Abstract
Main conclusion
The stilbene synthase gene VqSTS6, from Chinese wild type Vitis quinquangularis accession Danfeng-2, increases the resveratrol content and pathogen resistance of transgenic plants of V. vinifera Thompson Seedless.
This study successfully created transgenic plants of V. vinifera Thompson Seedless which overexpressed VqSTS6, cloned from Chinese wild type V. quinquangularis accession Danfeng-2. Western blot and qRT-PCR showed a variable range in transcript levels among transgenic lines. The resistance to powdery mildew (Uncinula necator) was particularly enhanced in lines most highly expressing VqSTS6. Compared with the non-transformed controls, trans-resveratrol and other stilbene compounds were significantly increased in the transgenic lines. The correlation between high resveratrol content and high pathogen resistance in transgenic grapes is discussed. We hypothesize that the fruit-specific, highly expressed gene VqSTS6 from Chinese wild V. quinquangularis accession Danfeng-2, is directly involved in the resveratrol synthesis pathway in grapes, and plays an important role in the plant’s defense against pathogens. Genetic transformation of VqSTS6 explored the potential of the wild Chinese grape species for use in breeding, the results of which would raise both the disease resistance and the fruit quality of V. vinifera grapevines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The grape (Vitis sp.) is an ancient fruit crop that is becoming increasingly important throughout the developed world. All along, the prominence of this crop has been based on its high economic value and also its cultural significance. More recently, however, the health benefits of its products are also being recognized (Bouquet et al. 2008). Significant research is now being focused on grape’s high resveratrol content—resveratrol having anti-inflammatory, antiplatelet, anticarcinogenic, antifungal, and antibacterial activities (Adrian and Jeandet 2006; Anekonda 2006; Athar et al. 2007; Barger et al. 2008; Jang et al. 1997; King et al. 2006; Saiko et al. 2008; Xu et al. 2014). While the European grapevine (Vitis vinifera L.) is the predominant grape species cultivated, most V. vinifera cultivars are very susceptible to fungal pathogens (Gomès and Coutos-Thévenot 2009). In particular, powdery mildew (Uncinula necator (Schw.) Burr.) severely impacts both the yield and the quality of grapes (He et al. 1991). However, most of the Chinese wild species of Vitis possess excellent resistance to powdery mildew (Wang and He 1997; Wang et al. 1995). Moreover, the high level of resveratrol contained in their ripe berries has been reported previously by our research group (Shi et al. 2014; Zhou et al. 2015). Among these Chinese Vitis species, Vitis quinquangularis accession Danfeng-2 (with white berries) is naturally highly resistant to powdery mildew and it is also rich in resveratrol (Shi et al. 2014; Wan et al. 2015). Therefore, it is very interesting to see if it is possible to use this Chinese wild germplasm Danfeng-2, to improve the disease resistance and resveratrol contents in some of the European V. vinifera grapevine cultivars. Due to the long juvenile stage, polyploidy and high level of heterozygosity (Gray et al. 1992; Nakano et al. 1994), conventional crossing between Chinese wild and European grapevines was severely limited. Precision breeding through genetic transformation enable to transfer fungi-resistant and high level resveratrol traits of Danfeng-2 into European grape cultivars without changing their horticultural traits (Bornhoff et al. 2005; Franks et al. 2006; Vidal et al. 2003; Yamamoto et al. 2000).
Stilbene synthase (STS), belonging to the polyketide synthase family, is a key enzyme in the biosynthesis of resveratrol (Rupprich and Kindl 1978). Since STS was first purified from cell suspension cultures in 1984 (Schöppner and Kindl 1984), a considerable amount of research has been carried out on it. In Hain et al. (1990), successfully introduced a complete STS gene from groundnut into tobacco. Later, they transformed more STS genes from V. vinifera into tobacco, which increased its resistance to Botrytis cinerea (Hain et al. 1993). Since then, grapevine STS genes have been transferred into a number of plants, including Arabidopsis (Christine et al. 2006), pea (Richter et al. 2006), lettuce (Liu et al. 2006), kiwifruit (Kobayashi et al. 2000), apple (Rühmann et al. 2006) and banana (Vishnevetsky et al. 2009), in each case resulting in significant improvements in pathogen resistance.
However, disease symptoms in transgenic lines are often only partially relieved (Delaunois et al. 2009; Hipskind and Paiva 2000; Serazetdinova et al. 2005). In some cases, fungal infection is merely delayed, not prevented, such as in transgenic papaya plants (Zhu et al. 2004). (Serazetdinova et al. 2005) suggest that different transgenic lines express varying resistance to the same pathogen. Moreover, in other cases, STS-overexpressing plants have shown no signs of increased resistance to pathogens (Kobayashi et al. 2000), even though the transformed plants produced higher amounts of stilbene (Giorcelli et al. 2004). Therefore, the relationship between pathogen resistance and resveratrol content in transgenic plants requires further investigation.
Previously, our research group has isolated and characterized a number of stilbene synthase genes from the wild Chinese grapevine species, V. pseudoreticulata and V. quinquangularis. Transcript abundances of six VpSTS genes cloned from V. pseudoreticulata ‘Baihe-35-1’ were significantly up-regulated by inoculation with the powdery mildew fungus, U. necator (Wang et al. 2007). One VpSTS gene was successfully transformed into V. vinifera L. cv. Thompson Seedless and this resulted in a marked increase in resveratrol concentration, compared to non-transformed plants (Fan et al. 2008). More recently, one stilbene synthase gene VpSTSgDNA2 was transferred to V. vinifera cv. Chardonnay and the transgenic plants exhibited increased levels of stilbene and H2O2 in comparison with wild type plants and, therefore, enhanced the transgenic plants’ resistance to U. necator (Dai et al. 2015). Our research group has also verified that all genotypes from V. quinquangularis have higher resveratrol contents than most V. vinifera grapevine cvs. This is especially true for cv. Danfeng-2, which contains much higher levels of resveratrol than other V. quinquangularis genotypes (Shi et al. 2014). Moreover, the expression of gene VqSTS6 from cv. Danfeng-2 was markedly higher than the other VqSTS genes in the six genotypes examined. Therefore, we suggest that VqSTS6 may be a key to achieving the best biological properties for the European grapevine, V. vinifera with respect to resistance to biotic stress.
In this paper, we generate transgenic V. vinifera Thompson Seedless plants overexpressing VqSTS6 isolated from Chinese wild V. quinquangularis accession Danfeng-2, through embryogenesis. Our interest focusses on the role of over-expressed VqSTS6 in influencing the resveratrol content and also tolerance to powdery mildew of the transgenic Thompson Seedless plants. Genes related to the resveratrol synthesis pathway were also analyzed. It is expected the findings of this study will stimulate interest in exploring the high intrinsic potential of the wild Chinese grape germplasm both for conventional breeding (hybridization) and also as a source of genes for transgenic work to enhance the disease resistance and resveratrol content of a wide range of table and wine grape cultivars.
Materials and methods
Plant materials
Chinese wild Vitis quinquangularis accession Danfeng-2 (leaves) and Vitis vinifera cv. Thompson Seedless (anthers) were harvested from vines grown under natural conditions in the grape germplasm resources orchard of Northwest A&F University, in Yangling, Shaanxi, People’s Republic of China.
Binary vector construction and genetic transformation of grape
Genetic transformation assays of Chinese wild V. quinquangularis accession Danfeng-2 STS cDNA (VqSTS6) (GenBank Accession No. JQ868692) were carried out using A. tumefaciens strain GV3101 harboring pART-CAM-S binary vector (Fig. 1). This vector embodies the VqSTS6 cDNA and nptII coding region, which confers kanamycin resistance as selectable marker.
Molecular analysis of transgenic lines of Vitis vinifera cv. Thompson Seedless. Schematic representation of the T-DNA region of the binary vector pART-CAM-S; LB left T-DNA border, RB right T-DNA border, Ter terminator, CaMV35S cauliflower mosaic virus 35S promoter nos promoter nopaline synthase promoter, NPTII neomycin phosphotransferase gene, FLAG a short, hydrophilic protein tag used for Western blot
Proembryonic masses (PEM) of V. vinifera Thompson Seedless which were initiated from immature anthers were used for genetic transformation of VqSTS6 (Fig. 2). Transformation was carried out via the Agrobacterium-mediated transformation system as previously described by our group (Dai et al. 2015; Fan et al. 2008; Zhou et al. 2014). Germinated embryos were transferred to 50 ml woody plant medium (Aguero et al. 2006), supplemented with 0.20 mg l−1 IBA, 0.15 mg l−1 BAP and 1.0 g l−1 activated charcoal (AC). Well-developed plants were transplanted into pots and kept in greenhouse conditions.
Genetic transformation of gene VqSTS6 via Agrobacterium tumefaciens and regeneration of Vitis vinifera cv. Thompson Seedless transgenic lines. a Pro-embryogenic masses (PEM) (bar: 1.5 cm); b co-culture of embryogenic callus and Agrobacterium tumefaciens (bar: 1.5 cm); c, d resistant embryos formed (bar: 1.5 cm); e, f resistant plantlet germination (bar: 1 cm); g transgenic plantlet propagation (bar: 8 mm); h transgenic lines grown in growth room (bar: 5 cm); i transgenic lines grown in greenhouse (bar: 10 cm)
DNA extraction and semi-quantitative RT-PCR
Grapevine DNA was isolated from 0.5 to 1.0 g of in vitro grown transformed grape plants (Thomas et al. 1993). Specific oligonucleotide primers for gene sequences were designed to identify the presence of VqSTS6 in genomic DNA. Primers for VqSTS6 gene (sense primer 5′-ATGGCTTCAGTTGAGGAATTTA-3′ and antisense primer 5′-TTAATTTGTAACTGTAGG AATG-3′) were used for the PCR reactions.
Protein extraction and Western blot
The protein extraction procedure was carried out according to the method of Méchin et al. (2007). About 20–25 µg of protein was used for SDS-PAGE and blotted onto a polyvinylidene fluoride membrane (Roche, Product No. 03010040001). Immunodetection was carried out using Anti-Flag Mouse Monoclonal Antibody (TransGen Biotech, Beijing, China) combined with a secondary Goat Anti-Mouse IgG (H + L) horseradish peroxidase-conjugate antibody (TransGen Biotech, Beijing, China). Detection was carried out using Pierce ECL Western Blotting Substrate (Thermo Scientific) and imaged on a ChemiDoc™ XRS + System with Image Lab™ Software (BIO-RAD).
Inoculation with powdery mildew fungus
Transgenic and non-transformed lines were assayed for powdery mildew U. necator resistance as described by Vidal et al. (2006). Three plants of each line were evaluated. Fully expanded leaves in the third or fourth positions from the shoot tip were chosen for inoculation. Symptoms of powdery mildew infection were recorded 24, 48, 72 and 168 h after inoculation. Leaves were fixed and prepared for microscopy by an established procedure (Vanacker et al. 2000).
Measurement of resveratrol contents
Fully expanded leaves collected from transgenic and non-transgenic V. vinifera cv. Thompson Seedless were used for resveratrol analysis by HPLC. Leaf samples (1.0 g) were ground to a fine powder in liquid nitrogen then transferred to a vacuum freeze drier (CS110-4, LaboGene, Denmark). After freeze drying for 48 h in the dark, samples were extracted in 1:20 (W/V) methanol overnight at 4 °C and centrifuged (J-LITE®JLA-16.250, Beckman Coulter Inc., CA, USA) for 15 min at 5500 rpm to remove insoluble debris. The supernatant was collected in separate glass tubes and filtered through a 0.22 µm membrane film before HPLC analysis. All these steps were performed under low-light conditions.
The extracts obtained were subjected to chromatographic analysis on an Agilent 1200 HPLC system with an Inertsil ODS-3 column (5.0 μm particle size, 4.6 × 250 mm; GL Sciences Inc., Tokyo, Japan). Elution was carried out using mobile phase A (acetonitrile) and mobile phase B (water). The following linear gradient was used: 0 min: 20 % A, 80 % B; 0–30 min: 75 % A, 25 % B; 30–32 min: 100 % A, 0 % B; 32–35 min: 100 % A, 0 % B; 35–36 min: 20 % A, 80 % B; 36–45 min: 20 % A, 80 % B. The flow rate was 1.0 ml/min. Injection volume was 5 µl with a diode array detector (Agilent Technology, Palo Alto, CA, USA) set to the absorbance wavelengths of 288 and 306 nm. The retention times for each of the components in the pool analyzed were compared to the following standards: ε-viniferin, pterostilbene, trans-resveratrol, cis-resveratrol, trans-piceid, and cis-piceid.
Quantitative real-time PCR
Total RNA was isolated from grape tissues using a small-scale method based on the RNeasy plant mini kit (Qiagen). DNase-treated total RNA (3 µg) was reversely transcribed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) using the Oligo (dT)20 primer according to the manufacturer’s instructions.
Specific primer pairs of six selected genes were designed (Table 1) using Primer Express 3.0 and tested by Real-time RT-PCR. Primers (10 μM) 5′-CCCTTGTCCTCCCAACTCT-3′ and 5′-CCTTCTCAGCACTGTCCCT-3′ were used for amplifying grapevine GAPDH (GenBank Accession No. GR883080) as an internal control. Quantitative real-time PCR was carried out using a SYBR Green method in a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative expression level of each gene analyzed was calculated by comparison with the internal control.
Accession numbers
VqSTS6 (GenBank Accession No. JQ868692).
GAPDH (GenBank Accession No. GR883080).
MYB14 (GenBank Accession No. NM_001281203).
MYB15 (GenBank Accession No. KC514110).
CHS (GenBank Accession No. JF808008).
STS (GenBank Accession No. JX673940).
PAL (GenBank Accession No. XM_002268737).
RSGT (GenBank Accession No. DQ832169).
Statistical analysis
Mean values of all experimental results were compared by one-way ANOVA and differences among results were tested by post hoc comparison test (Student–Newman–Keuls) at p < 0.05 with SPSS 21.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Molecular analysis of transformants
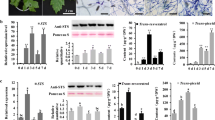
To further study the function of VqSTS6 in grapevine, transgenic V. vinifera Thompson Seedless plants with the overexpression construct VqSTS6 (Fig. 1) were obtained through embryogenesis via Agrobacterium-media transformation (Fig. 2). Gene introgression was confirmed by semi-quantitative RT-PCR analysis using specific primers for introduced STS and original genomic DNA in transgenic plants (Fig. 3). As VqSTS6 is homologous to VvSTS48 in V. vinifera and they both have an intron in the genes, PCR assay amplified two bands, including the genomic DNA model (1538 base pairs) and the complementary DNA model (1179 base pairs) from transgenic lines, while only the genomic DNA model appeared in wild type and only the complementary DNA model presents in samples with transformed plasmid being amplified template. Moreover, no PCR signals for foreign genes were detected in the water control. Putative transgenic plants were transferred to a greenhouse for further analysis.
VqSTS6 is fused with a short peptide DYKDDDDK, known as FLAG, to avoid non-specific binding with other STS proteins, when conducting a Western blot experiment to confirm the protein expression level of VqSTS6 in overexpression lines.
As shown in Fig. 4, Western blot analysis of leaf extracts with polyclonal antibody confirmed the presence of the expected proteins in these positive transgenic plants. Western blot results demonstrated various expression levels of STS in individual transgenic lines of V. vinifera cv. Thompson Seedless. Transgenic plants expressing STS could be recognized by the polyclonal antibody. No bands were detected from the non-transformed controls. A comparison of STS levels in transgenic plants with that in the positive control suggested that transgenic plants expressed more or less proteins than the positive control, and the transgenic plants (lines 2, 5 and 6) with higher expressions were selected for gene transcript analysis.
Assessment of transgenic plants for resistance to Uncinula necator
To investigate whether VqSTS6 overexpression enhances resistance to pathogens, the lines 2, 5, 6 and the control lines were inoculated with U. necator (Fig. 5). We conducted a study of conidial germination and hyphal development over a 7-day period to compare the characteristics of powdery mildew-induced symptoms in the transgenic and control plants. Images at 24, 48, 72 and 168 hpi (hours post inoculation) are presented in Fig. 6, when the degrees of infection were evaluated (Table 2). The percentage of powdery mildew occurrence on the leaf surface after inoculation was used to measure the extent of resistance in transgenic plants. Severity was recorded at six positions on each (inoculated) leaf. Conidia produced appressoria on both V. vinifera cv. Thompsom Seedless transgenic and control leaves at 24 hpi (Fig. 6a, b). Hyphae were generated in both transgenic and control leaves at 48 hpi (Fig. 6c, d). The numbers of U. necator conidia produced at 24, 48 and 72 hpi were not significantly different between the transgenic lines and the wild type (Table 2). Moreover, the severity of infection was no higher in the control leaves than in the transgenic ones at 24 and 48 hpi. Brown-colored cells were observed beneath appressoria that had developed from secondary hyphae at 72 hpi (Fig. 6e, f). The numbers of secondary hyphae growing on the leaves of transgenic plants at 72 hpi, were significantly lower than in the controls, revealing a lower susceptibility of the VqSTS6 overexpressors, compared with the non-transformed controls. The infection led to the formation of colonies with dense conidiophores on non-transformed leaves but only small colonies with conidiophores on transgenic leaves by 168 hpi (Fig. 6g, h). Furthermore, the number of conidiophores counted at 168 hpi showed that the difference between the VqSTS6 overexpressors and the non-transformed control was statistically significant. Conidiophore formation on transgenic lines was lower than on non-transformed plants. To sum up, these data clearly demonstrate that overexpression of VqSTS6 contributed to a significant reduction in the susceptibility of the transformed V. vinifera plants to U. necator.
Measurement of resveratrol contents of transgenic grapes
We used the optimized extraction conditions above coupled with HPLC analytical methods to compare the accumulation of resveratrol in leaves between transgenic lines (L2, L5, L6, L7 and L9) with non-transformed controls. We specifically quantified the resultant ε-viniferin, pterostilbene, trans-resveratrol, cis-resveratrol, trans-piceid and cis-piceid (Fig. 7). As analyzed from the chromatograms, neither cis-resveratrol nor cis-piceid was detected in extracts from either transgenic or wild type plants, whereas ε-viniferin, pterostilbene, trans-resveratrol and trans-piceid accumulations at various levels were observed in transgenic lines (Table 3). In transgenic lines 2, 5 and 6, over-expressed VqSTS6 greatly increased all the components of the stilbenes tested for, with ε-Viniferin being the predominant one. Line 5 had the highest trans-resveratrol concentration (108.94 µg/g DW), followed by lines 2 and 6 (80.04 and 77.90 µg/g DW, respectively). These were significantly higher than in lines 7, 9 and the controls. Pterostilbene and trans-piceid concentrations from lines 7 and 9 were very low, although the ε-viniferin concentrations of these two lines were significantly higher than in the controls. Moreover, lines 2, 5 and 6 had the highest resveratrol accumulations, which is in agreement with the Western blot result.
Quantitative real-time PCR analysis
To validate STS expression profiles, the steady-state transcript levels of six related genes were analyzed in transgenic lines 2, 5 and 6 using qRT-PCR. Among these, two genes (STS and RSGT) were up-regulated and two genes (PAL and MYB14) were down-regulated (Fig. 8). However, MYB15 and CHS transcripts were not significantly changed compared with the controls. GAPDH, shown to be stable in previous work (Guan et al. 2011), was chosen as a reference gene for data normalization.
Discussion
Grapevine (Vitis spp.) is one of the world’s most important fruit crops. Powdery mildew (Uncinula necator (Schw.) Burr.) is one of the most damaging fungal diseases of the European grapevines (Vitis vinifera L.). Resistance of grapevines to this serious fungal disease has been reported in Chinese wild Vitis germplasm (He et al. 1991; Wan et al. 2015; Wang et al. 1995, 1998). In addition to their potential as sources of disease resistance genes, resveratrol contents were also significantly higher in most Chinese wild grapevines than in European Vitis vinifera grapevine cvs (Shi et al. 2014). Fruit of the Chinese wild V. quinquangularis accession Danfeng-2 were shown to contain much higher levels of resveratrol than other Chinese wild samples tested. Moreover, ‘Danfeng-2’, which originates from north-west China, is highly resistant to powdery mildew (Wan et al. 2015).
Here, we report the successful transformation of the Chinese wild V. quinquangularis accession Danfeng-2 STS cDNA gene (VqSTS6) into the European V. vinifera grapevine cultivar Thompson seedless, to show that VqSTS6 plays a crucial role in enhancing the cultivar’s biological properties and its resistance to biotic stress. The expression of resistance genes in transgenic plants, resulting in enhanced resistance, is one more approach of value to breeding research which is both precise and methodical (Mitra 2001). Recently, a number of natural and synthetic resistance genes from foreign varieties were successfully transformed into transgenic Indica rice (Chen et al. 2005), broccoli (Zhao et al. 2003), tomato (Ruf et al. 2001) and tobacco (Lindbo et al. 1993), causing enhanced resistance to pathogen infection. Transgenic grapevines with improved pathogen resistance have been reported in previous research. Yamamoto et al. (2000) introduced the rice chitinase gene (RCC2) into the somatic embryos of grapevine (Vitis vinifera L. cv. Neo Muscat) by Agrobacterium infection, and an increased defense against powdery mildew caused by U. necator was found. ‘Freedom’ grape expressing a rpfF gene, encoding the synthase for diffusible signal factor (DSF), from Xylella fastidiosa, reduced the severity of Pierce’s disease and also pathogen mobility in the plant (Lindow et al. 2014). A biotech-based solution for controlling Root-knot nematodes (RKNs) by introducing RNA interference (RNAi) to silence RKN effector gene (16D10), was used to enhance nematode resistance in transgenic grape hairy roots (Yang et al. 2013). Grapevine rootstock of V. berlandieri × V. rupestris cv. ‘Richter 110’ was transformed with an Agrobacterium oncogene-silencing transgene to establish crown gall-resistant lines (Galambos et al. 2013). To overcome production losses caused by chilling and freezing, an identified grapevine C-repeat binding factor (CBF) gene, VvCBF4, was over-expressed in grape vine cv. ‘Freedom’, which exhibited improved freezing survival (Tillett et al. 2012). Whereas, transgenic grapevines over-expressing VvPIP2;4N, which is an aquaporin gene, improved growth performance by modifying water metabolism in the absence of water stress, but drought stress resistance was not induced in the research of Perrone et al. (2012). In the present study, we report the transformation of Thompson Seedless containing VqSTS6 gene, which was selected from Chinese wild V. quinquangularis accession Danfeng-2. Transgenic plants regenerated from transformed PEM conferred lower susceptibility to U. necator and higher resveratrol accumulation, compared with non-transformed plants. Transgenic plants also displayed enhanced disease resistance to phytopathogenic fungi, although sometimes they did not contain high levels of free resveratrol (Liu et al. 2006). In our work, field trials are currently underway to evaluate their powdery mildew resistance under natural conditions but this study will take a number of years to complete.
The HPLC analyses showed that trans-resveratrol was present in some transgenic Thompson Seedless lines at higher concentrations than in the non-transformed control lines. To date, trans-resveratrol has also been detected in transgenic apples (Szankowski et al. 2003), white poplars (Giorcelli et al. 2004) and grapes (Fan et al. 2008). Resveratrol also exists in the chemical form of trans-piceid. Kiwifruit (Kobayashi et al. 2000) and hop (Schwekendiek et al. 2007) transformed with a grape stilbene synthase gene produced piceid at concentrations reaching 182 μg/g fresh weight (kiwifruit) and 560 μg/g fresh weight (hop). In this study, however, the maximum amount of trans-piceid in transgenic leaves was only 93.9 μg/g fresh weight.
Induced expression of key genes and increased resveratrol levels strongly suggest the resveratrol synthesis pathway is activated in the transgenic V. vinifera cv. Thompson Seedless leaf tissue. We found different expressions of a set of genes, including transcription factor genes and enzyme genes. Transcription factors, MYB14 and MYB15, considered to interact with STS genes, were demonstrated to specifically activate the promoters of STS genes, regulating stilbene biosynthesis in grapevine at transcriptional levels (Höll et al. 2013). However, results obtained in this study show that, compared with non-transformed controls, transcript levels of MYB14 and MYB15 were not increased along with STS overexpression. This may find it explanation in that obvious feedback did not take place through this pathway from transcription factors, MYB14 and MYB15, to STS. A possible reason for this may be due to competition between the introduced STS and the endogenous CHS for the substrates 4-coumaroyl CoA and malonyl CoA. STS and CHS are key enzymes of the flavonoid biosynthesis pathway (Jez and Noel 2000; Suh et al. 2000). It is reasonable to assume that the expression of an exogenous STS may lead to substrate competition with CHS, which possibly decreases CHS activity or that of other related pathways (Delaunois et al. 2009). Such competition has also been observed between STS activity and resveratrol accumulation in transgenic white poplars (Giorcelli et al. 2004), and between anthocyanin activity and piceid accumulation in red-pigmented apple fruit (Rühmann et al. 2006). Similar results were found in this work. The transcript levels of chalcone synthase (CHS) in transgenic plants were not changed very much, although STS transcript levels were 22-times higher than in the controls. These results indicate that over-expression of STS affected secondary biosynthetic pathways in grapevine. The expression of RSGT in transgenic plants was significantly (30 %) higher than in non-transformed plants. Hall and De Luca (2007) found that Vitis labrusca grape berries accumulated both stilbene glucosides and hydroxycinnamic acid glucose esters, consistent with the bi-functional role of RSGT in stilbene and hydroxycinnamic acid modification. It may be that our over-expressed STS led to a rise in levels of hydroxycinnamic acid glucosyltransferase, with the result that accumulation of piceid was increased (Table 3). Stilbenes, including resveratrol, are synthesized via the phenylpropanoid pathway (Langcake and Pryce 1977). Phenylalanine ammonia-lyase (PAL) is the first enzyme in this pathway which catalyzes monooxidative deamination of phenylalanine (Phe) leading to cinnamate production (Jeandet et al. 2002). Our results indicate that the expression of PAL was down-regulated in the transgenic lines. A similar conclusion was reached in previous research. Resveratrol contents were increased in V. amurensis calli via an enhancement of expression of individual STS genes by coumaric acid (CA) treatment (Dubrovina et al. 2010). Meanwhile, the expression of PAL genes remained unchanged or was decreased by various concentrations of CA treatment, compared with non-transformed control plants (Shumakova et al. 2011). In summary, qRT-PCR analyses indicate that VqSTS6 was involved in the resveratrol synthesis pathway in transgenic plants (Fig. 8).
In conclusion, we demonstrate here the genetic transformation of VqSTS6 from Chinese wild V. quinquangularis accession Danfeng-2 into V. vinifera Thompson seedless plants. Following this successful integration of VqSTS6 into the grapevine genome, we also detected the expression of this gene, protein accumulation and metabolite product accumulations, as well as an interaction with pathogen resistance of transgenic Thompson seedless plants. Genetic transformation of VqSTS6 represents a significant step towards the goal of increasing the resveratrol content and pathogen resistance of V. vinifera grape cultivars—especially of the well-established winegrape cvs—for the improvement of their key horticultural traits.
Author contribution statement
YW designed research. XX, SC, YX, CZ, XW, JZ and YW performed experiments. XX, YW analyzed the results. XX wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- PEM:
-
Pro-embryogenic masses
- SE:
-
Somatic embryo
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- AC:
-
Activated charcoal
- BAP:
-
6-Benzylaminopurine
- PM:
-
Powdery mildew
- HPLC:
-
High-performance liquid chromatography
- DW:
-
Dry weight
References
Adrian M, Jeandet P (2006) Resveratrol as an antifungal agent. In: Aggarwal BB, Shishodia S (eds) Resveratrol in health and disease. CRC Press, Boca Raton, pp 475–497
Aguero CB, Meredith CP, Dandekar AM (2006) Genetic transformation of Vitis vinifera L. cvs Thompson Seedless and Chardonnay with the pear PGIP and GFP encoding genes. Vitis 45:1–8
Anekonda TS (2006) Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev 52:316–326
Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL (2007) Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharm 224:274–283
Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C (2008) A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3:e2264
Bornhoff B-A, Harst M, Zyprian E, Töpfer R (2005) Transgenic plants of Vitis vinifera cv. Seyval blanc. Plant cell reports 24:433–438
Bouquet A, Torregrosa L, Iocco P, Thomas MR (2008) Grapes. In: Kole C, Hall TC (eds) Compendium of transgenic crop plants: transgenic temperate fruits and nuts. Wiley-Blackwell, Oxford, pp 189–232. doi:10.1002/9781405181099.k0407
Chen H, Tang W, Xu C, Li X, Lin Y, Zhang Q (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet 111:1330–1337
Christine K, Lam CN, Springob K, Schmidt J, Chu IK, Lo C (2006) Constitutive accumulation of cis-piceid in transgenic Arabidopsis overexpressing a sorghum stilbene synthase gene. Plant Cell Physiol 47:1017–1021
Dai L, Zhou Q, Li R, Du Y, He J, Wang D, Cheng S, Zhang J, Wang Y (2015) Establishment of a picloram-induced somatic embryogenesis system in Vitis vinifera cv. chardonnay and genetic transformation of a stilbene synthase gene from wild-growing Vitis species. PCTOC 121:397–412
Delaunois B, Cordelier S, Conreux A, Clément C, Jeandet P (2009) Molecular engineering of resveratrol in plants. Plant Biotechnol J 7:2–12
Dubrovina AS, Manyakhin AY, Zhuravlev YN, Kiselev KV (2010) Resveratrol content and expression of phenylalanine ammonia-lyase and stilbene synthase genes in rolC transgenic cell cultures of Vitis amurensis. Appl Microbiol Biotechnol 88:727–736
Fan C, Pu N, Wang X, Wang Y, Fang L, Xu W, Zhang J (2008) Agrobacterium-mediated genetic transformation of grapevine (Vitis vinifera L.) with a novel stilbene synthase gene from Chinese wild Vitis pseudoreticulata. PCTOC 92:197–206
Franks T, Powell K, Choimes S, Marsh E, Iocco P, Sinclair B, Ford C, Van Heeswijck R (2006) Consequences of transferring three sorghum genes for secondary metabolite (cyanogenic glucoside) biosynthesis to grapevine hairy roots. Transgenic Res 15:181–195
Galambos A, Zok A, Kuczmog A, Oláh R, Putnoky P, Ream W, Szegedi E (2013) Silencing Agrobacterium oncogenes in transgenic grapevine results in strain-specific crown gall resistance. Plant Cell Rep 32:1751–1757
Giorcelli A, Sparvoli F, Mattivi F, Tava A, Balestrazzi A, Vrhovsek U, Calligari P, Bollini R, Confalonieri M (2004) Expression of the stilbene synthase (StSy) gene from grapevine in transgenic white poplar results in high accumulation of the antioxidant resveratrol glucosides. Transgenic Res 13:203–214
Gomès E, Coutos-Thévenot P (2009) Molecular aspects of grapevine-pathogenic fungi interactions. In: Grapevine molecular physiology and biotechnology. Springer, New York, pp 407–428
Gray S, Meredith C (1992) Grape. In: Hammerschlag F, Litz R (eds) Biotechnology of perennial fruit crops. CAB International, Wallingford, pp 229–262
Guan X, Zhao H, Xu Y, Wang Y (2011) Transient expression of glyoxal oxidase from the Chinese wild grape Vitis pseudoreticulata can suppress powdery mildew in a susceptible genotype. Protoplasma 248:415–423
Hain R, Bieseler B, Kindl H, Schröder G, Stöcker R (1990) Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol 15:325–335
Hain R, Reif H-J, Krause E, Langebartels R, Kindl H, Vornam B, Wiese W, Schmelzer E, Schreier PH, Stöcker RH (1993) Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 361:153–156
Hall D, De Luca V (2007) Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J 49:579–591
He P, Wang Y, Wang G, Ren Z, He C (1991) The studies on the disease-resistance of Vitis wild species originated in China. Sci Agr Sinica 24:50–56
Hipskind JD, Paiva NL (2000) Constitutive accumulation of a resveratrol-glucoside in transgenic alfalfa increases resistance to Phoma medicaginis. Mol Plant Microbe In 13:551–562
Höll J, Vannozzi A, Czemmel S, D’Onofrio C, Walker AR, Rausch T, Lucchin M, Boss PK, Dry IB, Bogs J (2013) The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 25:4135–4149
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Jeandet P, Douillet-Breuil A-C, Bessis R, Debord S, Sbaghi M, Adrian M (2002) Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 50:2731–2741
Jez JM, Noel JP (2000) Mechanism of chalcone synthase. Pka of the catalytic cysteine and the role of the conserved histidine in a plant polyketide synthase. J Biol Chem 275:39640–39646
King RE, Bomser JA, Min DB (2006) Bioactivity of resveratrol. Comp Rev Food Sci F 5:65–70
Kobayashi S, Ding C, Nakamura Y, Nakajima I, Matsumoto R (2000) Kiwifruits (Actinidia deliciosa) transformed with a Vitis stilbene synthase gene produce piceid (resveratrol-glucoside). Plant Cell Rep 19:904–910
Langcake P, Pryce R (1977) A new class of phytoalexins from grapevines. Experientia 33:151–152
Lindbo JA, Silva-Rosales L, Proebsting WM, Dougherty WG (1993) Induction of a highly specific antiviral state in transgenic plants: implications for regulation of gene expression and virus resistance. Plant Cell 5:1749–1759
Lindow S, Newman K, Chatterjee S, Baccari C, Lavarone AT, Ionescu M (2014) Production of Xylella fastidiosa diffusible signal factor in transgenic grape causes pathogen confusion and reduction in severity of pierce’s disease. Mol Plant Microbe In 27:244–254
Liu S, Hu Y, Wang X, Zhong J, Lin Z (2006) High content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J Agric Food Chem 54:8082–8085
Méchin V, Damerval C, Zivy M (2007) Total protein extraction with TCA-acetone. In: Thiellement H, Zivy M, Damerval C, Méchin V (eds) Plant Proteomics. Springer, New York, pp 1–8. doi:10.1385/1-59745-227-0:1
Mitra J (2001) Genetics and genetic improvement of drought resistance in crop plants. Curr Sci Bangalore 80:758–763
Nakano M, Hoshino Y, Mii M (1994) Regeneration of transgenic plants of grapevine (Vitis vinifera L.) via Agrobacterium rhizogenes mediated transformation of embryogenic calli. J Exp Bot 45:649–656
Perrone I, Gambino G, Chitarra W, Vitali M, Pagliarani C, Riccomagno N, Balestrini R, Kaldenhoff R, Uehlein N, Gribaudo I (2012) The grapevine root-specific aquaporin VvPIP2; 4N controls root hydraulic conductance and leaf gas exchange under well-watered conditions but not under water stress. Plant Physiol 160:965–977
Richter A, Jacobsen H-J, De Kathen A, De Lorenzo G, Briviba K, Hain R, Ramsay G, Kiesecker H (2006) Transgenic peas (Pisum sativum) expressing polygalacturonase inhibiting protein from raspberry (Rubus idaeus) and stilbene synthase from grape (Vitis vinifera). Plant Cell Rep 25:1166–1173
Ruf S, Hermann M, Berger IJ, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol 19:870–875
Rühmann S, Treutter D, Fritsche S, Briviba K, Szankowski I (2006) Piceid (resveratrol glucoside) synthesis in stilbene synthase transgenic apple fruit. J Agric Food Chem 54:4633–4640
Rupprich N, Kindl H (1978) Stilbene synthases and stilbenecarboxylate synthases. I Biol Chem 359:165–172
Saiko P, Szakmary A, Jaeger W, Szekeres T (2008) Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 658:68–94
Schöppner A, Kindl H (1984) Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut. J Biol Chem 259:6806–6811
Schwekendiek A, Spring O, Heyerick A, Pickel B, Pitsch NT, Peschke F, De Keukeleire D, Weber G (2007) Constitutive expression of a grapevine stilbene synthase gene in transgenic hop (Humulus lupulus L.) yields resveratrol and its derivatives in substantial quantities. J Agric Food Chem 55:7002–7009
Serazetdinova L, Oldach KH, Lörz H (2005) Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. J Plant Physiol 162:985–1002
Shi J, He M, Cao J, Wang H, Ding J, Jiao Y, Li R, He J, Wang D, Wang Y (2014) The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera). Plant Physiol Biochem 74:24–32
Shumakova OA, Manyakhin AY, Kiselev KV (2011) Appl Biochem Biotech 165:1427–1436
Suh DY, Kagami J, Fukuma K, Sankawa U (2000) Evidence for catalytic cysteine–histidine dyad in chalcone synthase. Biochem Biophys Res Co 275:725–730
Szankowski I, Briviba K, Fleschhut J, Schönherr J, Jacobsen H, Kiesecker H (2003) Transformation of apple (Malus domestica Borkh.) with the stilbene synthase gene from grapevine (Vitis vinifera L.) and a PGIP gene from kiwi (Actinidia deliciosa). Plant Cell Rep 22:141–149
Thomas M, Matsumoto S, Cain P, Scott N (1993) Repetitive DNA of grapevine: classes present and sequences suitable for cultivar identification. Theor Appl Genet 86:173–180
Tillett RL, Wheatley MD, Tattersall EA, Schlauch KA, Cramer GR, Cushman JC (2012) The Vitis vinifera C-repeat binding protein 4 (VvCBF4) transcriptional factor enhances freezing tolerance in wine grape. Plant Biotechnol J 10:105–124
Vanacker H, Carver TL, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol 123:1289–1300
Vidal J, Kikkert J, Wallace P, Reisch B (2003) High-efficiency biolistic co-transformation and regeneration of ‘Chardonnay’ (Vitis vinifera L.) containing npt-II and antimicrobial peptide genes. Plant Cell Rep 22:252–260
Vidal JR, Kikkert JR, Malnoy MA, Wallace PG, Barnard J, Reisch BI (2006) Evaluation of transgenic ‘Chardonnay’(Vitis vinifera) containing magainin genes for resistance to crown gall and powdery mildew. Transgenic Res 15:69–82
Vishnevetsky J, Flaishman M, Cohen Y, Elad I, Velcheva M, Hanania U, Perl A (2009) Transgenic disease resistant banana. World Patent
Wan Y, Schwaninger H, He P, Wang Y (2015) Comparison of resistance to powdery mildew and downy mildew in Chinese wild grapes. VITIS J Grapevine Res 46:132
Wang Y, He P (1997) Study on inheritance of leaves’ resistance to powdery mildew in Chinese native wild. VITIS 30:19–25
Wang Y, Liu Y, He P, Chen J, Lamikanra O, Lu J (1995) Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis species. Vitis 34:159–164
Wang Y, Liu Y, He P, Lamikanra O, Lu J (1998) Resistance of Chinese Vitis species to Elsinoë ampelina (de Bary) shear. HortScience 33:123–126
Wang X, Wang Y, Zhang C, Zhang J (2007) Isolation and characterization of cDNA encoding stilbene synthases from Chinese wild Vitis pseudoreticulata. VITIS 46:104–109
Xu C, Yagiz Y, Hsu W-Y, Simonne A, Lu J, Marshall MR (2014) Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J Agric Food Chem 62:6640–6649
Yamamoto T, Iketani H, Ieki H, Nishizawa Y, Notsuka K, Hibi T, Hayashi T, Matsuta N (2000) Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep 19:639–646
Yang Y, Jittayasothorn Y, Chronis D, Wang X, Cousins P, Zhong G-Y (2013) Molecular characteristics and efficacy of 16D10 siRNAs in inhibiting root-knot nematode infection in transgenic grape hairy roots. PLoS ONE 8:e69463
Zhao J, Cao J, Li Y, Collins HL, Roush RT, Earle ED, Shelton AM (2003) Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat Biotechnol 21:1493–1497
Zhou Q, Dai L, Cheng S, He J, Wang D, Zhang J, Wang Y (2014) A circulatory system useful both for long-term somatic embryogenesis and genetic transformation in Vitis vinifera L. cv. Thompson seedless. Plant Cell Tissue Organ Cult (PCTOC) 118:157–168
Zhou Q, Du Y, Cheng S, Li R, Zhang J, Wang Y (2015) Resveratrol derivatives in four tissues of six wild Chinese grapevine species. N Z J Crop Horticult Sci 43:1–9
Zhu YJ, Agbayani R, Jackson MC, Tang C, Moore PH (2004) Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta 220:241–250
Acknowledgments
This work was supported by the Key Laboratory of Horticultural Plant Biology and Germplasm Innovation in Northwest China. The research was done with Grants from the National Science Foundation of China (Grant No. 31372039) and from the Program for Innovative Research Team of Grape Germplasm Resource and Breeding (2013KCT-25). The authors thank Dr. Alexander (Sandy) Lang from RESCRIPT Co. (New Zealand) for useful comments and language editing which have greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cheng, S., Xie, X., Xu, Y. et al. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from Chinese wild Vitis quinquangularis . Planta 243, 1041–1053 (2016). https://doi.org/10.1007/s00425-015-2459-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2459-1