Abstract
Chronic kidney disease (CKD) represents a growing public health problem associated with loss of kidney function and cardiovascular disease (CVD), the main leading cause of morbidity and mortality in CKD. It is well established that CKD is associated with gut dysbiosis. Over the past few years, there has been a growing interest in studying the composition of the gut microbiota in patients with CKD as well as the mechanisms by which gut dysbiosis contributes to CKD progression, in order to identify possible therapeutic targets to improve the morbidity and survival in CKD. The purpose of this review is to explore the clinical evidence and the mechanisms involved in the gut-kidney crosstalk as well as the possible interventions to restore a normal balance of the gut microbiota in CKD. It is well known that the influence of the gut microbiota on the gut–kidney axis acts in a reciprocal way: on the one hand, CKD significantly modifies the composition and functions of the gut microbiota. On the other hand, gut microbiota is able to manipulate the processes leading to CKD onset and progression through inflammatory, endocrine, and neurologic pathways. Understanding the complex interaction between these two organs (gut microbiota and kidney) may provide novel nephroprotective interventions to prevent the progression of CKD by targeting the gut microbiota. The review is divided into three main sections: evidences from clinical studies about the existence of a gut microbiota dysbiosis in CKD; the complex mechanisms that explain the bidirectional relationship between CKD and gut dysbiosis; and reports regarding the effects of prebiotic, probiotic, and synbiotic supplementation to restore gut microbiota balance in CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) affects between 8 and 16% of the total population and represents a growing public health problem, since patients with not adequately controlled CKD progress to end-stage renal disease (ESRD) and often develop cardiovascular disease (CVD), the main leading cause of morbidity and mortality in CKD [13, 118]. A number of well-known risk factors have been described to promote cardiovascular complications in patients with CKD such as hypertension, dyslipidemia, obesity, and diabetes; however, in the last two decades, other novel risk factors have also been identified, such as chronic systemic inflammation and gut microbiota, which have risen as key factors in the pathogenesis and progression of CVD in CKD [69, 89]. The relationship between renal disease and the gut microbiota is recognized as an emerging spotlight of research. Gastrointestinal (GI) microbiota is composed by approximately 1 trillion of microorganism with thousands of species encoding more than 3 million of genes (150-fold more than human genome) [74]. The gut microbiota is mainly represented by 5 phyla: Firmicutes, Bacteriodetes, Actinobacteria, Verrucomicrobia, and Proteobacteria [86]. As an essential part of human health, a healthy gut microbiota provides beneficial effects to the host by regulating physiologic homeostasis including the immune system [46]. The concept of dysbiosis implies an imbalance in gut microbiota composition and its metabolic capacity that could promote chronic diseases including kidney disease. In this context, pathogenic bacteria predominate and synthetize different harmful substances and toxins causing chronic immune activation [62]. The kidney–gut crosstalk refers to the association between CDK, the GI environment, and changes in the gut epithelial barrier permeability [47]. Particularly, the influence of the gut microbiota on the gut–kidney crosstalk plays a fundamental role in CKD, acting in a reciprocal way: on the one hand, CKD significantly modifies the composition and functions of the gut microbiota and contributes to dysbiosis in humans [50, 111]. On the other hand, gut microbiota is able to manipulate the processes leading to CKD onset and progression through inflammatory, endocrine, and neurologic pathways [81]. The purpose of this review is to explore the clinical evidence and the mechanisms involved in the relationship between gut dysbiosis and CKD as well as the strategies to restore a normal balance in gut microbiota in CKD. Understanding the complex interaction between these two organs (gut microbiota and kidney) may provide novel nephroprotective interventions to prevent the progression of CKD by targeting the gut microbiota.
Gut microbiota dysbiosis in CKD: looking for clues and evidences
It is well established that CKD is associated with gut dysbiosis. In this way, there has been a growing interest in studying the composition and richness of the gut microbiota in patients with CKD as well as the mechanisms by which gut dysbiosis contributes to the progression of CKD, in order to identify possible therapeutic targets to improve the morbidity and survival of patients with CKD.
The existence of intestinal microbiota alterations such as decrease of microbial richness, diversity, and uniformity has been related to CKD [81]. Patients with CDK show a lower colonization of Bifidobacteriaceae families, mainly Bifidobacterium, Lactobacillaceae, Bacteroidaceae, and Prevotellaceae genera and higher intestinal levels of Enterobacteriaceae, particularly Enterobacter, Klebsiella, and Escherichia, and also increased levels of Enterococci and Clostridium perfringes [47, 100]. By using multiple independent datasets, Wilkins et al. determined the type of dysbiosis for a cluster of chronic diseases including kidney disease. The authors demonstrated that antibiotic-driven loss of gut microbiota diversity may increase the risk for kidney disease as well as other chronic conditions like CVD, obesity, and diabetes. In this study, the most frequent dysbiotic genera pattern associated with kidney disease were Bacteroides, Corynebacterium, Anaerococcus, Prevotella, Rothia, Sutterella, Eubacterium, Fusobacterium, Leptotrichia, Parabacteroides, Peptoniphilus, Porphyromonas, and Veillonella. According to this study, the dysbiosis associated with kidney disease is more likely due to a loss of diverse genera more than a gain of microbial genera [120]. Another study compared the fecal samples from patients with CKD and healthy control subjects and demonstrated that patients with CKD exhibited a significant reduction in gut microbiota richness and composition, with reduced abundance of Actinobacteria phylum and Akkermansia genera but increased abundance of Verrucomicrobia phylum and enrichment of the genera Lactobacillus, Clostridium IV, Paraprevotella, Clostridium sensustricto, Desulfovibrio, and Alloprevotella. Conversely, healthy control subjects exhibited higher abundance of Akkermansia and Parasutterella genera. The decrease in the abundance of Akkermansia, an important probiotic, in patients with CKD negatively correlated with plasma interleukin-10 (IL-10) levels, suggesting that an altered microbiota in CKD may promote chronic systemic inflammation [62]. Additionally, a systematic review carried out by Chung et al. included 11 clinical studies in order to characterize GI microbiota in patients with CKD. The authors reported that one-third of the patients with CKD exhibited enrichment of pathogen bacteria Escherichia coli and Enterobacter with reduced amounts of butyrate-producing bacteria Roseburia spp. In those patients with mild CKD, a relationship between early stages of impaired renal function and rising numbers of uremic toxin-producing bacteria was found [15]. In the subgroup of CKD patients with ESRD, Vaziri et al. found changes in 190 bacterial operational taxonomic units (OTUs) compared to healthy subjects, with special overgrowth of Actinobacteria, Proteobacteria, and Firmicutes (mainly Clostridia) [111]. Biruete et al. found in patients on hemodialysis (HD) that Firmicutes/Bacteroidetes ratio positively correlated with traditional cardiovascular risk factors like aortic and braquial systolic pressure [6]. Moreover, Faecalibacterium spp. was positively associated with carbohydrate intake and inversely associated with carotid-femoral pulse wave velocity, a surrogate marker of arterial stiffness. They also found that lipopolysaccharide (LPS) serum levels were inversely associated with butyrate-producing bacteria such as Ruminococcus and Oscillospira spp. These results open up the question whether targeting the gut microbiota could result in a lower burden for CVD in HD patients [6]. Considering the evidence to date, dysbiosis of the gut microbiota in patients with CKD is characterized by a decrease of bacterial species with saccharolytic fermentation activity such as Lactobacillus and Prevotella and an enrichment of bacterial species with proteolytic fermentation activity, Bacteroides and Clostridium among them, with increased levels of circulating uremic toxins from fermentation of nitrogen-containing compounds that result in a chronic inflammatory state in this group of patients [11].
Changes in gut microbiota in patients with CKD are not only limited to stool samples. Gut dysbiosis in CKD leads to high intestinal permeability which allows intestinal bacteria and their products to translocate into the host blood circulation. It has been reported that even healthy human donors carry on a circulating microbiome in blood [82]. A pilot study demonstrated that patients with CKD exhibit a different blood microbiome profile compared to healthy control patients with more variability in bacterial 16S rDNA quantity and a decrease in α diversity (bacterial taxa richness). At taxonomic level, it has been detected a total of 22 OTUs significantly different between both groups, with a high proportion of Proteobacteria at phylum level, Gammaproteobacteria at class level, and Enterobacteriaceae and Pseudomonadaceae at family level in the CKD group. Additional data point out that the proportion of Proteobacteria inversely correlates with glomerular filtration rate (GFR) [99]. Gut dysbiosis in a context of CKD can also lead to a poor clinical outcome due to its impact on cognitive function [56]. It has been proposed a possible link between gut microbiota, inflammatory cytokines, and neuronal network connectivity. Wang et al. demonstrated that ESRD patients exhibit gut dysbiosis, increased systemic inflammation, and disrupted topological organization with impaired network connectivity in brain and worse cognitive performance compared to control group [117]. This finding highlights the important role of establishing a normal balance in gut microbiota to keep a healthy gut-cerebral axis that favorably impacts on cognitive behavior.
Increasing evidence from recent years indicates that gut dysbiosis has a critical role in the pathogenesis of chronic systemic inflammation. In a context of gut dysbiosis, pathogen bacteria overwhelm beneficial bacteria and release large amounts of immunogen substances including LPS and peptidoglycans, which activate the intestinal mucosa immune system and disrupt intestinal permeability, with translocation of bacterial products into the host circulatory system, thereby favoring the production of inflammatory mediators like IL-6, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) [36, 95, 96]. Supporting this fact, patients with type 2 diabetes and CKD (stages 4 and 5 without dialysis) present a significant increase in gram-negative bacteria such as Proteobacteria, Verrucomicrobia, and Fusobacteria in fecal microbiota samples. Gram-negative bacteria exhibit in the outer membrane a potent endotoxin, LPS, recognized by cell surface receptor of immune cells like Toll-like receptor 4 (TLR4) which induces the production of pro-inflammatory cytokines via nuclear factor-κB (NF-κB) [10, 57]. Serum levels of LPS in this group of patients are significantly elevated when compared with healthy subjects and correlate with increased levels of inflammatory biomarkers such as TNFα, IL-6, and C-reactive protein (CRP) [97]. This chronic systemic inflammation state represents a major risk factor for CKD progression and cardiovascular complications [19].
Given the large number of species (around 35.000) composing our intestinal microbiota, the limited capacity of the studies to determine the prevalence of only some groups of microorganisms, and the lack of knowledge regarding the real contribution of each microorganism in the pathophysiology of CKD, it is clear that more clinical evidence will be needed to propose the use of gut microbiota as a new biomarker of prognosis for kidney disease.
Mechanisms of CKD-induced dysbiosis
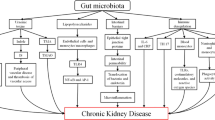
CKD is associated with diet restrictions, slow colonic transit, changes in the biochemical environment of the GI tract, and the use of certain medications such as antibiotics, phosphate binders, and iron-containing compounds [72]. All these factors contribute to the development of gut dysbiosis in CKD patients [2, 47] (Fig. 1).
Diet restrictions
CDK patients are characterized by decreased consumption of dietary fibers. Indigestible carbohydrates are essential nutrients for the gut saccharolytic microbiota, and the reduction of these substrates results in decreased production of short-chain fatty acids (SCFAs) by this group of bacteria. Lack of dietary fibers leads to increased amino nitrogen, which can be transformed into uremic toxins by the gut microbiota [111]. Patients with CDK are characterized by an imbalance between saccharolytic (fermentative) and proteolytic (putrefactive) microbiota in favor of the latter. The imbalance in favor of proteolytic species is related to detrimental effects and has also a fundamental role in the progression of CKD [123].
Slow colonic transit
A prolonged colonic transit reduces the availability of carbohydrates in the colon, facilitating increased protein fermentation by proteolytic bacteria. A slowing down in colonic transit time induces an upstream expansion in the number of proteolytic species, contributing to the imbalance between saccharolytic and proteolytic microbiota in patients with CKD [123]. This results in an increased production and uptake of end-products of bacterial protein fermentation [29].
Changes in the GI tract biochemical environment
Urea is the most abundant waste product retained in CKD patients [47]. It has been proved that the increased influx of urea into the GI lumen favors the overgrowth of bacteria expressing urease. This was confirmed by clinical studies, as patients with ESRD showed dominance of bacterial families possessing urease compared to healthy controls [59]. The hydrolysis of urea by gut microbes results in the formation of large quantities of ammonia. Ammonia raises luminal pH and alters the composition of the microbiota, leading to microbial dysbiosis [47].
Medications
CKD patients are commonly exposed to antibiotics to treat vascular accesses and other infections [111]. The use of antibiotics impacts the gut microbiota by loss of critical taxa necessary to maintain homeostasis, loss of biodiversity, changes in metabolic capacity, and expansion of pathogens [110]. On the other hand, long-term consumption of phosphate binders and iron-containing compounds can cause alterations in the luminal environment of the GI tract and affect the resident microbial flora, leading to dysbiosis [2, 47, 111].
Influence of microbial dysbiosis in CKD onset and progression
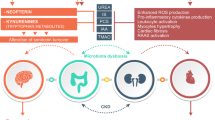
Microbial dysbiosis of the gut microbiota is characterized by a set of features associated to accumulation of microbiota-derived metabolites, neuroendocrine deregulation, chronic inflammation, and interruption of intestinal barrier function, all of which play a critical role in the pathogenesis of CKD and CKD-associated complications [49, 83] (Fig. 2).
Microbiota-derived metabolites
Uremic toxins
There are numerous evidences indicating that altered gut microbiota in CDK could contribute to the increased production of gut-derived uremic toxins [3, 113]. The origin of uremic toxins in CKD is multiple [61]. These toxic metabolites can be classified according to their origin in (1) uremic toxins derived from endogenous metabolism, (2) uremic toxins derived from microbial metabolism, or (3) uremic toxins derived from exogenous intake [54]. These products are normally eliminated by feces, although a part can be absorbed and eliminated by the kidneys, so they accumulate in CKD [16].
Patients with CKD usually present a gut microbiota imbalance that favors the growth of pathological bacteria with proteolytic activity, leading to the generation of uremic toxins like indoxyl sulfate (IS), p-cresyl sulfate (p-CS), indole-3-acetic acid (IAA), and trimethylamine n-oxidase (TMAO) [25, 72]. All these toxins often accumulate at the early stages of CKD and stimulate inflammation and oxidative stress, thereby contributing to the progression of kidney damage and increasing the cardiovascular risk in CKD patients [4, 21, 22, 26, 34]. IS is synthetized from dietary tryptophan metabolism while p-CS derives from phenylalanine and tyrosine catabolism by anaerobic gut bacteria. Both IS and p-CS are capable of inducing tubulointerstitial fibrosis and glomerular sclerosis, impaired renal function, and disease progression. IS also plays a key role in endothelial dysfunction by inducing pro-inflammatory cytokines and free radical production, inhibiting endothelium repair, and promoting proliferation of vascular smooth muscle cells [77, 79, 102].
In patients with CKD, there is an increase of bacterial species producing uremic toxins, such as Enterobacteriaceae, Clostridiaceae, Pseudomonadaceae, and Bacteroidiaceae, whereas beneficial species, such as Lactobacillaceae, Bifidobacteriaceae, and Prevotellaceae, are decreased [81]. Recently, Joossens et al. conducted a clinical study in patients with ESRD to determine the role of gut microbiota in the generation of precursors of specific uremic toxins associated with negative outcomes in those patients. The authors identified six taxa (Enteroccocus, Akkermancia, Dialester, Rominicoccus, Bacteroides, and Blautia) that correlated with increased levels of uremic toxins and would need further exploration as microbial targets to lower uremic toxin concentrations to improve outcomes in patients with CDK [49]. It has been proposed that the influx of uremic toxins and urea into the GI lumen applies a selective pressure that favors the overgrowth of bacteria that produce urease, uricase, indole, and p-cresol forming enzymes, generating a vicious circle of inflammation and oxidative stress at renal level [111].
The aryl hydrocarbon receptor (AhR), a transcriptional factor, has been postulated as the mediator in the renal inflammatory and oxidative effects of the uremic toxins in CKD patients. A cross-sectional study in patient with CKD showed that AhR protein expression positively correlated with IAA plasma levels and NF-κB protein expression in peripheral blood mononuclear cells, suggesting a possible role of AhR activation in the progression of renal inflammation induced by uremic toxins in CKD patients [8]. Except for TMAO, uremic toxins tightly bound to serum albumin, making them difficult to remove by HD [21]. The binding site on serum albumin (site II) by uremic toxins is shared with other ligands like fatty acids [22, 121]. Taking this fact into consideration, Kemp et al. demonstrated a negative correlation between p-CS plasma levels and specific polyunsaturated fatty acids (PUFAs) like docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and gamma-linolenic (GLA) in patients with CKD on HD. This result suggests that PUFAs could contribute to reduce uremic toxin plasma levels in patients with CKD undergoing HD [51].
TMAO is produced by bacterial metabolism of quaternary amines including betaine, l-carnitine, or phosphatidylcholine that releases trimethylamine [14]. TMAO is linked to renal function and is associated with the progression of CKD and with an increased risk of CVD, the leading cause of morbi-mortality in patients with CKD [98, 103]. TMAO is completely excreted by glomerular filtration without contribution of tubular secretion or tubular reabsorption at all stages of CKD. Pelletier et al. demonstrated that serum TMAO concentration negatively correlates with estimated glomerular filtration rate (eGFR), confirming high levels of serum TMAO in patient with CKD [85]. Several studies have demonstrated a proatherogenic role by TMAO as well as a kidney tubulointerstitial fibrosis promoting effect [53, 106, 107]. Therefore, subclinical detection of CVD, especially at the early stages of CKD, has a crucial relevance for the prognosis of CKD. In this way, TMAO metabolite has been proposed as a potential surrogate marker to detect early cardiovascular risk in patient with CKD [41]. Hsu et al. showed that urinary TMAO levels positively correlated with the abundance of the beneficial probiotic bacterial strains Bifidobacterium and Lactobacillus genera in gut microbiota of children with early-stage CKD (G1-G3). Moreover, a lower TMAO urinary level is associated with elevated pulse wave velocity (PWV) and abnormalities in the ambulatory blood pressure monitoring (ABPM) [41].
Another uremic derivate metabolite is indolepropionic acid (IPA), an aromatic amino acid synthetized by deamination by the microbiota. Recent evidence indicates that IPA exhibits beneficial effects since high serum levels of this product were associated with lower risk for develop type 2 diabetes and might serve as inhibitor of beta-amyloid fibril generation [5, 109]. Patients with elevated serum levels of IPA are more protected from a rapid renal function decline with lower risk in developing CKD [105]. A possible mechanism of its beneficial effects might be due to its anti-oxidative stress properties capable of suppressing renal inflammation and fibrosis triggered by uremic toxins [125].
Then, it is clear that the progression of symptoms and clinical complications in CKD is caused by accumulation of uremic toxins, especially in ESRD where HD or peritoneal dialysis can only partially remove them [86]. Therefore, attempts to reduce their production or accumulation by favoring their elimination from the human body through manipulation of gut microbiota seem to be a reasonable and novel therapeutic strategy to improve the survival of these patients.
Short-chain fatty acids
Short-chain fatty acids (SCFAs) are aliphatic carboxylic acids of low carbon number (C2–6) produced by bacterial fermentation of dietary fiber or via protein catabolism, being acetate (C2), propionate (C3), and butyrate (C4) the main contributors to total SCFA content [37, 66]. In kidneys, SCFAs regulate immune response, decrease inflammation, and exert anti-oxidant and anti-fibrotic actions. SCFAs also regulate blood pressure levels and metabolism by the activation of G protein-coupled receptors and the inhibition of histone acetylation [42, 63].
It has been proved that abundance of SCFAs-producing bacteria (Lactobacillaceae and Prevotellaceae) is reduced in patients with ESRD [37]. Roseburia intestinalis, Faecalibacterium prausnitzii, and some species of Clostridium and Eubacterium represent the main anaerobic bacteria in synthetize butyrate through saccharolytic fermentation activity from non-digestible carbohydrates [88]. Jiang et al. demonstrated a significant reduction in the abundance of the butyrate-producing species Roseburia and Faecalibacterium in patients with CKD in comparison to healthy controls [48]. Another recent study found that use of anaerobic antibiotics in patients with kidney transplant is associated with less gut abundance of butyrate-producing bacteria and thereby with higher risk for developing respiratory viral infections. Conversely, patients with higher butyrate-producing bacteria in gut microbiota were associated with lesser incidence of respiratory viral infections at post transplantation [60]. This finding supports the anti-inflammatory properties of butyrate beyond the improvement of intestinal barrier function and mucosal immunity.
Clinical studies investigating the potential of circulating SCFA measurements to serve as biomarkers in diagnosis, prognosis, and therapeutic monitoring of renal patients are still scarce. Recently, Wang et al. showed that the main SCFAs (acetate, propionate, and butyrate) and especially butyrate were reduced in the feces and serum of patients during CKD development [116]. In this study, most markers of renal function (cystatin C, creatinine rate, blood urea nitrogen [BUN], GFR and uric acid) showed a negative correlation with the concentration of butyrate [116]. Further research is needed to determine whether increasing levels of circulating SCFAs would provide any direct clinical benefit in patients with CDK.
Endocrine regulation
The gut microbiota acts like an endocrine organ by producing several hormones and neurotransmitters that affect intestinal endocrine activity and have the potential to regulate kidney function [81].
It has been proved that alterations in the gut microbiota can lead to the hypothalamic–pituitary–adrenal (HPA) axis activation and to increased secretion of serotonin and other neurotransmitters and neuroactive compounds [47]. The HPA axis can be stimulated either directly or via the activation of the immune system elicited by toxic substances produced by altered gut microbiota such as endotoxin and peptidoglycan [47]. Additionally, Lactobacillaceae, Prevotellaceae, and Bifidobacteriaceae species are able to synthesize neurotransmitters such as γ-aminobutyric acid (GABA) and acetylcholine (Ach) and to promote production of the intestinal incretins glucagon-like peptide 1 and 2 (GLP-1, GLP-2) and the gut hormone peptide YY (PYY) [81]. Propionate, a SCFA synthesized by gut microbiota, also stimulates the release of GLP-1 and PYY [123]. Recently, Cheema and Pluznick identified 12 metabolites in plasma and another 96 in feces that were significantly altered with angiotensin II (ANG II) infusion in conventional mice, but not in germ-free mice, suggesting that they are dependent on the gut microbiota and can be regulated by ANG II [12].
All these neurotransmitters and hormones are able to modulate the renal function [81]. It has been proved that GABA can stimulate natriuresis and suppress renal sympathetic nerve activity, Ach can increase the GFR by promoting renal vasodilatation, and GLP-1 can increase the GFR, diuresis, and natriuresis and reduce ANG II levels [30][48, 101, 119]. In CKD patients, there is a clear reduction of bacteria species that can exert renoprotective actions through reducing renin–angiotensin–aldosterone and renal sympathetic systems activity while increasing GFR, diuresis, and natriuresis. Ultimately, endocrine alterations in sodium and blood pressure hemostasis can contribute to CKD onset and progression [47]. In this way, gut dysbiosis can be considered a key feature for CKD progression via alteration of endocrine gut–kidney interactions [81].
Chronic inflammation
Altered gut microbiota is associated to the development of systemic inflammation [81, 83]. This association has been proved in patients with ESRD, who are characterized by increased levels of systemic inflammation markers such as CRP, pro-inflammatory cytokines, and activated complement [83]. The development of systemic inflammation in patients with CKD could be explained by the effects of uremic toxins produced by the gut microbiota, LPS-induced monocyte/macrophage activation, oxidative stress, and increased cytokine secretion.
Gut microbiota dysbiosis can stimulate the accumulation of uremic toxins, which, in turn, can increase the production of pro-inflammatory cytokines [39]. LPS, a product originated from the cell wall component of Gram negative bacteria, elicits a pro-inflammatory and oxidative stress response by activation of endothelial cells and monocytes/macrophages [83]. This generates a ring reaction in which inflammation is associated to a redox imbalance with increased reactive oxygen species (ROS). Increase in ROS in turn potentiates the pro-inflammatory response, generating a vicious circle of inflammation and oxidative stress at renal level. In the kidneys, inflammatory cytokines, pro-fibrotic factors, and ROS are able to induce inflammation, nephrotoxicity, cell injury, and impairment of renal function [100].
Gut barrier disruption
Urea toxicity, gut wall edema, inflammation, and oxidative stress are major mechanisms that drive the disintegration of the intestinal barrier [47, 124]. Elevated urea levels as a consequence of the expansion of bacteria with urease activity leads to increasing ammonium production in the gut lumen. This causes alterations in gut pH, mucosal irritation, and gut wall structural damage, contributing to increased intestinal permeability by the alteration of the tight enterocyte junctions [83, 90]. Increased permeability of the intestinal barrier in patients with CKD favors the translocation of bacterial products of intestinal origin, such as LPS, uremic toxins, and cytokines into the systemic circulation. The translocation of endotoxin and bacterial fragments leads to local inflammation via the activation of immune cells such as macrophages and T cells, the release of pro-inflammatory cytokines and chemokines, and the infiltration of circulating inflammatory cells [47].
Finally, the increase in circulating bacterial products of intestinal origin favors the development of an inflammatory chronic state associated with CKD. The immune response explains the systemic inflammation that contributes to the deterioration of kidney disease and increases the incidence of CVD and mortality in patients with CKD [16].
Beneficial effect of prebiotic, probiotic, and symbiotic therapies on chronic kidney disease
Patients with CKD usually have certain conditions that influence the composition and richness of gut microbial flora: they are recommended to follow a strict diet with limited ingestion of protein, fat, fiber, and food with high content of potassium and oxalate; they often require antibiotics to prevent infections; and also require phosphate-binding agents [62, 64, 94, 120]. Nutrient ingestion has a direct effect in regulating the composition and richness of gut microbial flora, for example non-digestible complex carbohydrates promote the overgrowth of saccharolytic fermentative bacteria and when this substrate is reduced, proteolytic bacteria growth is favored with increase production of toxic metabolites like ammonia, phenols, and indoles [62]. Since CKD is associated with an imbalance of gut microbiota, restoring gut microbiota by increasing the total dietary intake, especially with diets rich in fiber in order to alter the carbohydrate/protein ratio, may shift the gut microbiota to a fermentation profile that favors the production of SCFAs [36]. Therefore, prebiotic, probiotic, and synbiotic supplementations have emerged as a potential therapeutic intervention.
The concept of prebiotics implies those nutrients selectively used by gut microbiota with beneficial effect to the host [17]. Examples of prebiotics are complex carbohydrates, oligosaccharides, fructans, galactans, starch, and polyphenols [17, 33]. The term probiotics involves live microorganisms that confer a health benefit on the host when they are administered in adequate concentration through different mechanisms: catabolism of waste molecules, production of bacteriocins that suppress pathogen bacteria growth, immunomodulation, and anti-inflammatory effects [86]. Examples of probiotics are mainly bacterial strains, mostly Lactobacillus or Bifidobacterium. Finally, synbiotic term is defined as the combination of prebiotic plus probiotic in order to enhance the benefits of each one as food-based strategy [18]. Several experimental and clinical studies have showed the beneficial effects of prebiotic, probiotic, and synbiotic supplementation on gut microbiota-renal axis [73].
Table 1 shows the main findings of different clinical trials and studies in animal models regarding the use of probiotic, prebiotics, and synbiotics in CKD.
Prebiotic supplementation as intervention to attenuate gut dysbiosis in CKD
Prebiotics stimulate the growth of beneficial bacteria species in the gut such as Bifidobacteria and Lactobacilli at the cost of other strains of bacteria, such as Bacteroides species, Clostridia species, and enterobacteria [70, 83].
Supplementation with prebiotics exerts beneficial effects in animal models of CKD [114]. In this sense, the group of Vaziri et al. studied the effects of supplementation with high resistant starch on CKD progression in male Sprague–Dawley rats with CKD induced by a diet containing 0.7% adenine for 2 weeks. Rats were then fed diets supplemented with amylopectin (low-fiber control) or high fermentable fiber (amylose maize resistant starch, HAM-RS2) for 3 weeks. CKD rats with low fiber diet presented reduced creatinine clearance, interstitial fibrosis, inflammation, tubular damage, activation of NF-kB, upregulation of pro-inflammatory, pro-oxidant, and pro-fibrotic molecules, downregulation of antioxidant enzymes, and disruption of colonic epithelial tight junction. The high resistant starch diet significantly prevented all these abnormalities, retarding the progression of CKD [112]. In another study by Kieffer et al., male Sprague–Dawley rats with adenine-induced CKD consumed a semipurified low-fiber diet or a high-fiber diet [59% (wt/wt) HAMRS2] for 3 weeks (n = 9 rats/group). HAMRS2-fed rats showed an increased Bacteroidetes-to-Firmicutes ratio, associated with a healthy gut microbial community. Serum and urine IS levels were reduced by 36% and 66%, respectively, in HAMRS2-fed rats and urine PCS was reduced by 47% in HAMRS2-fed rats. Overall, dietary resistant starch had a protective effect on kidney function in CKD rats that takes place together with changes in gut microbe ecology and shifts in specific groups of gut bacteria [52]. The prebiotic lactulose is also able to modify gut microbiota and improve renal function by inhibiting the production of uremic toxins, in adenine-induced CKD Wistar/ST male rats of 10 weeks old. In doses of 3.0% and 7.5%, lactulose decreased serum creatinine and BUN levels and prevented CKD progression by suppressing tubulointerstitial fibrosis. Lactulose reduced species of gut microbiota which produced IS and therefore; this toxin levels in serum [104]. In a study by Hung et al., guar gum increased the Lactobacillus counts in adenine-induced CKD mice. In another study, xylooligosaccharide reduced the levels of six out of the nine CKD-associated bacterial genera in CKD mice. The authors concluded that prebiotic supplementation might be effective for the prevention or management of CKD by restoring colonic barrier integrity and microflora composition [43, 122].
Regarding the use of prebiotics in patients with CKD, a randomized placebo-controlled trial evaluated the effects of lactulose syrup as prebiotic on 32 patients (16 with CKD stages 3 or 4) for 8 weeks. The prebiotic significantly increased the number of Bifidobacteria and Lactobacilli in stool samples and significantly decreased creatinine plasma levels in patients with CKD [108]. Esgalhado et al. evaluated the effects of another prebiotic, resistant starch, on inflammatory and oxidative stress biomarkers in HD patients. They conducted a pilot randomized controlled trial on 31 HD patients for 4 weeks. The prebiotic supplementation was able to reduce IL-6, thiobarbituric acid reactive substances (TBARS), and IS plasma levels compared to placebo group, suggesting the use of prebiotic-resistant starch as a promising nutritional strategy to reduce inflammation, oxidative stress, and uremic toxins levels in CKD patients on HD [28]. The Medika Study was a prospective crossover-controlled trial that enrolled 60 patients with CKD (grades 3B-4) in order to evaluate the effects of two types of dietary regimens (very low protein and Mediterranean diet) on gut microbiota composition and uremic toxins production. The authors demonstrated that a very low protein diet increased Actinobacteria and reduced inflammatory Proteobacteria phyla; meanwhile, both Mediterranean and very low protein diets were able to decrease pathogen Enterobacteriaceae and increase butyrate-producer species like Lachnospiraceae, Ruminococcaceae, Prevotellaceae, and Bifidobacteriaceae. The very low protein diet also favored the growth of anti-inflammatory Blautia and Faecalibacterium, and butyrate-producer species Coprococcus and Roseburia, which correlated negatively with IS and PCS plasma levels [24]. These results confirm the role of very low protein diet to induce a significant reduction of urea and uremic milieu through modulation of gut microbiota in CKD patients [23]. Conversely, Poesen et al. performed a randomized, placebo-controlled, double-blind, cross-over study in 39 patients with CKD not yet on dialysis (eGFR between 15 and 45 ml/min/1.73 m2) to evaluate the influence of prebiotic arabinoxylan oligosaccharides and maltodextrin for 4 weeks on microbiota derived uremic toxins plasma and urinary levels. Although a limitation of the study was the lack of fecal samples to study the microbial composition, the authors could not demonstrate any effect of prebiotic arabinoxylan oligosaccharides on serum and 24 h urinary levels of p-CS, IS, p-cresyl glucuronide, and phenylacetylglutamine [87].
Probiotic supplementation as intervention to attenuate gut dysbiosis in CKD
Several experimental and clinical studies have evaluated the effects of different interventions based on probiotics to modify the gut microbiota composition and their bioproducts in CKD.
The group of Lippi I et al. has demonstrated that probiotic VSL#3 reduced the deterioration of GFR along time during a 2-month period in dogs with CKD, compared to a control group consisting of CKD dogs with prescribed diet and standard therapy. VSL#3 is a multi-strain probiotic containing viable lyophilized bacteria. It contains four strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus), three strains of Bifidobacterium (B. longum, B. breve, and B. infantis), and one strain of Streptococcus salivarius subsp. Thermophiles. A total of 60 dogs were used and the dose of probiotic was 12 to 225 × 109 lyophilized bacteria per 10 kg body weight [65]. In another study, Ranganathan et al. proved that probiotic supplementation with Bacillus pasteurii and Lactobacillus sporogenes reduced CKD progression and contributed to longer life in Sprague–Dawley rats undergoing nephrectomy [92]. Treatment with Sprosarcina pasteurii also improved renal function and was associated to longer life span in uremic rats, neutralizing the uremic toxin IS and reducing the progression of CKD [93].
On the other hand, metabolic syndrome is a highly prevalent entity worldwide, associated with low-grade systemic inflammation and insulin resistance, factors that may damage the kidney, leading to CDK. Related to this, the administration of the probiotic shubat in different doses [(6.97 × 106 lactic acid bacteria + 2.20 × 104 yeasts) colony forming unit (CFU)/mL, (6.97 × 107 lactic acid bacteria + 2.20 × 105 yeasts) CFU/mL, and (6.97 × 108 lactic acid bacteria + 2.20 × 106 yeasts) CFU/mL] was nephroprotective and improved carbohydrate and lipid metabolism in a rat model of type 2 diabetes induced by a high intake of glucose and fat for six weeks and a low dose of streptozotocin (30 mg/kg) [75]. Additionally, probiotic supplementation with Lactobacillus paracasei HII01 in a concentration of 1 × 108 CFU/ml given by oral gavage for 12 weeks to obese high fat rats alleviated kidney inflammation, endoplasmic reticule (ER) stress, and apoptosis, leading to improved kidney function. These benefits involve the attenuation of hyperlipidemia, systemic inflammation, and insulin resistance [115]. Moreover, fructose overload in the diet is a well-known model of metabolic syndrome associated to kidney dysfunction. The administration of Lactobacillus plantarum to male Wistar rats with metabolic syndrome by fructose overload in a concentration of 1 × 109 CFU per 100 g of body weight during 5 weeks, resulted in a reversion of the suppression of insulin signaling pathway, augmentation of inflammatory markers, and upregulation of sodium/glucose cotransporter 2 (SGLT2) induced by fructose overload [55].
Regarding the clinical use of probiotics, controversial results arise from different studies, some of them showing significant benefits in patients with kidney disease. In this way, the study conducted by Hida et al. demonstrated an increased number of anaerobic Clostridia perfringens with significantly decreased number of Bifidobacteria and high plasma levels of phenol, p-cresol, and indicant in 20 patients on HD compared to control (n = 12) before probiotic treatment. After 2 weeks of therapy with probiotic containing lactic acid bacteria Lactobacillus acidophilus, Bifidobacteria infantis, and Enterococcus faecalis, patients on HD showed a significant reduction of aerobic Enterobacteria, Klebsiella, and Clostridia perfringens, accompanied with a significant decrease in fecal p-cresol and indole as well as indicant in plasma [38]. On the other hand, a randomized, placebo-controlled study enrolled 22 patients with ESRD on HD treated with probiotic containing Streptococcus terhmophiles KB19, Lactobacillus acidophilus KB27, and Bifidobacterium longum KB31, did not observed significant variation either on inflammation or oxidative stress markers nor uremic toxins (IS and PCS) [78]. Additionally, Hyun et al. evaluated the effects of probiotics in pediatric patients with ESRD on peritoneal dialysis (PD) (n = 16) and HD (n = 20). The probiotic was administered for 12 weeks (dosage by age and weight) and contained a mix of Lactobacillus casei, L. plantarum, L. acidophilus, Streptococcus salivarius subsp. thermophiles, and L. delbrueckii subsp. Bulgaricus; Bifidobacterium longum, B. breve, and B. infantis. Results from this study demonstrated no significant differences in serum concentrations of PCS and IS after probiotic treatment in any cohort group [44].
Synbiotic supplementation as intervention to attenuate gut dysbiosis in CKD
Regarding synbiotic therapy in CKD, the group of Iwashita et al. carried out a study to investigate whether synbiotics modulate the gut microbiota and ameliorate kidney function using a rat model of CKD. Five out of six nephrectomy (Nx) rats were fed with glutamine, dietary fiber, oligosaccharide, and Bifidobacterium longum strain (GFOB) diet. GFOB diet decreased serum creatinine and blood urea nitrogen levels, compared to control rats, as well as the uremic toxin IS, consequently improving renal function. The authors concluded that restoring the gut microbiota using synbiotics improved kidney function and might be a pharmacological treatment for CKD-related mineral and bone disorder without any serious adverse events [45].
The SYNERGY (SYNbiotics Easing Renal failure by improving Gut microbiologY) was a randomized, double-blind, placebo-controlled study with crossover design, in which 31 predialysis adult patients with CKD stage 4 or 5 were under a synbiotic therapy for 6 weeks. The synbiotic therapy consisted in a mix of prebiotic components (fructo-oligosaccharides, high-molecular weight inulin, and galacto-oligosaccharides) and probiotic components (nine different strains of Lactobacillus, Streptococcus, and Bifidobacteria genera). The symbiotic therapy was able to reduce both nephrovascular uremic toxin, serum IS, and p-CS, in those patients who did not take antibiotics along the study. This effect was associated with changes in fecal microbiota consistent of Bifidobacterium spp. and Lachnospiraceae enrichment with Ruminococcaceae depletion [95]. Another randomized placebo-controlled study evaluated the effects of synbiotic therapy (inulin plus Lactobacillus acidophilus and Bifidobacterium bifidum with omega 3 fatty acids and vitamins B, C, and E) for 2 months in 18 patients on HD. The symbiotic group (n = 10) showed a significant increase in Bifidobacterium species with less GI symptoms scores compared to placebo group (n = 8) [18]. Furthermore, Pavan demonstrated the beneficial effects of probiotics, accompanied by prebiotics and a low protein intake, in CKD patients (stage 3 to 5). In spite of the fact that eGFR had been reduced during the 12-month period of treatment, pro/prebiotics prevented that reduction compared to control patients which only received the low protein diet [84].
Considering the variability in the design of the studies regarding the length of the study, doses of prebiotics/probiotics/synbiotics used, type of experimental animal models, exclusion and inclusion criteria for patients and taxonomic phylum, genus and species studied, additional studies are needed in order to support a solid conclusion about the benefits of these “biotics” as interventional therapy in CKD. Finally, it is worth to mention an elegant systematic review and meta-analysis carried out by McFarlane et al., in which the authors concluded the limited evidence to date to support the use of prebiotics, probiotics, and synbiotics in patients with CKD [76].
Conclusions
The gut microbiota-CKD crosstalk is a mutual relationship in which the own condition of CKD predisposes to loss of resident microbial flora on one side and the gut dysbiosis influences the progression of CKD on the other side. The setting of this crosstalk involves an imbalance between saccharolytic (fermentative) and proteolytic (putrefactive) microbiota in favor of the latter, with increased levels of circulating uremic toxin compounds and reduced levels of nephroprotective metabolites like butyrate, that result in a chronic inflammatory state that favors the progression of CKD and its complications. Attempts to reduce the production or accumulation of nephrotoxins and/or to stimulate the production of nephroprotective metabolites through manipulation of gut microbiota seem to be a reasonable and novel therapeutic strategy to improve the survival of these patients. The use of prebiotic, probiotic, and synbiotic supplementations have emerged as a potential therapeutic intervention to restore the imbalance of the gut microbiota. To date, the experimental evidences are promising, but we still need more support from clinical studies to confirm the efficacy and safety of the use of these “biotics” as a therapeutic tool for CKD.
Abbreviations
- Ach:
-
Acetylcholine
- AhR:
-
Aryl hydrocarbon receptor
- ANG II:
-
Angiotensin II
- BUN:
-
Blood urea nitrogen
- CFU:
-
Colony-forming unit
- CKD:
-
Chronic kidney disease
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated glomerular filtration rate
- ESRD:
-
End-stage renal disease
- GABA:
-
γ-aminobutyric acid
- GI:
-
Gastrointestinal
- GLP-1:
-
Glucagon-like peptide 1
- GLP-2:
-
Glucagon-like peptide 2
- GFOB:
-
Glutamine, dietary fiber, oligosaccharide and Bifidobacterium longum strain
- GFR:
-
Glomerular filtration rate
- HAM-RS2:
-
High amylose maize resistant starch
- HD:
-
Hemodialysis
- HPA:
-
Hypothalamic–pituitary–adrenal
- IPA:
-
Indolepropionic acid
- IS:
-
Indoxyl sulfate
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IAA:
-
Indole-3-acetic acid
- LPS:
-
Lipopolysaccharide
- NF-κB:
-
Nuclear factor-κB
- OTUs:
-
Operational taxonomic units
- p-CS:
-
p-cresyl sulfate
- PD:
-
Peritoneal dialysis
- PUFAs:
-
Poly-unsaturated fatty acids
- PYY:
-
Peptide YY
- ROS:
-
Reactive oxygen species
- SCFAs:
-
Short-chain fatty acids
- TMAO:
-
Trimethylamine n-oxidase
- TNF-α:
-
Tumor necrosis factor α
References
Ahrén IL, Xu J, Önning G, Olsson C, Ahrné S, Molin G (2015) Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin Nutr 34(4):719–726
Al Khodor S, Shatat IF (2017) Gut microbiome and kidney disease: a bidirectional relationship. Pediatr Nephrol 32(6):921–931
Aronov PA, Luo FJ-G, Plummer NS, Quan Z, Holmes S et al (2011) Colonic contribution to uremic solutes. J Am Soc Nephrol 22(9):1769–1776
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) (2009) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4:1551–1558
Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla MA (2002) Development of indole-3-propionic acid (OXIGON™) for alzheimer's disease. J Mol Neurosci 19:213–217
Biruete A, Allen JM, Kistler BM, Jeong JH, Fitschen PJ, Swanson KS, Wilund KR (2019) Gut microbiota and Cardiometabolic risk factors in hemodialysis patients: a pilot study. Top Clin Nutr 34(2):153–160
Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, Stockler-Pinto MB, Mafra D (2019) Effects of probiotic supplementation on Trimethylamine-N-oxide plasma levels in hemodialysis patients: a pilot study. Probiotics Antimicrob Proteins 11(2):648–654
Brito JS, Borges NA, Anjos JSD, Nakao LS, Stockler-Pinto MB, Paiva BR, Cardoso-Weide LC, Cardozo LFMF, Mafra D (2019) Aryl hydrocarbon receptor and uremic toxins from the gut microbiota in chronic kidney disease patients: is there a relationship between them? Biochemistry 58(15):2054–2060
Cani PD, Neyrinck AM, Maton N, Delzenne NM (2005) Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res 13(6):1000–1007
Carpenter S, O'Neill LA (2009) Recent insights into the structure of toll-like receptors and post-translational modifications of their associated signalling. Biochem J 422(1):1–10
Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C et al (2018) Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins (Basel) 10(7):300
Cheema MU, Pluznick JL (2019) Gut microbiota plays a central role to modulate the plasma and fecal Metabolomes in response to angiotensin II. Hypertension 74(1):184–193
Chen TK, Knicely DH, Grams ME (2019) Chronic kidney disease diagnosis and management: a review. JAMA 322(13):1294–1304
Chen Y, Chen D, Chen L, Liu J, Vaziri ND et al (2019) Microbiome–metabolome reveals the contribution of gut–kidney axis on kidney disease. J Transl Med 17:5
Chung S, Barnes JL, Astroth KS (2019) Gastrointestinal microbiota in patients with chronic kidney disease: a systematic review. Adv Nutr 10(5):888–901
Cigarran Guldris E, González Parra E, Cases Amenós A (2017) Gut microbiota in chronic kidney disease. Nefrología 37(1):9–19
Cremon C, Barbaro MR, Ventura M, Barbara G (2018) Pre- and probiotic overview. Curr Opin Pharmacol 43:87–92
Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, García-García G, Parra-Rojas I, Castro-Alarcón N (2014) Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr 24(5):330–335
Darisipudi MN, Knauf F (2016) An update on the role of the inflammasomes in the pathogenesis of kidney diseases. Pediatr Nephrol 31:535–544
Dehghani H, Heidari F, Mozaffari-Khosravi H, Nouri-Majelan N, Dehghani A (2016) Synbiotic supplementations for azotemia in patients with chronic kidney disease: a randomized controlled trial. Iran J Kidney Dis 10(6):351–357
Deltombe O, Van Biesen W, Glorieux G, Massy Z, Dhondt A et al (2015) Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins 7:3933–3946
Devine E, Krieter DH, Rüth M, Jankovski J, LHD (2014) Binding affinity and capacity for the uremic toxin indoxyl sulfate. Toxins 6:416–429
Di Iorio BR, Marzocco S, Bellasi A, De Simone E, Dal Piaz F et al (2018) Nutritional therapy reduces protein carbamylation through urea lowering in chronic kidney disease. Nephrol Dial Transplant 33(5):804–813
Di Iorio BR, Rocchetti MT, De Angelis M, Cosola C, Marzocco S et al (2019) Nutritional therapy modulates intestinal microbiota and reduces serum levels of total and free Indoxyl sulfate and P-cresyl sulfate in chronic kidney disease (Medika study). J Clin Med 8(9):E1424
Ding C, Han F, Xiang H, Wang Y, Li Y, Zheng J, Xue W, Ding X, Tian P (2019) Probiotics ameliorate renal ischemia-reperfusion injury by modulating the phenotype of macrophages through the IL-10/GSK-3β/PTEN signaling pathway. Pflugers Arch 471(4):573–581
Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, Fallague K, Brunet P, Calaf R, Dussol B, Mallet B, Dignat-George F, Burtey S (2015) The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 26:876–887
Eidi F, Poor-Reza Gholi F, Ostadrahimi A, Dalili N, Samadian F, Barzegari A (2018) Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-cresol) in hemodialysis patients: a double blind randomized clinical trial. Clin Nutr ESPEN 28:158–164
Esgalhado M, Kemp JA, Azevedo R, Paiva BR, Stockler-Pinto MB, Dolenga CJ, Borges NA, Nakao LS, Mafra D (2018) Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct 9(12):6508–6516
Evenepoel P, Poesen R, Meijers B (2017) The gut-kidney axis. Pediatr Nephrol 32(11):2005–2014
Fujimura S, Shimakage H, Tanioka H, Yoshida M, Suzuki-Kusaba M, Hisa H, Satoh S (1999) Effects of GABA on noradrenaline release and vasoconstriction induced by renal nerve stimulation in isolated perfused rat kidney. Br J Pharmacol 127:109–114
Furuse SU, Ohse T, Jo-Watanabe A, Shigehisa A, Kawakami K et al (2014) Galacto-oligosaccharides attenuate renal injury with microbiota modification. Phys Rep 2(7):e12029
García-Arroyo FE, Gonzaga G, Muñoz-Jiménez I, Blas-Marron MG, Silverio O, Tapia E, Soto V, Ranganathan N, Ranganathan P, Vyas U, Irvin A, Ir D, Robertson CE, Frank DN, Johnson RJ, Sánchez-Lozada LG (2018) Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS One 13(8):e0202901
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G (2017) Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14(8):491–502
Gryp T, Vanholder R, Vaneechoutte M, Glorieux G (2017) P-Cresyl sulfate. Toxins 9:E52
Guida B, Cataldi M, Memoli A, Trio R, di Maro M, Grumetto L, Capuano I, Federico S, Pisani A, Sabbatini M (2017) Effect of a short-course treatment with Synbiotics on plasma p-cresol concentration in kidney transplant recipients. J Am Coll Nutr 36(7):586–591
Hand TW, Vujkovic-Cvijin I, Ridaura VK, Belkaid Y (2016) Linking the microbiota, chronic disease, and the immune system. Trends Endocrinol Metab 27:831–843
Heaney LM, Davies OG, Selby NM (2019) Gut microbial metabolites as mediators of renal disease: do short-chain fatty acids offer some hope? Future Sci OA 5(4):FSO384
Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y (1996) Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74(2):349–355
Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL (2019) Chronic kidney disease and the gut microbiome. Am J Physiol Ren Physiol 316(6):F1211–F1217
Hsu CN, Yl T (2019) The good, the bad, and the ugly of pregnancy nutrients and developmental programming of adult disease. Nutrients 11(4):894
Hsu CN, Lu PC, Lo MH, Lin IC, Chang-Chien GP et al (2018) Gut microbiota-dependent trimethylamine N-oxide pathway associated with cardiovascular risk in children with early-stage chronic kidney disease. Int J Mol Sci 19(12):E3699
Huang W, Zhou L, Guo H, Xu Y, Xu Y (2017) The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 68:20–30
Hung TV, Suzuki T (2018) Dietary fermentable fibers attenuate chronic kidney disease in mice by protecting the intestinal barrier. J Nutr 148:552–561
Hyun HS, Paik KH, Cho HY (2013) P-Cresyl sulfate and indoxyl sulfate in pediatric patients on chronic dialysis. Korean J Pediatr 56(4):159–164
Iwashita Y, Ohya M, Yashiro M, Sonou T, Kawakami K, Nakashima Y, Yano T, Iwashita Y, Mima T, Negi S, Kubo K, Tomoda K, Odamaki T, Shigematsu T (2018) Dietary changes involving Bifidobacterium longum and other nutrients delays chronic kidney disease progression. Am J Nephrol 47(5):325–332
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D (2015) Role of the normal gut microbiota. World J Gastroenterol 21(29):8787–8803
Jazani NH, Savoj J, Lustgarten M, Lau WL, Vaziri ND (2019) Impact of gut dysbiosis on neurohormonal pathways in chronic kidney disease. Diseases 7(1):E21
Jiang S, Xie S, Lv D, Zhang Y, Deng J et al (2016) A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 109(10):1389–1396
Joossens M, Faust K, Gryp T, Nguyen ATL, Wang J, Eloot S, Schepers E, Dhondt A, Pletinck A, Vieira-Silva S, Falony G, Vaneechoutte M, Vanholder R, van Biesen W, Huys GRB, Raes J, Glorieux G (2019) Gut microbiota dynamics and uraemic toxins: one size does not fit all. Gut 68(12):2257–2260
Kanbay M, Onal EM, Afsar B, Dagel T, Yerlikaya A, Covic A, Vaziri ND (2018) The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol 50(8):1453–1466
Kemp JA, Esgalhado M, Macedo RA, Regis B, Damasceno NRT et al (2019) A possible link between polyunsaturated fatty acids and uremic toxins from the gut microbiota in hemodialysis patients: a hypothesis. Hemodial Int 23(2):189–197
Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, Nazertehrani S, Moore ME, Marco ML, Martin RJ, Adams SH (2016) Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Ren Physiol 310(9):F857–F871
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato J, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19(5):576–585
Koppe L, Fouque D, Soulage CO (2018) The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins (Basel) 10(4):E155
Korkmaz OA, Sumlu E, Koca HB, Pektas MB, Kocabas A et al (2019) Effects of Lactobacillus plantarum and Lactobacillus helveticus on renal insulin signaling, inflammatory markers, and glucose transporters in high-fructose-fed rats. Medicina (Kaunas) 55(5):E207
Kurella Tamura M, Yaffe K, Hsu CY, Yang J, Sozio S, Fischer M, Chen J, Ojo A, DeLuca J, Xie D, Vittinghoff E, Go AS, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators (2016) Cognitive impairment and progression of CKD. Am J Kidney Dis 68:77–83
Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN et al (2017) TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 5:E34
Laffin MR, Tayebi Khosroshahi H, Park H, Laffin LJ, Madsen K, Kafil HS, Abedi B, Shiralizadeh S, Vaziri ND (2019) Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: microbial analysis from a randomized placebo-controlled trial. Hemodial Int 23(3):343–347
Lau WL, Vaziri ND (2017) The leaky gut and altered microbiome in chronic kidney disease. J Ren Nutr 27(6):458–461
Lee JR, Huang J, Magruder M, Zhang LT, Gong C, Sholi AN, Albakry S, Edusei E, Muthukumar T, Lubetzky M, Dadhania DM, Taur Y, Pamer EG, Suthanthiran M (2019) Butyrate-producing gut bacteria and viral infections in kidney transplant recipients: a pilot study. Transpl Infect Dis 21(6):e13180
Lehto M, Groop P-H (2018) The gut-kidney axis: putative interconnections between gastrointestinal and renal disorders. Front Endocrinol 9(553):1–11
Li F, Wang M, Wang J, Li R, Zhang Y (2019) Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol 9:206
Li LZ, Tao SB, Ma L, Fu P (2019) Roles of short-chain fatty acids in kidney diseases. Chin Med J 132(10):1228–1232
Lin J, Hu FB, Curhan C (2010) Associations of diet with albuminuria and kidney function decline. Clin J Am Soc Nephrol 5:836–843
Lippi I, Perondi F, Ceccherini G, Marchetti V, Guidi G (2017) Effects of probiotic VSL#3 on glomerular filtration rate in dogs affected by chronic kidney disease: a pilot study. Can Vet J 58(12):1301–1305
Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X (2018) Butyrate: a double-edged sword for health? Adv Nutr 9(1):21–29
Lopes RCSO, de Lima SLS, da Silva BP, Toledo RCL, Moreira MEC, Anunciação PC, Walter EHM, Carvalho CWP, Queiroz VAV, Ribeiro AQ, Martino HSD (2018) Evaluation of the health benefits of consumption of extruded tannin sorghum with unfermented probiotic milk in individuals with chronic kidney disease. Food Res Int 107:629–638
Lopes RCSO, Theodoro JMV, da Silva BP, Queiroz VAV, de Castro Moreira ME et al (2018) Synbiotic meal decreases uremic toxins in hemodialysis individuals: a placebo-controlled trial. Food Res Int 116:241–248
Lu CY, Chen YC, Lu YW, Muo CH, Chang RE (2019) Association of Constipation with risk of end-stage renal disease in patients with chronic kidney disease. BMC Nephrol 20(1):304
Macfarlane GT, Macfarlane S (2011) Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 45(suppl):S120–S127
Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z (2018) Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct 9(9):4763–4770
Mafra D, Fouque D (2015) Gut microbiota and inflammation in chronic kidney disease patients. Clin Kidney J 8:332–334
Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L et al (2019) Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients 11(3):496
Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S (2017) The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother 93:412–419
Manaer T, Yu L, Zhang Y, Xiao XJ, Nabi XH (2015) Anti-diabetic effects of shubat in type 2 diabetic rats induced by combination of high-glucose-fat diet and low-dose streptozotocin. J Ethnopharmacol 169:269–274
McFarlane C, Ramos CI, Johnson DW, Campbell KL (2019) Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr 29(3):209–220
Meijers BK, Evenepoel P (2011) The gut kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant 26(3):759–761
Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A et al (2014) Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int 2014:568571
Neirynck N, Vanholder R, Schepers E, Eloot S, Pletinck A, Glorieux G (2013) An update on uremic toxins. Int Urol Nephrol 45:139–150
Noureen S, Riaz A, Arshad M, Arshad N (2019) In vitro selection and in vivo confirmation of the antioxidant ability of Lactobacillus brevis MG000874. J Appl Microbiol 126(4):1221–1232
Onal EM, Afsar B, Covic A, Vaziri ND, Kanbay M (2019) Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens Res 42(2):123–140
Païssé S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B (2016) Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 56:1138–1147
Pan W, Kang Y (2018) Gut microbiota and chronic kidney disease: implications for novel mechanistic insights and therapeutic strategies. Int Urol Nephrol 50(2):289–299
Pavan M (2016) Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol 68(2):222–226
Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S et al (2019) Elevation of Trimethylamine-N-oxide in chronic kidney disease: contribution of decreased glomerular filtration rate. Toxins (Basel) 11(11):E635
Plata C, Cruz C, Cervantes LG, Ramírez V (2019) The gut microbiota and its relationship with chronic kidney disease. Int Urol Nephrol 51(12):2209–2226
Poesen R, Evenepoel P, de Loor H, Delcour JA, Courtin CM et al (2016) The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: a randomized controlled trial. PLoS One 11(4):e0153893
Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ (2002) The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 217:133–139
Qian Q (2017) Inflammation: a key contributor to the genesis and progression of chronic kidney disease. Contrib Nephrol 191:72–83
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R et al (2016) Role of the gut micro-biome in uremia: a potential therapeutic target. Am J Kidney Dis 67(3):483–498
Ramos CI, Armani RG, Canziani MEF, Dalboni MA, Dolenga CJR et al (2019) Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrol Dial Transplant 34(11):1876–1884
Ranganathan N, Patel BP, Marczely J, Dheer R, Chordia T, Dunn SR, Friedman EA (2005) Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci World J 5:652–660
Ranganathan N, Patel BG, Ranganathan P, Marczely J, Dheer R, Pechenyak B, Dunn SR, Verstraete W, Decroos K, Mehta R, Friedman EA (2006) In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J 52(1):70–79
Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, Weston KS, Hawley CM, McWhinney B, Ungerer JP, Isbel N (2014) Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3–4 chronic kidney disease. Arch Med Res 45:309–317
Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto CC, McWhinney B, Ungerer JP, Campbell KL (2016) Synbiotics Easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 11(2):223–231
Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S et al (2015) Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant 30:924–933
Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL (2019) Dysbiosis of gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med 18(5):3461–3469
Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G et al (2017) Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 38(39):2948–2956
Shah NB, Allegretti AS, Nigwekar SU, Kalim S, Zhao S, Lelouvier B, Servant F, Serena G, Thadhani RI, Raj DS, Fasano A (2019) Bloodd microbiome profile in CKD: a pilot study. Clin J Am Soc Nephrol 14(5):692–701
Sircana A, De Michieli F, Parente R, Framarin L, Leone N et al (2019) Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol Res 144:390–408
Skov J (2014) Effects of GLP-1 in the kidney. Rev Endocr Metab Disord 15:197–207
Stockler-Pinto MB, Saldanha JF, Yi D, Mafra D, Fouque D, Soulage CO (2016) The uremic toxin indoxyl sulfate exacerbates reactive oxygen species production and inflammation in 3T3-L1 adipose cells. Free Radic Res 50:337–344
Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C et al Serum trimethylamine-N-Oxide is Elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 27:305–313
Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, Narita Y, Hirata S, Kadowaki D (2019) Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin Exp Nephrol 23(7):908–919
Sun CY, Lin CJ, Pan HC, Lee CC, Lu SC, Hsieh YT, Huang SY, Huang HY (2019) Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clin Nutr 38(6):2945–2948
Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584
Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL (2015) Gut microbiota dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116:448–455
Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, Moghaddaszadeh M, Parkhide S (2016) The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Renal Inj Prev 5(3):162–167
Tuomainen M, Lindstrom J, Lehtonen M, Auriola S, Pihlajamaki J, Peltonen M et al (2018) Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diabetes 8:35
Vangay P, Ward T, Gerber JS, Knights D (2015) Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17(5):553–564
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis T, Ni Z, Nguyen TH, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83(2):308–315
Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ (2014) High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9(12):e114881
Velasquez MT, Centron P, Barrows I, Dwivedi R, Raj DS (2018) Gut microbiota and cardiovascular uremic toxicities. Toxins (Basel) 10(7):E287
Wanchai K, Yasom S, Tunapong W, Chunchai T, Thiennimitr P, Chaiyasut C, Pongchaidecha A, Chatsudthipong V, Chattipakorn S, Chattipakorn N, Lungkaphin A (2018) Prebiotic prevents impaired kidney and renal Oat3 functions in obese rats. J Endocrinol 237(1):29–42
Wanchai K, Yasom S, Tunapong W, Chunchai T, Eaimworawuthikul S et al (2018) Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin Sci (Lond) 132(14):1545–1563
Wang S, Lv D, Jiang S, Jiang J, Liang M et al (2019) Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci (Lond) 133(17):1857–1870
Wang YF, Zheng LJ, Liu Y, Ye YB, Luo S et al (2019) The gut microbiota-inflammation-brain axis in end-stage renal disease: perspectives from default mode network. Theranostics 9(26):8171–8181
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389:1238–1252
Wierema TK, Houben AJ, de Leeuw PW (1997) Acetylcholine-induced vasodilatation in the human hypertensive kidney: inhibition by muscarinic receptor antagonism. J Hypertens 15:1649–1651
Wilkins LJ, Monga M, Miller AW (2019) Defining dysbiosis for a cluster of chronic diseases. Sci Rep 9(1):12918
Yamasaki K, Hyodo S, Taguchi K, Nishi K, Yamaotsu N et al (2017) Long chain fatty acids alter the interactive binding of ligands to the two principal drug binding sites of human serum albumin. PLoS One 12:e0180404
Yang J, Li Q, Henning SM, Zhong J, Hsu M et al (2018) Effects of prebiotic fiber Xylooligosaccharide in adenine-induced nephropathy in mice. Mol Nutr Food Res 62:1–34
Yang T, Richards EM, Pepine CJ, Raizada MK (2018) The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 14(7):442–456
Yang J, Lim SY, Ko YS, Lee HY, Oh SW et al (2019) Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol Dial Transplant 34(3):419–428
Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T (2017) Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci 79:477
Younan S, Sakita GZ, Coluna JGY, Rufino MN, Keller R, Bremer-Neto H (2019) Probiotic mitigates the toxic effects of potassium dichromate in a preclinical study: a randomized controlled trial. J Sci Food Agric 99(1):183–190
Funding
This work was supported by grants from the Universidad de Buenos Aires (UBACYT 20020170100621BA (2018–2022) and Sociedad Argentina de Hipertensión Arterial (Stimulus Grant for Research on Hypertension 2019–2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure statement
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rukavina Mikusic, N.L., Kouyoumdzian, N.M. & Choi, M.R. Gut microbiota and chronic kidney disease: evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflugers Arch - Eur J Physiol 472, 303–320 (2020). https://doi.org/10.1007/s00424-020-02352-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02352-x