Abstract
Intermittent hypoxic training (IHT) is a discrete cost-effective method for improving athletic performance and high altitude acclimatization. Unfortunately, IHT protocols widely vary in terms of hypoxia severity, duration, and number of cycles affecting physiological outcomes. In the present study, we evaluated the efficacy of a moderate normobaric IHT protocol (12% FiO2 for 4 h, 4 days) on acclimatization to high altitude (3250 m). Global plasma proteomics studies revealed that IHT elicited acute-phase response proteins like C-reactive protein (CRP), serum amyloid A-1 protein (SAA), and alpha-1-acid glycoprotein 2 (AGP 2) as well as altered levels of several apolipoproteins. On subsequent exposure to high altitude, the IH trained volunteers exhibited significant higher arterial oxygen saturation with concomitant lower incidences of acute mountain sickness (AMS) as compared to controls. Interestingly, IH trained subjects exhibited lower levels of positive acute-phase proteins like C-reactive protein (CRP), serum amyloid A-1 protein (SAA), and fibrinogen (FGA, FGB, and FGG) both after days 4 and 7 of high altitude ascent. High altitude exposure also decreased the levels of HDL, LDL, and associated proteins as well as key enzymes for assembly and maturation of lipoprotein particles like lecithin-cholesterol acyltransferase (LCAT), cholesteryl ester transfer protein (CETP), and phospholipid transfer protein (PLTP). In contrast, IHT curtailed hypoxia-induced alterations of HDL, LDL, Apo-AI, Apo-B, LCAT, CETP, and PLTP. Further validation of results also corroborated attenuation of hypoxia-induced inflammation and dyslipidemia by IHT. These results provide molecular evidences supporting the use of moderate IHT as a potential non-pharmacological strategy for high altitude acclimatization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reduced oxygen level in the inspired air results in hypoxia and can be divided into intermittent or chronic forms depending on duration of exposure. Thus, intermittent hypoxia (IH) is defined by episodic or periodic cycles of hypoxia as observed during mountain expeditions and obstructive sleep apnea (OSA). IH training (IHT) protocols find widespread use in sports medicine to enhance the aerobic capacity of athletes by increasing erythrocyte mass [12, 32, 33]. Additionally, IHT protocols also promote high altitude acclimatization process by promoting erythropoiesis and angiogenesis [4, 19, 30, 31]. Due to its unbeatable cost to efficiency ratio, IHT protocols and simulation chambers have witnessed a rapid rise in popularity among athletes. Moreover, IHT protocols also bypass the common inconveniences associated with high-altitude stay and training [5, 44]. Currently, IH finds widespread application as a non-pharmacological strategy for treating a wide range of pathophysiological states including chronic lung disease, bronchial asthma, hypertension, myocardial infarction, diabetes mellitus, conferring neuroprotection during ethanol withdrawal, Parkinson’s disease, emotional disorders, and radiation toxicity as well as for the prophylactic treatment of some occupational diseases [15, 22, 41, 44].
The IH protocols consisting of episodes of mild hypoxia (9–16% inspired oxygen) that are short in duration (15 s to 4 min), small in numbers (10 episodes) and have a short length of exposure (1 h or less) can enhance beneficial physiological processes, whereas high dose of hypoxia (2–8% of inspired oxygen) is associated with progressively pathological mechanisms [22, 25]. The IH protocols aimed at improving physical performance consist of daily exposure of single dose of mild prolonged hypoxia (more than 1 h). These protocols exert cellular effects primarily through hypoxia-inducible transcription factors (HIFs), the master transcriptional regulator of adaptive response genes during hypoxia [37]. HIF-1 is a heterodimer consisting of HIF-1α and HIF-1β subunits. Under normoxia, HIF-1α is present at very low levels. In presence of oxygen, iron and 2-oxoglutarate HIF-1α is hydroxylated, binds with the von Hippel–Lindau protein (vHL), and then undergoes ubiquitination and degradation. During hypoxia, HIF-1α translocate to the nucleus, binds with HIF-1β, and recruits coactivator proteins to activate transcription of genes involved in energy metabolism, angiogenesis, and many other genes whose protein products increase oxygen delivery to cells and facilitate metabolic adaptation to hypoxia [35, 36, 46]. However, the complex interaction between hypoxia-responsive molecular pathways and varied time frame response of different tissues and organs along with inter-individual differences to hypoxia limits benefits of IHT protocols involving real or simulated altitudes. Hence, a better understanding of molecular and cellular effects of IHT will benefit sports coaches, altitude acclimatization protocols, altitude training camps, and altitude competition events as well as find more application in hypoxia-associated human diseases [44].
In the present study, we used iTRAQ-LC MS/MS proteomics strategy to study alterations in human plasma proteome after a mild normobaric IHT protocol (12% FiO2, 4 h for 4 consecutive days). By comparing results with sea level plasma proteome, we identified proteins and molecular pathways modulated by IHT at sea level. Subsequently, we exposed the IH trained volunteers to hypobaric hypoxia (3250 m) along with control subjects and evaluated plasma proteome alterations after 4 and 7 days respectively. By comparing plasma proteomes, we observed attenuation of hypobaric hypoxia-induced alterations in lipoprotein metabolism, IL-6-induced acute phase response, and coagulation pathways in IH trained volunteers that were further validated.
Materials and methods
Materials
All reagents and chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) unless specified.
Experiment design and sample collection
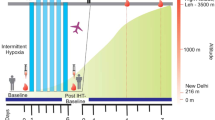
A total number of 40 male healthy volunteers (age 22–25 years, height 170 ± 4 cm, weight 65 ± 4 kg) were recruited for the present study and randomized to intermittent hypoxic training (IHT, n = 20) and control (SL, n = 20) groups. Volunteers under medication, smoking, and alcohol intake habits as well as high altitude exposure in the last 6 months were excluded. The study protocol was approved by an institutional ethical committee (IEC/DIPAS/09/DIP-251), which is in accordance with Helsinki declaration for human studies. All the participants were apprised for the scope of the study and informed written consent was obtained from all the volunteers. The basal parameters of the volunteers were recorded at Delhi (control, C) and the IHT volunteers were exposed to normobaric IH (12% FIO2 for 4 h per day for 4 consecutive days). Subsequently, both the C and the IHT volunteers were airlifted to Leh (3520 m), India, and monitored for 7 days. Physiological parameters like arterial oxygen saturation and Lake Louise Score (LLS) was recorded for each day at high altitude. Fasting venous blood samples were collected in EDTA-coated vacutainers at Delhi (C and IHT) as well as at Leh after 4 (HAD4 and IHT-HAD4) and 7 (HAD7 and IHT-HAD7) days during morning hour. Plasma was separated by centrifugation at 1000g for 15 min at 4 °C and stored at − 80 °C with mammalian protease inhibitor cocktail.
iTRAQ-based plasma proteomics studies
Approximately 50 μL plasma from each individual per experimental group was pooled to yield six different pools (C, IHT, HAD4, IHT-HAD4, HAD7, and IHT-HAD7) of 1 ml plasma each. High abundance plasma proteins were depleted using ProteoMiner protein enrichment kit (Cat. no.163–3006, Bio-Rad, USA) as per the manufacturer’s instructions and protein concentrations were determined by Bradford assay. For iTRAQ labeling, 100 μg of depleted plasma from each group were reduced, blocked on cysteines, and digested overnight with trypsin (V511QA, Promega, USA) at 37 °C as per the manufacturer’s instructions (Applied Biosystems, USA). Each pooled plasma sample was labeled with 113 (C), 114 (IHT), 115 (HAD4), 116 (IHT-HAD4), 117 (HAD7), and 118 (IHT-HAD7) mass tagged iTRAQ labels. Subsequently, all the labeled samples were pooled and fractionated using strong cation exchange (SCX) column. Fifteen top fractions were collected, vacuum dried, and reconstituted in 0.1% trifluoroacetic acid. Finally, these samples were desalted and subjected to LC-MS/MS analysis on a LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific, Germany) coupled with a 1200 nano-liquid chromatography system (Agilent Technologies, USA). Xcalibur 2.1 was used for data acquisition. Data-dependent acquisition was performed for the spectra and scans were acquired in Orbitrap mass analyzer at a mass resolution of 60,000 at m/z 400. The MS/MS data acquisition was done for the top 20 precursor fragments at a 15,000 resolution using high-energy collision-induced dissociation. Precursor ions with unassigned or single charge states were rejected. MS data was analyzed using Proteome Discoverer 1.3 (Thermo Scientific, Germany) software. Peptide mass tolerance of ± 20 ppm at MS and 0.1 Da at MS/MS was set during SEQUEST-mediated database searches with taxonomy set as Homo sapiens. A decoy database search was used to estimate false discovery rate (FDR) which came as 1% with search parameters having trypsin as protease with one missed cleavage, carbamidomethyl cysteine as a fixed modification, oxidation of methionine as dynamic modification, and iTRAQ modifications at N-terminus of the peptide and lysine were set as static modifications.
Biological network analysis
To identify biological processes and pathways associated with identified plasma proteomes, the MetaCore software suite (https://clarivate.com/products/metacore/) was used. The normalized datasets (with respect to control) was uploaded into MetaCore and analyzed using enrichment analysis of “process networks,” reflecting the content of a MetaCore database that was manually curated based on pathway maps and process networks of cellular processes along with metabolic networks.
Evaluation of CRP levels by ELISA
The concentration of CRP was evaluated in plasma samples by human C-reactive protein ELISA Kit (E0829h, EIAab Science, Wuhan, CHINA) as per manufacturer’s instructions. Briefly, 100 μl of plasma samples were added to pre-coated wells and incubated at 37 °C for 2 h followed by addition of 100 μl of detection reagent A. The plate was then incubated at 37 °C for 1 h. The plate was washed and 100 μl of detection reagent B was added followed by incubation for 1 h at 37 °C. Substrate solution was added to each well and incubated in the dark for 15 min at 37 °C. Finally, stop solution was added and color change was measured at wavelength of 450 nm. The concentrations of CRP in plasma samples were determined with respect to a standard curve.
Validation of proteomics results by Western blot analysis
Western blots were performed using Apo-AI (MIA1404, Invitrogen, USA), Apo-B (CSB-PA001918GA01HU, CUSABIO), Angiotensinogen (ab108294, Abcam, UK), C3 (WH000718M1, Sigma Aldrich, USA), and β-tubulin (ab134185, Abcam, UK) primary antibodies. In brief, 30-μg protein was separated on 10% SDS-PAGE and transferred to nitrocellulose membrane. The membranes were blocked overnight with 5% skimmed milk at 4 °C. After washing thrice with 1X PBST (10 min each), the membranes were incubated with primary antibodies for 2 h at room temperature. The membranes were washed thrice and subsequently incubated with respective secondary antibody (1:10,000 dilutions) for 1.5 h at room temperature. Images were acquired after adding chemiluminescent peroxidase substrate (CPS1A60, Sigma) on a gel documentation system (Biospectrum Imaging system, UVP, Cambridge, UK) and analyzed using image analysis software (Image J).
Plasma HDL and LDL estimation
Plasma lipid parameters including cholesterol, HDL, LDL, and triglycerides were measured using Randox Monaco clinical chemistry analyzer (Randox Laboratories, Crumlin, UK).
Statistical analysis
All the physiological and biochemical values were represented as mean ± SEM. Statistical analysis was performed using ANOVA with Newman Keuls post hoc tests with a significance of 0.05 (p < 0.05). All the analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software, CA, USA).
Results
Intermittent normobaric hypoxic training elicits inflammation and alters lipoprotein levels
IH training at sea level did not alter arterial oxygen saturation or any other significant physiological alterations (Fig. 1). The global plasma proteomics study using iTRAQ-LC MS/MS resulted in the identification of 233 proteins among the study groups (Supplementary information). Comparison of post-IHT with control plasma protein profile identified significant alterations in levels of 29 proteins (cut off ± 1.5-fold, p < 0.05), out of which abundance of 22 proteins increased while levels of 7 proteins decreased. These proteins were associated with biological functions like response to wounding, protein activation cascade, regulation of inflammatory response, and pathways for lipoprotein remodeling (Fig. 2a). Further analysis revealed higher abundance of acute-phase response proteins like C-reactive protein (CRP, 2.5-fold), serum amyloid A-1 protein (SAA, 2.1-fold), and alpha-1-acid-glycoprotein 2 (ORM2, 1.3-fold) for post-IHT as compared to sea level (Supplementary information). Evaluation of CRP levels also confirmed higher abundance after IHT at sea level suggesting activation of inflammatory pathways (Fig. 3a). IHT also differentially regulated apolipoprotein assembly and remodeling by altering several levels of apolipoproteins. The levels of Apo-AII (1.1-fold), Apo-CI (1.3-fold), Apo-D (1.6-fold), Apo-E (1.1-fold), and Apo-H (1.7-fold) was found to be increased while levels of Apo B-100 (− 1.4-fold), Apo-CII (− 1.1-fold), Apo-CIII (− 1.1-fold), Apo-F (− 1.1 fold), Apo-L1 (− 1.1-fold), and Apo-M (− 1.1-fold) was found to be decreased after IH training. Minor changes in the levels of several apolipoproteins like Apo-AI, Apo-AIV, Apo-AV, and Apo-CIV were also observed. In corroboration, minor higher level of cholesteryl ester transfer protein (CETP) along with lower levels of phosphatidylcholine-sterol acyltransferase (LCAT, − 1.1-fold) and phospholipid transfer protein isoform a (PLTP) were observed after IH training. Validation of results by western blotting also confirmed higher level of Apo-AI and lower level of Apo-B in IH-trained subjects further confirming alterations in lipoprotein assembly and maturation (Fig. 2b). Additionally, IHT also elicited higher levels of antioxidant proteins like SOD3 (1.3-fold) and PON1 (1.1-fold) at sea level.
Monitoring of physiological parameters. a Comparative representation of arterial oxygen saturation levels at sea level as well as at high altitude (3250 m). The values for both control and IH-trained groups were presented. b Prevalence of AMS at high altitude. The Lake-Louise Score for both control and IH-trained groups were plotted against each day of high altitude stay. * represents p < 0.5 and *** represents p < 0.001 compared to respective day matched control
Molecular effects of IHT at sea level. a All the proteins altered after IHT were analyzed for identification of altered GO processes. Top ten identified processes (p < 0.05) were presented. b Western blot-based analysis of Apo-AI and Apo-B levels depicting alterations in lipoprotein pathways. c Densitometry analysis. *** represents p < 0.001 as compared to control
Evaluation of plasma CRP, HDL, and LDL levels. a ELISA-based estimation of CRP in all the study groups. b, c. Plasma HDL and LDL levels respectively. * represents p < 0.05 and *** represents p < 0.001 with respect to control, # represents p < 0.05 with respect to HAD4, ^^ represents p < 0.01 with respect to HAD7
IHT improves arterial oxygen saturation at high altitude and lowers prevalence of AMS
The IH trained subjects were airlifted to an altitude of 3250 m after 18 h of completion of training along with control subjects. Monitoring of arterial oxygen saturation values revealed that IH-trained subjects possessed significant higher values during the first 6 days of the study period as compared to control subjects (Fig. 1a). During the first 3 days at high altitude, the IH-trained subjects possessed around 2% higher (p < 0.001) oxygen saturation than the control subjects. In corroboration, the incidence of AMS as measured by LLS was significantly lower in IH-trained subjects suggesting better acclimatization to high altitude (Fig. 1b).
IHT curtails hypobaric hypoxia-induced inflammation
In order to gain an insight of effects of IHT during high altitude acclimatization, we compared plasma proteomes of IH-trained volunteers with control volunteers on 4th and 7th day of high altitude ascent. Plasma proteome analysis of control subjects after day 4 of high altitude induction (HAD4) resulted in alteration of 40 proteins (± 1.5-fold) of which, 30 were high and 10 were lower abundant as compared to respective sea level proteome (Supplementary information). Positive acute-phase response proteins CRP (6.4-fold) and SAA1 (4.2-fold) were the top two highest abundant proteins after 4 days stays at high altitude. Higher levels of several other proteins like angiotensinogen (AGT, 2.7-fold), S100A8 (2.1-fold), and S100A9 (1.7-fold) which play prominent roles in vasoconstriction and regulation of inflammation were also observed. In contrast, lower levels of CRP (5.6-fold), SAA1 (4.3-fold), angiotensinogen (AGT, 1.3-fold), protein S100-A8 (S100A8, 1.1-fold) and protein S100-A9 (S100A9, 1.1-fold) were observed for IHT-HAD4 as compared to HAD4 respectively (Supplementary information). Additionally, lower levels of other acute phase response proteins like IGFBP1, ORM1, APCS, and C3 were also observed for IHT-HAD4 (Fig. 4a). Evaluation of CRP levels by ELISA (Fig. 3a), C3 and AGT levels by western blotting (Fig. 4b) also confirmed lower levels of these proteins for IHT-HAD4 further corroborating attenuation of hypoxia-induced inflammation and vasoconstriction. Chronic exposure of 7 days of hypobaric hypoxia (HAD7) resulted in higher levels of 43 proteins (± 1.5-fold) of which levels of 34 proteins were higher than control while 9 proteins were lower than control. Significant higher levels of S100 family of proteins involved in inflammatory and immunomodulation processes like S100A8 (fivefold), S100A9 (3.6-fold) and S100A7 (6.6-fold) were observed for HAD7. Higher levels of acute-phase response proteins like CRP (2.1-fold), C3 (1.3-fold), FGA (1.4-fold), and FGG (1.1-fold) were also observed for HAD7 as compared to control, suggesting persistence of inflammatory pathways. In contrast, lower level of S100A8 (2-fold), S100A9 (1.9-fold), S100A7 (5-fold), CRP (1.75-fold), and C3 (1.2-fold) were observed of IHT-HAD7 as compared to HAD7 suggesting curtailing of hypoxia-induced inflammation. ELISA-based estimation of CRP levels also corroborated lower levels for both IHT-HAD4 and IHT-HAD7 as compared to HAD4 and HAD7 respectively (Fig. 3a).
IHT curtails hypoxia-induced inflammation after 4 days of high altitude ascent. a Plasma proteomics and subsequent analysis identified IL-6-induced acute-phase response in hepatocytes as a major altered pathway. Thermometer-like icons beside proteins represent expression level of the protein for HAD4 (1) and IHT-HAD4 (2). Red color indicates higher level as compared to control and blue color indicates lower level as compared to control. b Western blot analysis for angiotensinogen and C3. c Densitometry analysis. *** represents p < 0.001 with respect to HAD4
IHT modulates lipoprotein assembly and maturation pathways during hypobaric hypoxia
Bioinformatics analysis identified lipoprotein-associated pathways like lipoprotein metabolism, HDL dyslipidemia in type 2 diabetes and metabolic syndrome X, low-density lipoproteins assembly, and remodeling at 4 and 7 days of high altitude ascent for both IHT and control groups (Supplementary information). Exposure to 4 days of hypoxia-altered HDL, LDL levels as well as associated proteins like Apo-AI, Apo-AII, Apo-AIV, Apo-AV, Apo-B, Apo-CI, Apo-CII, Apo-CIII, Apo-CIV, Apo-D, Apo-E, Apo-H, Apo-L, and Apo-M in control subjects (Fig. 3b, c, Supplementary information). Similar alterations were also observed for IHT-HAD4 and non-significant reductions for HDL-associated proteins like Apo-AI, Apo-AII, Apo-CI, Apo-CII, Apo-CIII, Apo-E, PON1, and PLTP was observed as compared to HAD4. Lower level of Apo-B, the major protein of LDL and chylomicrons was also found to be low in IHT-HAD4 (1.1-fold) as compared to HAD4. The differences between apolipoprotein levels became more significant after 7 days of high altitude ascent. Lower levels of HDL-associated proteins like Apo-AI (− 1.58 fold), Apo-AII (− 1.63-fold), Apo-CI (− 1.47-fold), and enzymes LCAT (− 2.45-fold), PLTP (− 1.53-fold), and PON1 (− 1.63-fold) were observed for HAD7 as compared to control. In contrast, significant higher level of Apo-AI (1.3-fold), Apo-AII (1.5-fold), Apo-CI (1.2-fold), and Apo-CII (2.1-fold) was observed for IHT-HAD7 group as compared to HAD7 though the levels were lower than control. Higher levels of HDL-associated enzymes essential for particle maturation like LCAT (twofold), PLTP (1.4-fold), and PON1 (1.6-fold) were also observed for IHT-HAD7 as compared to HAD7. Hypoxia exposure of 7 days also significantly decreased Apo-B levels (− 2.1-fold) in HAD7 as compared to control. In contrast, 1.9-fold higher LDL levels were observed for IHT-HAD7 as compared to HAD7 though the levels are lower than the control. Lipid profiling results for HDL and LDL (Fig. 3b, c) together with western blot-based analysis of Apo-AI and Apo-B (Fig. 5) also corroborated IHT-mediated curtailing of hypoxia-induced dyslipidemia.
Discussion
Use of limited duration, cyclic, and moderately intense hypoxia-reoxygenation cycles are being used as non-pharmacological strategy to improve athletic performance, pre-acclimatization to high altitude, increasing cardiac resistance to ischemia-reperfusion stress, neuroprotection, and many other pathological conditions. These protocols are fundamentally different from severe hypoxia-reoxygenation cycles of obstructive sleep apnea (OSA), a potential risk factor independently associated with several disorders, diminished quality of life, and morbidity [47]. Hence, it has been advocated that understanding the mode of action of beneficial IH protocols at molecular level will enlarge its therapeutic potential and augment its usage as a discrete non-pharmaceutical intervention [44]. In this respect, our global plasma proteomics studies after IH training and subsequent comparison with high altitude sojourner plasma proteome is a major effort toward understanding and establishing IH as a pre-acclimatization procedure for high altitude. Our studies report IH exposure prior to high altitude ascent curtails hypobaric hypoxia-induced inflammation and dyslipidemia at high altitude.
We used a mild IH training protocol (single exposure to 12% FiO2 for 4 h daily for 4 consecutive days) in the present study owing to the fact that single IH exposure per day lasting for more than 1 h improves athletic performance by inducing hypoxia-responsive genes and thus improving oxygen delivery to tissue [5, 22]. Using a similar IH exposure (12.3% FiO2 daily 1 h for 10 days), Taralov et al. have reported increased autonomic control and augmented parasympathetic nervous activity during subsequent hypoxia challenge [42]. The present IHT protocol did not alter arterial oxygen saturation at sea level and the values were similar with control subjects. Interestingly, the IHT volunteers exhibited approximately 2% higher arterial oxygen saturation during the first 6 days of high altitude ascent. Exposure to high altitude results in lower arterial oxygen saturation with a possibility of developing AMS [23, 40]. In contrast, climbers successfully maintaining oxygen saturation both at rest and exercise most likely do not develop AMS [16, 23]. Our present results of increased oxygen saturation of IH-trained subjects during hypobaric hypoxia are consistent with the observations of Taralov et al., who have reported significant increase in oxygen saturation after a 10 day 1 h daily IH training protocol during acute exogenous hypoxia [42]. As expected, the IH-trained subjects also exhibited lower symptoms of AMS that may be attributed to the higher oxygen saturation [16, 28]. These results suggest that IH training at sea level improves oxygen saturation during hypobaric hypoxia and thus attenuates the occurrence of AMS.
The plasma proteomics studies and subsequent analysis revealed that normobaric IH training induces acute-phase response proteins and IL-6-induced acute-phase response pathway at sea level. The abundance of two proteins of this pathway, CRP and SAA1, were significantly higher as compared to pre-training plasma levels. Hypoxia and inflammation share an interdependent relationship [6, 43]. Members of the nuclear factor κB (NF-κB) family along with hydroxylases (PHD1, PHD2, PHD3, and FIH) have been implicated in regulating inflammation during hypoxia by functionally interacting with HIF pathway [2, 6, 34]. Since IH protocols primarily function by activating HIFs, higher levels of inflammatory mediators was an expected observation. On the other hand, physiological indices of IH-trained participants were similar to their pre-training values and no uncomfort was reported during the entire training protocol. Higher levels of circulating inflammatory markers like IL-6, IL-6RA, and CRP have been reported for healthy volunteers at altitudes above 3400 m [13]. These previous observations along with our present results suggest that activation of inflammatory mediators by normobaric IHT is a normal physiological response to hypoxia. The IH training protocol induced minor alterations in the abundance of lipoproteins and associated pathways like lipoprotein metabolism, dyslipidemia, assembly, and remodeling of LDL as compared to pre-training levels. Severity of the hypoxic stimulus in IH protocols determine the degree of metabolic dysregulation and moderate IH reportedly does not induce hyperlipidemia as well as lipid peroxidation [20]. The alterations observed for lipoprotein levels did not result in any dyslipidemia though the HDL levels were lower in IHT volunteers. Hypoxia promotes increased formation of reactive oxygen species (ROS) and oxidative stress, both recognized as major pro-inflammatory mediators [20, 24]. Interestingly, the present IHT also activated antioxidant proteins like SOD3 and PON1 that reportedly confer protection during hypobaric hypoxia [26, 29]. These cumulative results suggest that the mild hypoxia dose of the present IH protocol elicits inflammation and alters lipid metabolism as well as evoke antioxidant enzymes.
Chronic hypobaric hypoxia exposure to 3250 m for 4 days exacerbated inflammation by activating positive acute-phase response proteins like CRP and SAA1 [8, 18] as well as other inflammatory proteins like C3, S100A8, and S100A9. During acute and chronic inflammatory conditions, IL-6 stimulates the production of acute-phase proteins in hepatocytes that were further secreted into circulation [8, 9]. Highest levels of these inflammatory proteins were observed on day 4 of high altitude ascent (HAD4) and further decreased at day 7 (HAD7). As mentioned earlier, hypoxia induces inflammation in healthy sojourners [13, 38] and persistent high level of inflammation has been linked with many high altitude disorders like AMS, HAPE, and HACE [3, 10]. Recent studies have delineated role of HIF1∝-induced NLRP3 inflammasome complex in potentiating venous thrombosis during hypobaric hypoxia [11]. These studies signify higher levels of inflammatory markers as potential risk factors for high altitude illnesses. In contrast, marked reductions of these proteins were observed for IH-trained volunteers after 4 days of high altitude stay. Further validation of CRP levels also confirmed attenuated levels of CRP in IHT-HAD4 plasma samples. Similarly, lower levels of CRP, C3, S100A8, and S100A9 were also observed after 7 days in IH-trained volunteers (IHT-HAD7) as compared to HAD7. Individuals mounting adequate anti-inflammatory response during hypoxia do not suffer from AMS [14] and pharmacological inhibition of inflammation reportedly confers protection during hypoxia [1]. A recent exploratory study has reported significantly lower levels of acute-phase proteins (haptoglobin, transferrin, and C3) inflammatory cytokines (IL-1β, IL-6, and TNF-α) in non-AMS group as compared to AMS group [45] supporting our present observations. These studies signify that amelioration of hypoxia-induced inflammation facilitates high altitude acclimatization and IHT is a non-pharmacological tool to curb hypoxia-induced inflammation and AMS.
Acute hypoxia inhibits lipoprotein lipase activity and consequently disrupts lipoprotein transport in the human adipose tissue [21]. Studying plasma metabolic and lipidomic profiles during an ascent to Everest Base Camp (5300 m), O’Brien et al. have recently reported substantial changes in plasma lipid metabolism [27]. In corroboration, we also observed reduced levels of VLDL, LDL, IDL, and HDL-associated lipoproteins during hypobaric hypoxia and this reduction was dependent on duration of exposure. The levels of Apo-AI, Apo-AII, Apo B-100, Apo-CI, and Apo-E significantly decreased after 7 days at high altitude. The key enzymes in lipoprotein metabolism pathway, LCAT, CETP, and PLTP, were significantly reduced after 7 days of hypoxia. Nascent plasma HDL (pre-β-HDL) particles are produced in liver or intestine that are spherical in shape and poor in lipids. Initial lipidation of these particles occurs at cellular membranes involving ABCA1-mediated efflux of cholesterol. Subsequently, LCAT-mediated cholesterol esterification generates large spherical HDL2 particles. These particles undergo further remodeling by PLTP-mediated surface remnant transfer. Large HDL2 particles can be converted to small HDL3 particles by CTEP-mediated transfer of cholesteryl esters form HDL to Apo B-100 containing lipoproteins generating triglyceride-rich HDL which can further undergo hydrolysis to generate small, triglyceride-rich HDL particles. All these proteins act sequentially for lipidation and catabolism of HDL particles [18, 39]. The observed reductions of LCAT, PLTP, CTEP, and Apo-AI along with lower plasma HDL levels indicate that HDL maturation is compromised during chronic hypoxia. In contrast, IH-trained volunteers (IHT-HAD7) possessed higher levels of Apo-AI, Apo-B, LCAT, CETP, and PLTP as compared to HAD7 though the levels were lower than sea level. Concerted actions of acute phase response and inflammation are associated with marked changes in abundance, structure, and composition of lipoproteins affecting lipid homeostasis [17, 18]. During chronic inflammatory states, decreases production of Apo-AI in liver leads to replacement of Apo-AI by SAA in HDL particles. This replacement by SAA impairs the intrinsic cholesterol efflux capacity of HDL since cellular cholesterol efflux is largely mediated by Apo-AI containing HDL particles [7]. All the observed alterations in HDL composition during chronic hypoxia (decreased Apo-AI, PON1, LCAT, and increased SAA) reportedly attenuate anti-inflammatory and anti-oxidative activities of HDL. In contrast, IHT-trained subjects these alterations are less evident further suggesting IHT facilitates high altitude acclimatization by restricting hypoxia-induced inflammation and inflammatory modification of lipoproteins.
The present results should be interpreted for severe IHT protocols (2–8% oxygen) with a caution. Such protocols result in pathological outcomes and associated with many disease states. In the future, similar molecular studies are required to understand unfavorable effects of these severe IHT protocols. It is noteworthy that the lipoprotein particles are highly heterogeneous differing in size, shape, lipid, and protein composition. The compositional and structural variations of these particles impart highly defined diverse biological functions. Hence, understanding the particle composition, dynamics, and interaction with other proteins will provide new dimensions to the understanding of role of lipoprotein particles in hypoxia biology. The study is also constrained by using plasma proteomics studies for deciphering IHT-associated molecular pathways. The high dynamic range of plasma proteins is a potential technical challenge to identify low-abundance proteins. Though we have depleted high-abundance plasma proteins in the present study, it is possible to identify more plasma proteins that may depict additional molecular pathways for high altitude acclimatization.
In conclusion, the present study highlights the importance of moderate IH training protocols as a discrete non-pharmacological intervention for high altitude acclimatization. Using physiological, plasma proteomics, and real high altitude ascent, the study reports curtailing of inflammatory mediators and pathways by normobaric IHT at sea level. Additionally, the study also reports diminished high altitude–induced dyslipidemia in IH-trained volunteers. These observations will provide molecular support for IHT as an economical tool for efficient high altitude acclimatization. Results of the study will benefit thousands of tourists and professionals at high altitude in terms of cost and convenience while maximizing operational efficiency. Additionally, the study will also benefit sports personnel and coaches in devising better training programs.
References
Arya A, Sethy NK, Singh SK, Das M, Bhargava K (2013) Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int J Nanomedicine 8:4507–4520. https://doi.org/10.2147/IJN.S53032
Bartels K, Grenz A, Eltzschig HK (2013) Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A 110:18351–18352. https://doi.org/10.1073/pnas.1318345110
Basnyat B, Murdoch DR (2003) High-altitude illness. Lancet 361:1967–1974. https://doi.org/10.1016/S0140-6736(03)13591-X
Casas M, Casas H, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J, Rodriguez FA, Viscor G (2000) Intermittent hypobaric hypoxia induces altitude acclimation and improves the lactate threshold. Aviat Space Environ Med 71:125–130
Dale EA, Ben Mabrouk F, Mitchell GS (2014) Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29:39–48. https://doi.org/10.1152/physiol.00012.2013
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364:656–665. https://doi.org/10.1056/NEJMra0910283
Esteve E, Ricart W, Fernandez-Real JM (2005) Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 24:16–31. https://doi.org/10.1016/j.clnu.2004.08.004
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. https://doi.org/10.1056/NEJM199902113400607
Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H (1987) Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A 84:7251–7255
Grocott M, Montgomery H, Vercueil A (2007) High-altitude physiology and pathophysiology: implications and relevance for intensive care medicine. Crit Care 11:203. https://doi.org/10.1186/cc5142
Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ (2017) Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A 114:4763–4768. https://doi.org/10.1073/pnas.1620458114
Hamlin MJ, Lizamore CA, Hopkins WG (2018) The effect of natural or simulated altitude training on high-intensity intermittent running performance in team-sport athletes: a meta-analysis. Sports Med 48:431–446. https://doi.org/10.1007/s40279-017-0809-9
Hartmann G, Tschop M, Fischer R, Bidlingmaier C, Riepl R, Tschop K, Hautmann H, Endres S, Toepfer M (2000) High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12:246–252. https://doi.org/10.1006/cyto.1999.0533
Julian CG, Subudhi AW, Wilson MJ, Dimmen AC, Pecha T, Roach RC (2011) Acute mountain sickness, inflammation, and permeability: new insights from a blood biomarker study. J Appl Physiol 111:392–399. https://doi.org/10.1152/japplphysiol.00391.2011
Jung ME, Mallet RT (2018) Intermittent hypoxia training: powerful, non-invasive cerebroprotection against ethanol withdrawal excitotoxicity. Respir Physiol Neurobiol 256:67–78. https://doi.org/10.1016/j.resp.2017.08.007
Karinen HM, Peltonen JE, Kahonen M, Tikkanen HO (2010) Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt Med Biol 11:325–332. https://doi.org/10.1089/ham.2009.1060
Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45:1169–1196. https://doi.org/10.1194/jlr.R300019-JLR200
Kontush A, Chapman MJ (2006) Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58:342–374. https://doi.org/10.1124/pr.58.3.1
Levine BD, Stray-Gundersen J (1997) Living high-training low: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol 83:102–112. https://doi.org/10.1152/jappl.1997.83.1.102
Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY (2007) Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102:557–563. https://doi.org/10.1152/japplphysiol.01081.2006
Mahat B, Chasse E, Mauger JF, Imbeault P (2016) Effects of acute hypoxia on human adipose tissue lipoprotein lipase activity and lipolysis. J Transl Med 14:212. https://doi.org/10.1186/s12967-016-0965-y
Mallet RT, Manukhina EB, Ruelas SS, Caffrey JL, Downey HF (2018) Cardioprotection by intermittent hypoxia conditioning: evidence, mechanisms, and therapeutic potential. Am J Phys Heart Circ Phys 315:H216–H232. https://doi.org/10.1152/ajpheart.00060.2018
Mandolesi G, Avancini G, Bartesaghi M, Bernardi E, Pomidori L, Cogo A (2014) Long-term monitoring of oxygen saturation at altitude can be useful in predicting the subsequent development of moderate-to-severe acute mountain sickness. Wilderness Environ Med 25:384–391. https://doi.org/10.1016/j.wem.2014.04.015
McGarry T, Biniecka M, Veale DJ, Fearon U (2018) Hypoxia, oxidative stress and inflammation. Free Radic Biol Med 125:15–24. https://doi.org/10.1016/j.freeradbiomed.2018.03.042
Navarrete-Opazo A, Mitchell GS (2014) Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307:R1181–R1197. https://doi.org/10.1152/ajpregu.00208.2014
Nozik-Grayck E, Woods C, Taylor JM, Benninger RK, Johnson RD, Villegas LR, Stenmark KR, Harrison DG, Majka SM, Irwin D, Farrow KN (2014) Selective depletion of vascular EC-SOD augments chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307:L868–L876. https://doi.org/10.1152/ajplung.00096.2014
O'Brien KA, Atkinson RA, Richardson L, Koulman A, Murray AJ, Harridge SDR, Martin DS, Levett DZH, Mitchell K, Mythen MG, Montgomery HE, Grocott MPW, Griffin JL, Edwards LM (2019) Metabolomic and lipidomic plasma profile changes in human participants ascending to Everest Base camp. Sci Rep 9:2297. https://doi.org/10.1038/s41598-019-38832-z
Oliver SJ, Sanders SJ, Williams CJ, Smith ZA, Lloyd-Davies E, Roberts R, Arthur C, Hardy L, Macdonald JH (2012) Physiological and psychological illness symptoms at high altitude and their relationship with acute mountain sickness: a prospective cohort study. J Travel Med 19:210–219. https://doi.org/10.1111/j.1708-8305.2012.00609.x
Padhy G, Sethy NK, Ganju L, Bhargava K (2013) Abundance of plasma antioxidant proteins confers tolerance to acute hypobaric hypoxia exposure. High Alt Med Biol 14:289–297. https://doi.org/10.1089/ham.2012.1095
Ricart A, Casas H, Casas M, Pages T, Palacios L, Rama R, Rodriguez FA, Viscor G, Ventura JL (2000) Acclimatization near home? Early respiratory changes after short-term intermittent exposure to simulated altitude. Wilderness Environ Med 11:84–88
Richalet JP, Bittel J, Herry JP, Savourey G, Le Trong JL, Auvert JF, Janin C (1992) Use of a hypobaric chamber for pre-acclimatization before climbing Mount Everest. Int J Sports Med 13(Suppl 1):S216–S220. https://doi.org/10.1055/s-2007-1024644
Rodriguez FA, Casas H, Casas M, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J, Viscor G (1999) Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc 31:264–268
Rodriguez FA, Ventura JL, Casas M, Casas H, Pages T, Rama R, Ricart A, Palacios L, Viscor G (2000) Erythropoietin acute reaction and haematological adaptations to short, intermittent hypobaric hypoxia. Eur J Appl Physiol 82:170–177. https://doi.org/10.1007/s004210050669
Scholz CC, Cavadas MA, Tambuwala MM, Hams E, Rodriguez J, von Kriegsheim A, Cotter P, Bruning U, Fallon PG, Cheong A, Cummins EP, Taylor CT (2013) Regulation of IL-1beta-induced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci U S A 110:18490–18495. https://doi.org/10.1073/pnas.1309718110
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408. https://doi.org/10.1016/j.cell.2012.01.021
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454
Serebrovska TV, Portnychenko AG, Drevytska TI, Portnichenko VI, Xi L, Egorov E, Gavalko AV, Naskalova S, Chizhova V, Shatylo VB (2017) Intermittent hypoxia training in prediabetes patients: beneficial effects on glucose homeostasis, hypoxia tolerance and gene expression. Exp Biol Med 242:1542–1552. https://doi.org/10.1177/1535370217723578
Siervo M, Riley HL, Fernandez BO, Leckstrom CA, Martin DS, Mitchell K, Levett DZ, Montgomery HE, Mythen MG, Grocott MP, Feelisch M, Caudwell Xtreme Everest Research G (2014) Effects of prolonged exposure to hypobaric hypoxia on oxidative stress, inflammation and gluco-insular regulation: the not-so-sweet price for good regulation. PLoS One 9:e94915. https://doi.org/10.1371/journal.pone.0094915
Silva Afonso M, Marcondes Machado R, Ferrari Lavrador MS, Carlos Rocha Quintao E, Moore KJ, Lottenberg AM (2018) Molecular pathways underlying cholesterol homeostasis. Nutrients 10. doi:https://doi.org/10.3390/nu10060760
Soria R, Egger M, Scherrer U, Bender N, Rimoldi SF (2016) Pulmonary artery pressure and arterial oxygen saturation in people living at high or low altitude: systematic review and meta-analysis. J Appl Physiol 121:1151–1159. https://doi.org/10.1152/japplphysiol.00394.2016
Tan H, Lu H, Chen Q, Tong X, Jiang W, Yan H (2018) The effects of intermittent whole-body hypoxic preconditioning on patients with carotid artery stenosis. World Neurosurg 113:e471–e479. https://doi.org/10.1016/j.wneu.2018.02.059
Taralov ZZ, Terziyski KV, Dimov PK, Marinov BI, Kostianev SS (2018) Assessment of the impact of 10-day intermittent hypoxia on the autonomic control measured by heart rate variability. Physiol Int 105:386–396. https://doi.org/10.1556/2060.105.2018.4.31
Taylor CT (2008) Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol 586:4055–4059. https://doi.org/10.1113/jphysiol.2008.157669
Viscor G, Torrella JR, Corral L, Ricart A, Javierre C, Pages T, Ventura JL (2018) Physiological and biological responses to short-term intermittent hypobaric hypoxia exposure: from sports and mountain medicine to new biomedical applications. Front Physiol 9:814. https://doi.org/10.3389/fphys.2018.00814
Wang C, Jiang H, Duan J, Chen J, Wang Q, Liu X, Wang C (2018) Exploration of acute phase proteins and inflammatory cytokines in early stage diagnosis of Acute Mountain sickness. High Alt Med Biol 19:170–177. https://doi.org/10.1089/ham.2017.0126
West JB (2017) Physiological effects of chronic hypoxia. N Engl J Med 376:1965–1971. https://doi.org/10.1056/NEJMra1612008
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165:1217–1239
Acknowledgements
The authors acknowledge all the study participants and The Indian Army for facilitating this study. The technical help provided by Dr. Dishari Ghosh, Harish Kumar, Sanjiva Kumar, and Deepak Das is highly appreciated. AG is recipient of Senior Research Fellow from DST-INSPIRE, India, and Pooja is a recipient of Senior Research Fellowship from UGC, India.
Funding
The study was supported by research grants (Project DIP-251) from DRDO
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experiments were conducted in accordance with the Indian Council for Medical Research (ICMR) guidelines (National ethical guidelines for biomedical and health research involving human participants) and were approved by the Defence Institute of Physiology and Allied Sciences Ethics Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4995 kb)
Rights and permissions
About this article
Cite this article
Gangwar, A., Pooja, Sharma, M. et al. Intermittent normobaric hypoxia facilitates high altitude acclimatization by curtailing hypoxia-induced inflammation and dyslipidemia. Pflugers Arch - Eur J Physiol 471, 949–959 (2019). https://doi.org/10.1007/s00424-019-02273-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-019-02273-4