potassium (Table 2).
Transcript levels of different subunits of G protein in RGG1 over expressing T2 transgenic plants under 200 mM NaCl stress
Under control conditions (unstressed; 0 mM NaCl), the level of RGG1 transcript in T2 transgenic plants (L1–L5) increased considerably. The qRT-PCR showed 12- to 14-fold increases in the transcript level of RGG1 in contrast to WT and VC plants under normal conditions (Supplementary Fig. S2c). However, in similar unstressed conditions, transcript levels of other subunits (RGA, RGB, RGG2, DEP1 and GS3) were not induced in T2 transgenic plants (L1-L5) (Supplementary Fig. S2 a, b, d–f). In the 200 mM NaCl stress condition, the relative level of RGG1 transcript increased 10- to 14-fold increase in WT and VC plants, but in all transgenics, the relative fold change was around 19- to 20-fold (Fig. S2c). Similar results were found for RGG2. The WT, VC, as well as all the transgenic lines showed 10- to 13.5-fold increase in their relative transcript level (Fig. S2d). However, under 200 mM NaCl stress condition, there was no significant increase in the transcript level of RGA subunit in WT, VC and transgenic lines (Supplementary Fig. S2a). In case of RGB, the WT and VC did not show any significant increase in transcript level but all transgenic lines (L1–L5) showed a 12- to 14-fold increase in their relative transcript levels (Supplementary Fig. S2b). The relative expression of DEP1 and GS3 in WT and VC plants in 200 mM NaCl stress did not significantly change but all transgenic lines (L1–L5) showed 11- to 14.9-fold changes (Supplementary Fig. S2 e, f).
Gene expression of antioxidative enzymes
Manifestation of different stresses is mediated by ROS. Plants protect themselves from ROS by upregulating the gene expression of different antioxidant enzymes. To ascertain if ROS detoxifying enzymes are modulated by salt stress in WT, VC and transgenic plants, their gene expression was monitored in control and stressful environment.
Catalase (CATa) As compared to control conditions, the gene expression of CAT, responsible for destruction of H2O2, increased by twofold in both WT and VC and around sixfold in different T2 transgenic lines in saline environment (Fig. 4a).
Relative gene expression and antioxidant activities of T1 plants. a Relative gene expression of CAT in WT, VC and different transgenic lines (L1–L5) in H2O as well as in 200 mM NaCl stress. b Relative gene expression of APX in WT, VC and different transgenic lines (L1–L5) in H2O as well as in 200 mM NaCl stress. c Relative gene expression of GR in WT, VC and different transgenic lines (L1–L5) in H2O as well as in 200 mM NaCl stress. d Catalase (CAT) activity, one unit of enzyme activity defined as 1 μmol H2O2 oxidised min−1. e Ascorbate peroxidase (APX) activity, one unit of enzyme activity defined as 1 μmol of ascorbate oxidised min−1. f Glutathione reductase (GR) activity, one unit of enzyme activity is defined as 1 μmol of GS-TNB formed min−1 due to reduction of DTNB
Ascorbate peroxidase (APX1) In response to salinity stress, the relative gene expression of APX increased by twofold in WT and VC and fivefold in various T2 transgenic lines (Fig. 4b).
Glutathione reductase (GR2) The relative expression of GR with respect to control conditions increased in salt-stressed samples by 1.9-fold in both WT and VC and 5.5-fold in various T2 transgenic lines (Fig. 4c).
Analysis of antioxidant enzymes activity and response of malondialdehyde (MDA), H2O2 and ion leakage in T2 RGG1 transgenic plants
In agreement with gene expression results, the overexpression of RGG1 resulted in increased enzymatic activities of CAT, APX and GR due to salt treatment (200 mM NaCl) in RGG1 transgenic plants (Fig. 4d–f). This resulted in decreased accumulation of ROS, i.e. H2O2 in the transgenic plant in stressful environment. The changes induced by the presence of salt in the accumulation of malondialdehyde (MDA), H2O2, ion leakage, relative water content (RWC), proline and antioxidant machineries in T2 transgenic lines (L1–L5) were compared with that ones in WT and VC seedlings. The levels of MDA, H2O2 and ion leakage were significantly reduced while RWC and proline content were increased in RGG1 transgenic lines as compared to WT and VC under salt stress (200 mM NaCl) (Fig. 5a–e). The increased detoxification of ROS led to reduced membrane lipid peroxidation, i.e. MDA production and membrane damage as indicated by electrolyte leakage.
Biochemical analysis of RGG1 over expressing T1-transgenic lines (L1–L5), WT and empty vector control (VC) plants exposed to 24 h salinity stress (200 mM NaCl). a Lipid peroxidation expressed in terms of malondialdehyde (MDA) content. b Hydrogen peroxide (H2O2) content. c Percent electrolytic leakage. d Percent relative water content (RWC). e Level of proline accumulation. Error bars indicate the standard errors (SEs) calculated from three independent experiments
Endogenous soluble sugars and hormone content of T2 RGG1 transgenic plants
The T2 RGG1 transgenic plants (L1–L5) accumulated almost twice the amount of glucose and 3.5-fold more fructose in both their roots and shoots as weighed against the WT and VC plants during salinity stress (Fig. 6a–d). The T2 RGG1 transgenic lines L1–L5 illustrated high endogenous content of plant hormones as compared with the WT and VC plants (Fig. 6e–g). In this generation, GA3 content in the shoots of RGG1 transgenic rice lines (L1–L5) was relatively higher (ranging from 2.5 to 3.2 μg/gFW) as compared to the WT (1.49 μg/gFW) and VC (1.46 μg/g FW) plants. GA3 content ranged from 1.6 to 2.2 μg/gFW in the roots and 0.3–0.5 μg/gFW in the leaves of RGG1 lines as compared to roots (0.9 μg/gFW) and leaves (0.1 μg/gFW) of WT and VC plants (Fig. 6e). In transgenic plants, the value of zeatin averaged between 0.8–1.5 μg/gFW, while that of WT and VC plants contained 0.3–0.7 μg/gFW of zeatin in roots, shoots and leaves (Fig. 6f). The endogenous amount of IAA in leaves, shoots and roots of transgenic lines ranged between 2–3.2 μg/gFW against the values of 1.5–1.7 μg/gFW in WT and VC plants (Fig. 6g).
Soluble sugar content in roots and shoots of RGG1 over expressing rice T2-transgenic lines (L1–L5) compared to WT and empty vector control (VC) plants exposed to 24 h salinity stress (200 mM NaCl). a Glucose content in roots. b Fructose content in roots. c Glucose content in shoots. d Fructose content in shoots. e Endogenous content (μg/g FW) of GA3 in leaf, stem and root of WT and RGG1 T2 transgenic lines (L1–L5) under 200 mM NaCl stress. f Endogenous content (μg/g FW) of zeatin in leaf, stem and root of WT and RGG1 T2 transgenic lines (L1–L5) under 200 mM NaCl stress. g Endogenous content (μg/g FW) of IAA in leaf, stem and root of WT and RGG1 T2 transgenic rice lines (L1–L5) under 200 mM NaCl stress. The significant difference between the mean values (n = 3) of rice plants WT and RGG1 over expressing transgenic rice lines (L1–L5) was determined by one-way analysis of variance (ANOVA) using SPSS 10.0 (SPSS, Inc., now IBM, http://www-01.ibm.com/software/analytics/spss). The WT and transgenic lines at P < 0.05 and P < 0.001 were considered statistically significant
Isolation of interacting partners of RGG1 protein through yeast two-hybrid system
The interacting partners of RGG1 were isolated by yeast two-hybrid (Y2H) screening assay. About one hundred positive colonies were selected randomly and transferred to 3 DO-SD media and those which evidenced better growth even after 16 days were moved onto 3 DO-SD + 5 mM 3-AT (3-amino-1,2,4-triazole) media. Ten interacting partners were isolated and their putative functions have been presented (Table 4).
Validation of yeast two-hybrid interaction candidates using bi-molecular fluorescence complementation (BiFC) assay
The interacting proteins of RGG1 identified in yeast two-hybrid screening technique were further validated by BiFC assay. No signals were detected in the onion epidermal cells when the cells were co-bombarded with pSY728 and pSY738 and also when bombarded with pSY728- RGG1 and pSY738; pSY728 and pSY738-RGG1 and were used as negative control (Fig. 7). The BiFC signals were found for all the interacting proteins. The expression of YFP fusion protein was distinguished in the plasma membrane system and cytoplasm of cells, signifying the interaction of the candidate interacting partner with RGG1 protein (Fig. 7).
BiFC-based analysis of the interaction of RGG1 with other interacting partners in transiently transformed onion epidermal cells. After bombardment, onion epidermal pieces were incubated in darkness for 24–30 h followed by a 5-min exposure to white light before observation by confocal-laser microscopy. a The construct pairs of pSY728 and pSY738-RGG; pSY738-RGG1 and pSY728; pSY738 and pSY728-RGG1, respectively, were used as negative controls. b Rhodanese-like domain containing protein. c Enolase. d G-Beta. e Glyceraldehyde 3 phosphate dehydrogenase (G3PDH). f Receptor protein kinase. g Plasma membrane Ca-ATPase (PMCA). h Calcium-dependant protein kinase (CDPK). i Peptidyl prolyl isomerise (PIP). j Pyridoxal-dependant decarboxylase. k Cytochrome P450 (cyt P450). All images were taken at the same magnification, scale bars 17 µm
Discussion
The rice Gγ subunits are potential targets for crop improvement but their complete functions are still to be elucidated (Botella 2012). RGG1 and RGG2 are up-regulated in response to salinity, harsh temperature and ABA treatments (Yadav et al. 2012). AGG1 and AGG2 are found to be involved in osmotic stress during germination and auxin control of root development, and AGG3 in ABA signalling pathways (Chakravorty et al. 2011; Thung et al. 2013). Similarly, the soybean GγIII subunit is involved in ABA-dependent lateral root development (Roy Choudhury and Pandey 2013). Here, we have demonstrated that overexpression of RGG1 promotes tolerance of rice plants to salinity stress. It is unravelled that RGG1 interacts with stress-responsive proteins that confer salinity tolerance in rice via detoxification of stress-induced ROS.
The RGG1 transgenic lines also exhibited a higher content of endogenous nutrients under saline stress. Similar findings have been reported for OsSUV3 overexpressing rice transgenic lines (Tuteja et al. 2013). The overexpressing RGG1 seedlings grew in the constitutive presence of saline stress suggesting that the established trait is stable and functional in the transgenic plants. Enhanced control over the photosynthetic machinery under saline stress is perhaps due to the withholding of chlorophyll in these transgenic lines. The H2O2 production was significantly less in RGG1 transgenic lines under salinity stress and these data are in agreement with the previous studies where a decreased level of H2O2 production under saline stress has been reported in various overexpressing rice lines such as OsMYB2, OsPDH45 and OsSUV3 (Yang et al. 2012; Gill et al. 2013; Tuteja et al. 2013).
For protection from the injurious effects of H2O2, plants produce more APX from the ascorbate glutathione (AsA-GSH) cycle, where APX consumes ascorbate as the hydrogen donor and the NADPH-dependent reduction of GSSG (oxidised form) to GSH (reduced form) is catalysed by GR thus maintaining the high ratio of GSH/GSSG (Gill and Tuteja 2010). As compared to VC and WT, the gene expression of antioxidant enzymes, i.e. catalase, APX and GR, in transgenics were up-regulated by salt. It is likely that increase in the expression of antioxidative enzymes is mediated by signalling events induced by G-proteins, i.e. RGG1. Consequently, H2O2 content of salt-stressed transgenics was lower than in VC resulting in reduced damage of the plasma membrane causing minimal increase in solute leakage. Similarly, reduced membrane lipid peroxidation in transgenics caused lower accumulation of malondialdehyde (MDA). Thus, G-protein-induced signalling events that confer tolerance to salinity stress are mediated by increased gene expression and activity of the enzymes scavenging the ROS.
Sugars like glucose and fructose play a key role in the detoxification of ROS (Bohnert and Jensen 1996; Bentsink et al. 2000; Roy et al. 2005). In this study, the RGG1 transgenic plants exhibit saline tolerance by the accumulation of sugar alcohols, amino acids and its derivatives (Rathinasabapathi 2000). All of these were found to accumulate in increased quantities in the RGG1 transgenic rice plants thus helping them to adapt better to the changing environment. The increased glucose content plays a role in improving the negative effects of the culprit Na+ ions (Colaneri et al. 2014). The higher content of sugar in RGG1 plants supports results reported earlier (Pattanagul and Thitisaksakul 2008; Cha-um et al. 2009; Tuteja et al. 2015), for Triticum aestivum (Tammam et al. 2008), Lactuca sativa (Hasaneen et al. 2009) and Solanum lycopersicum (Mohamed and Ismail 2011).
It could be possible that overexpression of RGG1 may have pleiotropic effects and high levels of RGG1 protein in the cell may have secondary effects of sequestering all available beta molecules to form a dimer with RGG1 (beta/RGG1). The phenotypic transformations observed in this study could be mainly due to alteration in the level of the RGG1 in stres condition. In RGG1 transgenic lines in salinity, the transcript levels of RGG2, RGB, RGA, DEP1 and GS3 also increased in addition to RGG1.
A novel function of G-proteins in cell wall modification and its regulation has been suggested in the G-protein interactome study in Arabidopsis (Klopffleisch et al. 2011). The interacting partners of Gβ subunit of G-proteins in pea have a role in stress signal transduction and developmental pathways (Bhardwaj et al. 2012). The different interacting proteins of RGG1 such as Gβ, receptor-like protein kinase, plasma membrane Ca-ATPase (PMCA), calcium/calmodulin dependent protein kinases (CDPK), cyt P450, rhodanase-like domain containing protein, peptidyl prolyl isomerase (PPI), enzymes involved in glycolysis like glyceraldehyde 3-phosphate dehydrogenase (G3PDH) and enolase are obtained from the yeast two-hybrid assay (Table 4). Earlier reports suggest that AtRGS1- associated proteins identified by yeast two-hybrid system interact with other proteins. The co-purification of enolase 2 known to interact with AGB1 indicates its multiple functions (Klopffleisch et al. 2011). These observations suggest that RGG1 interacts with various proteins having distinct biochemical properties and possibly modulates various cellular and physiological processes.
A hypothetical model unfolding the role of isolated effectors of RGG1 in stress adaptation has been proposed although there is no direct evidence for these interactions (Fig. 8). The salinity stress tolerance is guarded by intricate mechanisms involving activation of calcium signal decoders such as calmodulin-like proteins (CaM) and calcium/calmodulin-dependent protein kinases (CDPKs). In the present study, we have shown the interaction of RGG1 with CDPKs which are known to regulate the production of ROS during salinity tolerance (Kobayashi et al. 2007), thus confirming the involvement of ROS scavenging machinery, which may finally lead to stress adaptation (Fig. 8). The increase in GA3 content is positively regulated by IAA (Dolley and Leyton 1968; Nelissen et al. 2012) and therefore it might be possible that the RGG1 may be indirectly involved in GA3 biosynthesis through cyt P450 which may help to increase the biomass of the transgenic plants leading to stress adaptation (Fig. 8). The CDPKs and cyt P450 are involved in other important biological pathways like transcription and signalling in which RGG1 is implicated. The role of PPI is evident under salinity-induced perturbation as the protein folding process is affected and proteins usually undergo mis-folding or aggregation. Folded proteins are less prone to aggregation and are necessary in a wide array of cellular pathways (Thomas and Sheena 2007). RGG1 interacts with a wide range of enzymes involved in glucose metabolism, e.g. glyceraldehyde 3-phosphate dehydrogenase (G3PDH) and enolase, thus indicating its role in various pathways involved in cellular metabolisms which may ultimately help the transgenic plants in stress adaptation (Fig. 8). The intracellular signal transduction is probably mediated via glycolysis with the involvement of G3PDH and enolase for the compensation of the energy need under salinity stress. Thus, the energy need of the cell for various metabolic processes is met by the de novo synthesis of glucose in response to the salinity stress in the RGG1 transgenic lines.
Hypothetical model for the role of RGG1 in plant stress adaptation. In response to extracellular signals including stress, the G-protein transduces the signal inside the cell resulting in the dissociation of Gα from Gβγ subunits. These Gα and Gβγ dimers can activate downstream signalling through interaction with their respective effectors. The interacting partners of RGG1 may directly or indirectly help in stress adaptation. G3PDH glyceraldehyde 3-phosphate dehydrogenase, CDPK calcium-dependant protein kinase, RLPK receptor-like protein kinase, cyt p450 cytochrome p450, PPI peptidyl prolyl isomerise, PMCA plasma membrane calcium ATPase
The exact mechanism of stress tolerance of RGG1 is not known yet; it may be attributed to improved maintenance of photosynthesis, antioxidant machinery, increase of proline and cellular sugar levels in transgenic plants under salinity stress possibly by interacting with stress-responsive proteins. Overall, the emerging function of RGG1 in promoting saline tolerance, without adversely influencing the yield, will help in understanding the G-protein-mediated stress tolerance in plants.
Author contribution statement
NT planned the experiment, DMS, RKS and VKS performed the experiments, NT, RT and BCT analyzed the data, NT and RT contributed reagents or other essential materials, and NT and RT wrote the paper.
Abbreviations
- AGG1 (2, 3):
-
Arabidopsis G-protein gamma subunit 1 (2, 3)
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DEP1:
-
Dense and erect panicle
- GR:
-
Glutathiol reductase
- GS3:
-
Grain size 3
- OsGGC2:
-
G-protein gamma subunit type C number 2 of rice
- RGA:
-
Rice G-protein alpha subunit
- RGB:
-
Rice G-protein beta subunit
- RGG1 (2):
-
Rice G-protein gamma subunit 1 (2)
- ROS:
-
Reactive oxygen species
- WT:
-
Wild type
- VC:
-
Vector control
References
Alvarez S, Roy Choudhury S, Sivagnanam K, Hicks LM, Pandey S (2015) Quantitative proteomics analysis of Camelina sativa seeds overexpressing the AGG3 gene to identify the proteomic basis of increased yield and stress tolerance. J Proteome Res 14:2606–2616. doi:10.1021/acs.jproteome.5b00150
Assmann SM (2002) Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell 14:S355–S373
Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SPC, Koornheef M (2000) Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol 124:1595–1604
Bhardwaj D, Sheikh AH, Sinha AK, Tuteja N (2011) Stress induced β subunit of heterotrimeric G-proteins from Pisum sativum interacts with mitogen-activated protein kinase. Plant Signal Behav 6:287–292
Bhardwaj D, Lakhanpaul S, Tuteja N (2012) Wide range of interacting partners of pea Gβ subunit of G-proteins suggests its multiple functions in cell signalling. Plant Physiol Biochem 58:1–5
Bohnert HJ, Jensen RG (1996) Strategies for engineering water stress tolerance in plants. Trends Biotechnol 14:89–97
Botella JR (2012) Can heterotrimeric G proteins helps to feed the world? Trends Plant Sci 17:563–568
Bracha-Drori K, Shichrur K, Katz A, Oliva M, Angelovici R, Yalovsky S, Ohad N (2004) Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J 40:419–427
Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Assmann SM, Botella JR (2011) An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J 67:840–851
Chakravorty D, Gookin TE, Milner MJ, Yu Y, Assmann SM (2015) Extra-large G proteins expand the repertoire of subunits in Arabidopsis heterotrimeric G protein signalling. Plant Physiol 169:512–529
Cha-um S, Charoenpanich A, Roytrakul S, Kirdmanee C (2009) Sugar accumulation, photosynthesis and growth of two indica rice varieties in response to salt stress. Acta Physiol Plant 31:477–486
Chen JG, Du XM, Zhao HY, Zhou X (1996) Fluctuation in levels of endogenous plant hormones in ovules of normal and mutant cotton during flowering and their relation to fibre development. J Plant Growth Regul 15:173–177
Cheng Z, Li JF, Niu Y, Zhang XC, Woody OZ, Xiong Y, Djonović S, Millet Y, Bush J, McConkey BJ, Sheen J, Ausubel FM (2015) Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521:213–216
Cho Y, Yu CY, Iwasa T, Kanehara K (2015) Heterotrimeric G protein subunits differentially respond to endoplasmic reticulum stress in Arabidopsis. Plant Signal Behav 10:e1061162. doi:10.1080/15592324.2015.1061162
Choudhury SR, Bisht NC, Thompson R, Todorov O, Pandey S (2011) Conventional and novel Gγ protein families constitute the heterotrimeric G-protein signaling network in soybean. PLoS One 6(8):e23361. doi:10.1371/journal.pone.0023361
Colaneri A, Ozdemir M, Huang J, Jones A (2014) Growth attenuation under saline stress is mediated by the heterotrimeric G protein complex. BMC Plant Biol 14:129–139
Dolley D, Leyton L (1968) Effects of growth regulating substances and water potential on the development of secondary xylem in fraxinus. New Phytol 67:579–594
Domagalska MA, Schomburg FM, Amasino RM, Vierstra RD, Nagy F (2007) Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering. Development 134:2841–2850
Dupre DJ, Robitaille M, Rebois RV, Hebert TE (2009) The role of Gbc subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol 49:31–56
Garg B, Jaiswal JP, Misra S, Tripathi BN, Prasad MA (2012) A comprehensive study on dehydration-induced antioxidative responses during germination of Indian bread wheat (Triticum aestivum L. emThell) cultivars collected from different agroclimatic zones. Physiol Mol Biol Plants 18:217–228
Ge XM, Cai LH, Lei X, Zhou X, Yue M, He JM (2015) Heterotrimeric G protein mediates ethylene-induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant J 82:138–150
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–939
Gill SS, Tajrishi M, Madan M, Tuteja N (2013) A DESD-box helicase functions in salinity stress tolerance by improving photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. PB1). Plant Mol Biol 82:1–22
Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649
Gu C, Zhang X, Jiang J, Guan Z, Zhao S, Fang W, Liao Y, Chen S, Chen F (2014) Chrysanthemum CmNAR2 interacts with CmNRT2 in the control of nitrate uptake. Sci Rep 4:5833. doi:10.1038/srep05833
Hasaneen MNA, Younis ME, Tourky SMN (2009) Plant growth, metabolism and adaptation in relation to stress conditions (Lactuca sativa L.). Plant Omics 2:60–69
Hurkman W, Tanaka C (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81:802–806
Jones AM (2002) G-protein-coupled signaling in Arabidopsis. Curr Opin Plant Biol 5:402–407
Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5:572–578
Karkacier M, Erbas M, Uslu MK, Aksu M (2003) Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC. J Chromatogr Sci 41:331–333
Klopffleisch K, Phan N, Augustin K, Bayne RS, Booker KS, Botella JR et al (2011) Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol Syst Biol 7:532. doi:10.1038/msb.2011.66
Knip M, Hiemstra S, Sietsma A, Castelein M, de Pater S, Hooykaas P (2013) DAYSLEEPER: a nuclear and vesicular-localized protein that is expressed in proliferating tissues. BMC Plant Biol 13:211–222
Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19:1065–1080
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the \( 2^{{ - \Delta \Delta C_{\text{t}} }} \) method. Methods 25: 402–408
Mahajan S, Sopory SK, Tuteja N (2006) Cloning and characterization of CBL-CIPK signaling components from a legume (Pisum sativum). FEBS J 273:907–925
Maruta N, Trusov Y, Brenya E, Parekh U, Botella JR (2015) Membrane-localized extra-large G proteins and Gβγ of the heterotrimeric G proteins form functional complexes engaged in plant immunity in Arabidopsis. Plant Physiol 167:1004–1016
Merlet F, Weinstein LS, Goldsmith PK, Rarick T, Hall JL, Bisson JP, de Mazancourt P (1999) Identification and localization of G protein subunits in human spermatozoa. Mol Hum Reprod 5:38–45
Misra S, Wu Y, Venkataraman G, Sopory SK, Tuteja N (2007) Heterotrimeric G-protein complex and G-protein-coupled receptor from a legume (Pisum sativum): role in salinity and heat stress and cross-talk with phospholipase C. Plant J 51:656–669
Mohamed AN, Ismail MR (2011) Changes in organic and inorganic solutes of in vitro tomato cultivars under NaCl stress. Aust J Crop Sci 5:939–944
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Vanlijsebettens M, Kamiya Y, Inze D, Beenster GT (2012) A local maximum in gibberellin levels regulates maize leave growth by spatial control of cell division. Curr Biol 22:1183–1187
Pattanagul W, Thitisaksakul M (2008) Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J Exp Biol 46:736–742
Perfus-Barbeoch L, Jones AM, Assmann SM (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7:719–731
Rathinasabapathi B (2000) Metabolic engineering for stress tolerance: installing osmo protectant synthesis pathways. Ann Bot 86:709–716
Roy Choudhury S, Pandey S (2013) Specific subunits of heterotrimeric G-proteins play important roles during nodulation in soybean. Plant Physiol 162:522–533
Roy P, Niyogi K, SenGupta DN, Ghosh B (2005) Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt sensitive rice cultivars. Plant Sci 168:583–591
Sahoo RK, Tuteja N (2012) Development of Agrobacterium-mediated transformation technology for mature seed-derived callus tissues of indica rice cultivar IR64. GM Crops Food 3:123–128
Sanan-Mishra N, Pham XH, Sopory SK, Tuteja N (2005) Pea DNA helicase 45 overexpression in tobacco confers high salinity tolerance without affecting yield. Proc Natl Acad Sci USA 102:509–514
Singh A, Singh V, Singh S, Pandian R, Ellur R, Singh D, Bhowmick P, Krishnan SG, Nagarajan M, Vinod K, Singh U, Prabhu K, Sharma T, Mohapatra T, Singh AK (2012) Molecular breeding for the development of multiple disease resistance in Basmati rice. AoB Plants 2012:pls029. doi:10.1093/aobpla/pls029
Subramaniam G, Trusov Y, Lopez-Encina C, Hayashi S, Batley J, Botella JR (2016) Type B heterotrimeric G protein γ subunit regulates auxin and ABA signaling in tomato. Plant Physiol 170:1117–1134
Tammam AA, AbouAlhamd MF, Hemeda MM (2008) Study of salt tolerance in wheat (Triticum aestivum L.) cultivar Banysoif 1. Aust J Crop Sci 3:115–125
Thomas RJ, Sheena ER (2007) Folding versus aggregation: polypeptide conformations on competing pathway. Arch Biochem Biophys 469:100–117
Thung L, Trusov Y, Chakravorty D, Botella JR (2012) Gγ1 + Gγ2 + Gγ3 = Gβ: the search for heterotrimeric G-protein γ subunits in Arabidopsis is over. J Plant Physiol 169:542–545
Thung L, Chakravorty D, Trusov Y, Jones A, Botella JR (2013) Signaling specificity provided by the Arabidopsis thaliana heterotrimeric G-protein γ-subunits AGG1 and AGG2 is partially but not exclusively provided through transcriptional regulation. PLoS One 8:e58503
Trusov Y, Botella JR (2012) New faces in plant innate immunity: heterotrimeric G-proteins. J Plant Biochem Biotechnol 21:40–47
Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen JG, Jones AM, Botella JR (2007) Heterotrimeric G protein γ subunits provide functional selectivity in G βγ dimer signaling in Arabidopsis. Plant Cell 19:1235–1250
Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR (2009) Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J 58:69–81
Trusov Y, Chakravorty D, Botella JR (2012) Diversity of heterotrimeric G-protein γ subunits in plants. BMC Res Notes 5:608
Tuteja N (2009) Signaling through G protein coupled receptors. Plant Signal Behav 4:942–947
Tuteja N, Sopory SK (2008) Plant signaling in stress: G-protein coupled receptors, heterotrimeric G-proteins and signal coupling via phospholipases. Plant Signal Behav 3:79–86
Tuteja N, Sahoo RK, Garg B, Tuteja R (2013) OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64). Plant J 76:115–127
Tuteja N, Sahoo RK, Huda KMK, Tula S, Tuteja R (2015) OsBAT1 augments salinity stress tolerance by enhancing detoxification of ROS and expression of stress-responsive genes in transgenic rice. Plant Mol Biol Rep 33:1192–1209. doi:10.1007/s11105-014-0827-9
Urano D, Chen JG, Botella JR, Jones AM (2013) Heterotrimeric G protein signalling in the plant kingdom. Open Biol 3:120186. doi.10.1098/rsob.120186
Yadav DK, Islam S, Tuteja N (2012) Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signal Behav 7:733–740
Yang A, Dai X, Zhang WH (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot 63:2541–2556
Yu Y, Assmann SM (2015) The heterotrimeric G-protein β subunit, AGB1, plays multiple roles in the Arabidopsis salinity response. Plant Cell Environ 38:2143–2156. doi:10.1111/pce.12542
Zhang W, He SY, Assmann SM (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56:984–996
Acknowledgements
We thank Dr. Dinesh Yadav for his help in initial cloning of RGG1 gene. Work on signal transduction and plant stress signalling in N.T.’s laboratory is partially supported by Department of Science and Technology (DST), Government of India. We thank Dr. Sharat Kumar Pradhan, principal scientist of ICAR-National Rice Research Institute, Cuttack for providing IR64 rice seeds. We thank Dr. Meerambika Mishra for critical review of the manuscript. We do not have any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gene Bank Accession Number of RGG1: GU111573.1; Locus: GU111573.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swain, D.M., Sahoo, R.K., Srivastava, V.K. et al. Function of heterotrimeric G-protein γ subunit RGG1 in providing salinity stress tolerance in rice by elevating detoxification of ROS. Planta 245, 367–383 (2017). https://doi.org/10.1007/s00425-016-2614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2614-3