Abstract
Main conclusion
Solanum tuberosum tropinone reductase I reduced tropinone in vivo. Suppression of tropinone reductase II strongly reduced calystegines in sprouts. Overexpression of putrescine N -methyltransferase did not alter calystegine accumulation.
Calystegines are hydroxylated alkaloids formed by the tropane alkaloid pathway. They accumulate in potato (Solanum tuberosum L., Solanaceae) roots and sprouting tubers. Calystegines inhibit various glycosidases in vitro due to their sugar-mimic structure, but functions of calystegines in plants are not understood. Enzymes participating in or competing with calystegine biosynthesis, including putrescine N-methyltransferase (PMT) and tropinone reductases (TRI and TRII), were altered in their activity in potato plants by RNA interference (RNAi) and by overexpression. The genetically altered potato plants were investigated for the accumulation of calystegines and for intermediates of their biosynthesis. An increase in N-methylputrescine provided by DsPMT expression was not sufficient to increase calystegine accumulation. Overexpression and gene knockdown of StTRI proved that S. tuberosum TRI is a functional tropinone reductase in vivo, but no influence on calystegine accumulation was observed. When StTRII expression was suppressed by RNAi, calystegine formation was severely compromised in the transformed plants. Under phytochamber and green house conditions, the StTRII RNAi plants did not show phenotypic alterations. Further investigation of calystegines function in potato plants under natural conditions is enabled by the calystegine deprived StTRII RNAi plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calystegines are nortropane alkaloids with two to five hydroxyl groups found in Convolvulaceae (Tepfer et al. 1988; Schimming et al. 1998), Solanaceae (Nash et al. 1993; Dräger et al. 1994; Asano et al. 1995; Bekkouche et al. 2001; Biastoff and Dräger 2007), Moraceae (Asano et al. 1994a, b; Kusano et al. 2002), Brassicaceae (Brock et al. 2006), and Erythroxylaceae (Brock et al. 2005). Calystegines are formed by the tropane alkaloid pathway (Fig. 1), including putrescine N-methyltransferase (PMT) and pseudotropine forming tropinone reductase (TRII). PMT produces N-methylputrescine, which is the first specific metabolite for tropane and nortropane alkaloids and for nicotine. TRII forms pseudotropine which, after N-demethylation, is the common precursor for the various calystegines. Tropine forming tropinone reductase (TRI) catalyzes a tropinone reduction competing with TRII. Tropine formation is essential in the course of the biosynthesis of the medicinal tropine alkaloids atropine and scopolamine that are not found in potato.
Similarities with monosaccharides are evident in the molecular structure of calystegines (Fig. 1). It is not surprising, therefore, that calystegines inhibit various glycosidases by occupying the substrate-binding site of the enzymes (Gloster et al. 2007). Many glycosidases catalyze glycosides cleavage by proton transfer from an acidic amino-acid residue onto the glycosidic oxygen. When calystegines are bound in the active site, the ring-bound nitrogen is protonated instead of the glycosidic oxygen, but without subsequent destabilisation (Borges de Melo et al. 2006; Gloster et al. 2006). Ring-bound nitrogen and several hydroxyl groups are common structural elements for many alkaloid-like glycosidase inhibitors, some of which are used as drugs against diabetes, e.g., miglitol (Campo et al. 2013). On the other hand, glycosidase inhibitors must be considered as potential toxins for mammals. If they are absorbed after ingestion, they can inhibit lysosomal glycosidases in human and animal cells. Sugar-mimic alkaloids, such as swainsonine and castanospermine, are plant-derived toxins for cattle, because they inhibit lysosomal glycosidases involved in glycoprotein processing (Elbein et al. 1981; Dibello et al. 1983; Saul et al. 1985) and cause symptoms similar to lysosomal storage diseases, such as bovine spongiform encephalitis (BSE) with vacuolated cells in different organs and neuronal damage (Molyneux et al. 1995; de Balogh et al. 1999). Armien and co-workers (Armien et al. 2007) reported an ataxia syndrome in cattle, whose occurrence was implicated with calystegine-containing Ipomoea species (Convolvulaceae) that grew in the area of poisoning events. The Ipomoea species that were investigated contain calystegines but also swainsonine, an alkaloidal glycosidase inhibitor with potent toxicity. Isolated calystegines were investigated for their toxicity in mice (Stegelmeier et al. 2008). After injection, they proved rather non-toxic on whole animals; however, histological examination revealed liver cell alterations with high doses (140 mg kg−1 day−1) of calystegine A3. Swainsonine (10 mg kg−1 day−1), in comparison, exhibited mouse toxicity macroscopically and in organ sections, although mice as rodents are less sensitive toward glycosidase inhibitor toxicity and demand higher doses than ruminants, such as sheep and goats.
Calystegines occur in many plants used for human nutrition, mostly in vegetables from Solanaceae. Eggplant, green peppers, potatoes, and physalis berries are examples (Asano et al. 1997). Potato tubers (Solanum tuberosum L.) are a major carbohydrate source consumed worldwide. Calystegines accumulate in young tissues, such as potato sprouts, but they were also reported in the edible parts of the vegetables that are ingested. Obviously, they are non-toxic in general, and rare intoxications observed with potato consumption were attributed to the steroidal alkaloids in potatoes after inappropriate storage, e.g., solanine and chaconine (Barceloux 2009). Calystegines are not destroyed by boiling potatoes or other vegetables; they are heat-resistant. Non-toxicity of calystegines in food possibly results from non-absorption in the intestine. In Caco-2 cell cultures widely used as model system for intestinal transport, calystegines remained on the luminal side representing the intestinal lumen (Jockovic et al. 2013). Calystegine B2, however, inhibited sucrase, one of the major intestinal enzymes responsible for carbohydrate digestion. Inhibition was visible in concentrations of calystegine B2 achievable by a regular serving of potato tubers, indicating that tubers may possibly retard carbohydrate digestion in the diet (Jockovic et al. 2013).
Bacterial, plant, and mammalian glycosidases are among those affected by calystegines (Asano et al. 2001). In spite of their clearly defined biochemical actions, functions for calystegines in plants are poorly understood. Calystegine accumulation in subterranean plant organs may attract microorganisms that are beneficial for plant rhizosphere, such as Rhizobia (Reitz et al. 2000; Unno et al. 2015). Rhizobium meliloti that utilizes calystegines as carbon and nitrogen source (Tepfer et al. 1988) grows preferably in the rhizosphere of calystegine-producing plants (Goldmann et al. 1996; Guntli et al. 1999). For potato, the organ-specific accumulation was investigated in detail. Highest calystegine accumulation was found in below-ground organs, those are roots and sprouting tubers (Keiner and Dräger 2000). A role in plant defence was assumed as for many glycosidase inhibitors; however, a proof was not provided up to now. Investigation on endophytic fungi in leaves of calystegines accumulating plants revealed no growth reduction of fungal biomass and no inhibition of fungal invertase by calystegines. Plant invertase, however, was inhibited by calystegine B2 (Höke and Dräger 2004). Different from defence, calystegines may be excreted into the soil by plant tissues and contribute to a rhizosphere beneficial for plant growth. Calystegines serve as carbon source for Rhizobium meliloti (Tepfer et al. 1988). Rhizobia were shown to increase in the rhizosphere of potato plants under macro-nutrient deficiency suggesting that by excreting compounds, potato plants may be able to create an altered rhizosphere and favour rhizobia growth (Unno et al. 2015).
As calystegines are compounds interesting for plant growth improvement and for human nutrition, it is important to investigate their role in potato growth and development. Gene expression of enzymes participating in or competing with calystegine biosynthesis was altered by RNA interference and by overexpression. The genetically altered potato plants were investigated regarding their accumulation of calystegines.
Materials and methods
Cloning of full-length and RNAi constructs, generation of transgenic plants
The coding regions of Solanum tuberosum TRI (StTRI, AJ307584) and Datura stramonium PMT (DsPMT, AJ583514) were amplified and cloned into pENTR/D-TOPO (Invitrogen). Full-length constructs were transferred via LR recombination (Invitrogen) to pGWB14 (Nakagawa et al. 2007). For cloning, the StTRI RNAi fragment primers (5′-GCTTATCATTTATCTCAAATTGC-3′ and 5′-CACCAGCTATTACTGCTGAAGCTTCTTC3′) and the StTRII RNAi fragment primers (5′-GCTTACCACTTATCTGTACTTGC-3′ and 5′-CACCTGCAAC AACTGCTGCAAGTTC3′) were used to amplify fragments from full-length genes S. tuberosum TRI and TRII (AJ292343). RNAi fragments of StTRI and StTRII were cloned into pENTR/D-TOPO (Invitrogen) and transferred to pHELLSGATE8 via LR recombination (Wesley et al. 2001). Leaves of potato plants (Solanum tuberosum cv Désirée) were transformed with Agrobacterium tumefaciens AGL0 (Lazo et al. 1991), containing the vectors with the constructs. Transgenic plants were regenerated from transformed and control potato leaves as described (Rocha-Sosa et al. 1989; Feltkamp et al. 1995). Potato cultivar Désirée was kindly provided by E. Tacke, Bioplant, Ebstorf, Germany.
Plant cultivation and feeding experiments
Sterile potato plants (Solanum tuberosum cv Désirée) were grown in tissue culture in a phytochamber for 3 weeks under long-day conditions (16 h light, 140 μmol photons) at 22 °C. The plants were transferred into sterilized soil and grown in a phytochamber for 3–4 weeks under long-day conditions (16 h light, 140 μmol photons) at 18–20 °C and 70% relative humidity. For tropinone feeding, three mature leaves of a plant were cut off and put into a glass of 20 ml tropinone (5 mM, buffered with HCl to pH 7.4). Control leaves were placed in 20 ml water. Tropinone, 99% pure, (Sigma-Aldrich) did not contain tropine or pseudotropine (Keiner and Dräger 2000). When the liquids in the glass decreased, tropinone solution or water was refilled. Tropinone application was completed after 8 days in the phytochamber. The leaf stems (petioles) were cut off and discarded to reduce tropinone content in the samples, as a very high tropinone content interferes with the separation of tropinone, tropine, and pseudotropine by GC–MS. The leaf base was rinsed with water to remove residual outer tropinone and the whole leaf was frozen in liquid nitrogen for tropine and pseudotropine analysis.
Assessment of plant phenotypes
Plant phenotypes were monitored after sterile potato plants from tissue culture were transferred into soil in 110 mm diameter pots. Plant growth, colour, and shape of the leaves were monitored for the whole life cycle of the plant. The formation and shape of flowers were judged after 3- to 4-week-old plants were transferred into large pots (320 mm diameter) in the greenhouse. For tuber formation, 3- to 4-week-old plants were grown in the greenhouse and tubers were formed after further 8 weeks. Tubers were harvested and judged by weight, size, number, and shape. Representative samples of tubers were stored at 7 °C in the dark until sprouting initiated after 5 months. Equivalent tuber samples were stored at 7 °C for 4 weeks and transferred to room temperature until sprouting initiated after another 2 months. Tuber sprouts (5–10 mm) were clipped off the tubers each week for 4 months and collected for analysis of gene expression and calystegines.
RNA isolation and cDNA synthesis
Total RNA was isolated from potato leaves, roots, and tuber sprouts using the Trizol method (Chomczynski and Sacchi 1987). Samples containing 2 μg total RNA were treated with 1 U DNAse (RNase-Free DNase Set, Qiagen) to avoid DNA contaminations. After 30 min incubation at 37 °C, 1 μl 25 mM EDTA was added and samples were heated to 65 °C for 10 min. The total RNA was transcribed into cDNA (RevertAid H Minus First Strand cDNA Synthesis Kit, Thermo Scientific) according to the manufacturers’ instructions and diluted 1:10 with water.
Expression analysis (Northern blot, qRT-PCR)
Total RNA was isolated as described above. 20 μg of total RNA were separated on a formaldehyde gel and blotted to a positively charged nylon membrane (Roche). cDNA full-length fragments of StTRI (795 bp) and DsPMT (1135 bp) were labelled with α-32P-dATP using the Megaprime DNA labelling system (Amersham Pharmacia Biotechnology). For hybridization, membrane and labelled probe were incubated in hybridization buffer (0.9 M NaCl, 50 mM NaH2PO4, 5 mM EDTA, 0.1% SDS, 0.1% PVP, 0.1% Ficoll, 0.1% BSA, 50% (v/v) formamide, 100 μg/ml denatured salmon sperm DNA) for 24 h at 42 °C. The membrane was washed in 50 ml 3× SSC, 0.5% (v/v) SDS at 65 °C three times.
Quantitative PCR was measured on an Mx3005P qPCR system (Agilent). Reactions were performed in triplicates using the Maxima Probe qPCR Master Mix (Thermo Scientific) and Roche Universal Probe Library, Probe #33 for amplification of StTRI (primers 5′-CTGAAGAAGTTTCAGCAGTAA-3′ and 5′-GCCCATATAATTTGGCCTGTAA-3′), Probe #45 for StTRII (primers 5′-CAAAGGGGCAATGTTGTCTT-3′ and 5′-TTTGGTTGCTCCATAAACAGC-3′), and Probe #162 for the endogenous elongation factor StEF1α (primers 5-CACTGCCCAGGTCATCATC-3′ and 5′-GTCGAGCACTGGTGCATATC-3′). Relative transcript levels were calculated using the MxPro qPCR software (Agilent).
Polyamine analysis
Putrescine, N-methylputrescine, spermidine, and spermine were extracted from potato leaves. Plant material was homogenized and extracted with 1.5 ml of 5% (v/v) perchloric acid and incubated at 4 °C for 2 h in the dark. After centrifugation, the supernatant was used to determine free, soluble polyamines. 100 μl supernatant were derivatized with dansyl chloride by a modified method of Flores and Galston (1982). The extract was mixed with 100 μl saturated sodium carbonate and 400 μl dansyl chloride (7.5 mg/ml in acetone). The mixture was shaken in the dark at 60 °C for 60 min. After adding 100 μl proline (1 mg/ml) and incubation for 30 min in the dark, the dansylated polyamines were extracted in 500 μl toluol triply. The extracted organic phases were evaporated and resuspended in acetonitrile. For HPLC separation (LaChrom, Merck), a LiChrocart reverse phase C18 column, 5 μm particle size, 250 mm length, and 4 mm diameter (Merck) were used with an acetonitrile/water gradient from 10 to 90% at 40 °C and a flow rate of 1 ml/min. Detection was performed concurrently by a fluorescence detector (365 nm extinction, 510 nm emission) and diode array detector (220 nm). Dansylated putrescine, N-methylputrescine, spermidine, and spermine with retention times of 6.4, 8.0, 10.4, and 14.6 min were quantified by fluorescence detection. The limit of quantification was 5 ng/ml for each polyamine. N-methylputrescine was provided by T. Hashimoto, Nara Institute of Science and Technology, Nara, Japan. Putrescine, spermidine, and spermine were purchased from Sigma-Aldrich.
Tropinone, tropine, and pseudotropine analysis
Tropinone, tropine, and pseudotropine were extracted from potato leaves. Homogenized plant material was extracted twice with distilled water by shaking for 1 min and incubation in an ultrasonic bath for 10 min. After centrifugation, the supernatant was concentrated to 1 ml, mixed with 100 μl 25% NH3, and centrifuged. The extract was applied on a 1 g Extrelut® NT (Merck) column. After an exposure of up to 20 min, elution was performed by 2 × 3 ml chloroform and 3 ml of chloroform/methanol 85:15 (v/v). The eluate was dried by sodium sulphate, evaporated, and resuspended by ethyl acetate. The ethyl acetate samples were separated and quantified on a GC–MS 2010 system (Shimadzu) equipped with a FS-Supreme-5 ms column, 0.1 μm film thickness, (5% phenyl polysilphenylene-siloxane), 300 mm length, and 0.25 mm inner diameter (Chromatographie Service) at a constant flow rate of 1 ml/min helium. One μl sample was injected splitless at 110 °C and passed the temperature program: 60 °C (1 min), 10 °C/min up to 180 °C, 180 °C (1 min), 15 °C/min to 300 °C, and 300 °C (2 min). The mass spectrometer (QP2010S, Shimadzu) was set to the parameters: transfer line temperature 250 °C, ion source temperature 200 °C, EI ionisation 70 eV, and detector voltage 1.2 kV. Two scans per second were taken in the range of 50–350 amu. Retention times were 6.9, 7.1, and 7.3 min for tropinone, tropine (Sigma-Aldrich), and pseudotropine, respectively (synthesized—as described in Nickon and Fieser 1952). Quantification limits were 1 μg/ml for tropinone and 5 μg/ml for tropine and pseudotropine.
Calystegine analysis
Potato sprout tissue was homogenized an extracted twice with 5 ml methanol/water 1:1 (v/v) by shaking and sonication for 10 min. After centrifugation, calystegine samples were purified and concentrated by a cation exchange column (Chromabond® SA, 6 ml/1000 mg, Macherey–Nagel). Solvent flow rate was set to 1 ml/min. After column conditioning with 5 ml methanol/water 1:1 (v/v), the sample was loaded and the column was washed with 10 ml water. Elution was performed with 4 ml 2 N ammonia and 1 ml water. The eluate was freeze-dried and silylated by an adapted procedure of Fleet et al. (1990). Standards and samples were dissolved in 30 μl anhydrous pyridine, mixed with 40 μl hexamethyl disalazane and 10 μl trimethyl chlorsilane, and incubated at 65 °C for 20 min. n-Hexane and azobenzol as internal standard were added to a final concentration of 200 ng/μl. GC–MS analysis (instrument and column as described in tropine and pseudotropine analysis) was performed with the temperature program: 50 °C (2.5 min), 20 °C/min to 160 °C, 160 °C (2 min), 5 °C/min to 240 °C, 240 °C (2 min), 10 °C/min to 330 °C, and 330 °C (2 min). Mass spectra in the EI mode with two scans per second were taken from 50 to 500 amu. Retention times were 11.0, 12.3, and 14.4 min for silylated calystegines A3, B4, and B2. For calystegine quantification, castanospermine (Tocris Bioscience, Bristol, UK) was used as reference standard. The quantification limit was 10 μg/ml sample. Identity of calystegines was confirmed by comparison to calystegine reference standards, provided by N. Asano, Tohuku University, Kanazawa, Japan (Dräger 1995; Molyneux et al. 1996).
Statistical analysis
Experimental repetitions are indicated in the legends of each graph. Average values and standard deviations or standard deviations of the mean were calculated by and indicated for each experiment specifically.
Results
DsPMT expressing potato plants
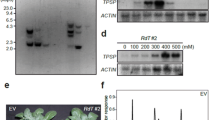
Putrescine N-methyltransferase from Datura stramonium catalyzes the methylation of putrescine (Teuber et al. 2007). To assess the effect of increased levels of N-methylputrescine levels in potato, the coding region of DsPMT was cloned behind the 35S promoter in the binary vector pGWB14 (Nakagawa et al. 2007). Potato plants were transformed via Agrobacterium tumefaciens-mediated leaf disk transformation. The phenotypes of all regenerated plants did not differ from those of control plants (data not shown). Varying levels of DsPMT transcripts were detected in leaves of transgenic potato plants by Northern blotting (Suppl. Fig. S1). In control and empty-vector-transformed plant leaves, PMT transcripts were not detected. Five plant lines were selected which showed strongest DsPMT expression. In these plants, the soluble fraction of N-methylputrescine was analyzed, as well as the polyamines spermidine, spermine, and putrescine, the latter being the precursor of both, higher polyamines and of tropane alkaloids. N-methylputrescine was hardly detectable in non-transformed and empty vector control plants (Fig. 2a). This is in accordance with a screening for N-methylputrescine in potato organs that revealed the metabolite only in spouts of potato tubers (Stenzel et al. 2006). Leaves of DsPMT expressing plants contained N-methylputrescine up to 8 µg/g fresh weight (Fig. 2a). Yet, the considerable flux of putrescine into N-methylputrescine in DsPMT expressing plants did not impair the accumulation of putrescine and that of the derived spermidine and spermine (Fig. 2b–d). Possibly, the levels of polyamines are kept constant by anapleurotic reactions confirming the notion that these amines have vital regulatory functions in plants (Tiburcio et al. 2014). In three independent experiments, the levels of the tropane alkaloid precursors tropinone, tropine, and pseudotropine, which derive from N-methylputrescine, were at the detection limit in both DsPMT expressing and control plants (data not shown), suggesting that enhancing N-methylputrescine is not sufficient for enabling potato leaves to produce more tropane alkaloid precursors. Tropinone, tropine, and pseudotropine levels as metabolites, however, are mostly low in potato tissues, irrespective whether calystegines are accumulated (Richter et al. 2007).
Leaves of DsPMT overexpressing plants (DsPMT, lines B–I) contained higher levels of the tropane alkaloid precursor N-methylputrescine (NMP) (a), but non-altered levels of polyamines putrescine, spermidine, and spermine (b–d). NMP and polyamines are given as free, soluble compounds. WT wildtype, EV empty vector; B–I selected transformants overexpressing DsPMT. FW fresh weight. Error bars represent standard deviation. Asterisks indicate significant difference using Student’s t test ***P ≤ 0.001, n = 26 (WT, EV), n = 10 (DsPMT overexpressing lines), two independent experiments
Overexpression and RNAi of StTRI
To analyze the function of StTRI, transgenic plants with reduced and enhanced expression of StTRI were generated. Overexpression of StTRI under the control of the 35S promoter was demonstrated by Northern blotting, and four lines with strong transcript signals were selected for further investigation (Suppl. Fig. S2). The effect of expression of an RNAi construct targeted against StTRI was quantified by qRT-PCR in leaves. Four lines with strongly suppressed StTRI expression were selected (Fig. 3). A possible effect of StTRI down-regulation on the expression of StTRII was checked by qRT-PCR in potato sprouts and in roots, as these are the tissues of strongest StTRII expression (Keiner et al. 2002). No alteration of StTRII expression in StTRI RNAi plants was detectable in these tissues (Suppl. Fig. S3).
Transcript levels of StTRI in leaves of StTRI RNAi lines (a–g) and control plants (WT wildtype, EV empty vector). RNA was isolated from leaves of 4-week-old potato plants, reverse transcribed, and analyzed for StTRI transcripts by quantitative PCR. StEF1α expression was used as a reference. Error bars represent standard error of the mean. Asterisks indicate statistically different values compared with WT and EV by Student’s t test, **P ≤ 0.01; ***P ≤ 0.001; n = 22 (WT, EV), n = 12 (StTRI RNAi lines). Data are derived from two independent experiments
Potato plants with suppressed and increased StTRI expression were used to explore the activity of StTRI in vivo. Tropinone was applied to leaves of the transformed plant lines and both reduction products, tropine and pseudotropine, were monitored. When no external substrate was applied, tropinone concentration in potato tissues was very low, and reduction products of potentially elevated enzyme activity did not accumulate. Upon exogenous tropinone application, the formation of tropine increased in StTRI overexpressing leaves, suggesting that StTRI protein was synthesized in the tissues and that in vivo StTRI is an active tropine-forming tropinone reductase (Fig. 4a). Correspondingly, in StTRI RNAi leaves, only traces of tropine were detected after tropinone feeding. Levels of tropinone (visible in the gas chromatograms) also varied somewhat from leaf to leaf, and the reduction product levels thus may reflect not only the enzyme activity in situ, but also the transport and local availability of tropinone. All tropinone-fed leaves contained higher concentrations of pseudotropine than of tropine (Fig. 4b). Compared with hardly detectable tropine and pseudotropine levels in untreated leaves (data not shown), tropinone feeding strongly increased pseudotropine levels in control and transformed leaves. Together with the tropine levels, the results show that StTRI is a functional tropine-forming enzyme in vivo, but the pseudotropine-forming StTRII is the stronger tropinone reductase in potato leaves producing more products.
Tropine (a) and pseudotropine (b) levels after feeding leaves of 4-week-old potato plants with 5 mM tropinone for 8 days. Control leaves were from wildtype (WT) and empty vector-transformed (EV) plants. Transformed plants were suppressed for StTRI expression (StTRI RNAi, lines a–g) or overexpressed StTRI (StTRI OE, lines O–Z). FW fresh weight. Error bars represent standard deviation, n = 24 (WT, EV), n = 12 (StTRI RNAi and StTRI OE lines). Asterisks indicate statistically different values compared with WT and EV by Student’s t test, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; n = 22 (WT, EV), n = 12 (StTRI RNAi lines). Data are derived from two independent experiments
Knockdown of StTRII
To elucidate StTRII function in vivo, StTRII expression was suppressed by RNAi. Transgenic potato plants expressing an RNAi construct targeted against StTRII were analyzed for alterations in StTRII expression in roots and sprouts by qRT-PCR. All lines showed a significant reduction in StTRII transcript levels; however, in sprouts of lines l and n, transcripts levels were reduced only threefold (Fig. 5).
Reduced transcript levels of StTRII in roots and sprouts of StTRII RNAi lines (j–n) compared with control plants (WT wildtype; EV empty vector). RNA was isolated from leaves of 4-week-old potato plants, reverse transcribed and analyzed for StTRII transcripts by quantitative PCR. StEF1α expression was used as a reference. Error bars represent standard deviation. Asterisks indicate statistically different values compared with WT and EV by Student’s t test, ***P ≤ 0.001; n = 20 (WT, EV), n = 8 (StTRII RNAi lines). Data are derived from two independent experiments
To test for reduced TRII enzyme activity in tissues, potato leaves were supplemented with tropinone and both reduction products, tropine and pseudotropine, were measured. While tropine levels were not different among StTRII RNAi and control lines, pseudotropine increased strongly in control lines but remained below tropine levels in the StTRII-RNAi lines (Fig. 6). Thus, reducing the expression of StTRII correlated with a strongly reduced ability to synthesize pseudotropine from exogenously applied tropinone. These results suggest that, in vivo, StTRII is the main enzyme catalyzing the synthesis of pseudotropine from tropinone.
Tropine and pseudotropine levels after feeding leaves of 4-week-old potato plants with 5 mM tropinone for 8 days. Control leaves were from wildtype (WT) and empty vector-transformed (EV) plants. Transformed plants were suppressed for StTRII expression (StTRII RNAi, lines j–n). FW fresh weight. Error bars represent standard deviation, n = 10 (WT, EV), n = 5 (StTRII RNAi). Asterisks indicate statistically different values compared with WT and EV by Student’s t test, ***P ≤ 0.001. Data are derived from two independent experiments
Phenotypes and calystegine levels
For all transgenic lines, plant phenotypes, growth, and development, and the accumulation of calystegines in potato sprouts were monitored. Potato plants regenerated from transformed calli did not differ in phenotype or growth and development from wild-type plants and from plants transformed with empty vector. All plants formed tubers after 8 weeks of cultivation. Potato tubers from all transgenic lines were compared. Differences in tuber size, shape, or number between control and transgenic plants were not observed. After 5 months of tuber dormancy at 7 °C or after 4 weeks at 7 °C and 2 months tuber storage at room temperature, all tubers began to sprout simultaneously.
Tuber sprouts were investigated for their calystegine accumulation. The major calystegines in tubers of the cultivar Désirée used in this study were calystegines B2, A3, and, at lower levels, calystegine B4 (Fig. 1). This is in accordance with the pattern of calystegines reported from other potato cultivars (Keiner and Dräger 2000; Friedman et al. 2003). Levels of calystegines varied considerably between transformed lines (Fig. 7). Sprouts from wild-type plants and from those transformed with empty vector accumulated up to 3 mg total calystegines per gram FW, corresponding to earlier findings in other cultivars (Keiner and Dräger 2000). Transformed lines expressing DsPMT did not show significantly higher calystegine concentrations. N-methylputrescine is essential for the biosynthesis of tropinone (Hashimoto et al. 1989), and tropinone is incorporated into calystegines (Scholl et al. 2001); however, in potato, N-methylputrescine levels in leaves do not appear to be rate-limiting for calystegine accumulation in sprouts.
Calystegine levels in sprouts of DsPMT and StTRI overexpressing lines (DsPMT OE, combined lines B, C, D, H, I as in Fig. 2; StTRI OE, combined lines O, Q, R, Z as in Fig. 4), StTRI RNAi and StTRII RNAi lines (StTRI RNAi, combined lines a, d, f, g as in Fig. 4; StTRII RNAi, combined lines j, k, l, m, n as in Fig. 5), control plants from wildtype (WT) and empty vector (EV). FW fresh weight. Error bars represent standard deviation, n ≥ 20 (control plants, DsPMT OE, StTRI OE, StTRI RNAi), n ≥ 12 (StTRII RNAi). Asterisks indicate statistical difference of the sum of all three calystegines B2, A3, B4 between WT/EV and the transgenic lines by Kruksal–Wallis test **P ≤ 0.01; ***P ≤ 0.001. Data are derived from two to three independent experiments per construct
When StTRI gene expression was suppressed, the effect on calystegine accumulation was not prominent. Higher availability of tropinone, when StTRI gene expression is reduced, and enhanced formation of pseudotropine, that may result in a higher calystegine accumulation, were not evident (Fig. 7). Inversely, it was tested whether ectopic and overexpression of StTRI might reduce tropinone availability to the extent that the flux through pseudotropine was reduced and resulted in lower calystegine levels. However, only a slight reduction in calystegine levels was observed.
A strong reduction of calystegines, however, appeared in the StTRII RNAi lines j, k, and m (Fig. 7). This suggests that endogenous pseudotropine levels were reduced in these StTRII RNAi lines not only in detached leaves (Fig. 6), but also in sprouts. Lines l and n accumulated calystegines; in line l, calystegine accumulation was comparable to wildtype (Fig. 7). This correlates with higher StTRII transcript levels, compared with the other RNAi lines, in sprouts (Fig. 5). Taken together, the reduction of StTRII expression correlates with a decreased ability to reduce tropinone to pseudotropine and to synthesize calystegines. These results strongly argue for a role of StTRII in calystegine formation in potato sprouts.
Discussion
N-Methylputrescine is the first specific metabolite for calystegine formation (Biastoff et al. 2009), but an increase of N-methylputrescine in leaves provided by PMT expression was not sufficient to increase calystegine accumulation in potato sprouts. This is different from tobacco, where increased PMT activity augmented nicotine content (Sato et al. 2001). In Atropa belladonna, a tropane alkaloid accumulating plant, PMT overexpression did not result in increased atropine or scopolamine formation, and calystegine levels equally were not enhanced (Rothe et al. 2003). Obviously, further steps downstream of N-methylputrescine in the subsequent biosynthetic pathway are rate-limiting for tropane alkaloid accumulation.
StTRI overexpressing plants proved that StTRI is a functional tropinone reductase in vivo, but its role in potato metabolism is not clear. Tropine does not normally accumulate in potato tissues, possibly due to high StTRII activity that prevents tropinone accumulation and tropinone availability for StTRI. The two enzymes are synthesized in different tissues (Kaiser et al. 2006) and may have different access to tropinone. StTRs, like many short chain dehydrogenases and ketone reductases (Oppermann et al. 2003), accept a range of substrates (Keiner et al. 2002; Kaiser et al. 2006). It remains a question, under which metabolic or stress conditions StTRI will exert tropinone reducing activity in potato tissues. Irrespective whether StTRI was genetically enhanced or repressed, it did not interfere with tropinone reduction by StTRII.
Decrease of StTRII expression reduced pseudotropine accumulation and calystegine levels considerably, but not all StTRII RNAi lines were equally affected. It is evident from feeding tropinone to wildtype and transformed potato leaves that StTRII is a very active enzyme that is not rate-limiting for calystegine formation. Our results suggest that only if TRII activity is suppressed below a certain threshold, pseudotropine levels become limiting and calystegine accumulation is severely decreased.
The transgenic potato lines provided in this study with various levels of calystegines did not reveal differences in sprouting time or intensity. Potato tuber sprouting demands carbohydrate relocation, in particular degradation of starch and formation of sucrose for translocation into the sprouts (Farre et al. 2001; Hajirezaei et al. 2003). These processes might be responsive to calystegine-induced glycosidase inhibition, but transgenic tubers obtained in this study did not differ phenotypically when storage and sprouting were observed under controlled conditions (Suppl. Fig. S4). It must be considered, however, that tuber sprouting in field agriculture is influenced by many parameters, which are not encountered by isolated tubers that are maintained in a controlled atmosphere.
Sprouting tubers in the soil are subjected to a number of infections and pests. Rhizoctonia solani, for example, is a soil-borne fungus that attacks tuber sprouts (Lahlali and Hijri 2010) and also roots and stolons of potato plants by forming pores on the subterranean organ surface (Chamoun et al. 2014). The density and virulence of Rhizoctonia solani are influenced by the plant rhizosphere. Soil microorganisms able to reduce Rhizoctonia growth, such as Bacterium subtilis, are, therefore, used as biocontrol agents against Rhizoctonia infections, and furthermore, beneficial microorganisms for potato cultivation are intensively searched for (Lahlali and Hijri 2010; Ghyselinck et al. 2013). Among the microorganisms that induce a beneficial rhizosphere for potato plants are Rhizobia (Reitz et al. 2000; Unno et al. 2015), which catabolize calystegines (Tepfer et al. 1988). It is hypothesized that calystegines are important metabolites for potato plant health in the field, for example by inhibiting growth of pathogenic microorganisms, such as Rhizoctonia. The generation of transgenic potato plants with reduced StTRII expression thus should permit functional analyses of calystegines with regard to sprout development, pest resistance, and stress tolerance.
Greenhouse cultivation of transgenic potato plants may permit to compare the rhizosphere microbiome in pots with StTRII RNAi and wild-type plants. Addition of growth-supportive and pathogenic microorganisms to the pots and observation of their development in the presence and absence of calystegine-producing potato plants may support the assumption of a function of calystegines in the rhizosphere. Potato sprouts and potato roots contain steroidal glycoalkaloids (Friedman and Dao 1992; Asano et al. 1996; Friedman et al. 2003), which may also be present in the rhizosphere of potato plants. It will be interesting to investigate whether these alkaloids with membrane penetrating properties influence the microbiome and whether they interact with calystegines. Subsequent experiments ideally should involve governmentally approved field trials to judge the performance of calystegine-free plants under highly diverse conditions. Exposure to potential pathogens and predators and the presence of a more natural and potentially highly complex rhizosphere may provide further hints for the function of calystegines in potato plants.
Author contribution statement
BD and SR conceived and designed research. NK designed and conducted experiments and analyzed data. NK contributed new analytical tools. NK and BD wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- Ds:
-
Datura stramonium
- PMT:
-
Putrescine N-methyltransferase
- RNAi:
-
RNA interference
- St:
-
Solanum tuberosum
- TRI (II):
-
Tropinone reductase I (II)
References
Armien AG, Tokarnia CH, Peixoto PV et al (2007) Spontaneous and experimental glycoprotein storage disease of goats induced by Ipomoea carnea subsp fistulosa (Convolvulaceae). Vet Pathol 44:170–184. doi:10.1354/vp.44-2-170
Asano N, Oseki K, Tomioka E et al (1994a) N-containing sugars from Morus alba and their glycosidase inhibitory activities. Carbohydr Res 259:243–255
Asano N, Tomioka E, Kizu H et al (1994b) Sugars with nitrogen in the ring isolated from the leaves of Morus bombycis. Carbohydr Res 253:235–245
Asano N, Kato A, Oseki K et al (1995) Calystegins of Physalis alkekengi var. Francheti (Solanaceae). Structure determination and their glycosidase inhibitory activities. Eur J Biochem 229:369–376
Asano M, Goto N, Isshiki K (1996) Simple and rapid analysis of potato glycoalkaloids. Nippon Shokuhin Kogaku Kaishi 43:593–597. doi:10.3136/nskkk.43.593
Asano N, Kato A, Matsui K et al (1997) The effects of calystegines isolated from edible fruits and vegetables on mammalian liver glycosidases. Glycobiology 7:1085–1088
Asano N, Yokoyama K, Sakurai M et al (2001) Dihydroxynortropane alkaloids from calystegine-producing plants. Phytochemistry 57:721–726. doi:10.1016/S0031-9422(01)00131-5
Barceloux DG (2009) Potatoes, tomatoes, and solanine toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis Mon 55:391–402. doi:10.1016/j.disamonth.2009.03.009
Bekkouche K, Daali Y, Cherkaoui S et al (2001) Calystegine distribution in some solanaceous species. Phytochemistry 58:455–462. doi:10.1016/S0031-9422(01)00283-7
Biastoff S, Dräger B (2007) Calystegines. In: Cordell GA (ed) The alkaloids: chemistry and biology, vol 64, pp 49–102. doi:10.1016/S1099-4831(07)64002-4
Biastoff S, Brandt W, Dräger B (2009) Putrescine N-methyltransferase—the start for alkaloids. Phytochemistry 70:1708–1718. doi:10.1016/j.phytochem.2009.06.012
Borges de Melo E, da Silveira Gomes A, Carvalho I (2006) α- and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 62:10277–10302. doi:10.1016/j.tet.2006.08.055
Brock A, Bieri S, Christen P et al (2005) Calystegines in wild and cultivated Erythroxylum species. Phytochemistry 66:1231–1240. doi:10.1016/j.phytochem.2005.04.017
Brock A, Herzfeld T, Paschke R et al (2006) Brassicaceae contain nortropane alkaloids. Phytochemistry 67:2050–2057. doi:10.1016/j.phytochem.2006.06.024
Campo VL, Aragao-Leoneti V, Carvalho I (2013) Glycosidases and diabetes: metabolic changes, mode of action and therapeutic perspectives. In: Rauter AP, Lindhorst T (eds) Carbohydrate Chemistry, vol 39, pp 181–203. doi:10.1039/9781849737173-00181
Chamoun R, Samsatly J, Pakala SB et al (2014) Suppression subtractive hybridization and comparative expression of a pore-forming toxin and glycosyl hydrolase genes in Rhizoctonia solani during potato sprout infection. Mol Genet Genomics. doi:10.1007/s00438-014-0962-x
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi:10.1016/0003-2697(87)90021-2
de Balogh KK, Dimande AP, van der Lugt JJ et al (1999) A lysosomal storage disease induced by Ipomoea carnea in goats in Mozambique. J Vet Diagn Invest 11:266–273
Dibello IC, Dorling P, Winchester B (1983) The storage products in genetic and Swainsonine-induced human mannosidosis. Biochem J 215:693–696
Dräger B (1995) Identification and quantification of calystegines, polyhydroxyl nortropane alkaloids. Phytochem Analysis 6:31–37. doi:10.1002/pca.2800060105
Dräger B, Funck C, Höhler A et al (1994) Calystegines as a new group of tropane alkaloids in Solanaceae. Plant Cell Tiss Org 38:235–240. doi:10.1007/BF00033882
Elbein AD, Solf R, Dorling PR et al (1981) Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci USA 78:7393–7397. doi:10.1073/Pnas.78.12.7393
Farre EM, Bachmann A, Willmitzer L et al (2001) Acceleration of potato tuber sprouting by the expression of a bacterial pyrophosphatase. Nat Biotechnol 19:268–272. doi:10.1038/85726
Feltkamp D, Baumann E, Schmalenbach W et al (1995) Expression of the mannopine synthase promoter in roots is dependent on the mas elements and correlates with high transcript levels of mas-binding factor. Plant Sci 109:57–65. doi:10.1016/0168-9452(95)04154-M
Fleet GW, Fellows LE, Winchester B (1990) Plagiarizing plants: amino sugars as a class of glycosidase inhibitors. In: Wiley J (ed) Bioactive compounds from plants. Ciba F Symp 154:112–125
Flores HE, Galston AW (1982) Analysis of polyamines in higher-plants by high-performance liquid-chromatography. Plant Physiol 69:701–706. doi:10.1104/Pp.69.3.701
Friedman M, Dao L (1992) Distribution of glycoalkaloids in potato plants and commercial potato products. J Agr Food Chem 40:419–423. doi:10.1021/jf00015a011
Friedman M, Roitman JN, Kozukue N (2003) Glycoalkaloid and calystegine contents of eight potato cultivars. J Agr Food Chem 51:2964–2973. doi:10.1021/jf021146f
Ghyselinck J, Velivelli SL, Heylen K et al (2013) Bioprospecting in potato fields in the Central Andean Highlands: screening of rhizobacteria for plant growth-promoting properties. Syst Appl Microbiol 36:116–127. doi:10.1016/j.syapm.2012.11.007
Gloster TM, Madsen R, Davies GJ (2006) Dissection of conformationally restricted inhibitors binding to a β-glucosidase. ChemBioChem 7:738–742. doi:10.1002/cbic.200600005
Gloster TM, Madsen R, Davies GJ (2007) Structural basis for cyclophellitol inhibition of a beta-glucosidase. Org Biomol Chem 5:444–446. doi:10.1039/b616590g
Goldmann A, Message B, Tepfer D et al (1996) Biological activities of the nortropane alkaloid, aalystegine B2, and analogs: structure–function relationships. J Nat Prod 59:1137–1142. doi:10.1021/np960409v
Guntli D, Heeb M, Moenne-Loccoz Y et al (1999) Contribution of calystegine catabolic plasmid to competitive colonization of the rhizosphere of calystegine-producing plants by Sinorhizobium meliloti Rm41. Mol Ecol 8:855–863. doi:10.1046/J.1365-294x.1999.00640.X
Hajirezaei MR, Bornke F, Peisker M et al (2003) Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). J Exp Bot 54:477–488. doi:10.1093/Jxb/Erg040
Hashimoto T, Yukimune Y, Yamada Y (1989) Putrescine and putrescine N-methyltransferase in the biosynthesis of tropane alkaloids in cultured roots of Hyoscyamus albus: II. Incorporation of labeled precursors. Planta 178:131–137. doi:10.1007/bf00392536
Höke D, Dräger B (2004) Calystegines in Calystegia sepium do not inhibit fungal growth and invertase activity but interact with plant invertase. Plant Biol 6:206–213. doi:10.1055/s-2004-817797
Jockovic N, Fischer W, Brandsch M et al (2013) Inhibition of human intestinal alpha-glucosidases by calystegines. J Agr Food Chem 61:5550–5557. doi:10.1021/jf4010737
Kaiser H, Richter U, Keiner R et al (2006) Immunolocalisation of two tropinone reductases in potato (Solanum tuberosum L.) root, stolon, and tuber sprouts. Planta 225:127–137. doi:10.1007/s00425-006-0335-8
Keiner R, Dräger B (2000) Calystegine distribution in potato (Solanum tuberosum) tubers and plants. Plant Sci 150:171–179. doi:10.1016/S0168-9452(99)00184-3
Keiner R, Kaiser H, Nakajima K et al (2002) Molecular cloning, expression and characterization of tropinone reductase II, an enzyme of the SDR family in Solanum tuberosum (L.). Plant Mol Biol 48:299–308
Kusano G, Orihara S, Tsukamoto D et al (2002) Five new nortropane alkaloids and six new amino acids from the fruit of Morus alba LINNE growing in Turkey. Chem Pharm Bull 50:185–192. doi:10.1248/Cpb.50.185
Lahlali R, Hijri M (2010) Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiol Lett 311:152–159. doi:10.1111/J.1574-6968.2010.02084.X
Lazo GR, Stein PA, Ludwig RA (1991) A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Nat Biotechnol 9(10):963–967
Molyneux RJ, McKenzie RA, O’Sullivan BM et al (1995) Identification of the glycosidase inhibitors swainsonine and calystegine B2 in Weir vine (Ipomoea sp. Q6 [aff. calobra]) and correlation with toxicity. J Nat Prod 58:878–886
Molyneux RJ, Nash RJ, Asano N (1996) The chemistry and biological activity of calystegines and related nortropane alkaloids. In: Pelletier SW (ed) Alkaloids: chemical and biological perspectives, vol 11, pp 303–343. doi:10.1016/S0735-8210(96)80008-2
Nakagawa T, Kurose T, Hino T et al (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104:34–41. doi:10.1263/jbb.104.34
Nash RJ, Rothschild M, Porter EA et al (1993) Calystegines in Solanum and Datura species and the deaths-head hawk-moth (Acherontia-Atropus). Phytochemistry 34:1281–1283. doi:10.1016/0031-9422(91)80016-T
Nickon A, Fieser LF (1952) Configuration of tropine and pseudotropine. J Am Chem Soc 74:5566–5570. doi:10.1021/ja01142a005
Oppermann U, Filling C, Hult M et al (2003) Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chem-Biol Interact 143–144:247–253. doi:10.1016/S0009-2797(02)00164-3
Reitz M, Rudolph K, Schroder I et al (2000) Lipopolysaccharides of Rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Appl Environ Microb 66:3515–3518. doi:10.1128/Aem.66.8.3515-3518.2000
Richter U, Sonnewald U, Dräger B (2007) Calystegines in potatoes with genetically engineered carbohydrate metabolism. J Exp Bot 58:1603–1615. doi:10.1093/jxb/erl295
Rocha-Sosa M, Sonnewald U, Frommer W et al (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8:23–29
Rothe G, Hachiya A, Yamada Y et al (2003) Alkaloids in plants and root cultures of Atropa belladonna overexpressing putrescine N-methyltransferase. J Exp Bot 54:2065–2070. doi:10.1093/jxb/erg227
Sato F, Hashimoto T, Hachiya A et al (2001) Metabolic engineering of plant alkaloid biosynthesis. P Natl Acad Sci USA 98:367–372. doi:10.1073/pnas.98.1.367
Saul R, Ghidoni JJ, Molyneux RJ et al (1985) Castanospermine inhibits alpha-glucosidase activities and alters glycogen distribution in animals. P Natl Acad Sci USA 82:93–97
Schimming T, Tofern B, Mann P et al (1998) Distribution and taxonomic significance of calystegines in the Convolvulaceae. Phytochemistry 49:1989–1995. doi:10.1016/S0031-9422(98)00430-0
Scholl Y, Höke D, Dräger B (2001) Calystegines in Calystegia sepium derive from the tropane alkaloid pathway. Phytochemistry 58:883–889. doi:10.1016/S0031-9422(01)00362-4
Stegelmeier BL, Molyneux RJ, Asano N et al (2008) The comparative pathology of the glycosidase inhibitors swainsonine, castanospermine, and calystegines A3, B2, and C1 in mice. Toxicol Pathol 36:651–659. doi:10.1177/0192623308317420
Stenzel O, Teuber M, Drager B (2006) Putrescine N-methyltransferase in Solanum tuberosum L., a calystegine-forming plant. Planta 223:200–212. doi:10.1007/s00425-005-0077-z
Tepfer D, Goldmann A, Pamboukdjian N et al (1988) A plasmid of Rhizobium meliloti 41 encodes catabolism of 2 compounds from root exudate of Calystegium sepium. J Bacteriol 170:1153–1161
Teuber M, Azemi ME, Namjoyan F et al (2007) Putrescine N-methyltransferases—a structure-function analysis. Plant Mol Biol 63:787–801. doi:10.1007/s11103-006-9126-7
Tiburcio A, Altabella T, Bitrián M et al (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240:1–18. doi:10.1007/s00425-014-2055-9
Unno Y, Shinano T, Minamisawa K et al (2015) Bacterial community shifts associated with high abundance of Rhizobium spp. in potato roots under macronutrient-deficient conditions. Soil Biol Biochem 80:232–236. doi:10.1016/J.Soilbio.2014.10.002
Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27(6):581–590
Acknowledgements
The authors wish to thank Prof. Dr. Naoki Asano (Hokuriku University, Kanazawa, Japan) for the gift of isolated calystegines. We thank Prof. Dr. Takashi Hashimoto (Nara Institute of Science and Technology, Nara, Japan) for providing N-methylputrescine. Technical assistance by Ulrike Smolka and Anja Wodak is gratefully acknowledged. Financial support by the German Research Foundation (DFG, Grant DR227/10-2) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2016_2610_MOESM1_ESM.tiff
Suppl. Fig. S1 DsPMT transcript levels were determined by Northern analysis in leaves of potato wildtype (WT), empty vector (EV), and lines transformed with DsPMT overexpression construct (DsPMT, lines A–I). RNA was isolated from potato leaves, subjected to RNA blot analysis and hybridized with a 32α-dATP labelled fragment of DsPMT cDNA (1135 bp). As a loading control, rRNA was stained with ethidium bromide. Strong DsPMT transcripts were observed in lines C, D, H, and I (TIFF 1066 kb)
425_2016_2610_MOESM2_ESM.tiff
Suppl. Fig. S2 StTRI transcript levels were determined by Northern analysis in leaves of potato wildtype (WT), empty vector (EV), and lines transformed with StTRI overexpression construct (StTRI, lines J–Q and R–Z). RNA was isolated from potato leaves, subjected to RNA blot analysis and hybridized with a 32α-dATP labelled fragment of StTRI cDNA (795 bp). As a loading control, rRNA was stained with ethidium bromide. Strong StTRI transcripts were observed in lines K, O, Q, R, T, X, and Z (TIFF 1891 kb)
425_2016_2610_MOESM3_ESM.tif
Suppl. Fig. S3 Transcript levels of StTRII in sprouts (a) and roots (b) of StTRI RNAi lines (a–g) compared with control plants (WT, wildtype; EV, empty vector). RNA was isolated from 5–10 mm-long sprouts or roots of 4-week-old potato plants, reverse transcribed, and analyzed for StTRII transcripts by quantitative PCR. StEF1α expression was used as a reference. Error bars represent standard deviation, n = 21 (WT, EV), n = 6 (StTRI RNAi lines). Data are derived from two independent experiments (TIFF 109 kb)
425_2016_2610_MOESM4_ESM.tif
Suppl. Fig. S3 Transcript levels of StTRII in sprouts (a) and roots (b) of StTRI RNAi lines (a–g) compared with control plants (WT, wildtype; EV, empty vector). RNA was isolated from 5–10 mm-long sprouts or roots of 4-week-old potato plants, reverse transcribed, and analyzed for StTRII transcripts by quantitative PCR. StEF1α expression was used as a reference. Error bars represent standard deviation, n = 21 (WT, EV), n = 6 (StTRI RNAi lines). Data are derived from two independent experiments (TIFF 112 kb)
425_2016_2610_MOESM5_ESM.tiff
Suppl. Fig. S4 Sprouting of S. tuberosum tubers 4 weeks after onset of sprouting. WT, wildtype; EV, empty vector; A, O, T, V, Y—StTRII RNAi lines. For tuber formation, 3–4-week-old plants were grown in the greenhouse and tubers were formed after 8 weeks. Tubers were harvested and kept in the dark at 7 °C until sprouting initiated after 2 months. The sprout length ranged from 5–10 mm. One photo represents all tubers harvested from one potato plant. Data are representative for three independent experiments (TIFF 7014 kb)
Rights and permissions
About this article
Cite this article
Küster, N., Rosahl, S. & Dräger, B. Potato plants with genetically engineered tropane alkaloid precursors. Planta 245, 355–365 (2017). https://doi.org/10.1007/s00425-016-2610-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2610-7