Abstract
Using the double whole-cell patch-clamp technique, we found that the absence of intracellular ATP led to gap junction uncoupling in cochlear-supporting Hensen cells. The uncoupling was observed as a progressive reduction of the gap junctional electrical conductance from a starting value of approximately 40 nS to less than 0.04 nS within 10–20 min. The conductance rundown was partly avoided by at least 3 mM ATP and completely suppressed by 5 mM ATP or 5ʹ-adenylyl-imidodiphosphate (AMP-PNP), the non-hydrolysable ATP analog, in the pipette filling solution, suggesting that ATP was needed as ligand and not as a hydrolysable energy supplier or substrate for enzymatic reactions. The effect of intracellular ATP was mimicked by the external application of barium, a nonselective blocker of inwardly rectifying K+ (Kir) channels, and glibenclamide, an inhibitor of the ATP-sensitive Kir channels (KATP). Moreover a Ba2+-sensitive whole-cell inward current was observed in absence of internal ATP. We propose that the internal ATP kept the KATP channels in a closed state, thereby maintaining the gap junction coupling of Hensen cells. The immunostaining of guinea pig cochlear tissue revealed for the first time the expression of the KATP channel subunits Kir6.1 and SUR1 in Hensen cells and supported the proposed hypothesis. The results suggest that KATP channels, as regulator of the gap junction coupling in Hensen cells, could be the physiological link between the metabolic state of the supporting cells and K+ recycling in the organ of Corti.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gap junction channels link the cytoplasm of adjacent cells in animal tissues, allowing the exchange of ions and small metabolites (generally less than 2 kDa) between neighboring cells [18]. In vertebrates, gap junction channels are formed by two docked connexons or hemichannels in adjacent cells. Each connexon is a hexameric assembly of the protein subunits known as connexins (Cx). So far, 20 and 21 genes encoding connexins were found in the mouse and human genomes, respectively [6, 32, 40]. In the mammalian inner ear, four different connexins (Cx26, Cx30, Cx31, and Cx43) have been identified. Cx26 is the predominant connexin expressed between the non-sensory cells of the cochlea, including the supporting cells [19]. Mutations in Cx26 have been attributed to syndromic and non-syndromic hearing impairment [4, 8, 15], suggesting a crucial role of gap junction coupling in the physiology of the inner ear.
Non-sensory supporting cells play a key role in the maintenance of epithelial barrier integrity in the cochlea. They ensure that fluid compartments are separated and that ion homeostasis is preserved [9]. Hensen cells and Deiters cells are the major supporting cell populations in the mammalian organ of Corti. They are situated at the basilar membrane adjacent to the outer row of outer hair cells and are coupled by gap junctions, thus forming a cytoplasmic syncytium providing ion and metabolic connectivity [30, 45]. They modify cochlear mechanics and control hearing sensitivity [7, 46]. Furthermore, it has been demonstrated that supporting cells can buffer potassium ions in the space between outer hair cells and Deiters cells and provide a pathway for an intercellular K+ transport from hair cells towards the spiral ligament [34]. The K+-buffering function of supporting cells is important for hearing because K+ represents the major cation in the endolymph and the main charge carrier for sensory transduction. For this K+-buffering function, outward rectifying K+ channels [22, 43] as well as the gap junction coupling between supporting cells [3, 16, 30] are involved (for review, see [30, 47]). K+ transport channels contribute to the generation of the endocochlear potential (EP) and the corresponding high K+ concentration of the cochlear endolymph. The existence of the Kir4.1 channel (KCNJ10) has been shown in cochlear-supporting cells and the marginal cells of the stria vascularis [3, 12]. Mutant mice lacking KCNJ10 (Kir4.1) did not exhibit a significant EP and were deaf [20]. The involvement of ATP-sensitive inwardly rectifying K+ channels in cochlear physiology was first indirectly demonstrated 20 years ago by experiments which showed that inhibitors of KATP channels reduced the amplitude of the anoxia-sensitive negative potential (ASNP), while openers of the channels enhanced the ASNP [17].
Several factors regulate gap junction coupling in vertebrates, including voltage, intracellular Ca2+ concentration, H+ concentration and phosphorylation [2, 28]. In the inner ear, membrane tension, temperature [29, 45], aminoglycosides, hydrogen peroxide [36, 37], and calmodulin [1] have also been reported to modulate gap junction coupling. In the present paper, we combined electrophysiological, pharmacological and immunostaining experiments and show that functional KATP channels are expressed in guinea pig Hensen cells and are involved in the modulation of gap junction coupling in these cells.
Materials and methods
Materials
If not otherwise stated, the chemicals used were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
Cell preparation
Animal keeping, handling, and killing were performed in accordance with the German “Law on Protection of Animals” and with the European Communities Council Directive 86/609/EEC for the protection of animals used for experimental purposes and with the permission of the local ethics committee.
Pigmented young guinea pigs (250–400 g) with a positive Preyer’s reflex were sacrificed via a lethal intraperitoneal dose of phenobarbital (200 mg/kg). The bullae were removed, and the organ of Corti was dissected from the cochlea under binocular microscope. Pieces of tissue containing Hensen cells recognizable on the basis of their characteristic lipid droplets were carefully isolated. The tissue pieces were transferred in a nominally Ca2+-free Hank’s balanced salt solution (HBSS) and digested for 15–30 min with 500 U/ml collagenase II (Worthington, Berlin, Germany) or with 0.12 % trypsin. For electrophysiological recordings, Hensen cells were settled on poly-lysine-coated coverslips in a perfusion chamber mounted on Axiovert 10 inverted microscope (Zeiss, Oberkochen, Germany). For the observation of the cells, the Hamamatsu digital camera C4742-95 controlled by the software Aquacosmos (Hamamatsu Photonics Deutschland GmbH, Herrsching am Ammersee, Germany) was used.
Electrophysiological recording
The measurement of gap junctional conductance by double whole-cell patch-clamp analysis in isolated Hensen cells was performed as previously described [36]. The cells were washed with HBSS and transferred into the perfusion chamber. After a resting period of approximately 30 min, a morphologically intact pair of Hensen cells was selected. A double whole-cell patch-clamp configuration [23] was established using borosilicate glass pipettes, containing a pipette solution composed of (in mM): 135 K-gluconate, 5 NaCl, 0.1 cAMP, 5 ethyleneglycol-bis(2-aminoethylether)-N,N,Nʹ,Nʹ-tetraacetic acid (EGTA), 10 HEPES, 2 MgCl2, and 0.3 CaCl2 at pH 7.3. Depending on the experiments, ATP (1, 3, or 5 mM) or the ATP analog 5ʹ-adenylyl-imidodiphosphate (AMP-PNP, 5 mM) was added to the pipette solution. The pipettes filled with the different pipette solutions had a resistance of 2–5 MΩ. Whole-cell configuration with series resistances not exceeding 15 MΩ was considered. The cell membrane resistance ranged from 200 MΩ to 2 GΩ. These resistances were derived from corresponding current measurements by the application of simultaneous pulses of −20 to +20 mV to each pipette in a double whole-cell mode. The resistances were determined at the beginning of the experiment. They were accessed at several time points during the recordings and at the end of each experiment. The determination of junction conductance was performed as previously described [24]. After achieving the double whole-cell configuration, the current clamp modus was used to measure the membrane potential of the cells. We found that cells had a membrane potential which varied between −27 and −43 mV. For gap junction-coupled cells, the membrane potential difference between the cells did not exceed 2 mV. For all experiments, we therefore decided to clamp both cells at −40 mV during the measurements. The membrane potential of the cells was checked at different time points of the experiments. Changes of the membrane potential of more than 5 mV were not observed. Every minute, test pulses to ±20 mV were applied to one cell for 50 ms. The evoked currents were amplified by two EPC 7 (List Medical, Darmstadt, Germany) connected to a Macintosh computer via an interface ITC 16 (Instrutech, MN, USA). The gap junctional conductance (G j) was calculated from the registered currents as described elsewhere [5, 24]. For registration and evaluation of the data, Pulse Pulsefit software (HEKA Electronics, Lamprecht, Germany) was used. Data are given as average ± SEM, and n denotes the number of independent cell pairs. For each treatment, cells prepared from at least three animals were used. For statistical analysis, the Student’s t-test was applied with * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
To measure the whole-cell currents, a whole-cell configuration was established on isolated Hensen cells. HBSS was used as external solution and ATP or ATP-free pipette filling solution as described earlier was used. The cells were clamped at −40 mV. Whole-cell currents were measured 10–20 min after establishment of the whole-cell configuration by application of voltage pulses from −140 to +30 mV in 10 mV steps with duration of 250 ms for each pulse. The amplitude of the evoked current was measured at the end of the voltage pulse. For a better comparison between the cells, the currents measured in each cell were normalized to the capacity of the cell. The data were plotted against the voltage in a current density/voltage plot. Data are given as average ± SEM, and n denotes the number of independent experiments. For each treatment, cells prepared from at least three animals were used. For statistical analysis, the Student’s t-test was applied with * for p < 0.05, ** for p < 0.01, and *** for p < 0.001.
Immunohistochemical staining of Kir6.1 channels and SUR1
The cochleae were harvested from hearing guinea pigs (350–500 g) and prepared for histology. The animals were deeply anesthetized and sacrificed. They were fixed by a transcardial perfusion with phosphate buffer saline (PBS) followed by 4 % paraformaldehyde solution (PFA). The head was post-fixed in 4 % PFA for several days. The temporal bones were removed and incubated for 4–6 weeks in a 20 % ethylenediaminetetraacetic acid (EDTA) solution. The solution was renewed every day for the first 2 weeks. After removal of the semicircular ducts, the solution was exchanged every 2 days until a satisfying state of decalcification characterized by the softness of the bones was achieved. Thereafter, the cochleae were dehydrated in ethanol (50–100 %) followed by an overnight incubation in benzyl benzoate (Merck, Darmstadt, Germany) and embedding in paraffin (Merck). The embedded cochleae were cut in 10 μm slices with a microtome (SLEE Medical GmbH, Mainz, Germany) and mounted on microscope slides. The slices were deparaffinated using xylene and a decreasing alcohol series. Heat-induced antigen retrieval was performed using 10 mM citrate buffer, pH 6.0 for 20 min in a water bath heated to 96 °C. The slides were removed from the heat and cooled to room temperature for 30 min. For immunostaining, cochlear sections were washed with PBS composed of (in mM): 137 NaCl, 2.8 KCl, 10 Na2HPO4, and 1.8 KH2PO4 (pH 7.4, 295 mOsmol/l) [35]. Slides were incubated for 20 min in freshly prepared 1 % NaBH4 in PBS for autofluorescence correction and blocked for 1 h in 10 % normal serum in 0.3 % Triton X-100 in PBS. Antibodies were incubated in antibody solution containing 1 % normal serum in 0.3 % Triton X-100 in PBS. Primary rabbit antibodies, raised against human ATP-sensitive Kir6.1 channels or sulfonylurea receptor (SUR) proteins, were applied overnight at 4 °C. Secondary goat anti-rabbit Atto532 antibodies were applied for 2 h at room temperature. Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (DAPI). Cochlear sections were rinsed with water, incubated with 1 mM CuSO4 in 50 mM ammonium acetate (pH 5.0) for further autofluorescence correction and rinsed again with water. Sections were mounted in Mowiol (Carl Roth, Karlsruhe, Germany). As negative control, cochlear sections were incubated with antibody solution without primary antibody and afterwards with secondary antibody.
Results
Suppression of gap junctional uncoupling by internal ATP
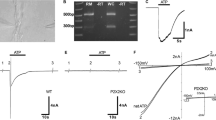
The double whole-cell patch-clamp configuration was established on isolated guinea pig Hensen cells. Absence of ATP in the pipette solution caused a rundown of gap junction electrical conductance (Gj), leading to a complete closure of gap junctional channels within 10–20 min (Fig. 1a, b). The rundown of the gap junction coupling was not due to an increase in intracellular free calcium concentration ([Ca2+]i) because the intracellular space was flooded by the pipette filling solution containing 5 mM EGTA and no Ca2+. To exclude an effect of voltage gating on gap junction coupling, the cells were clamped to −40 mV, the approximate membrane potential that was found at the beginning of the experiments. The addition of 1 mM ATP to the pipette solution did not stabilize gap junction coupling (not shown). ATP at 3 mM could not completely avoid a rundown of the gap junction coupling (Fig. 1). With 5 mM ATP, a stable gap junctional conductance could be measured for a recording time of up to 60 min. Pharmacological experiments were then performed to elucidate the link between the presence of intracellular ATP and stable gap junction coupling.
Electrical gap junction conductance (G j) in Hensen cells. a The time-dependent rundown of the conductance in absence of ATP in the pipette filling solution (empty circles, n = 10) was avoided by the addition of at least 5 mM ATP to the pipette solution (filled squares, n = 9). The addition of 1 mM ATP could not suppress the rundown (for the sake of clarity the data are not shown). Addition of 3 mM ATP to the pipette solution (empty triangles, n = 5) slowed the rundown, but could not completely suppress it. For a clear representation, the gap junction conductance (G j) was normalized to the original value (G o) of 40.04 ± 3.96 nS. b Statistical comparison of the degree of gap junction coupling between cells 15 min after the beginning of the experiments. For each experiment, the gap junction conductance measured 15 min after the beginning of the experiment was normalized to the conductance measured at the beginning of the experiment. The results are given as average; the error bars represent the SEM. The results were obtained from at least three animals for each treatment. The conductance after 15 min was compared to the values at the beginning of the experiment for each respective treatment, indicated by asterisk (*), or compared to 0 mM ATP at the respective time point, indicated by number sign (#). The significance of the difference was evaluated by the Student’s t-test (**, ## for p < 0.01; ***, ### for p < 0.001)
Pharmacological stabilization of the gap junction coupling
ATP is a cellular energy source, a substrate for enzymatic reactions and a ligand for many proteins, such as the regulatory SUR subunits of the ATP-sensitive Kir channels. As a source of energy or a substrate for enzymatic reactions, the action of ATP is based on its hydrolysis. As a ligand, ATP can be replaced by non-hydrolysable analogs such as the ATP analog 5′-adenylyl-imidodiphosphate (AMP-PNP). In our experiments, we found that 5 mM AMP-PNP in the pipette solution could avoid the gap junction coupling rundown (Fig. 2a, c) when double whole-cell patch-clamp experiments were performed in the absence of internal ATP. The effect of AMP-PNP on gap junction coupling was comparable to that observed for 5 mM ATP, indicating that ATP was needed as a ligand but not as a hydrolysable substrate.
Pharmacological stabilization of gap junctional conductance in cells in the absence of ATP in the pipette filling solution. a The absence of ATP in the pipette filling solution induced a rundown of the gap junction coupling (empty circles). This rundown was blocked by 5 mM of the non-hydrolyzable ATP analog AMP-PNP in the pipette filling solution (filled squares). b The external presence of Ba2+ (empty squares, 0.1 mM) or glibenclamide (glib., filled squares, 10 μM), both blockers of ATP-dependent K+ channels, also suppressed the decline of gap junction conductance induced by the absence of ATP in the pipette filling solution. c Statistical comparison of the degree of gap junction coupling between cells 15 min after the beginning of the experiments. For each experiment, the gap junction conductance measured 15 min after the beginning of the experiment normalized to the conductance measured at the beginning of the experiment is shown. The result are given as average, the error bars represent the SEM for at least five independent experiments using at least three animals for each treatment. Significant differences between 0 and 15 min of each respective treatment are marked with asterisk (*), significant differences to 0 mM ATP after 15 min are marked with number sign (#). The significance of the difference was evaluated by the Student’s t-test (**, ## for p < 0.01; ***, ### for p < 0.001)
As a ligand, ATP regulates many cellular functions, including ATP-sensitive Kir channels (KATP channels). These channels are opened upon the reduction of the internal ATP concentration. Kir channels are blocked by external Ba2+ and are specifically inhibited by sulfonylureas such as glibenclamide [13]. We therefore analyzed whether the pharmacological inhibition of the KATP channels affected the gap junction coupling. The results showed that when double whole-cell patch-clamp experiments were performed with ATP-free pipette solution but in the presence of external Ba2+ (0.1 mM) or glibenclamide (10 μM), the gap junction coupling rundown related to the absence of ATP in the pipette solution was suppressed (Fig. 2b, c). Tetraethylammonium (TEA) (20 mM), an inhibitor of voltage-dependent K+ channels, did not suppress the decline of gap junction conductance induced by the absence of internal ATP (results not shown). Moreover, the whole-cell patch-clamp showed a Ba2+ (0.1 mM) sensitive inward current in the cells. This current was only found when ATP was absent in the pipette filling solution (Fig. 3). Taken together, the results showed a possible involvement of ATP-sensitive Kir channels in the reduction of gap junction coupling induced by the depletion of internal ATP in cochlear Hensen cells.
Electrophysiological analysis of isolated Hensen cells. a The current–voltage (I (V)) plots of the whole-cell currents induced in isolated cells when the whole-cell patch-clamp configuration was established in absence (empty circles) or in presence of ATP (filled squares, 5 mM) in the pipette filling solution. b In presence of ATP (filled squares) in the pipette filling solution, Ba2+ (empty squares, 0.1 mM) did not change the current of the cells. c In contrary Ba2+ (empty squares) strongly reduced the inward current which was observed when ATP (empty circles) was absent in the pipette filling solution. The result are given as average, the error bars represent the SEM for five independent experiments using at least three animals for each treatment. The significance of the difference was evaluated by the Student’s t-test (* for p < 0.05; ** for p < 0.01; *** for p < 0.001)
Immunohistochemical staining of cochlear tissue
To see whether ATP-sensitive Kir channels were expressed in Hensen cells, we performed immunocytochemistry using fixed organ slices. The differential interference contrast (DIC) images of the stained slices allowed us to recognize the Hensen cells in the cochlea by their characteristic granular structures (Fig. 4). The fluorescent images and the images generated by merging the fluorescent and the DIC images showed a clear staining of Kir6.1 in the Hensen cells. The staining was also found in the hair cells (Fig. 4a). Functional ATP-sensitive channels are composed of the pore-forming Kir and the ATP-binding SUR subunits. We therefore examined whether a SUR isoform was also expressed in the Hensen cells. As for Kir6.1, the immunostaining experiments showed the clear presence of SUR1 in Hensen cells and hair cells (Fig. 4b). Together with the whole-cell patch-clamp experiments (Fig. 3), the staining data gives evidence that functional Kir6.1/SUR1 channels were present in the cochlear Hensen cells of the guinea pig.
Expression of Kir6.1 channels (a) and SUR1 (b) in the organ of Corti of the guinea pig. On the left side, DIC images reveal the cell distribution in the organ of Corti, with inner hair cells (green arrow), supporting cells (yellow arrow), basilar membrane (green asterisk), and tectorial membrane (yellow asterisk). Hensen cells with their characteristic granular structures can be identified as the most lateral supporting cells. Images on the right side show the immunofluorescent staining of the organ of Corti. Nuclei were stained with DAPI (blue). Kir6.1 (a) and SUR1 (b) positive cells are shown in red. Comparison of negative and positive immunofluorescent staining verifies that Kir6.1 and SUR1 were expressed in hair cells and in the supporting cells, particularly in the cells with the granular structures (Hensen cells). The images are representative of three animal preparations and three slides from each animal. The scale bar represents 50 μm
Discussion
Gap junction coupling of the cochlear-supporting cells has been related to the potassium recirculation from the organ of Corti back to the scala media [16, 34]. The gap junction channels of the supporting cells are mainly composed of the Cx26 and Cx30 isoforms. The physiological significance of the gap junction coupling between supporting cells is stressed by the observation that mutations in the connexins Cx26 and Cx30 were linked to different forms of juvenile deafness [11, 15, 42, 44]. It is therefore important to understand the physiological mechanisms that regulate the gap junction-dependent cell-to-cell communication in the supporting cells.
In the present report, we show that under a double whole-cell patch-clamp configuration with ATP-free pipette solution, a rundown of gap junction conductance between isolated Hensen cells of the guinea pig was induced (Fig. 1a, b). Internal ATP depletion can lead to an increase in the intracellular free calcium concentration ([Ca2+]i) as a result of the inhibition of the Ca2+-ATPases in the cell membrane and the membrane of the endoplasmic reticulum or the inhibition of the mitochondrial respiratory chain [27, 39, 41]. The increase in [Ca2+]i can in turn lead to the closure of gap junction channels in supporting cells [30]. However, in our experiments, a general intracellular rise in [Ca2+]i was made unlikely by the addition of 5 mM EGTA to a nominal Ca2+-free pipette solution [31].
An interesting finding of our experiments was that the rundown could be avoided by the addition of ATP to the pipette solution (Fig. 1a, b). It could be argued that ATP was needed for a continuous endogenous supply of ATP for the activity of protein kinases such as protein kinase A, as there is overwhelming evidence that changes in the connexin phosphorylation state can modulate cell-to-cell communication [2, 33]. However, it was shown that neither activation of protein kinase A nor protein kinase C affected the gap junction coupling of Hensen cells [1]. Furthermore, it is known that Cx26, the most expressed connexin in Hensen cells, is not phosphorylated [4]. It can therefore be hypothesized that ATP hydrolysis is not the key mechanism for the observed results (Fig. 1a, b). This independence from ATP hydrolysis and connexin phosphorylation is supported by our finding that the rundown of gap junctional coupling was suppressed by the internal presence of a non-hydrolysable ATP analog (AMP-PNP) (Fig. 2a, c), suggesting that ATP in this context played a role as ligand to sustain the gap junction coupling of the cochlear Hensen cells.
ATP as a ligand is known to regulate many cellular functions, including closing the ATP-sensitive Kir channels, also known as KATP channels. We hypothesize that the rundown of the gap junction coupling when ATP was absent from the pipette solution (Fig. 1a, b) was related to the opening of the KATP channels. This assumption agrees with pharmacological experiments showing that the inhibitors of KATP channels, Ba2+ and glibenclamide, could avoid the rundown of the gap junction conductance induced when the double whole-cell patch-clamp experiments were performed in the absence of internal ATP (Fig. 2b, c). The assumption that KATP channels were involved in the regulation of the gap junction coupling in Hensen cells was also supported by electrophysiological and immunostaining experiments. With whole-cell patch-clamp experiments, we showed a strong Ba2+-sensitive inward current which was only observed when ATP was absent in the pipette filling solution (Fig. 3). The immunostaining of Kir6.1 and SUR1 proteins showed that Hensen cells expressed the KATP channels with both the pore-forming Kir and the regulatory SUR subunits, suggesting, in combination with the physiological experiments, that the channels were present and functional (Fig. 4). To our knowledge, this is the first time that functional KATP channels (Kir6.1/SUR1) in the cochlear Hensen cells have been clearly demonstrated. It is noteworthy that the staining of Kir6.2 with anti-Kir6.2 antibody or SUR2 with anti-SUR2 antibody did not give conclusive results (results not shown). It might be that Kir6.2 or SUR2 were not expressed, but it is also possible that the antibodies, which were raised against human Kir6.2 and SUR2, did not recognize the proteins in the guinea pig.
In guinea pig, it was shown that glibenclamide inhibited the generation of the anoxia-sensitive negative potential (ASNP) in the cochlea, suggesting a role for KATP channels in K+ recirculation [17]. The present report combined physiological, pharmacological, and immunohistochemical data to identify the cells expressing the KATP channels. We further showed that the activity of the KATP channels is involved in the modulation of the gap junction coupling in Hensen cells. A similar modulation of the gap junction coupling by KATP channels was found in pancreatic ß cells [26], astrocytes [10, 14, 38], and cardiomyocytes [21]. However, the signaling mechanism involved remains elusive. From the physiological perspective, because KATP channels are known as sensors of the cellular metabolic state, our findings suggest that the modulatory effect of KATP channels on gap junction coupling links the metabolic state in the epithelial-like Hensen cells and the K+ recirculation in the cochlea [25]. Moreover, the results offer a rationale for testing KATP channel modulator drugs for the pharmacological treatment of hearing impairments.
References
Blödow A, Ngezahayo A, Ernst A, Kolb HA (2003) Calmodulin antagonists suppress gap junctional coupling in isolated Hensen cells of the guinea pig cochlea. Pflugers Arch 446:36–41
Bruzzone R, White TW, Paul DL (1996) Connections with connexins: the molecular basis of direct intercellular signalling. Eur J Biochem 244:1–27
Chen J, Zhao HB (2014) The role of an inwardly rectifying K+ channel (Kir4.1) in the inner ear and hearing loss. Neuroscience 0:137–146
Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C (2002) Targeted ablation of connexin26 in the inner ear epithelium gpa junction network causes hearing impairment and cell death. Curr Biol 12:1006–1111
Donaldson PJ, Dong Y, Roos M, Green C, Goodenough DA, Kistler J (1995) Changes in lens connexin expression lead to increased gap junctional voltage dependence and conductance. Am J Physiol 69:C590–C600
Evans HW, Martin PE (2002) Gap junctions: structure and function (review). Mol Membr Biol 19:121–136
Flock A, Flock B, Fridberger A, Scarfone E, Ulfendahl M (1999) Gap junctions and connexin expression in the inner ear. Novartis Found Symp 219:134–136
Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nickel R (2002) Connexins and gap junctions in the inner ear. Audiol Neurootol 7:141–145
Gale JE, Jagger DJ (2010) Cochlear supporting cells. In: Fuchs PA (ed) The Oxford handbook of auditory science: the ear. Oxford UP, Oxford, pp 307–327
Granda B, Tabernero A, Sanchez-Abarca LI, Medina JM (1998) The K-ATP channel regulates the effect of Ca2+ on gap junction permeability in cultured astrocytes. FEBS Lett 427:41–45
Grifa A, Wagner CA, D’Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P (1999) Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet 23:16–18
Hibino H, Horio Y, Ianobe A, Doi K, Ito M, Yamada M, Gotow T, Uchiyama Y, Kawamura M, Kubo T, Kurachi Y (1997) An ATP-dependent inwardly rectifying potassium channel, KAB-2 (Kir4.1), in cochlear stria vascularis of inner ear: its specific subcellular localization and correlation with the formation of endocochlear potential. J Neurosci 17:4711–4721
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366
Jiang K, Wang J, Zhao C, Feng M, Shen Z, Yu Z, Xia Z (2011) Regulation of gap junctional communication by astrocytic mitochondrial K(ATP) channels following neurotoxin administration in in vitro and in vivo models. Neurosignals 19:63–74
Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM (1997) Connexin 26 mutations in heredirary nonsyndromic sensorineural deafness. Nature 387:80–83
Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi (2000) Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc 33:51–56
Kitano I, Mori N, Matsunaga T (1995) Role of ATP-sensitive K+ channels in anoxia-sensitive negative potential of endolymph. Hear Res 90:24–30
Kumar NM, Gilula NB (1996) The gap junction communication channel. Cell 84:381–388
Lautermann J, ten Cate WJF, Altenhoff P, Gruemmer R, Traub O, Frank HG, Jahnke K, Winterhager E (1998) Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 294:415–420
Marcus DC, Wu T, Wangemann P, Kofuji P (2002) KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol 282:C403–C407
Naitoh K, Ichikawa Y, Miura T, Nakamura Y, Miki T, Ikeda Y, Kobayashi H, Nishihara M, Ohori K, Shimamoto K (2006) MitoKATP channel activation suppresses gap junction permeability in the ischemic myocardium by an ERK-dependent mechanism. Cardiovasc Res 70:374–383
Nenov AP, Chen C, Bobbin RP (1998) Outward rectifying potassium currents are the dominant voltage activated currents present in Deiters’ cells. Hear Res 123:168–182
Neyton J, Trautmann A (1985) Single-channel currents of an intercellular junction. Nature 317:331–335
Ngezahayo A, Altmann B, Kolb HA (2003) Regulation of ion fluxes, cell volume and gap junctional coupling by cGMP in GFSHR-17 granulosa cells. J Membrane Biol 194:165–176
Nichols CG (2006) KATP channels as molecular sensors of cellular metabolism. Nature 440:470–476
Rocheleau JV, Remedi MS, Granada B, Head WS, Koster JC, Nichols CG, Piston DW (2006) Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol 4(2):e26, Epub 2006 Jan 17
Saez JC, Spray DC, Nairn AC, Hertzberg E, Greengard P, Bennett MVL (1986) cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci 83:2473–2477
Santos-Sacchi J (1985) The effects of cytoplasmic acidification upon electrical coupling in the organ of Corti. Hear Res 19:207–215
Santos-Sacchi J (1986) The temperature dependence of electrical coupling in the organ of Corti. Hear Res 21:205–211
Santos-Sacchi J (2000) Cell coupling in the organ of Corti. Brain Res Rev 32:167–171
Sato Y, Santos-Sacchi J (1994) Cell coupling in the supporting cells of Corti’s organ: sensitivity to intracellular H+ and Ca++. Hear Res 80:21–24
Söhl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62:228–232
Solan JL, Lampe PD (2005) Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta 1711:154–163
Spicer SS, Schulte BA (1996) The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res 100:80–100
Spitzer N, Sammons GS, Price EM (2011) Autofluorescent cells in rat brain can be convincing impostors in green fluorescent reporter studies. J Neurosci Methods 197:48–55
Todt I, Ngezahayo A, Ernst A, Kolb HA (1999) Inhibition of gap junctional coupling in cochlear supporting cells by gentamicin. Pflugers Arch 438:865–867
Todt I, Ngezahayo A, Ernst A, Kolb HA (2001) Hydrogenperoxide inhibits gap junctional coupling and modulates intracellular free calcium in cochlear Hensen-cells. J Membr Biol 181:107–114
Velasco A, Tabernero A, Granda B, Medina JM (2000) ATP-sensitive potassium channel regulates astrocytic gap junction permeability by a Ca2+-independent mechanism. J Neurochem 74:1249–1256
Vera B, Sanchez-Abarca LI, Bolanos JP, Medina JM (1996) Inhibition of astrocyte gap junctional communication by ATP depletion is revered by calcium sequestration. FEBS 392:225–228
Willecke K, Eiberger J, Degen J, Eckhardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl U (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Wollbold J, Jaster R, Müller S, Rateitschak K, Wolkenhauer O (2014) Anti-inflammatory effects of reactive oxygen species—a multi-valued logical model validated by formal concept analysis. BMC Syst Biol 8:101
Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ (1998) Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet 20:370–373
Yang J, Wang J (2002) Possible function of outward potassium currents in isolated Deiters’cells of guinea pig cochlea. Chin Med J 115:264–267
Zelante L, Gasparini P, Estivill X, Melchionda S, D’Agruma L, Govea N, Milá M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, Surrey S, Fortina P (1997) Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet 6:1605–1609
Zhao HB, Santos-Sacchi J (1998) Effect of membrane tension on gap junctional conductance of supporting cells in Corti’s organ. J Gen Physiol 112:447–455
Zhao HB, Yu N, Fleming CR (2005) Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A 102:18724–18729
Zhao HB, Kikuchi T, Ngezahayo A, White TW (2006) Gap junction and cochlear homeostasis. J Membr Biol 209:177–186
Acknowledgments
The authors thank Ina G. Siller for the help with imaging the cochlear slides. We thank Dr. Kathrin Rübensam, ZTL, MH Hannover, and Dr. Henning Vogt, HNO-Klinik, MH Hannover for their kind help with the animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blödow, A., Begandt, D., Bader, A. et al. ATP-sensitive K+ channels (Kir6.1/SUR1) regulate gap junctional coupling in cochlear-supporting cells. Pflugers Arch - Eur J Physiol 468, 1215–1222 (2016). https://doi.org/10.1007/s00424-016-1815-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1815-8