Abstract
Crystallin zeta (CRYZ) is a phylogenetically restricted water-soluble protein and provides cytoprotection against oxidative stress via multiple mechanisms. Increasing evidence suggests that CRYZ is high abundantly expressed in the kidney where it acts as a transacting factor in increasing glutaminolysis and the Na+/K+/2Cl– cotransporter (BSC1/NKCC2) expression to help maintain acid–base balance and medullary hyperosmotic gradient. However, the mechanism by which CRYZ is regulated in the kidney remains largely uncharacterized. Here, we show that CRYZ is a direct target of farnesoid X receptor (FXR), a nuclear receptor important for renal physiology. We found that CRYZ was ubiquitously expressed in mouse kidney and constitutively expressed in the cytoplasm of medullary collecting duct cells (MCDs). In primary cultured mouse MCDs, CRYZ expression was significantly upregulated by the activation and overexpression of FXR. FXR-induced CRYZ expression was almost completely abolished in the MCD cells with siRNA-mediated FXR knockdown. Consistently, treatment with FXR agonists failed to induce CRYZ expression in the MCDs isolated from mice with global and collecting duct–specific FXR deficiency. We identified a putative FXR response element (FXRE) on the CRYZ gene promoter. The luciferase reporter and ChIP assays revealed that FXR can bind directly to the FXRE site, which was further markedly enhanced by FXR activation. Furthermore, we found CRYZ overexpression in MCDs significantly attenuated hypertonicity-induced cell death possibly via increasing Bcl-2 expression. Collectively, our findings demonstrate that CRYZ is constitutively expressed in renal medullary collecting duct cells, where it is transcriptionally controlled by FXR. Given a critical role of FXR in MCDs, CRYZ may be responsible for protective effect of FXR on the survival of MCDs under hypertonic condition during dehydration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crystallins are water-soluble proteins discovered by Morner in 1894 as the main structural proteins of the vertebrate eye lens [17]. There are two main classes of these proteins. The first class is constituted by α (alpha), β (beta), and γ (gamma) crystallins, important for the refractive index and transparency of the vertebrate eye lens. The second class is phylogenetically restricted and taxon-specific. It consists of ζ (zeta), λ (lambda), ρ (rho), η (eta), τ (tau), μ (mu), ω (omega), ι (iota), υ (ypsilon), ɛ (epsilon), and δ (delta) crystallins, important for both eye lens and other tissues [6]. Among phylogenetically restricted crystallins, crystallin ζ (CRYZ) is expressed in the lens and many tissues out of the lens [14], suggesting CRYZ may play an important role in many physiological processes.
CRYZ is endowed with enzyme and nucleic acid–binding activities. CRYZ is unique due to its ability to act as a moonlighting protein with NADPH: quinone oxidoreductase and mRNA stabilizing activity [14]. As an enzyme, CRYZ is structurally related to alcohol dehydrogenase [4] but functionally related to NADPH:quinone oxidoreductase (QOR) [19]. Upon binding to the NADPH, CRYZ preserves the reducing environment and provides protection from oxidizing agents and quinones [3, 16, 20]. It has been recently reported that CRYZ also exerts protective role against lipid peroxidation products [18]. As an mRNA-binding protein, CRYZ can bind and stabilize Bcl-xL and Bcl-2 mRNA to provide resistance to apoptosis [8, 9, 13], suggesting that CRYZ may protect cells against various stress-induced cell death.
In addition to the lens, CRYZ is highly expressed in the kidney, where it may play an important role in regulating renal function [14]. It has been reported that CRYZ increases glutaminolysis via binding and stabilizing glutaminase (GLS) mRNA to increase NH4+ production and secretion and HCO3− biosynthesis and reabsorption in response to acidosis in the proximal tubules [24]. CRYZ also increases the expression and activity of the Na+/K+/2Cl− cotransporter BSC1 by binding and enhancing its mRNA stability in the medullary thick ascending limbs (mTALs) [23], therefore increasing NH4+ reabsorption in mTALs and helping maintain the medullary hyperosmotic gradient. Therefore, CRYZ may be critical in the regulation of the acid–base balance and urine concentration.
Although it has been previously reported that CRYZ can be repressed at transcriptional level by p53 [2], the underlying mechanisms involved in the regulation of CRYZ expression in the kidney remains largely uncharacterized. As a ligand-activated transcription factor, farnesoid X receptor (FXR) belongs to the superfamily of nuclear hormone receptor [28]. FXR is expressed at high level in liver and small intestine, where it controls lipid, glucose, and bile acid metabolism [1, 5]. FXR is also highly expressed in renal tubules and plays an important role in renal physiology [11, 29]. We and other groups have demonstrated that FXR activation protects kidney from ischemia reperfusion damage via reducing inflammation and oxidative stress [7] and increases the survival of the medullary collecting duct cells (MCDs) under hypertonic stress condition via decreasing cell apoptosis [27]. Importantly, It has been previously reported that crystallin α-A is a direct target gene of FXR in human hepatocytes [10]. These findings suggest that FXR may induce CRYZ expression to exert its antioxidative and antiapoptotic effect in renal tubular cells.
In the present study, we show that CRYZ is constitutively expressed in renal collecting ducts. FXR activation induces CRYZ expression in primary cultured medullary collecting duct cells (MCDs) isolated from wild-type mice, but not from the mice with global and collecting duct–specific FXR gene knockout. We also provide evidence that CRYZ is a novel FXR target gene in the MCDs and its overexpression significantly attenuates hypertonicity-induced cell death possibly via increasing Bcl-2 expression. These findings uncover a previously unknown function of FXR in the regulation of CRYZ expression in the kidney and suggest CRYZ may be responsible for protective effect of FXR on the survival of MCDs under hypertonic condition during dehydration.
Methods and materials
Chemicals and reagents
Chenodeoxycholic acid (CDCA), GW4064, supplements, and media for cell culture were bought from Sigma-Aldrich (St Louis, MO, USA). Primary antibodies used in this study were those against FXR (orb156973, Biorbyt, Cambridge, United Kingdom), CRYZ (NBP2-16017, Novus Biologicals, CO, USA), Bax (ab182734, Abcam, Cambridge, United Kingdom), Bcl-2 (3498S, CST, Danvers, MA, USA), and β-actin (BM0627, Boster, Wuhan, China). FXR siRNA was synthesized by Genepharma (Shanghai, China), while Lipofectamine 3000 reagent kit was obtained from ThermoFisher Scientific (Waltham, MA, USA).
Animals and genotyping
All the animals (C57BL/6 mice) used in the present study were maintained on a 12-h dark/12-h light cycle at a control temperature of 22–24 °C and 50–60% humidity. Mice were free to access food and water ad libitum. Wild-type (WT), global FXR knock-out (FXR−/−), and AQP2-cre-mediated collecting duct–specific FXR knockout (CD-FXR−/−) mice were generated and genotyped as previously reported [27, 28] (Supplementary Figure 1). Male mice aged 10–14 weeks were used for primary culture of MCDs. Animal experiments were approved by the Ethical Committee of the Dalian Medical University and conformed to international guidelines for animal usage in research.
Culture and treatment of primary medullary collecting duct cells
Primary medullary collecting duct cells (MCDs) were isolated from wild-type and global and collecting duct–specific FXR gene knockout mice according to an optimized protocol of our group [28]. The cells were then grown in DMEM/F12 (1:1) medium which contains 5 pM 3,3,5-triiodo-l-thyronine, 50 nM hydrocortisone, 5 mg/L transferrin, 1 nM sodium selenate, and 20% FBS. The osmolality of the control medium was 300 mOsm, and the hyperosmotic medium was 600 mOsm (80 mM NaCl and 120 mM urea). At 80–90% confluence, the cells were treated for 12 h with the synthetic FXR agonist GW4064 or naturally occurring agonist CDCA at various concentrations, or transfected with a CRYZ expression vector.
Real-time PCR
Reagent of TRIZOL (Biotek, Beijing, China) was used for the extraction of total RNA from mouse primary MCD cells. Total RNA (2 μg) was used for cDNA synthesis by using the RevertAidTM cDNA Synthess Kit (Fermentas, USA) according to the protocol of manufacturer’s. The cDNA was used as a template for RT-PCR along with SYBR Green Mix (Bio-Rad, Hercules, CA, USA). The optimized reaction conditions were as following: an initial denaturation at 95C for 5 min, and then 36 cycles of 94 °C incubation 30 s, 60 °C annealing for 30 s and 72 °C extension for 30 s, with a final extension for 5 min at 72 °C. For loading normalization, an internal control (GAPDH) was used, and the quantification was obtained based on the threshold cycle number (Ct). Each sample was run in triplicate, and each experiment was done three times. The list of primer pairs used for amplifying mouse genes interested was shown in Supplementary Table 1.
Western blot analysis
The RIPA lysis buffer containing the protease inhibitor and PMSF was used to extract proteins from mouse primary MCD cells. The concentrations of protein were determined with the use of the Protein Assay kit (Pierce BCA Thermo Scientific, Waltham, MA, USA). Equivalent amounts of sample protein (30∼60 μg) mixed with 5× or 6× loading buffer were run on 10–12% SDS/PAGE gel and then transferred to a nitrocellulose membrane (Thermo Scientific, Waltham, MA, USA). The nonspecific binding were block by incubation in 5% skimmed milk powder at room temperature for 1 h. After blocking, the membranes were incubated with selected primary antibodies (1:1000) at 4 °C overnight. Primary antibody incubation step was followed by three times of washing with TBS-T buffer for 5 min. The membrane was then incubated with HRP-conjugated secondary antibodies (ABclonal Technology, Woburn, MA, USA) for 1 h. After washed for three times with TBST, the signals were detected with the chemiluminescence substrate (sc-2048, Santa Cruz Biotechnology, Dallas, TX, USA). For densitometric analysis and protein expression level quantification, the Image J software (Rawak Software Inc. Germany) was used.
Immunohistochemistry
Mouse kidneys were isolated and fixed with 4% paraformaldehyde (PFA), dehydrated, and embedded in paraffin. Thin sections of 3 μm were prepared from the blocks. After dewaxing and rehydration, the sections were quenched in 3% H2O2 to remove endogenous hydroxyl peroxidase activity. Incubation with 5% BSA for 30 min was performed to block nonspecific binding. After overnight incubation at 4 °C with indicated primary antibody, secondary antibody conjugated with a horseradish peroxidase (Zhong-shan Golden Bridge, Beijing, China) was added and incubated for 30 min at 37 °C. Counterstaining with hematoxylin was performed, and for the detection of the antigen–antibody interaction, peroxidase diaminobenzidine (DAB) was used.
Immunofluorescence
MCD Cells were grown at an appropriate confluence, incubated with 4% PFA for 15 min on a shaking platform at room temperature for fixation. After washed with PBS, the cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min. Blocking by 5% BSA for 10 min was proceeded by the incubation with the primary antibody overnight at 4 °C. After washing, the cells were incubated with appropriate dyLight 488- (green) or dyLight 594 (red)–conjugated secondary antibodies for 30 min at 37 °C. For nucleus staining, DAPI (4', 6'-diamidino-2 phenylindole) was used. Images were taken using a confocal microscope.
Infection with adenovirus and transfection with siRNA
Mouse primary MCD cells were culture in 6-well plates at 60–70% confluency. To overexpress FXR, the cells were infected with an adenovirus carrying a cDNA encoding a full-length human FXRα2 (Ad-hFXRα2) or an adenovirus carrying a cDNA encoding GFP (Ad-GFP) for 24 h at 15 MOI (multiplicity of infection) dose [26] (Supplementary Figure 2). To knockdown the endogenous FXR mRNA expression, the cells were transfected with either FXR siRNA (FXR-siRNA) or scramble siRNA (NC-siRNA) (100 nM) with Lipofectamine® 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the standard protocol. After 24 h of transfection, the cells were isolated for RNA and protein extraction. The oligonucleotides of siRNA specific for FXR were designed and synthesized by Suzhou GenePharma Co., Ltd (Suzhou, China). The siRNA sequences were as follows: (1) NC-siRNA: sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′, antisense 5′-ACG UGA CAC GUU CGG AGA ATT-3′; (2) FXR-siRNA: sense 5′-GCC GUG UAC AAG UGU AAG ATT-3′, antisense 5′-UCU UAC ACU UGU ACA CGG CTT-3.
Luciferase reporter assay
Mouse CRYZ gene promoter–driven luciferase reporter was constructed. Briefly, mouse CRYZ promoter region was amplified from tail-derived genomic DNA of a C57BL/6 mouse. The mouse CRYZ promoter region containing the fragment – 1000 to approximately − 10 bp was amplified by PCR with the forward primer 5′-CCA CAG GTT CCA GGA GTT CTT-3′ and the reverse primer 5′- CGC GCT AAC CCA ATC ACT GT-3′. The amplified fragment was cloned into the luciferase reporter gene vector PGL3 Basic (Promega, Madison, WI, USA), and the resultant construct designated CRYZ-Luc was sequenced to validate the orientation and sequence. MCD cells were allowed to grow to 60–70% confluence and were transfected with the CRYZ-Luc reporter for 24 h using the Lipofectamine® 3000 Transfection Reagent. Following 6-h incubation with GW4064 (2.5 μM) or CDCA (50 μM), the cells were harvested in the luciferase lysis buffer provided by the manufacture for the detection of luciferase activity by a luminometer (Turner BioSystems), which was normalized with renilla luciferase activity. Transfection experiments were performed in triplicate. Data were represented as fold induction over reporter gene alone.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was done using the SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) (#9005 Cell Signaling Technology, Boston, MA, USA) based on the manufacturer’s standard protocol. The primary MCD cells were treated with GW4064 (2.5 μM) for 12 h and then incubated with 1% formaldehyde at 37 °C for 10 min to fixed the cells. The cells were scratched pelleted, and lysed in lysis buffer provided the manufacture. DNA was cut by sonication to fragments of 200–900 bp and diluted in the dilution buffer. Positive control (Input) was kept from 1% of the diluted cell supernatant. The chromatin was precleared with enzyme and was incubated at 4 °C overnight with an antibody directed against FXR (PP-A9033A, Perseus Proteomics, Shanghai, China). The immuno-complexes were eluted and treated to reverse histone-DNA crosslinks. The purified DNA sample was used as a template. The region between − 889 and − 708 bp of mouse CRYZ gene promoter was amplified using the following primers: 5′-AGG AGG CTG GAA GTT TCA CTC A-3′ (sense), 5′-TGT AAC CTG CAA TCC TTT CCT CAT T-3′ (antisense). Two percent agarose gel was used for the visualization of 181 bp of the PCR products.
Cell viability assay
Cell viability of the MCDs was evaluated by the CCK-8 assay kit obtained from Dojindo (Kumamoto, Japan). Ten microliters of CCK-8 reagent was added to the cells, which were then seeded in a 96-well plate in a humidified 5% CO2 atmosphere at 37 °C for 2 h. The optical density (OD) was measured with a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) at 450 nm.
Statistical analysis
Statistical analysis was performed using PRISM. Results were presented as means ± standard error of the mean (SEM). Comparisons were performed by t test, with statistical significance set at a p value of < 0.05. Variations between the groups were examined by means of one way ANOVA.
Results
CRYZ is constitutively expressed in mouse renal medullary collecting ducts
Real-time PCR and western blot assays were utilized to determine the expression levels of CRYZ in mouse renal cortex and medulla. The results showed that CRYZ was ubiquitously expressed in mouse kidney (Fig. 1a and b). Immunohistochemistry study further revealed that CRYZ is constitutively localized in many renal tubules, especially medullary collecting duct cells (Fig. 1c). These findings suggest that CRYZ is constitutively express in medullary collecting duct cells where it might play an important role in these cells.
CRYZ is constitutively expressed in renal medullary collecting ducts. C57BL/6 mouse kidney was used for detecting CRYZ expression. a Real Time-PCR analysis of CRYZ mRNA expression in renal cortex, outer medulla (OM), and IM (inner medulla). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 vs. cortex. n = 4. b Western blot assay showing CRYZ protein levels in renal cortex, OM, and IM. c Immunohistochemistry study demonstrating the localization of CRYZ protein in renal medullary collecting ducts. Aquaporin 2 (AQP2) staining was used as a marker for the collecting ducts. IgG staining was used as a negative control. Arrows indicate renal tubules with CRYZ expression (left) and AQP2 expression (right)
CRYZ is expressed in primary cultured medullary collecting duct cells
Mouse primary medullary collecting duct (MCD) cells were cultured and used for confirming constitutive CRYZ expression. CRYZ mRNA and protein expression was observed in three preparations of mouse MCDs as assessed by RT-PCR (Fig. 2a) and immunoblot assays (Fig. 2b). Immunofluorescence analysis further revealed that CRYZ protein was mainly expressed in the cytoplasm of MCD cells (Fig. 2c). These findings demonstrate that CRYZ is constitutively present in MCDs where FXR is also coexpressed.
CRYZ is expressed in primary cultured medullary collecting duct (MCD) cells. Mouse primary MCD cells were cultured and used for detecting CRYZ expression. a RT-PCR analysis of CRYZ mRNA expression, n = 3. b Western blot assay showing CRYZ protein expression, n = 3. c Immunofluorescence analysis showing cytoplasmic localization of CRYZ protein (green). β-actin was used as a loading control. DAPI was used to stain the nuclei (blue)
FXR activation and overexpression induces CRYZ expression in primary MCD cells
In order to test whether FXR regulates CRYZ expression in MCDs, we treated the cells with an endogenous FXR agonist CDCA (50 μM) and a synthetic specific FXR agonist GW4064 (2.5 μM) for 12 h. Activation of FXR by either CDCA or GW4064 significantly increased CRYZ expression at both mRNA and protein levels as compared with the vehicle control (DMSO) (Fig. 3a–c). Consistently, adenovirus-mediated FXR overexpression (Supplementary Figure 2) also markedly upregulated CRYZ mRNA and protein expression as compared with control Ad-GFP (Fig. 3d–f). These findings demonstrate that FXR activation by its agonists CDCA and GW4064 and FXR overexpression dramatically increases CRYZ expression in MCD cells.
FXR activation and overexpression induce CRYZ expression in primary cultured medullary collecting duct cells. Primary MCD cells were treated with CDCA (50 μM) and GW4064 (2.5 μM) for 12 h, or infected with Ad-hFXRα2 (15 MOI) for 24 h. a Real-time PCR analysis showing the mRNA levels of CRYZ in the MCDs treated with GW4064 and CDCA. ***p < 0.001 vs. DMSO, n = 6; b Western blot assay showing the protein levels of CRYZ in the MCDs treated with CDCA and GW4064. c Quantification of CRYZ levels in (b) was performed. ***p < 0.001 vs. DMSO, n = 3; (d) Real-time PCR assay demonstrating that FXR overexpression induced CRYZ expression. ***p < 0.001 vs. control, n = 6; e Western blot analysis and f quantification of CRYZ protein expression in the MCDs infected with Ad-hFXRα2. ***p < 0.001 vs. Ad-GFP, n = 6. Data were presented as mean ± SEM, β-actin was used as an internal control
FXR silencing reduces CRYZ expression in primary MCDs
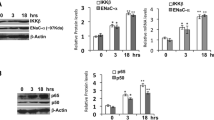
To test whether FXR knockdown can decrease the expression of CRYZ, we used siRNA-mediated approach to specific knockdown FXR mRNA expression. As shown in Fig. 4, siRNA-mediated FXR silencing markedly reduced FXR expression at mRNA (Fig. 4a) and protein (Fig. 4b, c) levels. As expected, FXR silencing significantly decreased CRYZ mRNA expression as assessed by real-time PCR (Fig. 4a). Consistently, FXR knockdown markedly suppressed CRYZ protein expression as determined by western blot assay (Fig. 4b, c) and immunofluorescence (Fig. 4d), respectively. These findings suggest that reduced FXR expression leads to decreased CRYZ abundance in MCD cells.
Effect of FXR silencing on the expression of CRYZ. Primary cultured medullary collecting duct cells were transfected with FXR-siRNA or scrambled siRNA (NC-siRNA) (100 nM) for 24 h. a Quantitative PCR demonstrating that FXR-siRNA effectively silenced FXR expression and reduced CRYZ expression levels. b Western blot assay showing that both FXR and CRYZ protein levels were markedly decreased after FXR-siRNA treatment. c Quantification of FXR and CRYZ protein levels in (b); d Immunofluorescence study showing a marked reduction in CRYZ protein levels (green). Data are represented as the mean ± SEM. *p < 0.05 vs. siRNA-NC, n = 4. β-actin was used as an internal control
FXR activation fails to induce CRYZ expression in primary MCDs isolated from conventional and renal collecting duct-specific FXR−/− mice
To further confirm FXR can directly induce CRYZ expression in MCDs, conventional FXR gene knockout mice (FXR−/−) and renal collecting duct-specific FXR knockout mice (CD-FXR−/−) mice were generated as we previously reported [28] (Supplementary Figure 1). We then cultured primary MCDs from wild-type (WT) mice and FXR−/− and CD-FXR−/− mice and treated with CDCA (50 μM) and GW4064 (2.5 μM) for 12 h. As expected, CDCA and GW4064 treatment induced CRYZ expression in MCDs cultured from the WT (FXR+/+) mice but failed to induce CRYZ expression at both mRNA and protein levels in MCDs cultured from FXR−/− and CD-FXR−/− mice (Fig. 5a–c). Similarly, although CDCA and GW4064 treatment increased CRYZ mRNA and protein expression in control (FXRf/f) mice, it had little effect in CD-FXR−/− mice (Fig. 5d–f). Together, these data indicate that treatment of CDCA and GW4064 induces CYRZ expression in an FXR-dependent manner.
FXR activation fails to induce CRYZ expression in primary cultured MCDs of conventional and renal collecting duct–specific FXR−/− mice. Primary MCDs were isolated from wild-type mice (FXR+/+), FXR knockout mice (FXR−/−), FXRf/f mice and renal collecting duct–specific FXR−/− mice (CD-FXR−/−). The MCDs were treated with GW4064 (2.5 μM) or CDCA (50 μM) for 12 h. a Real-time PCR analysis showing that FXR activation by CDCA and GW4064 significantly induced CRYZ expression in FXR+/+ mice, but not FXR−/− mice. **p < 0.01 vs. FXR+/+ DMSO, n = 4; b Immunoblot analysis and c quantification of CRYZ protein levels. FXR activation increased CRYZ protein levels in FXR+/+ mice, but not in FXR−/− mice. ***p < 0.001 vs. FXR+/+ DMSO, n = 4. d Real-time PCR analysis showing that FXR activation by CDCA and GW4064 significantly induced CRYZ expression in FXRf/f mice, but not CD-FXR−/− mice. **p < 0.01 vs. FXRf/f DMSO, n = 4; e Western analysis and f quantification of CRYZ protein levels. FXR activation increased CRYZ protein levels in FXRf/f mice, but not in CD-FXR−/− mice. ***p < 0.001 vs. FXR+/+ DMSO, n = 4. Data were presented as mean ± SEM. β-actin was used as an internal control
CRYZ is a direct target gene of FXR
The PROMO 3.0 software was used for analyzing potential FXR binding sites in mouse CRYZ gene promoter region. A putative FXRE sequence between − 759 and approximately − 748 bp upstream to the transcription start site was identified (Fig. 6a). To confirm the binding of FXR to the CRYZ gene promoter, the plasmid of CRYZ-Luc was constructed and transfected into MCD cells in the absence or presence of GW4064 or CDCA. The luciferase reporter assay showed that both CDCA and GW4064 treatment significantly increased the transcription activity of the CRYZ-Luc reporter (Fig. 6b), suggesting FXR activation may increase the transcription of CRYZ gene. To further confirm the binding of FXR to the CRYZ gene promoter, we utilized the oligonucleotide-directed site-specific mutagenesis technique and generated a mutant CRYZ gene promoter–driven luciferase reporter (mutant CRYZ-Luc) with the potential FXRE site between − 759 and approximately − 748 bp disrupted (Fig. 6a). As expected, both GW4064 and CDCA failed to increase the activity of the mutant CRYZ-Luc (Fig. 6b). In support, the ChIP assay further revealed a single band with the estimated size which perfectly matches the sequence between − 759 and approximately − 748 bp, indicating FXR indeed directly binds to the consensus FXRE sequence located within the CRYZ gene promoter. In addition, GW4064 treatment significantly enhanced the binding of FXR to the CRYZ gene promoter (Fig.6c and d). These findings indicate that CRYZ represents a novel target gene of FXR.
FXR activation significantly enhances the activity of mouse CRYZ gene promoter. a Schematic structure of CRYZ gene and sequence comparison between wild-type and mutated FXRE site. DNA sequence analysis revealing a potential FXRE-like site within the proximal promoter region of CRYZ gene located between − 759 and − 748 bp upstream from the transcription start site. The wild-type promoter fragment and the promoter fragment with the FXRE site mutation were used for the construction of the CRYZ-Luc reporter and mutant CRYZ-Luc reporter, respectively. b GW4064 (2.5 μM) and CDCA (50 μM) treatment for 6 h significantly increased the luciferase activity of the wild-type CRYZ-Luc reporter in primary MCD cells, but not the mutated CRYZ-Luc reporter. **p < 0.01, ***p < 0.001 vs. DMSO, n = 6; c ChIP assay showing FXR binding to the FXRE-like site in mouse CRYZ promoter. A predicted PCR amplification band of 181 bp was evident and confirmed by sequencing. Note: GW4064 treatment significantly increased FXR binding to the CYRZ gene promoter. Input, positive control; FXR, anti-FXR antibody precipitated DNA; IgG, IgG precipitated DNA as negative control; d GW4064 treatment significantly increased the binding of FXR to the FXRE site in the MCDs as assessed by semiquantitative PCR. **p < 0.01 vs. DMSO, n = 4. Data are represented as the mean ± SEM
CRYZ protects MCDs from apoptosis under hypertonic condition
To further investigate the CRYZ function in MCDs, we overexpressed CRYZ under normal or hypertonic conditions (Fig. 7c). The overexpression of CRYZ markedly improved the morphological changes induced by hypertonic stress (600 mOsm) (Fig. 7a). In addition, the CCK-8 assay showed CRYZ overexpression significantly reduced hypertonicity-induced cell death in MCDs (Fig. 7b). To elucidate the underlying mechanism, the Bcl-2-associated apoptotic pathway was determined by western blot assay (Fig. 7c). The results showed that hypertonic condition significantly decreased Bcl-2 protein level and increased Bax expression, which was largely reversed by CRYZ overexpression. These observations support the cytoprotective effect of CRYZ under hypertonic stress in MCDs.
CRYZ protects MCDs from apoptosis under hypertonic condition. a Effect of CRYZ overexpression on cell morphology in MCDs. b CCK-8 analysis showing the difference of cell viability between control (mock) and CRYZ overexpression groups under normal (300 mOsm) and hypertonic conditions. Data represent the mean ± SEM. n = 6 for each group. ****p < 0.0001, the CRYZ group compared with the mock group under normal condition; #### p < 0.0001, the mock group under hypertonic condition vs. the mock group under normal condition; ††††p < 0.0001, the CRYZ group compared with the mock group under hypertonic condition. c Western blot analysis demonstrating the effect of CRYZ overexpression on the protein levels of Bcl-2 and Bax
Discussion
In the present study, we show that CRYZ is constitutively expressed in renal collecting ducts, where FXR is also colocalized [27, 28]. In normal renal MCD cells, FXR activation via its specific agonists or FXR overexpression via an adenovirus-mediated approach induces CRYZ expression, while siRNA-mediated FXR silencing markedly reduces CRYZ expression. In addition, in renal MCD cells isolated from global FXR gene deficient mice or renal collecting duct–specific FXR knockout mice, FXR agonist treatment has little effect on CRYZ expression. Mechanistically, FXR can directly bind to a putative FXRE site located in the promoter region of CRYZ gene, thereby increasing CRYZ transcription. Given the critical role of FXR in maintaining renal medullary cell survival under hypertonic conditions and antiapoptotic properties of CRYZ, our findings suggest that FXR may exert its protective effect in renal medulla via inducing CRYZ expression in medullary collecting ducts.
FXR is a member of the superfamily of nuclear receptor and activated by endogenous bile acids [15]. FXR is abundantly expressed in the liver and small intestine, where it exerts a variety of biological functions important for bile acid, glucose, and lipid metabolism [30]. FXR is also found to be expressed at high level in the kidney, especially medullary collecting ducts [27, 28]. We and others have previously shown that collecting duct FXR is critical in the maintenance of sodium and fluid homeostasis [12, 22, 28]. Recently, it has been reported that FXR is critical for the survival of MCD cells under hyperosmotic stress with the underlying mechanism incompletely characterized [27]. We also provide evidence that CYRZ is a novel FXR target gene in the kidney. In addition, we found that CRYZ act as an antiapoptotic protein under hypertonic condition in medullary collecting duct cells. Our findings may help uncover the underlying mechanisms by which FXR protects the cells in hypertonic renal medulla through the induction of CRYZ expression.
Unlike most of other crystallins, CRYZ is endowed with enzyme and nucleic acid–binding activities [14]. As an enzyme, CRYZ is structurally related to alcohol dehydrogenase [4] but functionally related to NADPH:quinone oxidoreductase (QOR) [19]. Upon binding to the NADPH, CRYZ provides protection from oxidizing agents and quinones [3, 16, 20] and lipid peroxidation products [18]. As an mRNA-binding protein, CRYZ can bind and stabilize Bcl-xL and Bcl-2 mRNA to protect the cell against apoptosis under various stresses [8, 9, 13]. In the present study, we show that CRYZ is expressed in medullary collecting duct cells, which are vulnerable to hypoxic and hyperosmotic stress. Due to its antioxidant and antiapoptotic activities, CRYZ may play an important role in maintaining renal MCD cell viability under dehydration condition. This issue warrants further investigation.
CRYZ has been previously reported to be expressed in both eye lens and other tissues [14]. In tissues out of the lens, the kidney has the most abundant CRYZ expression. It has been previously shown that CRYZ is localized in the proximal tubule cells, where it promotes glutaminolysis to help produce HCO3− and NH4+ in response to acidosis [21, 24, 25]. CRYZ is also expressed in medullary thick ascending limbs (mTALs) and increases the expression and activity of the Na+/K+/2Cl− cotransporter BSC1 via a posttranscriptional mechanism [23] thereby increasing NH4+ reabsorption in mTALs and maintaining the medullary hyperosmotic gradient. The present study reports that CRYZ is also constitutively expressed in the medullary collecting ducts. Given an important role of the BSC2/NKCC1 in NH4+ excretion in the medullary collecting ducts, it would be helpful to determine the cell types with CRYZ expression and whether CRYZ induces BSC2/NKCC1 expression as well under conditions with chronic metabolic acidosis.
In summary, in the present study, we report that CRYZ is constitutively expressed in renal medullary collecting ducts. FXR activation and overexpression induce CRYZ expression in primary cultured medullary collecting duct cells (MCDs) isolated from wild-type mice but not from the mice with global and collecting duct–specific FXR gene knockout. We also show that CRYZ is a novel FXR target gene in the MCDs where it protects MCDs from hypertonicity-induced cell death. These findings uncover a previously unknown function of FXR in the regulation of CRYZ expression in the kidney and suggest CRYZ may be responsible for protective effect of FXR on the survival of MCDs under hypertonic condition in response to dehydration.
References
Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ (2001) Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 276:28857–28865. https://doi.org/10.1074/jbc.M011610200
Bansal N, Kadamb R, Mittal S, Vig L, Sharma R, Dwarakanath BS, Saluja D (2011) Tumor suppressor protein p53 recruits human Sin3B/HDAC1 complex for down-regulation of its target promoters in response to genotoxic stress. PLoS One 6:e26156. https://doi.org/10.1371/journal.pone.0026156
Bazzi MD, Rabbani N, Duhaiman AS (2001) High-affinity binding of NADPH to camel lens zeta-crystallin. Biochim Biophys Acta 1544:283–288. https://doi.org/10.1016/s0167-4838(00)00228-4
Borras T, Persson B, Jornvall H (1989) Eye lens zeta-crystallin relationships to the family of "long-chain" alcohol/polyol dehydrogenases. Protein trimming and conservation of stable parts. Biochemistry 28:6133–6139. https://doi.org/10.1021/bi00441a001
Chiang JY, Kimmel R, Weinberger C, Stroup D (2000) Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem 275:10918–10924. https://doi.org/10.1074/jbc.275.15.10918
Fujii Y, Watanabe K, Hayashi H, Urade Y, Kuramitsu S, Kagamiyama H, Hayaishi O (1990) Purification and characterization of rho-crystallin from Japanese common bullfrog lens. J Biol Chem 265:9914–9923
Gai Z, Chu L, Xu Z, Song X, Sun D, Kullak-Ublick GA (2017) Farnesoid X receptor activation protects the kidney from ischemia-reperfusion damage. Sci Rep 7:9815. https://doi.org/10.1038/s41598-017-10168-6
Kadamb R, Mittal S, Bansal N, Batra H, Saluja D (2013) Sin3: Insight into its transcription regulatory functions. Eur J Cell Biol 92:237–246. https://doi.org/10.1016/j.ejcb.2013.09.001
Lapucci A, Lulli M, Amedei A, Papucci L, Witort E, Di Gesualdo F, Bertolini F, Brewer G, Nicolin A, Bevilacqua A, Schiavone N, Morello D, Donnini M, Capaccioli S (2010) zeta-Crystallin is a bcl-2 mRNA binding protein involved in bcl-2 overexpression in T-cell acute lymphocytic leukemia. FASEB J 24:1852–1865. https://doi.org/10.1096/fj.09-140459
Lee FY, Kast-Woelbern HR, Chang J, Luo G, Jones SA, Fishbein MC, Edwards PA (2005) Alpha-crystallin is a target gene of the farnesoid X-activated receptor in human livers. J Biol Chem 280:31792–31800. https://doi.org/10.1074/jbc.M503182200
Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA (2006) FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res 47:201–214. https://doi.org/10.1194/jlr.M500417-JLR200
Li S, Qiu M, Kong Y, Zhao X, Choi HJ, Reich M, Bunkelman BH, Liu Q, Hu S, Han M, Xie H, Rosenberg AZ, Keitel V, Kwon TH, Levi M, Li C, Wang W (2018) Bile acid G protein-coupled membrane receptor TGR5 modulates aquaporin 2-mediated water homeostasis. J Am Soc Nephrol 29:2658–2670. https://doi.org/10.1681/ASN.2018030271
Lulli MLR, Lapucci A, Papucci L, Capaccioli S, Schiavone N (2016) Involvement of ζ-crystallin in acidic microenvironment of melanoma: In: 3rd Joint meeting of pathology and laboratory medicine. Am J Pathol S18
Lulli M, Nencioni D, Papucci L, Schiavone N (2019) Zeta-crystallin: a moonlighting player in cancer. Cell Mol Life Sci 77:965–976. https://doi.org/10.1007/s00018-019-03301-3
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365. https://doi.org/10.1126/science.284.5418.1362
Malik A, Albogami S, Alsenaidy AM, Aldbass AM, Alsenaidy MA, Khan ST (2017) Spectral and thermal properties of novel eye lens zeta-crystallin. Int J Biol Macromol 102:1052–1058. https://doi.org/10.1016/j.ijbiomac.2017.04.101
Mörner CT (1894) Untersuchungen der Proteinsubstanzen in den lichtbrechenden Medien des Auges. Z Physiol Chem 18:223–256
Porte S, Moeini A, Reche I, Shafqat N, Oppermann U, Farres J, Pares X (2011) Kinetic and structural evidence of the alkenal/one reductase specificity of human zeta-crystallin. Cell Mol Life Sci 68:1065–1077. https://doi.org/10.1007/s00018-010-0508-2
Rao PV, Krishna CM, Zigler JS Jr (1992) Identification and characterization of the enzymatic activity of zeta-crystallin from guinea pig lens. A novel NADPH:quinone oxidoreductase. J Biol Chem 267:96–102
Rao PV, Horwitz J, Zigler JS Jr (1994) Chaperone-like activity of alpha-crystallin. The effect of NADPH on its interaction with zeta-crystallin. J Biol Chem 269:13266–13272
Schroeder JM, Liu W, Curthoys NP (2003) pH-responsive stabilization of glutamate dehydrogenase mRNA in LLC-PK1-F+ cells. Am J Physiol Ren Physiol 285:F258–F265. https://doi.org/10.1152/ajprenal.00422.2002
Su W, Cao R, Zhang XY, Guan Y (2020) Aquaporins in the kidney: physiology and pathophysiology. Am J Physiol Ren Physiol 318:F193–F203. https://doi.org/10.1152/ajprenal.00304.2019
Szutkowska M, Vernimmen C, Debaix H, Devuyst O, Friedlander G, Karim Z (2009) Zeta-crystallin mediates the acid pH-induced increase of BSC1 cotransporter mRNA stability. Kidney Int 76:730–738. https://doi.org/10.1038/ki.2009.265
Tang A, Curthoys N (2001) Identification of -crystallin/NADPH:quinone reductase as a renal glutaminase mRNA pH response element-binding protein. J Biol Chem 276:21375–21380. https://doi.org/10.1074/jbc.M101941200
Taylor L, Curthoys NP (2004) Glutamine metabolism: role in acid-base balance*. Biochem Mol Biol Educ 32:291–304. https://doi.org/10.1002/bmb.2004.494032050388
Wang B, Zhang H, Luan Z, Xu H, Wei Y, Zhao X, Xing M, Huo X, Zhang J, Su W, Guan Y, Zhang X (2020) Farnesoid X receptor (FXR) activation induces the antioxidant protein metallothionein 1 expression in mouse liver. Exp Cell Res:111949. https://doi.org/10.1016/j.yexcr.2020.111949
Xu S, Huang S, Luan Z, Chen T, Wei Y, Xing M, Li Y, Du C, Wang B, Zheng F, Wang N, Guan Y, Gustafsson J-Å, Zhang X (2018) Farnesoid X receptor is essential for the survival of renal medullary collecting duct cells under hypertonic stress. Proc Natl Acad Sci U S A 115:5600–5605. https://doi.org/10.1073/pnas.1803945115
Zhang X, Huang S, Gao M, Liu J, Jia X, Han Q, Zheng S, Miao Y, Li S, Weng H, Xia X, Du S, Wu W, Gustafsson JA, Guan Y (2014) Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci U S A 111:2277–2282. https://doi.org/10.1073/pnas.1323977111
Zhang XY, Wang B, Guan YF (2016) Nuclear receptor regulation of aquaporin-2 in the kidney. Int J Mol Sci 17. https://doi.org/10.3390/ijms17071105
Zhu Y, Liu H, Zhang M, Guo GL (2016) Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B 6:409–412. https://doi.org/10.1016/j.apsb.2016.07.008
Funding
This work was supported by the National Natural Science Foundation of China Grants 81722010, 81970606 (to X.Z.); 91639201 and 81970595 (to Y.G.); 81601174 (to Z. L.); 81900267 (to H.X.) and the Liaoning BaiQianWan Talents Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 629 kb).
Rights and permissions
About this article
Cite this article
Alam, G., Luan, Z., Gul, A. et al. Activation of farnesoid X receptor (FXR) induces crystallin zeta expression in mouse medullary collecting duct cells. Pflugers Arch - Eur J Physiol 472, 1631–1641 (2020). https://doi.org/10.1007/s00424-020-02456-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02456-4