Abstract
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. After release from presynaptic nerve terminals, glutamate is quickly removed from the synaptic cleft by a family of five glutamate transporters, the so-called excitatory amino acid transporters (EAAT1–5). EAATs are prototypic members of the growing number of dual-function transport proteins: they are not only glutamate transporters, but also anion channels. Whereas the mechanisms underlying secondary active glutamate transport are well understood at the functional and at the structural level, mechanisms and cellular roles of EAAT anion conduction have remained elusive for many years. Recently, molecular dynamics simulations combined with simulation-guided mutagenesis and experimental analysis identified a novel anion-conducting conformation, which accounts for all experimental data on EAAT anion currents reported so far. We here review recent findings on how EAATs accommodate a transporter and a channel in one single protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ion movement across biological membranes is mediated and precisely controlled by membrane proteins commonly classified into ion channels, transporters, and pumps. These three classes of transport proteins mediate structurally and thermodynamically distinct transport processes. Ion channels exhibit an aqueous conduction pathway, which allows passive ionic diffusion across the membrane. Diffusion through ion channels is fast and plays—among many other cellular tasks—an important role in electrical signaling of excitable cells. Channels can only mediate passive ion movement; i.e., ions can only move along electrical or chemical gradients. The generation of such gradients requires proteins that transport ions against their electrochemical gradients. In ion pumps, the required energy is provided by the hydrolysis of ATP, whereas secondary active transporters thermodynamically couple the energetic upward movement of one substrate to the downward flow of other substrates along their electrochemical gradients [9, 10, 30]. Transmembrane ion movement by transporters and pumps is based on conformational changes of the proteins, and transport rates are usually at least an order of magnitude smaller than for ion channels.
For decades, channels and transporters have been treated as separate entities. However, there are multiple transporters that can also function as channels [13, 36]. One of the first examples in which such dual function was fully appreciated is a class of glutamate transporters belonging to the solute carrier 1 family, the excitatory amino acid transporters (EAATs). In glia or in neurons, EAATs mediate the re-uptake of synaptically released glutamate via the coupled co-transport of three Na+, one H+, and one glutamate, in counter-transport to one K+. This transport process is electrogenic, but very slow, and results in only small transport currents in cells expressing such transporters. In 1995, studies on oocytes heterologously expressing EAATs [16, 74] and on native salamander photoreceptors [53] demonstrated that these transporters also function as anion-selective channels.
We here review recent progress in understanding this novel class of anion-selective channels. Surprisingly, members of another class of glutamate transporters, vesicular glutamate transporters, were also proposed to function as anion channels [59]. While being involved in similar physiological processes, the two protein families are not related, and we would like to refer interested readers to Shigeo Takamori’s review on these transporters in our special issue.
Slips, leaks, or channels?
Many transporters mediate transport processes that deviate from stoichiometrically coupled co-transport [13, 43, 44]. To account for such behavior, three terms have been mainly used: slips, leaks, and transporter-associated ion channels. Although the discussion about these terms is semantic, they are related with different concepts underlying ion fluxes. We will shortly introduce how we would use these terms and why we believe that—in the case of EAATs—only the term anion channel is appropriate.
Secondary active transporters might perform transport cycles that deviate from the normal transport stoichiometry [48], and in extreme cases, coupled symporters or antiporters may transport one substrate uncoupled from the movement of other substrates across the membrane. Although there exists no description of such processes at the structural level, uncoupled substrate movement might arise from imperfect coordination of certain conformational changes of the transporter, permitting ion slippage through an occluded intermediate state. Slippage can only occur for ions that are transport substrates. To describe crossing of ions that are usually not transported in the regular mode of function of transporters, some researchers employ the term leak. This expression implies diffusion through variable, non-optimized pathways, for example by transient openings of leak pathways during conformational changes of the transporter proteins. Slips and leaks designate defective states of a transporter, rather than an evolutionarily optimized transport function.
The notions of slips and leaks do not apply to EAAT anion conduction. Anions pass through EAATs along a defined, aqueous conduction pathway [32, 40, 68] that is perfectly selective [46, 75] and precisely gated [5, 25, 41, 51, 58, 61, 62]. EAAT anion channels exhibit unitary current amplitudes, which are small but in the range of specialized anion channels [60]. The EAAT anion conductance is evolutionarily preserved in all EAAT glutamate transporters that have been studied so far [16, 56, 74], and there is increasing evidence that EAAT anion channels fulfill important physiological functions [73, 77, 78]. All these results demonstrate that EAAT glutamate transporters can operate as anion channels, making EAAT anion channels another example for the variability that nature has created in the field of anion-selective channels [49].
EAAT anion channel gating is tightly coupled to the glutamate transport cycle
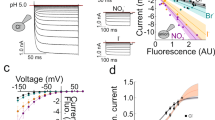
Figure 1a depicts a representative whole-cell patch-clamp recording from a mammalian cell expressing rat EAAT4. The use of NO3 − as main permeant anion increases EAAT4 anion currents to levels that greatly exceed the small background currents of these cells. Since electrogenic glutamate transport—but not the glutamate-induced activation of the anion conductance—requires K+-bound re-translocation of the transporter, substitution of intracellular K+ by Na+ specifically abolishes glutamate uptake currents. Taken together, these two maneuvers permit recording anion currents in isolation. EAAT4 anion currents are small in the absence of glutamate or aspartate and are greatly increased by addition of transport substrates such as glutamate or aspartate. Transport substrates do not only increase the whole-cell amplitude, but also modify the time and voltage dependence of EAAT anion currents [41, 45, 68, 69].
The time, voltage, and substrate dependence of EAAT anion currents reveals a tight coupling between anion channel gating and the glutamate transport cycle. a Representative whole-cell anion current responses of a cell expressing rat EAAT4 to voltage steps between −170 and +180 mV in the absence or presence of saturating concentrations of l-glutamate or l-aspartate. Symmetric NaNO3 concentrations were used for the pipette and the bath. b Kinetic state diagram of EAAT glutamate transport and anion channel gating. Anion-conducting states are illustrated as branching channel states (Ch). c Simulated EAAT4 anion current traces upon voltage steps under the same conditions as in a. The current traces were calculated by solving the differential equations defined by b as described in [41]. Figure partially reprinted from [41] with permission
Such experimental results [32, 35, 41, 45], together with analyses of time-dependent changes in EAAT anion currents upon fast application of transport substrates [5, 17, 20, 50, 65–67], demonstrated a tight coupling between the glutamate transport cycle and the EAAT anion currents. The interplay between glutamate transport and anion channel activation becomes particularly evident in experiments performed to pharmacologically separate these two transport functions. Blockers of glutamate transport prevent progression of the uptake cycle and thus interfere with opening of anion channels [1, 28, 53]. Removal of transport substrates, such as in experiments recording anion currents in the absence of intracellular K+ (Figs. 1 and 2), modifies voltage- and time-dependent gating of EAAT anion channels [35].
Isoform-specific differences in EAAT transport properties result in distinct time and voltage dependences of anion currents. a–e Representative current responses of HEK293T cells expressing hEAAT1, hEAAT2, hEAAT3, rEAAT4, or mEAAT5 to voltage steps between −150 and +150 mV. Cells were dialyzed with NaNO3-based pipette solutions and externally perfused with NaNO3-based solution containing 0.5 mM l-glutamate. Figure partially reprinted from [41, 60] with permission
For many years, EAAT anion currents have been described with kinetic schemes which are based on the glutamate transport cycle and in which certain states are either assumed to be anion conducting by themselves or to be linked with open anion channel states. In order to account for glutamate-, Na+-, and K+-dependent changes in macroscopic EAAT anion currents, the different states of the glutamate uptake cycle need to exhibit different unitary current amplitudes in the first case [65]. However, experimental evidence argues against the existence of separate states with multiple distinct unitary conductances [32, 39, 40]. Anion channel opening therefore needs to be represented by additional states branching from the glutamate uptake cycle all with the same conductance [32, 41, 50]. The notion of a distinct conformational gating transition, which opens or forms the anion conduction pathway, is further corroborated by the finding that EAAT anion channel activation is delayed by a Na+-independent and electrogenic reaction of the glutamate transport cycle [20, 50, 76].
Kinetic models that consider the states of the glutamate uptake cycle as closed channels and additionally feature branching pathways to open channel states (Fig. 1b) qualitatively reproduce the voltage dependence of EAAT anion and transport currents with surprising accuracy (Fig. 1c). Such models were developed before structural information about anion conduction was available. Our current understanding that open EAAT anion channels are associated with conformations that are intermediate to inward- and outward-facing states of the transporter (see below) will require novel kinetic schemes.
Isoform-specific variation in EAAT anion conduction
There are five different EAAT isoforms expressed in the mammalian brain as well as in certain absorptive epithelia [12]. EAAT1 and EAAT2 are predominantly expressed in glia, whereas EAAT3, EAAT4, and EAAT5 are neuronal glutamate transporters. EAAT3 is furthermore responsible for glutamate and aspartate reabsorption in the renal proximal tubule [4]. The distinct EAAT isoforms differ in the extent to which they function as glutamate transporter or as anion channel. In one of the first descriptions of EAAT functions, Wadiche and colleagues compared human EAAT1, EAAT2, and EAAT3 heterologously expressed in Xenopus oocytes [74]. They measured glutamate-elicited currents at different holding potentials and observed pronounced isoform-specific differences in the reversal potentials between these currents. Whereas EAAT2 currents reversed at positive potentials close to values expected for secondary active glutamate transport, reversal potentials of EAAT1 and EAAT3 currents were much more negative in standard external solutions and changed upon altering the external Cl− concentration [74]. These results were interpreted by assuming that distinct EAAT isoforms differ in individual transport rates and/or unitary anion current amplitudes. Experiments with EAAT4 and EAAT5, also expressed in Xenopus oocytes, revealed that these transporters predominantly function as anion channels [3, 16]. During evolution, certain EAAT glutamate transporters obviously specialized into isoforms that mainly operate as glutamate uptake carriers (EAAT1, EAAT2, EAAT3) and others that represent glutamate-gated anion channels (EAAT4, EAAT5).
We now know that these differences in glutamate transport efficacy are mainly due to isoform-specific variations of rate constants within the glutamate uptake cycle [17, 47, 60]. Due to the tight linkage of anion channel opening to the uptake cycle, these differences result in distinct time and voltage dependences of EAAT1 to EAAT5 anion currents (Fig. 2) [47].
Functional properties of EAAT anion channels
The observation of EAAT anion currents that are not stoichiometrically coupled to secondary active glutamate transport does not provide information about the anion transport mechanism. Possible mechanism included channel-like anion permeation or carrier-mediated anion transport. A quantitative parameter that distinguishes channel- from carrier-mediated ion fluxes is a large unitary current amplitude [24].
Initial approaches to determine single EAAT anion channel current amplitudes through noise analysis resulted in large differences between individual studies. Larsson and colleagues [33] demonstrated that power spectra of glutamate transporter-associated current fluctuations measured by whole-cell patch-clamp recordings on tiger salamander cones obey Lorentzian functions [33]. This result indicated that these currents generate noise by the random opening and closing events of individual channels and that current fluctuations can be used to estimate the current amplitude and the opening frequency of such underlying individual events. Non-stationary noise analysis based on modifying anion channel open probabilities through changes in external [Glu−] revealed a unitary conductance of 0.7 pS at symmetric [Cl−] and an absolute open probability above 0.5 [33]. These single-channel amplitudes are too high to be accounted for by carrier-mediated transport, and these data thus establish channel-like anion conduction by EAAT glutamate transporters.
After this first determination of single channel amplitudes, two studies appeared that reported very different estimates of this parameter. Noise analysis on human EAAT1 in excised patches from injected oocytes [75] resulted in currents that were three orders of magnitude smaller than those determined on salamander cones, i.e., single-channel conductances between 0.63 and 1.0 fS. There are significant differences in experimental conditions between these two studies; however, they cannot fully account for such pronounced divergence in apparent unitary conductance. One potential explanation is that low expression levels resulted in very small current noise that cannot be correctly separated from background noise. This notion is underlined by power spectra of the induced currents that did not conform to Lorentzian functions. Palmer and colleagues performed noise analysis on bipolar cell terminals and obtained a single channel conductance of 13.3 pS together with a maximum absolute open probability of 1 with symmetric chloride [52]. In these experiments, glutamate transporter-associated anion currents were elicited by electrical synapse stimulation, and currents and variances measured before this stimulus were subtracted as background values. Since a significant fraction of EAAT anion channels is already active prior to synaptic activation, this procedure might have underestimated EAAT anion currents and variances and thus overestimated unitary current amplitudes.
Our group compared the unitary conductance of all mammalian EAATs, ranging from EAAT1 [78] to EAAT5 [60], in transiently transfected HEK293T cells. Non-stationary or stationary noise analysis employing voltage-dependent alterations of EAAT anion currents [32, 39, 40, 45, 68] robustly provided unitary conductances around 1 pS. There were slight isoform-specific variations in unitary current amplitudes, with EAAT5 having the highest [60] and EAAT4 the lowest single channel amplitude [40]. The most effective glutamate transporters EAAT2 and EAAT3 exhibit anion channels with intermediate unitary conductances [60, 68]. These data demonstrate that the differences in macroscopic anion current amplitudes between EAAT1, EAAT2, EAAT3, EAAT4, and EAAT5 are not caused by large variations in single-channel conduction rates.
In addition to the amplitudes of individual current events, noise analysis is also often used to determine the fraction of time that the transporters assume an open anion channel, i.e., the absolute open probability of the anion channel. In the majority of non-stationary noise analyses on EAAT anion channels, absolute open probabilities above 0.5 were determined [32, 33, 45, 68, 78], suggesting that transporters dwell most of the time in anion conducting states. However, whereas non-stationary noise analysis is known to reliably provide unitary current amplitudes, this method potentially underestimates the number of channels, resulting in incorrectly high absolute open probabilities. Fast transitions between different protein conformations, which are typical for ion transporters, might result in semi-equilibria between these states. These reactions will thus underrate the number of channels under study and produce incorrectly high absolute open probabilities [2].
To test the accuracy of the absolute open probability determination by noise analysis, we applied an alternative method that is based on the comparison of glutamate uptake current and macroscopic anion current amplitudes. Figure 3 shows an example of this analysis for EAAT2. In the absence of permeant anions, glutamate-induced whole-cell currents from cells expressing EAAT2 are entirely mediated by electrogenic glutamate transport. Since individual transport rates are known for EAAT2 (54.3 s−1, corresponding to a transport current of 1.74·10−17 A, at −88 mV [51]), the whole-cell uptake current can be used to determine the number of EAAT2 subunits in the surface membrane of the cell under observation. Glutamate uptake currents are pronouncedly inward rectifying such that measuring the current amplitude at positive potentials, after applying a NaNO3-based solution at the external side, specifically yields the anion current component mediated by this number of EAAT2 transporters. This value together with the corresponding unitary current amplitude (21.5 fA at +70 mV [60]) provides an absolute open probability in the range of 0.002. A comparison of transport rates and anion current/uptake current ratios of the distinct mammalian EAATs suggests that absolute open probabilities of the other EAAT anion channels were in the same range. Thus, while being very useful in the determination of EAAT unitary conductances, noise analysis dramatically overestimates the probability of EAAT anion channel opening.
Comparing glutamate uptake currents with anion currents demonstrates very small absolute open probabilities of EAAT anion channels. Representative current responses of a HEK293T cell expressing hEAAT2 to consecutive voltage steps to +70 and −70 mV. The cell was dialyzed with a potassium gluconate (KGluc)-based pipette solution and then externally perfused with NaGluc-based solution with or without l-glutamate and finally with a NaNO3-based solution containing 0.5 mM l-glutamate
All EAAT anion channels exhibit a lyotropic or Hofmeister selectivity sequence [3, 32, 45, 74, 75]. Substitution of the main physiological anion Cl− by NO3 −, I−, ClO4 −, or SCN− results in significant alterations of macroscopic anion currents. Such selectivity sequences indicate that the major determinant of anion selectivity and conduction is the energetic cost for anion dehydration, rather than the energy released by the association of the dehydrated anion to binding sites within the anion conduction pathway [79]. Wadiche and Kavanaugh employed anion substitution experiments to estimate the minimum pore diameter pore to about 5 Å [75].
Taken together, these data demonstrate that EAAT anion channels exhibit unitary current conductances in the low picosiemens range and absolute open probabilities in the sub-per mill range. Functional properties support the notion of the EAAT anion conduction pathway being a rather wide pore, with mostly hydrophobic side chains interacting with the permeating anions.
Structural basis of EAAT glutamate transport
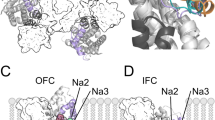
In recent years, crystal structures of two prokaryotic members of the EAAT family, GltPh [7, 54, 71, 72, 80] and GltTk [29], in various functional states provided insights into EAAT glutamate transport at atomic resolution. EAAT/Glt transporters were shown to assemble as bowl-shaped trimers with each subunit containing eight transmembrane domains (TM) and two hairpin loops (HP) that control access of transport substrate to its binding site from both sides of the membrane [18, 23, 27, 80]. Transport is initiated by association of aspartate/glutamate to an outward-facing conformation (OFC) with open external hairpin HP2 [22, 27]. Its closure after amino acid association enhances the tightness of binding, resulting in an induced fit mechanism of substrate binding [15, 21]. Subsequently, the transporter translocates into an inward-facing conformation (IFC) via a large-scale (∼18 Å) rotational–translational movement of the substrate-harboring transport domain relative to the static trimerization domain. This elevator-like movement was simultaneously suggested by the theoretical analysis of internal structural symmetry found in the GltPh monomer [11] and by comparing crystal structures of GltPh in the OFC [7] and the IFC [54]. Recently, all-atom MD simulations, solely relying on the OFC and IFC structures, sampled a possible OFC–IFC transition path [40, 64]. These MD simulations [40] were able to reproduce the recently crystallized intermediate GltPh structure [71] within 1.3 Å RMSD and confirmed the above-described elevator-like substrate translocation. Opening of the internal HP1 allows release of the substrate into the cytoplasm [23], and subsequent HP1 closure and retranslocation complete the transport cycle. In marked contrast, none of the X-ray structures captured a transporter in its anion-conducting conformation or permitted the identification of anion density within the protein.
Early attempts to define pore-forming residues of EAAT anion channels
Vandenberg and colleagues were the first to systematically employ site-directed mutagenesis, functional analysis, and chemical modification to identify side chains that alter chloride permeation [8, 26, 57]. They demonstrated that mutations in the second transmembrane domain (TM2) affect relative anion permeabilities and/or the magnitude of whole-cell anion currents and that some of these residues are accessible to hydrophilic cysteine-reactive methanethiolsulphonates [57]. Later, additional mutations in TM 5 and 7 [26] and in HP1 and TM7 [8] were reported to affect the substrate-activated anion conductance. In all these studies, effects on relative anion permeability and on macroscopic current amplitudes were chosen as parameters reporting on alterations of the anion conduction pathway.
Testing the effects of side chain substitutions on relative cation permeability has been extremely successful in identifying the molecular basis of K+, Na+, and Ca2+ selectivity in voltage- and ligand-gated cation channels [42]. The unique mechanisms of EAAT anion conduction and channel gating render this approach much more complicated for this class of transport proteins. Since channel opening is only possible from certain transporter conformations, mutations that do not directly modify the anion conduction pathway, but affect the transport cycle, will indirectly alter the channel’s open probability as well [5, 40, 50]. Hence, macroscopic current amplitudes in mutants do not necessarily report on changes in unitary conductance. Furthermore, the anion conduction pathway is dynamically formed by EAATs during normal operation, and long-range effects by residues that influence these conformational changes also need to be taken into account. The lyotropic selectivity sequence of EAAT anion channels indicates that anion selectivity is mainly determined by the dehydration energy. Many mutations therefore rather affect relative anion selectivities by modifying the pore geometry than by altering the interaction of the permeating anion with the particular side chain.
Our group re-analyzed one of the mutations with the most pronounced effects on anion conduction in an earlier study [57] in the background of another transporter, rat EAAT4. This mutation, D117A EAAT4, affects not only relative anion permeabilities, but also the unitary current amplitude [32], a parameter that is often believed to reliably report on changes in pore-forming residues. However, since D117A removes a negative charge and decreases the single channel amplitude, a close contact of permeating anions with the Asp117 side chain seems unlikely, suggesting indirect effects of this mutation on the EAAT4 anion conduction pathway. In a subsequent voltage clamp fluorometry study, the homologous mutation was shown to modify translocation of EAAT3 [25], thereby illustrating an intimate relationship between substrate translocation and the conformational changes required for or preceding anion channel opening [58, 61].
These studies demonstrated that mutations can cause major functional alterations of the EAAT anion conduction pathway by modifying the translocation of glutamate and that structure–function analysis alone is insufficient to identify pore-forming residues in EAATs. We therefore decided to employ molecular simulations to resolve the anion-conducting conformation in GltPh and to guide electrophysiological and biochemical experiments to verify that such conformations are responsible for anion conduction in EAATs.
Molecular dynamics simulations resolve an anion-conducting conformation and identify the mechanisms of anion selectivity and conduction
We employed all-atom MD simulations of GltPh [27, 63] in presence of a transmembrane voltage in order to directly simulate Cl− permeation [40]. We found that neither the substrate-bound outward- nor the inward-facing structures were conductive to anions even at high voltages ranging from +800 mV up to 1.6 V. Since experimental evidence suggested that substrate translocation intermediates might be anion conductive [25, 58, 61], we simulated the outward–inward transition of GltPh. However, none of the intermediate conformations during translocation encompasses molecular voids or tunnels connecting the external and internal side with the dimensions required to account for the experimentally observed anion conduction properties (>5 Å as reported by [75]; Fig. 4a–c).
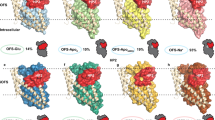
Structural basis of EAAT anion conduction. a–c Illustration of the substrate-bound part of the glutamate uptake cycle. For clarity, GltPh monomers are shown in cartoon representation in side view; the perspective is from the center of the trimeric protein (yellow, trimerization domain; blue, transport domain except HPs; orange and cyan, HP1 and HP2, respectively; red spheres, aspartate; blue spheres, Na+ ions; R276 is shown as sticks). An elevator-like substrate transport mechanism becomes obvious by comparison of the transport domains [OFC outward-facing conformation (PDB 2NWX), IC cen centrally located translocation intermediate [40], IFC inward-facing conformation (PDB 3KBC)]. d, e Lateral movement of the transport domain in ICcen leads to the channel conformation (ChC) with an aqueous anion conduction pathway. d Superposition of IC and ChC in top view (blue, transport domain in ICcen; lime, transport domain in the anion-conducting ChC). e Illustration of the observed anion permeation pathway (red mesh, Cl− density at σ = 0.2). f Simulated Cl− and I− permeation events through the ChC GltPh anion pore at +800 or −900 mV (dashed lines). g, h Pore profile of anion hydration numbers and pore diameter (g) and Poisson–Boltzmann energies (h) for Na+ and Cl− of WT or R276S GltPh and for WT GltPh after removal of aspartate and Na1/2+ (in ChC). Hydration numbers are integrals of Cl−/hydrogen radial distribution functions to the first minimum. Figure partially reprinted from [40] with permission from Elsevier
From several of these “translocation intermediates,” fully reversible conformational changes were observed within 60 to hundreds of nanoseconds in the presence of membrane voltage (Fig. 4d). Lateral movement of the mobile substrate transport domain and subsequent water influx formed an anion pore at the interface between the transport and trimerization domain, close to the tip of HP1. Analysis of the chloride permeation events in these simulations defined an hourglass-shaped aqueous anion conduction pathway (Fig. 4e). Simulations with different salts such as NaCl or NaI reproduced perfect anion over cation selectivity and the preference of I– over Cl− known for EAAT anion channels (Fig. 4f). Transport substrates such as aspartate were impermeant, and simulated anion permeation rates were consistent with experimental EAAT unitary anion current amplitudes. Lastly, this mechanism permits formation of anion permeation pathways in each of the three subunits of functional transporters, thus accounting for the experimentally demonstrated co-localization of glutamate translocation and anion currents within individual subunits [19, 31, 34]. The simulated anion channel accounts for all experimental data on the EAAT/GltPh anion conduction and also for water permeation through these proteins [37, 38, 70].
The hydrated anion conduction pathway has a minimum diameter about 5.6 Å and is almost perpendicular to the membrane with large cavities on the extracellular and intracellular entrance (Fig. 4e, g). Most side chains lining the pore center are hydrophobic, with the exception of one positively charged arginine R276 that protrudes from the tip of HP1 into the Cl− density. R276 is the major determinant of the positive electrostatic potential within the anion pore, and its removal results in a loss of anion-over-cation selectivity in our simulations (Fig. 4h) and experiments [6, 40, 55]. This residue is not conserved in the primary sequence of EAATs; however, in mammalian EAATs, there is an arginine at the position corresponding to M395 in TM8 of GltPh that projects its side chain to the same location in the anion conduction pathway and fulfills the same functional role. In general, residues that project into the GltPh conduction pathway are rather well conserved across the EAAT family, suggesting that the same conduction path accounts for mammalian EAAT anion conduction. Simulated ion permeation was similar for transporters with bound Na+/aspartate or without transport substrate, in agreement with the indistinguishable unitary conductances of EAAT4 anion channels in the presence and in the absence of glutamate [32].
A large in silico mutagenesis screen of all pore-forming mutants revealed that the anion conductance was rather robust against individual changes of most side chains. MD simulations identified several mutations that either increase or decrease Cl− currents. Surprisingly, three mutations were found that also allowed Na+ permeation, thus converting the anion pore to a non-selective anion/cation channel. In patch-clamp recordings of homologous mutations in EAAT2 and EAAT4, we observed changes in single-channel conductance or Cl−/Na+ selectivity that were comparable to those in the GltPh simulations.
The anion conduction pathway resolved by the MD simulations was tested by fluorescence spectroscopy experiments on single-tryptophan mutants in GltPh (Fig. 5a). Tryptophan fluorescence is collisionally quenched by iodide anions, and fluorescence spectroscopy thus permits testing which residues come in close contact to permeating anions (Fig. 5b). Locations close to the simulated conduction pathway showed a significant decrease in tryptophan fluorescence upon application of I−, whereas regions apart from the simulated anion pore were not accessible to I− in these experiments. A recent single-molecule study demonstrated a significant increase in the occupation of intermediate confirmations by GltPh upon substrate application which can also correspond to open anion channel conformations [14]. The macroscopically observed activation of the anion conductance upon substrate may thus be directly related to an increased population of translocation intermediates, which in turn allow for anion pore opening. Taken together, these data demonstrate that EAATs/GltPh can assume this anion-conducting conformation under experimental conditions and that anion permeation through this class of glutamate transporters occurs along the conserved anion conduction pathway identified by MD simulations.
Iodide fluorescence quenching reports on spatial proximity to the anion conduction pathway. a Localization of single GltPh tryptophan insertions with side chains color-coded according to the ratio of the fluorescence intensity in the absence of I− (F 0) by the corresponding values at [I−] = 350 mM. The red mesh represents the Cl− permeation path from the simulations (Fig. 4e). b Iodide-dependent changes of representative fluorescence spectra of WT, V51W, and S65W GltPh. The inset provides the concentration dependence of V51W fluorescence lifetimes and intensities in a Stern–Volmer plot, indicating a collisional quenching mechanism. Figure reprinted from [40] with permission from Elsevier
The anion conduction pathway resolved by MD simulations differs from earlier suggestions on the basis of mutagenesis studies that hypothesized residues mainly positioned in TM7 and the carboxy-terminal limb of HP1 and centered around S65 (GltPh numbering) to form an aqueous cavity during substrate translocation [8]. While these residues are accessible to water in MD simulation, they are not reached by anions (Fig. 5) [40]. Due to their location near the transport/trimerization domain interface, modifications of these residues most likely affect the conformational changes underlying channel opening, i.e., the lateral movement of the transport domain and hydration of the anion pathway along the tip of HP1. As explained above, the degree of pore hydration is important since the lyotropic anion selectivity depends on differences in dehydration energy, and the discrimination between anions can be altered if the pore hydration is changed.
Summary and outlook
EAATs combine two thermodynamically and structurally distinct transport functions in a single protein. In recent years, detailed information about the molecular and structural basis of EAAT glutamate transport and anion conduction has been obtained by crystallographic, spectroscopic, and electrophysiological studies as well as molecular dynamics simulations. It is well established that EAAT/Glt transporters isomerize from outward- to inward-facing conformation via an elevator-like motion of the transport domain to deliver glutamate from the extracellular space to the cytoplasm. EAAT anion channel opening occurs via lateral movement of the transport domain from intermediate conformations reached during this substrate translocation. The resulting anion conduction pathway is structurally and functionally conserved among the EAAT isoforms. Anion-over-cation selectivity is ensured by a single positively charged arginine protruding into a pathway, which gets reversibly filled by water molecules concomitant with anion channel opening.
The anion channel gating mechanism requires that the two EAAT transport functions are mutually exclusive: at a certain time point, an individual EAAT transporter is either transporting glutamate or conducting anions. Since glutamate transport is only possible if the anion channel is closed, EAATs have to dwell only a small fraction of time in the anion-conducting mode to permit effective glutamate transport.
Whereas the functional and structural basis of anion permeation is now well understood, we are still beginning to appreciate the cellular roles of EAAT anion channels. Recent progress in understanding EAAT anion conduction at the structural level and the identification of mutations that significantly alter the function of EAAT anion channels will help designing new experiments to study cellular roles of these channels. These future studies will hopefully clarify the biological impact of combining chloride channels and neurotransmitter transporters in this fascinating class of membrane transport proteins.
References
Abrahamsen B, Schneider N, Erichsen MN, Huynh TH, Fahlke C, Bunch L, Jensen AA (2013) Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J Neurosci 33:1068–1087. doi:10.1523/jneurosci.3396-12.2013
Alekov A, Fahlke C (2009) Channel-like slippage modes in the human anion/proton exchanger ClC-4. J Gen Physiol 133:485–496
Arriza JL, Eliasof S, Kavanaugh MP, Amara SG (1997) Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94:4155–4160
Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, Auray-Blais C, Vandenberg RJ, Broer S, Rasko JE (2011) Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J Clin Invest 12:446–453. doi:10.1172/jci44474
Bergles DE, Tzingounis AV, Jahr CE (2002) Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters. J Neurosci 22:10153–10162
Borre L, Kanner BI (2004) Arginine-445 controls the coupling between glutamate and cations in the neuronal glutamate transporter EAAC-1. J Biol Chem 279:2513–2519
Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E (2007) Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445:387–393
Cater RJ, Vandenberg RJ, Ryan RM (2014) The domain interface of the human glutamate transporter EAAT1 mediates chloride permeation. Biophys J 107:621–629. doi:10.1016/j.bpj.2014.05.046
Christensen HN, Riggs TR (1952) Concentrative uptake of amino acids by the Ehrlich mouse ascites carcinoma cell. J Biol Chem 194:57–68
Crane RK (1977) The gradient hypothesis and other models of carrier-mediated active transport. Rev Physiol Biochem Pharmacol 78:99–159
Crisman TJ, Qu S, Kanner BI, Forrest LR (2009) Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats. Proc Natl Acad Sci USA 106:20752–20757
Danbolt NC (2001) Glutamate uptake. Progr Neurobiol 65:1–105
DeFelice LJ, Goswami T (2007) Transporters as channels. Annu Rev Physiol 69:87–112
Erkens GB, Hanelt I, Goudsmits JM, Slotboom DJ, van Oijen AM (2013) Unsynchronised subunit motion in single trimeric sodium-coupled aspartate transporters. Nature 502:119–123. doi:10.1038/nature12538
Ewers D, Becher T, Machtens JP, Weyand I, Fahlke C (2013) Induced fit substrate binding to an archeal glutamate transporter homologue. Proc Natl Acad Sci USA 110:12486–12491. doi:10.1073/pnas.1300772110
Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG (1995) An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375:599–603
Gameiro A, Braams S, Rauen T, Grewer C (2011) The discovery of slowness: low-capacity transport and slow anion channel gating by the glutamate transporter EAAT5. Biophys J 100:2623–2632
Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, Schmalzing G, Hidalgo P, Fahlke C (2004) A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. J Biol Chem 279:39505–39512
Grewer C, Balani P, Weidenfeller C, Bartusel T, Tao Z, Rauen T (2005) Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry 44:11913–11923
Grewer C, Watzke N, Wiessner M, Rauen T (2000) Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc Natl Acad Sci USA 97:9706–9711
Hänelt I, Jensen S, Wunnicke D, Slotboom DJ (2015) Low affinity and slow Na+-binding precedes high affinity aspartate binding in GltPh. J Biol Chem. doi:10.1074/jbc.M115.656876
Heinzelmann G, Bastug T, Kuyucak S (2011) Free energy simulations of ligand binding to the aspartate transporter GltPh. Biophys J 101:2380–2388. doi:10.1016/j.bpj.2011.10.010
Heinzelmann G, Bastug T, Kuyucak S (2013) Mechanism and energetics of ligand release in the aspartate transporter GltPh. J Phys Chem B 117:5486–5496. doi:10.1021/jp4010423
Hille B (2001) Ion channels of excitable membranes, 3rd edn. Sinauer Associates Inc., Sunderland, MA
Hotzy J, Machtens JP, Fahlke C (2012) Neutralizing aspartate 83 modifies substrate translocation of excitatory amino acid transporter 3 (EAAT3) glutamate transporters. J Biol Chem 287:20016–20026
Huang S, Vandenberg RJ (2007) Mutations in transmembrane domains 5 and 7 of the human excitatory amino acid transporter 1 affect the substrate-activated anion channel. Biochemistry 46:9685–9692
Huang Z, Tajkhorshid E (2008) Dynamics of the extracellular gate and ion-substrate coupling in the glutamate transporter. Biophys J 95:2292–2300
Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L (2015) Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr Opin Pharmacol 20:116–123. doi:10.1016/j.coph.2014.10.008
Jensen S, Guskov A, Rempel S, Hanelt I, Slotboom DJ (2013) Crystal structure of a substrate-free aspartate transporter. Nat Struct Mol Biol 20(10):1224–1226. doi:10.1038/nsmb.2663
Kaback HR, Jung K, Jung H, Wu J, Prive GG, Zen K (1993) What’s new with lactose permease. J Bioenerg Biomembr 25:627–636
Koch HP, Brown RL, Larsson HP (2007) The glutamate-activated anion conductance in excitatory amino acid transporters is gated independently by the individual subunits. J Neurosci 27:2943–2947
Kovermann P, Machtens JP, Ewers D, Fahlke C (2010) A conserved aspartate determines pore properties of anion channels associated with excitatory amino acid transporter 4 (EAAT4). J Biol Chem 285:23676–23686
Larsson HP, Picaud SA, Werblin FS, Lecar H (1996) Noise analysis of the glutamate-activated current in photoreceptors. Biophys J 70:733–742
Leary GP, Stone EF, Holley DC, Kavanaugh MP (2007) The glutamate and chloride permeation pathways are colocalized in individual neuronal glutamate transporter subunits. J Neurosci 27:2938–2942
Leinenweber A, Machtens JP, Begemann B, Fahlke C (2011) Regulation of glial glutamate transporters by C-terminal domains. J Biol Chem 286:1927–1937
Lester HA, Cao Y, Mager S (1996) Listening to neurotransmitter transporters. Neuron 17:807–810
Li J, Shaikh SA, Enkavi G, Wen PC, Huang Z, Tajkhorshid E (2013) Transient formation of water-conducting states in membrane transporters. Proc Natl Acad Sci USA 110:7696–7701. doi:10.1073/pnas.1218986110
MacAulay N, Gether U, Klaerke DA, Zeuthen T (2001) Water transport by the human Na+-coupled glutamate cotransporter expressed in Xenopus oocytes. J Physiol 530:367–378
Machtens JP, Fahlke C, Kovermann P (2011) Noise analysis to study unitary properties of transporter-associated ion channels. Channels 5:468–472
Machtens JP, Kortzak D, Lansche C, Leinenweber A, Kilian P, Begemann B, Zachariae U, Ewers D, de Groot BL, Briones R, Fahlke C (2015) Mechanisms of anion conduction by coupled glutamate transporters. Cell 160:542–553. doi:10.1016/j.cell.2014.12.035
Machtens JP, Kovermann P, Fahlke C (2011) Substrate-dependent gating of anion channels associated with excitatory amino acid transporter 4. J Biol Chem 286:23780–23788. doi:10.1074/jbc.M110.207514
MacKinnon R (2004) Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture). Angew Chem (Intern ed in English) 43:4265–4277. doi:10.1002/anie.200400662
Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA (1994) Conducting states of a mammalian serotonin transporter. Neuron 12:845–859
Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA (1993) Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron 10:177–188
Melzer N, Biela A, Fahlke C (2003) Glutamate modifies ion conduction and voltage-dependent gating of excitatory amino acid transporter-associated anion channels. J Biol Chem 278:50112–50119
Melzer N, Torres-Salazar D, Fahlke C (2005) A dynamic switch between inhibitory and excitatory currents in a neuronal glutamate transporter. Proc Natl Acad Sci USA 102:19214–19218
Mim C, Balani P, Rauen T, Grewer C (2005) The glutamate transporter subtypes EAAT4 and EAATs 1-3 transport glutamate with dramatically different kinetics and voltage dependence but share a common uptake mechanism. J Gen Physiol 126:571–589
Nelson N, Sacher A, Nelson H (2002) The significance of molecular slips in transport systems. Nat Rev Mol Cell Biol 3:876–881
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Otis TS, Jahr CE (1998) Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosc 18:7099–7110
Otis TS, Kavanaugh MP (2000) Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci 20:2749–2757
Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H (2003) Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J Neurosci 23:4831–4841
Picaud SA, Larsson HP, Grant GB, Lecar H, Werblin FS (1995) Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol 74:1760–1771
Reyes N, Ginter C, Boudker O (2009) Transport mechanism of a bacterial homologue of glutamate transporters. Nature 462:880–885
Ryan RM, Kortt NC, Sirivanta T, Vandenberg RJ (2010) The position of an arginine residue influences substrate affinity and K+ coupling in the human glutamate transporter, EAAT1. J Neurochem 114:565–575. doi:10.1111/j.1471-4159.2010.06796.x
Ryan RM, Mindell JA (2007) The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat Struct Mol Biol 14:365–371
Ryan RM, Mitrovic AD, Vandenberg RJ (2004) The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J Biol Chem 279:20742–20751
Ryan RM, Vandenberg RJ (2002) Distinct conformational states mediate the transport and anion channel properties of the glutamate transporter EAAT-1. J Biol Chem 277:13494–13500
Schenck S, Wojcik SM, Brose N, Takamori S (2009) A chloride conductance in VGLUT1 underlies maximal glutamate loading into synaptic vesicles. Nat Neurosci 12:156–162. doi:10.1038/nn.2248
Schneider N, Cordeiro S, Machtens JP, Braams S, Rauen T, Fahlke C (2014) Functional properties of the retinal glutamate transporters GLT-1c and EAAT5. J Biol Chem 289:1815. doi:10.1074/jbc.M113.517177
Seal RP, Shigeri Y, Eliasof S, Leighton BH, Amara SG (2001) Sulfhydryl modification of V449C in the glutamate transporter EAAT1 abolishes substrate transport but not the substrate-gated anion conductance. Proc Natl Acad Sci USA 98:15324–15329
Shabaneh M, Rosental N, Kanner BI (2014) Disulfide cross-linking of transport and trimerization domains of a neuronal glutamate transporter restricts the role of the substrate to the gating of the anion conductance. J Biol Chem 289:11175–11182. doi:10.1074/jbc.M114.550277
Shrivastava IH, Jiang J, Amara SG, Bahar I (2008) Time-resolved mechanism of extracellular gate opening and substrate binding in a glutamate transporter. J Biol Chem 283:28680–28690. doi:10.1074/jbc.M800889200
Stolzenberg S, Khelashvili G, Weinstein H (2012) Structural intermediates in a model of the substrate translocation path of the bacterial glutamate transporter homologue GltPh. J Phys Chem B 116:5372–5383
Tao Z, Grewer C (2007) Cooperation of the conserved aspartate 439 and bound amino acid substrate is important for high-affinity Na+ binding to the glutamate transporter EAAC1. J Gen Physiol 129:331–344
Tao Z, Rosental N, Kanner BI, Gameiro A, Mwaura J, Grewer C (2010) Mechanism of cation binding to the glutamate transporter EAAC1 probed with mutation of the conserved amino acid residue Thr101. J Biol Chem 285:17725–17733
Tao Z, Zhang Z, Grewer C (2006) Neutralization of the aspartic acid residue Asp-367, but not Asp-454, inhibits binding of Na+ to the glutamate-free form and cycling of the glutamate transporter EAAC1. J Biol Chem 281:10263–10272
Torres-Salazar D, Fahlke C (2007) Neuronal glutamate transporters vary in substrate transport rate but not in unitary anion channel conductance. J Biol Chem 282:34719–34726
Torres-Salazar D, Jiang J, Divito CB, Garcia-Olivares J, Amara SG (2015) A mutation in transmembrane domain 7 (TM7) of excitatory amino acid transporters disrupts the substrate-dependent gating of the intrinsic anion conductance and drives the channel into a constitutively open state. J Biol Chem 290:22977–22990. doi:10.1074/jbc.M115.660860
Vandenberg RJ, Handford CA, Campbell EM, Ryan RM, Yool AJ (2011) Water and urea permeation pathways of the human excitatory amino acid transporter EAAT1. Biochem J 439:333–340. doi:10.1042/bj20110905
Verdon G, Boudker O (2012) Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog. Nat Struct Mol Biol 19:355–357
Verdon G, Oh S, Serio RN, Boudker O (2014) Coupled ion binding and structural transitions along the transport cycle of glutamate transporters. eLife 3:e02283. doi:10.7554/eLife.02283
Veruki ML, Morkve SH, Hartveit E (2006) Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci 9:1388–1396
Wadiche JI, Amara SG, Kavanaugh MP (1995) Ion fluxes associated with excitatory amino acid transport. Neuron 15:721–728
Wadiche JI, Kavanaugh MP (1998) Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J Neurosci 18:7650–7661
Watzke N, Bamberg E, Grewer C (2001) Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J Gen Physiol 117:547–562
Wersinger E, Schwab Y, Sahel JA, Rendon A, Pow DV, Picaud S, Roux MJ (2006) The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J Physiol 577:221–234
Winter N, Kovermann P, Fahlke C (2012) A point mutation associated with episodic ataxia 6 increases glutamate transporter anion currents. Brain 135:3416–3425. doi:10.1093/brain/aws255
Wright EM, Diamond JM (1977) Anion selectivity in biological systems. Physiol Rev 57:109–156
Yernool D, Boudker O, Jin Y, Gouaux E (2004) Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature 431:811–818
Acknowledgments
These studies were supported by the Deutsche Forschungsgemeinschaft (FA301/9 to ChF). The authors gratefully acknowledge the computing time granted on the supercomputers JUROPA and JURECA at Jülich Supercomputing Centre (JSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fahlke, C., Kortzak, D. & Machtens, JP. Molecular physiology of EAAT anion channels. Pflugers Arch - Eur J Physiol 468, 491–502 (2016). https://doi.org/10.1007/s00424-015-1768-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1768-3