Abstract
Ischemic stroke is an urgent public health concern and one of the major causes of deaths and disabilities over the world. MicroRNA (miRNA) has become a key mediator of cerebral ischemia-reperfusion (I/R) injuries. However, whether miR-190 is involved in cerebral I/R-induced neuronal damage remains unknown. This study was to investigate the role of miR-190 in the brain I/R injury. We divided the rats into sham, I/R, control, and miR-190-mim (miR-190 mimics) groups. Quantitative real-time polymerase chain reaction (qRT-PCR), Nissl staining, flow cytometry, and western blot were conducted to examine the expression of miR-190 and cell apoptosis in different groups. The results showed that the expression of miR-190 was greatly decreased in rats suffering with I/R. Overexpression of miR-190 significantly reduced the increased neurological scores, brain water contents, infarct volumes, and neuronal apoptosis in rats suffering with I/R. In addition, we found that the expression of RhoA and Rho kinase was greatly elevated in rats suffering with I/R. Bioinformatics analysis indicated that Rho was a target of miR-190. Moreover, overexpression of miR-190 significantly downregulated the increased mRNA and protein expression of Rho/Rho kinase and cell apoptosis, while inhibition of miR-190 further upregulated the increased mRNA and protein expression of Rho/Rho kinase and cell apoptosis in rats suffering with I/R. Furthermore, knockdown of Rho significantly downregulated the increased mRNA and protein expression of Rho/Rho kinase and cell apoptosis, while these effects were inhibited by miR-190 inhibitors in rats suffering with I/R. These results indicate that miR-190 confers protection against brain I/R damage by modulating Rho/Rho-kinase signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is an urgent public health concern and one of the major causes of deaths and disabilities over the world [12, 22]. An abnormal promotion in cell apoptosis may lead to neuronal death after acute ischemic brain injuries, but its underlying mechanism is not fully understood [6, 33]. The diagnosis of stroke depends on clinical manifestations and is confirmed by CT or magnetic resonance examination [3, 11]. The symptoms of early ischemic stroke are mild and difficult to distinguish from other neurological and non-neurological disorders, which limit the chances for early diagnostics [9]. Therefore, accurate diagnostic methods are critical for the treatment of ischemic stroke patients. As a multifactorial disease that is affected by multiple genes and environmental factors, ischemia stroke has attracted much attention to molecular biomarkers for ideal diagnosis for stroke [34].
MicroRNAs (miRNAs), consisting of about 20 to 22 nucleotides, are small non-coding RNAs that regulate post-transcriptional levels of gene expressions [18]. They act as a vital role in hemostasis and thrombosis, which could occlude cerebral arteries and cause an ischemic stroke [24]. Previous researchers have shown that miRNAs are greatly related with the pathogenesis of stroke by regulating oxidative stress/inflammation, apoptosis, and vascular endothelial function [2, 13]. For example, microRNA-124 was reported to protect neurons against apoptosis in cerebral ischemic stroke [15, 25]. MiR-190 has been reported to modulate cell apoptosis. For instance, miR-190 was upregulated in Epstein-Barr virus type I latency and modulated cell mRNAs for cell survivals [7]. However, whether miR-190 can be utilized as a biomarker for stroke diagnosis is largely unknown. In this study, we focused to investigate its expression and role in ischemia stroke tissue samples and cells.
The small GTPase Rho and its downstream effector Rho kinase could help agonist-induced vascular contraction through Ca2+ sensitization of the contractile system [4]. This activation could inhibit endothelial nitric oxide synthase and affect nitric oxide generation. Massive studies have shown that these procedures are associated with the pathogenesis of vascular diseases and ischemia stroke [21]. Previous researches demonstrated that inhibiting Rho kinase could attenuate nephrosclerosis and improve survivals in hypertensive stroke-prone rat [20]. The bioinformatics study in our preliminary experiment illustrated that miR-190 could target Rho. Therefore, we will study the association of miR-190 and Rho in the regulation of ischemic stroke. Our results might provide a new therapeutic direction for the treatment of ischemic stroke.
Materials and methods
Focal cerebral I/R injury model in rats
Six-week-old male SD rats (200 g) were purchased from Shandong University. All animals were reared at room temperature with a half day and half night cycle, and they were free to eat or drink. After 7 days’ acclimation, the animals were divided to 3 groups: normal, sham operation, and model (I/R). The animals were anesthetized by intraperitoneal injection with 3% sodium pentobarbital (30 mg/kg), and the head and limbs were fixed on a stainless-steel operating table. A midline cervical incision was conducted after shaving and sterilization. After the left internal carotid artery (ICA) was exposed, a nylon wire was inserted. ICA was ligated. After occlusion for 2 h, the line was removed for complete reperfusion for 24 h, and a rat middle cerebral artery occlusion-reperfusion model (MACO/R) was made. Animals in the sham operation had ICA isolation, but no nylon surgical wire was inserted, while rats in the normal group were not treated. After anesthesia, neurological deficit was evaluated. Rats with a score of 1 to 3 were employed in the experiment. The animal use and experimental procedures in the research was approved by the ethics committee of Shandong Provincial ENT Hospital and conducted in accordance with the animal care and guidelines of Shandong Provincial ENT Hospital.
Animal grouping and treatment
All rats were divided into five groups: normal (untreated normal rats), sham operation (CCA, ICA, and ECA, but un-ligated CCA), I/R (un-transfected MCAO/R rats), control group (MCAO/R rats injected with miR-190 NC), and miR-190-mim (MCAO/R rats injected with miR-190-mim). We injected miR-190-mim/control into the right ventricle using a micro-syringe to inject at a rate of 0.2 μl/min (lateral side). MiR-190-mim and miR-190 negative control (NC/normal) were purchased from RiBoBio, China. Injections were conducted at 2 days before MCAO/R. According to Zea Longa 5-point scale (Table 1), the neurologic impairment of rats in each group was scored [5].
Determination of infarct volumes
After 24-h reperfusion, the rats were anesthetized, and the brain was dissected and sectioned. Five 1.5-mm thick sections were cut into thin slices and stained with 1% triphenyl tetrazolium chloride (TTC) for half an hour and fixed in 4% paraformaldehyde. Quantitative analysis was conducted for lesion areas that were not stained. The infarct volume was calculated by utilizing the slice’s thickness and determined area of the lesions.
TUNEL staining
Tissues of the normal and the model groups were dewaxed and dehydrated. After washing, the tissues were treated by proteinase K in a wet chamber at 37 °C for 30 min. A total of 50 μl TUNEL was added. Transcription activator protein was added and incubated for 30 min. Tissues were then stained by 3,3′-diaminobenzidine (DAB), dehydrated, permeabilized, and sealed. Using a microscope, 5 non-overlapping areas were selected in the ischemic area, and the number of cells was measured.
QRT-PCR
RNA was extracted from the hippocampus utilizing miRNeasy Kit (217004, Qiagen, Germany). The cells were stored at − 80 °C and reversely transcribed to cDNA utilizing the TaqMan Kit (4427975, Applied Biosystems, USA). The reaction conditions for PCR were pre-denaturation at 95 °C for 15 min, denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s, and extension at 72 °C for 20 s in 40 cycles utilizing β-actin as an internal reference. QRT-PCR was conducted and 2-ΔΔCt represented the relationship of target gene expressions. The primers were displayed in Table 2.
Western blot
In each group, proteins were extracted from rat hippocampus, and then quantified utilizing the BCA kit (20201ES76, Yisheng Bio., Shanghai). A total of 25 μg protein samples was separated by 12% SDS-PAGE and transferred to PVDF membrane. After blocking with 5% BSA, the membrane was treated with anti-RhoA (ab54835; 1:1000; Abcam, USA), anti-Rho kinase (ab45171; 1:2000; Abcam, USA), and anti-Rho kinase β (ab134181; 1:1000; Abcam, USA). The membrane was treated with a goat anti-rabbit (C86-SSA004; 1:1000, Canspec, China). Chemiluminescence was subsequently conducted (Biomiga, USA).
Hippocampal neuron
The hippocampus of newborn SD rats (J001, Bate, China) in 24 h was isolated and digested. The mixture of tissue pieces was transferred to a centrifuge tube, centrifuged at 1000 r/min for 20 min. The hippocampal tissue was resuspended in 7 ml of DMEM/F12. After filtration, we observed cell suspension using a microscope (×100). After 48 h, the medium was refreshed with a serum-free medium DMEM/F12 medium. After 96 h, 0.005 g/L cytarabine was pipetted to suppress cell growth. After 24 h, the mediums were refreshed. Immunohistochemical staining was utilized after 8 days.
Assessment of neurobehavioral parameters
The neurological deficits (N = 6) in rats subjected to cerebral I/R were evaluated blinded using the scales as previously described [16]: 0 point, rats behave normally; 1 point, rats cannot fully stretch their left front legs; 2 points, rats turn around into a circle; 3 points, rats fall down to the left side; and 4 points, rats cannot move by themselves, losing their consciousness.
Cell treatment
Logarithmic growth stage rat hippocampal neuron cells were inoculated. When the confluence got 60‑80%, the cells had transfection. We diluted 100 pmol miR-190-mim (miR-190 mimics), miR-190-inh (miR-190 inhibitor), siRNA-Rho, and miR-190-inh+siRNA-Rho negative. The negative control group was mixed and incubated for 5 min. We diluted 5 μl Lipofectamine 2000 with 250 μl serum-free Opti-MEM, mixed them gently, and incubated them at room temperature for 5 min. The two dilutions were then mixed and incubated for 20 min at room temperature before being added to the plate wells. After culturing in 37% 5% CO2 for 6‑8 h, the mixture was cultured for 24‑48 h. Cells were divided as untreated normal hippocampal neuron cell, blank group with un-transfected oxygen-glucose deprivation/reperfusion (OGDR)-treated hippocampal neuron cell, and NC group of miR-190 negative control sequence transfected OGD-treated hippocampal neuron cell, miR-190-mim group, miR-190-inh group, siRNA-Rho group, and miR-190-inh+siRNA-Rho group. All reagents were purchased from Gene Pharmaceutical, China. To mimic ischemia-like diseases in vitro, hippocampal neuron cell had 2-h OGD/24-h reoxygenation. The cells were transferred to an anaerobic chamber (Thermo Electron LED GmbH, Germany) at 37 °C under hypoxic conditions. The culture medium was replaced with glucose-free DMEM (Gibco, USA) and the cells were maintained in a hypoxic chamber for 2 h. After 2 h of OGD exposure, the cells were reoxygenated for 24 h in a growth medium maintained under normoxic conditions. The normal control group was stored in a growth medium under normoxic conditions.
Detection of luciferase reporter gene
We inserted the Rho 3′-UTR fragment containing the miR-190 binding site or the corresponding mutant generated by mutating the miR-190 region binding site into the pmirGLO dual-luciferase miRNA target expression vector (Promega, USA). The recombinant construct was co-transfected with miR-190-mim or NC miRNA. Luciferase activity was determined utilizing a luminometer.
Nissl staining
The tissue paraffin sections were dewaxed and hydrated. After washing with distilled water, the sections were stained with 0.25% toluidine blue (Sinopharm Group; 71041284) at 60 °C for 3 h. The remaining dye solution was quickly washed with ultra-pure water, and then the sections were washed with 95% ethanol, dehydrated with absolute ethanol, made transparent with xylene (Sinopharm Group; 10023418), and then mounted with neutral gum (Sinopharm Group; 10004160). The sections were observed, and images were captured under an upright fluorescence microscope (Olympus BX53, Tokyo, Japan). The number of Nissl bodies/mm2 was analyzed by the ImageJ 5.0 software (Rawak Software Inc., Stuttgart, Germany). The above analysis was completed by three researchers who were blinded to the treatments.
Flow cytometry
At 48-h transfections, cells were trypsinized without EDTA and collected in a flow tube, and had centrifugation. It was then washed and centrifuged again to remove the supernatant. Annexin V-FITC (K201-100, BioVision, USA), PI, and HEPES buffers were utilized at a ratio of 1:2:50. Cells were resuspended in 100 μl staining solution and incubated for 15 min after 1 ml of HEPES. The signal was observed by the flow cytometer.
Statistical analysis
Shapiro-Wilk test was used to test whether the data distribution conforms to normality. The normally distributed variables were described as mean ± SD. Otherwise, variables were described as median (Q1, Q3). Independent t test or Mann-Whitney U test was performed as appropriate to compare the differences in scale or ordinal variables between the two groups. Statistical analysis was conducted utilizing SPSS 21.0. Difference between 2 groups was analyzed by t test, and > 2 groups were evaluated by one-way ANOVA. A value of < 0.05 was statistically significant.
Results
MiR-190 inhibited brain I/R injuries
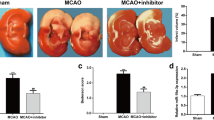
To investigate the neuroprotective effects of miR-190, we firstly examined the expression of miR-190 in rats suffering from ischemic stroke. The results illustrated that miR-190 expression was decreased in MCAO-treated rats compared with normal or sham operation group (p < 0.01), while miR-190 expression was increased in rats treated with miR-190-mim compared with normal or sham group (p < 0.01) (Fig. 1a). The results illustrated that miR-190-mim effectively reduced neurological scores, brain water content, infarct volume, and neuronal apoptosis in comparison with I/R or the control group (Fig. 1b‑e, p < 0.05, p < 0.01). Our data provided solid supports for the neuroprotection effects of miR-190 on the brain I/R injuries.
Neuroprotection effects of miR-190 on the brain I/R injuries in vivo. a Expression of miR-190 in rats measured by qRT-PCR. b Neurobehavioral results measured by the Zea Longa 5-point scale. c Brain water content. d Infarct volume measured by TTC staining. e The apoptosis rate of neurons in the rat hippocampus measured by TUNEL staining (× 400). Rod = 50 μm. In contrast to the normal or sham operation group, N = 6 per group, * p < 0.05, ** p < 0.01; # p < 0.05, ## p < 0.01 vs. I/R or control group
Rho was a direct target of miR-190
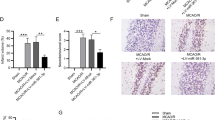
The qRT-PCR results illustrated no obvious variation in mRNA expression of RhoA and Rho kinase in sham operation and normal group. For the I/R group, the expression of RhoA and Rho kinase was greatly elevated (Fig. 2a, p < 0.05). Western blot illustrated no obvious variation in the protein expression of RhoA and Rho kinase in the sham operation and the normal group. For the I/R group, the expression of RhoA and Rho kinase was greatly elevated in comparison with the normal and sham group (Fig. 2b, p < 0.05, p < 0.01). The microRNA.org bioinformatics showed that miR-190 could target Rho (Fig. 2c). We conducted a double luciferase reporter assay. Rho 3′-UTR containing wild-type (WT) or mutant (MT) putative miR-190 binding site was inserted into a reporter vector co-transfected with miR-190-mim into 293 T cells. The results revealed that overexpression of miR-190 greatly reduced the luciferase activity of the reporter vector containing WT Rho 3′-UTR (Fig. 2d, p < 0.01). However, overexpression of miR-190 had no obvious effect on the luciferase activity of reporter vectors containing mutant Rho 3′-UTR (Fig. 2d). These results indicated that miR-190 bound directly to Rho 3′-UTR by predicting the binding site.
Rho might be a target gene for miR-190. a MRNA expression of RhoA and Rho kinase in rat measured by qRT-PCR. b Protein expression of RhoA and Rho kinase in rats measured by western blot. c Binding sequences of miR-190 and Rho-3′-UTR. d Luciferase activities of Rho WT and Rho MT in the transfection groups. N = 6 per group, * p < 0.05, ** p < 0.01 vs. normal, false, or NC
Expression of miR-190 and Rho/Rho kinase after transfection
We found the effect of miR-190 on the mRNA and protein expression of RhoA and Rho kinase related to the Rho/Rho-kinase pathway. Hippocampal neurons had OGD/R for 2 h and then recovered for 24 h to simulate I/R-like conditions. RT-PCR revealed that miR-190 expression was greatly reduced, while Rho expression was greatly elevated in all other groups compared with the normal group (Fig. 3a, p < 0.05, p < 0.01). Compared with the blank and the NC group, miR-190 expression in the miR-190-mim was greatly elevated (p < 0.01), and miR-190 expression in the miR-190-inh was greatly reduced (p < 0.01). In addition, the expression of Rho, Rho kinase α, and Rho kinase β in miR-190-mim and siRNA-Rho was greatly reduced (p < 0.05), and Rho expression in the miR-190-inh group was greatly elevated (p < 0.05), while there was no obvious variation in the miR-190-inh+siRNA-Rho group (Fig. 3a, p > 0.05). The results of western blots (Fig. 3b) revealed that in contrast to the normal group, the protein expression of Rho, Rho kinase α, and Rho kinase β in the other group was elevated (p < 0.01). Compared with the NC group, the expression of Rho, Rho kinase α, and Rho kinase β in the miR-190-mim group and siRNA-Rho group was greatly reduced (p < 0.05); Rho expression in the miR-190-inh group was greatly elevated (p < 0.05). There was no obvious variation in the miR-190-inh+siRNA-Rho group (p > 0.05). Our data indicated that miR-190-inh could greatly elevate the expression of Rho, Rho kinase α, and Rho kinase β.
Expression of miR-190 and Rho/Rho kinase after transfection in rat hippocampal neuron cell. a MiR-190 expression measured by qRT-PCR and mRNA expression of Rho/Rho-kinase pathway-related molecules. b Protein expression of Rho/Rho-kinase pathway-related molecules measured by western blot. Data are expressed as mean ± SD. N = 6 per group, * p < 0.05, ** p < 0.01 vs. normal group; # p < 0.05, ## p < 0.01 with blank or NC group
Nissl staining of transfected neurons between different groups
Then, the molecular mechanism of neuroprotection of miR-190 against brain I/R injuries was further studied with Nissl staining. The results illustrated that in the normal group, hippocampal neurons were closely packed. In the miR-190-inh+siRNA-Rho group, many Nissl corpses were ruptured, apoptosis occurred, and live neurons were greatly reduced. In the miR-190-mim and siRNA-Rho, neurons were compact. In the miR-190-inh group, neurons were scattered, many Nissl corpses are ruptured, and many hippocampal neuron cells undergo apoptosis (Fig. 4). Compared with the blank group and the NC group, the average optical density of the miR-190-mim and the siRNA-Rho group was greatly reduced, while that of miR-190-inh group was greatly elevated (p < 0.05). Blank, NC, and miR-190-inh+siRNA-Rho had no obvious variation in density (p > 0.05). Our data illustrated that overexpression of miR-190 or inhibition of Rho greatly decreased neuronal deaths.
Nissl staining of transfected neurons in different groups in rat hippocampal neuron cell. Nissl staining of neurons in 7 groups of normal, blank, NC, miR-190-mim, miR-190-inh, si-Rho, and miR-190-inh+si-Rho (400 times). Rod = 50 μm. Data are expressed as mean ± SD. * p < 0.05, ** p < 0.01 vs. normal group; # p < 0.05 with blank or NC group
Apoptosis of neurons transfected between different groups
From Fig. 5, results illustrated that the apoptotic rates of the 7 groups (normal, blank, NC, miR-190-mim, miR-190-inh, siRNA-Rho, and miR-190-inh+siRNA-Rho) were as follows: 8.97 ± 1.16%, 19.59 ± 2.21%, 18.83 ± 1.16%, 13.47 ± 1.55%, 27.99 ± 2.39%, 13.28 ± 1.76%, and 18.29 ± 1.74%. Compared with the normal group, the apoptotic neurons of the other groups were greatly elevated (p < 0.05, p < 0.01). In contrast to the blank group and the NC group, the miR-190-mim group and the siRNA-Rho group demonstrated a great decrease (p < 0.05), while the miR-190-inh group illustrated a promotion (p < 0.01). There was no obvious variation in the apoptosis rate of the inhibitor+siRNA-Rho group. It was revealed that miR-190 could enhance the cell apoptosis but si-Rho attenuates this effect.
Cell apoptosis of transfected neurons in different groups. Comparison of apoptotic rates after the transfection of the 7 groups of cells in normal, blank, NC, miR-190-mim, miR-190-inh, si-Rho, and miR-190-inh+si-Rho. Data are expressed as mean ± SD. N = 3 per group, * p < 0.05, ** p < 0.01 vs. normal group; # p < 0.05, ## p < 0.01 with blank or NC group
Discussion
In this study, we confirmed the important role of miR-190 in stroke caused by ischemia reperfusion. The results showed that the expression of miR-190 was greatly decreased in rats suffering with I/R. Overexpression of miR-190 significantly reduced the increased neurological scores, brain water contents, infarct volumes, and neuronal apoptosis in rats suffering with I/R. The results of bioinformatics analysis and luciferase analysis indicated that Rho was a direct target of miR-190. Further results showed that miR-190 inhibited cell apoptosis through the Rho/Rho-kinase pathway.
Studies have shown that miRNAs can play a role in regulating gene expression by regulating the translation process of their target mRNAs, thereby participating in various biological phenomena such as cell differentiation, proliferation, and vascular regeneration [18]. Altering miRNAs have been implicated in the pathogenesis of various diseases including stroke [8]. MiR-190 has been confirmed to be dysregulated in many tumors [14, 28, 30,31,32]. Previous studies have shown that MiR-190 is dysregulated in patients with Alzheimer’s disease [23], Parkinson’s disease [26], and chronic heart failure [27], and plays an important role in resisting neuronal damage and promoting vascular endothelial proliferation. In the brain injury model, miR-190 was significantly downregulated [17]. Ischaemic stroke, the most universal kind of stroke, may arise while a blood vessel or neuronal damage in the brain is occluded. Numerous evidences prompt that miR-190 may be a potential target for the treatment of stroke. In I/R group, miR-190 was significantly downregulated, suggesting that miR-190 may be related to stroke caused by ischemia reperfusion.
MACO/R is used to establish ischemic stroke model. In our study, it was found that the overexpression of miR-190 mitigated cerebral infarct size and improved neurological function in cerebral I/R. Therefore, our subsequent experiments were performed to identify the specific mechanisms of miR-190-mim treatment.
To further investigate the potential mechanisms of miR-190 in cerebral ischemia reperfusion, the expression of proteins in RhoA signaling pathway was detected. We identified that the inhibition of RhoA signaling pathway by miR-190 contributed to anti-apoptosis post ischemia reperfusion. The RhoA/Rho-kinase signaling pathway is an important way to block regeneration in the adult central nervous system (CNS) [10, 19] and neuron apoptosis [29] after stroke. Bioinformatics and luciferase analysis showed that miR-190 targeted RhoA. After ischemia stroke, the excessive production of free radicals, Ca2+ overload, and excitatory toxicity might lead to neuron cell apoptosis [1]. The small GTPase Rho and its downstream effector Rho kinase could contribute to agonist-induced vascular contraction via Ca2+ sensitization of the contractile system [4]. In the acute phase of the disease, neurons of the ischemic lesion die quickly, while other neuron groups in the ischemic penumbra are vulnerable to secondary injury. In this study, when the expressions of miR-190 and Rho were both inhibited, the hippocampal neuronal cell damage and apoptosis rate of hippocampal neurons caused by the inhibition of miR-190 were reduced.
There were several limitations in this study. Rho was predicted as a target of miR-190 using the microRNA.org bioinformatics, which was then confirmed by the double luciferase reporter assay. In terms of our study, the results provided by the research indicated that miR-190 conferred protection against brain I/R damage by modulating Rho/Rho-kinase signaling. Of course, considering the regulatory functional complexity of miRNAs in a range of organisms, miR-190 may also interact with other mRNAs during cerebral I/R, which remains to be further explored.
Conclusion
This study indicates that miR-190 confers protection effects against brain I/R damage by modulating Rho/Rho-kinase signaling.
References
Agam Bansal RP, Gupta S, Chaurasia A, Chaudhary P (2019) Role of microRNAs in stroke recovery. J Family Med Prim Care 8:1850
Bam M, Yang X, Sen S, Zumbrun EE, Dennis L, Zhang J, Nagarkatti PS, Nagarkatti M (2018) Characterization of dysregulated miRNA in peripheral blood mononuclear cells from ischemic stroke patients. Mol Neurobiol 55:1419–1429
Brainin M, Heiss W-D (2019) Textbook of stroke medicine, 3rd ed. Cambridge University Press, Cambridge. https://doi.org/10.1017/9781108659574
Budzyn K, Marley PD, Sobey CG (2006) Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci 27:97–104
Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, Wang SX, Zhang Y, Zeng WG, Ni HJ, Zhao B, Chen YF, Tang ZP (2012) Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther 18:847–854. https://doi.org/10.1111/j.1755-5949.2012.00382.x
Cheon SY, Kim EJ, Kim SY, Kim JM, Kam EH, Park J-K, Koo B-N (2018) Apoptosis signal-regulating kinase 1 silencing on astroglial inflammasomes in an experimental model of ischemic stroke. Neuroscience 390:218–230
Cramer EM, Shao Y, Wang Y, Yuan Y (2014) miR-190 is upregulated in Epstein–Barr Virus type I latency and modulates cellular mRNAs involved in cell survival and viral reactivation. Virology 464:184–195
Eyileten C, Wicik Z, De Rosa S, Mirowska-Guzel D, Soplinska A, Indolfi C, Jastrzebska-Kurkowska I, Czlonkowska A, Postula M (2018) MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke—a comprehensive review and bioinformatic analysis. Cells 7:249
Fernandes LF, Bruch GE, Massensini AR, Frézard F (2018) Recent advances in the therapeutic and diagnostic use of liposomes and carbon nanomaterials in ischemic stroke. Front Neurosci 12:453
Fujita Y, Yamashita T (2014) Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci 8:338. https://doi.org/10.3389/fnins.2014.00338
Hasan TF, Rabinstein AA, Middlebrooks EH, Haranhalli N, Silliman SL, Meschia JF, Tawk RG (2018) Diagnosis and management of acute ischemic stroke. Mayo Clin Proc 4. Elsevier:523–538
Jensen M, Thomalla G (2019) Causes and secondary prevention of acute ischemic stroke in adults. Hämostaseologie 40(1):22–30. https://doi.org/10.1055/s-0039-1700502
Jickling G, Ander B, Stamova B, Liu D, Sharp F (2018) Regulation of HMGB1 inflammation in patients with ischemic stroke by microRNA. AAN Enterprises 90(15 Supplement):S15.006
Jin Z, Piao L, Sun G, Lv C, Jing Y, Jin R (2020) Long non-coding RNA PART1 exerts tumor suppressive functions in glioma via sponging miR-190a-3p and inactivation of PTEN/AKT pathway. Onco Targets Ther 13:1073–1086. https://doi.org/10.2147/OTT.S232848
Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao H, Yan F, Li S, Ji X (2013) MicroRNA-124–mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke 44:1973–1980
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.str.20.1.84
Meissner L, Gallozzi M, Balbi M, Schwarzmaier S, Tiedt S, Terpolilli NA, Plesnila N (2016) Temporal profile of microRNA expression in contused cortex after traumatic brain injury in mice. J Neurotrauma 33:713–720. https://doi.org/10.1089/neu.2015.4077
Mirzaei H, Momeni F, Saadatpour L, Sahebkar A, Goodarzi M, Masoudifar A, Kouhpayeh S, Salehi H, Mirzaei HR, Jaafari MR (2018) MicroRNA: relevance to stroke diagnosis, prognosis, and therapy. J Cell Physiol 233:856–865
Nahm M, Lee M, Baek SH, Yoon JH, Kim HH, Lee ZH, Lee S (2006) Drosophila RhoGEF4 encodes a novel RhoA-specific guanine exchange factor that is highly expressed in the embryonic central nervous system. Gene 384:139–144. https://doi.org/10.1016/j.gene.2006.07.024
Nishikimi T, Koshikawa S, Ishikawa Y, Akimoto K, Inaba C, Ishimura K, Ono H, Matsuoka H (2007) Inhibition of rho-kinase attenuates nephrosclerosis and improves survival in salt-loaded spontaneously hypertensive stroke-prone rats. J Hypertens 25:1053–1063
Nunes KP, Rigsby CS, Webb RC (2010) RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci 67:3823–3836
Powers W, Rabinstein A, Ackerson T, Adeoye O, Bambakidis N, Becker K, Biller J, Brown M, Demaerschalk B, Hoh B (2018) 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke 49(3):e46–e99
Serpente M, Fenoglio C, D’Anca M, Arcaro M, Sorrentino F, Visconte C, Arighi A, Fumagalli GG, Porretti L, Cattaneo A, Ciani M, Zanardini R, Benussi L, Ghidoni R, Scarpini E, Galimberti D (2020) MiRNA profiling in plasma neural-derived small extracellular vesicles from patients with Alzheimer’s disease. Cells 9. https://doi.org/10.3390/cells9061443
Sohrabji F, Selvamani A (2019) Sex differences in miRNA as therapies for ischemic stroke. Neurochem Int 127:56–63
Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X (2013) MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther 19:813–819
Sun Q, Wang S, Chen J, Cai H, Huang W, Zhang Y, Wang L, Xing Y (2019) MicroRNA-190 alleviates neuronal damage and inhibits neuroinflammation via Nlrp3 in MPTP-induced Parkinson’s disease mouse model. J Cell Physiol 234:23379–23387. https://doi.org/10.1002/jcp.28907
Sun B, Meng M, Wei J, Wang S (2020) Long noncoding RNA PVT1 contributes to vascular endothelial cell proliferation via inhibition of miR-190a-5p in diagnostic biomarker evaluation of chronic heart failure. Exp Ther Med 19:3348–3354. https://doi.org/10.3892/etm.2020.8599
Xie B, Deng Z, Pan Y, Fu C, Fan S, Tao Y, Zhou J, Xiao D (2018) Post-transcriptional regulation DPC4 gene by miR-190 in colorectal cancer cells. J Cancer Res Ther 14:838–843. https://doi.org/10.4103/jcrt.JCRT_577_17
Xie Y, Ge CL, Zhang ZY, Fei GX (2020) Oxycodone inhibits myocardial cell apoptosis after myocardial ischemia-reperfusion injury in rats via RhoA/ROCK1 signaling pathway. Eur Rev Med Pharmacol Sci 24:6371–6379. https://doi.org/10.26355/eurrev_202006_21535
Yu Y, Cao XC (2019) miR-190-5p in human diseases. Cancer Cell Int 19:257. https://doi.org/10.1186/s12935-019-0984-x
Yu Y, Luo W, Yang ZJ, Chi JR, Li YR, Ding Y, Ge J, Wang X, Cao XC (2018) miR-190 suppresses breast cancer metastasis by regulation of TGF-beta-induced epithelial-mesenchymal transition. Mol Cancer 17:70. https://doi.org/10.1186/s12943-018-0818-9
Yu Y, Yin W, Yu ZH, Zhou YJ, Chi JR, Ge J, Cao XC (2019) miR-190 enhances endocrine therapy sensitivity by regulating SOX9 expression in breast cancer. J Exp Clin Cancer Res 38:22. https://doi.org/10.1186/s13046-019-1039-9
Zhang K, Tu M, Gao W, Cai X, Song F, Chen Z, Zhang Q, Wang J, Jin C, Shi J (2019) Hollow Prussian blue nanozymes drive neuroprotection against ischemic stroke via attenuating oxidative stress, counteracting inflammation, and suppressing cell apoptosis. Nano Lett 19:2812–2823
Zhu M, Li N, Luo P, Jing W, Wen X, Liang C, Tu J (2018) Peripheral blood leukocyte expression of lncRNA MIAT and its diagnostic and prognostic value in ischemic stroke. J Stroke Cerebrovasc Dis 27:326–337
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
Ethical approval was obtained from the Ethics Committee of Shandong Provincial ENT Hospital. All procedures performed in studies involving animals were conducted in accordance with the animal care and guidelines of Shandong Provincial ENT Hospital.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, C., Dong, N., Feng, J. et al. MiRNA-190 exerts neuroprotective effects against ischemic stroke through Rho/Rho-kinase pathway. Pflugers Arch - Eur J Physiol 473, 121–130 (2021). https://doi.org/10.1007/s00424-020-02490-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02490-2