Abstract
Mutant forms of connexin40 (Cx40) exist in the human population and predispose carriers to atrial fibrillation. Since endothelial expression of Cx40 is important for electrical and chemical communication within the arterial wall, carriers of mutant Cx40 proteins may be predisposed to peripheral arterial dysfunction and dysregulation of blood pressure. We have therefore studied mice expressing either a chemically dysfunctional mutant, Cx40T202S, or wild-type Cx40, with native Cx40, specifically in the endothelium. Blood pressure was measured by telemetry under normal conditions and during cardiovascular stress induced by locomotor activity, phenylephrine or nitric oxide blockade (Nɷ-nitro-l-arginine methyl ester hydroxide, L-NAME). Blood pressure of Cx40T202STg mice was significantly elevated at night when compared with wild-type or Cx40Tg mice, without change in mean heart rate, pulse pressure or locomotor activity. Analysis over 24 h showed that blood pressure of Cx40T202STg mice was significantly elevated at rest and additionally during locomotor activity. In contrast, neither plasma renin concentration nor pressor responses to phenylephrine or L-NAME were altered, the latter indicating that nitric oxide bioavailability was normal. In isolated, pressurised mesenteric arteries, hyperpolarisation and vasodilation evoked by SKA-31, the selective modulator of SKCa and IKCa channels, was significantly reduced in Cx40T202STg mice, due to attenuation of the SKCa component. Acetylcholine-induced ascending vasodilation in vivo was also significantly attenuated in cremaster muscle arterioles of Cx40T202STg mice, compared to wild-type and Cx40Tg mice. We conclude that endothelial expression of the chemically dysfunctional Cx40T202S reduces peripheral vasodilator capacity mediated by SKCa-dependent hyperpolarisation and also increases blood pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic variations in the gap junction protein, connexin40 (Cx40), are prevalent in the human population, and a single mutant allele can predispose carriers to atrial fibrillation and ventricular arrhythmias [16, 28, 42]. This is because Cx40 is necessary for movement of electrical signals along the conduction pathways of the heart [28, 38]. Cx40 is also important for movement of chemical and electrical signals within arteries and is highly expressed in the endothelium [23, 29, 43], the main source of vasodilatory factors that regulate vascular tone and peripheral resistance. However, human carriers of dysfunctional Cx40 proteins have not been examined for arterial disease, which might result from interference with chemical or electrical coupling within the vascular wall.
To assess the impact of a dysfunctional endothelial Cx40 on arterial function, we have created a transgenic mouse strain that expresses a mutant Cx40, in addition to native Cx40, specifically in the endothelium [5]. This mutant, Cx40T202S, contains a threonine to serine substitution at amino acid 202. This site was chosen as it lies in the centre of a short six-amino acid sequence shown to be critical for gap junction function [6, 17, 40]. While this particular mutation has not been associated with human pathology to date, there are increasing reports of links between Cx40 gene mutations and cardiovascular dysfunction in humans [1, 14, 16].
Our previous data showed that inclusion of the Cx40T202S mutant caused gap junctions to become impermeable to chemicals, without affecting electrical conductance [5]. At the vascular level, mesenteric arteries from Cx40T202S transgenic (Cx40T202STg) mice exhibited increased arterial stiffness and a greater contractile sensitivity to intraluminal pressure, due to reduced vasodilator capacity following loss of endothelium-derived hyperpolarisation (EDH; [5]). These data were consistent with previous studies showing indirect activation of EDH by chemical feedback from the overlying smooth muscle cells via myoendothelial gap junctions following smooth muscle constriction [17, 25, 27, 37] and localisation of Cx40 expression at myoendothelial gap junctions [18, 22, 29, 34].
In spite of these physiological alterations to vascular function of Cx40T202STg mice, we saw no change in daytime blood pressure using tail cuff plethysmography and concluded that the homeostatic mechanisms that regulate blood pressure must be sufficient to buffer the arterial dysfunction [5]. However, carriers of mutant Cx40 may have reduced tolerance to stresses that normally increase blood pressure during the active cycle. A striking example of reduced tolerance comes from mice that lack endothelial nitric oxide synthase (eNOS; [26]). When fed a high salt diet, the blood pressure in eNOS knockout mice was found to rise by approximately 40 mmHg while little effect was seen in wild-type mice fed the same diet [26]. We therefore hypothesised that carriers of mutant Cx40T202S may be more sensitive to cardiovascular stressors, such as locomotor activity and substances that induce pressor responses.
Since mice are nocturnal, measurements of blood pressure in the present study were made continuously using radiotelemetry. We now report that expression of mutant Cx40T202S amongst wild-type Cx40 in the endothelium leads to mild hypertension, as well as augmentation of the blood pressure increase found during physical activity, without significant alteration of heart rate, pulse pressure or nitric oxide. With data from our vascular studies in vivo and in vitro, we propose that impairment to chemical coupling through the mutant endothelial Cx40T202S leads to a deficit in activation of the small conductance, calcium-activated potassium channels (SKCa) which, with intermediate conductance IKCa channels, underpin EDH and this in turn impairs ascending vasodilation. These data provide an important functional link between endothelial Cx40 and SKCa channels that might contribute to the observed increase in blood pressure.
Materials and methods
Animals
All experimental procedures were approved by the Animal Experimentation Ethics Committee of the Australian National University under the protocol A2011/72 and carried out in accordance with the Guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council of Australia).
Experiments were carried out on 2 to 4-month-old male wild-type or transgenic C57BL/6 mice created to express either a single amino acid mutant form of Cx40, Cx40T202S (Cx40T202STg), or wild-type Cx40 (Cx40Tg), both controlled by the endothelium-specific promoter TIE2 (see [5], for details). The Cx40Tg mice were used to control for effects that were not specific to the Cx40T202S transgene. If an observed phenotype was due to the T202S mutation, the same phenotype would not be expected in Cx40Tg mice. Since both transgenes would be indistinguishable from endogenously expressed Cx40 protein using Cx40 antibodies, transgenic constructs were designed to express mutant or wild-type Cx40, in addition to an independently translated reporter, EGFP, from the same TIE2 promoter. Endothelial specificity of EGFP expression was demonstrated previously in arteries and arterioles from both Cx40T202STg and Cx40Tg, but not wild-type mice [5, 30].
A second approach was used to control for artefacts arising from the site of integration of the Cx40T202S transgene into the genome. This approach enabled transgene expression to be regulated through use of the modified Lac operon system [8, 33] and was achieved through addition of Lac operator (LacO) binding sites to the TIE2 promoter [30]. When Cx40T202STg mice were bred to mice ubiquitously expressing the Lac inhibitor protein, LacI R (Cx40T202STg-LacI), transgene expression from the TIE2 promoter was totally repressed [30]. Physiological phenotypes observed in Cx40T202STg mice could therefore be compared with those in Cx40T202STg-LacI mice, where transgene expression was repressed. If no phenotype was found in Cx40T202STg-LacI mice, then observations in Cx40T202STg mice must have resulted from the transgene and not the site of integration of the transgene into the genome.
Blood pressure measurement
Mice were anaesthetised by spontaneous inhalation of isoflurane (2–2.5 % in air) and implanted with a radiotelemetry blood pressure device (TA11PA-C10; Data Sciences International, St Paul, MN, USA), as described earlier [4]. Mice were allowed to recover for 8 to 10 days prior to commencing experiments. Blood pressure (mmHg), heart rate (beats min−1; BPM) and locomotor activity data (number of changes in mouse location within the cage per min; counts min−1; CPM) were recorded using DataQuest ART data acquisition system (Data Sciences International). Telemetric activity measurements have been verified as reliable indicators of locomotor activity, by comparison of activity curves obtained simultaneously with those obtained from external infrared cage motion sensors [15]. Data samples were collected during 20-s periods every 2 min throughout the study. Blood pressure was measured continuously for 5 days before mice were treated with the nitric oxide synthase (NOS) inhibitor Nɷ-nitro-l-arginine methyl ester hydrochloride (L-NAME, 40 mg kg−1 day−1; Sigma-Aldrich, St Louis, MO, USA) in their drinking water for a further 6 days.
Following cessation of L-NAME treatment, the blood pressure in mice was allowed to normalise for 24 or 48 h. After this period, pressor responses to phenylephrine (Sigma-Aldrich, St Louis, MO, USA) were examined. First, a vehicle solution containing 0.9 % NaCl and 0.2 % ascorbic acid was injected into the peritoneum of mice and blood pressure was monitored for 24 h. The following day, mice were injected with phenylephrine (5 mg kg−1) dissolved in the vehicle solution and injected as described above. Blood pressure was again monitored for 24 h after injection. Mice were euthanised by CO2 inhalation, probes were removed and pressure recordings were again made to determine the accuracy of recordings and probe integrity.
Measurement of plasma renin concentration
Mice were lightly anaesthetised with isoflurane and a 200-μL blood sample was immediately collected from the retro-orbital sinus into heparinised glass tubes (Natelson, Oxford Labware, St Louis USA) and transferred to tubes containing 4 μL of a protease inhibitor cocktail (Na2EDTA 50 mmol L−1, aprotinin 21 U L−1, N-ethylmaleimide 200 mmol L−1, leupeptin 10.5 μmol L−1 and pepstatin-A 1.5 μmol L−1; Sigma-Aldrich, St Louis, MO, USA). The plasma fraction was removed after centrifugation and frozen. Plasma renin concentration was determined using radio immunoassay (ProSearch International Australia Pty Ltd, Malvern, VIC, Australia) from samples collected at the same time each day. Exogenous substrate was added to the reaction to avoid rate limitation and data expressed as a measure of angiotensin I production [24].
Assessment of potassium channel function in mouse small mesenteric arteries in vitro
We assessed potassium channel function in small, third-order mesenteric resistance arteries, which were removed from mice after deep anaesthesia with isoflurane and decapitation. Arteries were placed into ice cold physiological salt solution (PSS) containing, in millimole per litre: NaCl, 120; KCl, 5; NaHCO3, 25; CaCl2, 2; MgSO4, 2; NaH2PO4, 1; and glucose, 11, removed from their surrounding fatty tissue and cannulated with glass micropipettes in a chamber containing calcium-free PSS. They were then pressurised to 50 mmHg (Living Systems Instruments, Burlington, VT, USA), stretched by 33 % from their initial isolated length in order to regain their in vivo length and superfused at 37 °C with calcium-containing PSS, gassed with 5 % CO2 in air, for 1 h, during which time the arteries developed myogenic tone. Intraluminal pressure was then increased to 70 mmHg and arterial tone was allowed to stabilise for 20 min.
Vasodilator responses were measured with a line-detection programme [31] to successive concentrations of SKA-31, a selective allosteric modulator of SKCa (small conductance) and IKCa (intermediate conductance) potassium channels [35]. Stock solutions of SKA-31 were made in DMSO and diluted in PSS, with the maximum concentration of DMSO in the final solution being 1:3333. Vehicle control experiments demonstrated that this concentration of DMSO had no effect on vessel diameter. Maximal vessel diameter (D Max) was determined at the end of the experiments by incubation in calcium-free PSS containing 10 mmol L−1 ethylene glycol tetra-acetic acid. The amplitude of SKA-induced vasodilation is expressed as a percentage of the dilator capacity: 100 × (D SKA − D 0)/(D Max − D 0), where D SKA is the diameter after application of SKA-31 and D 0 is the resting diameter. The role of SKCa and IKCa channels in the actions of SKA-31 was determined by pre-incubation for 30 min in UCL1684 (1 μm L−1), followed by a combination of UCL1684 (1 μm L−1) and TRAM-34 (1 μm L−1; 30 min) to block SKCa and IKCa channels, respectively.
Membrane potential measurements were made in similarly isolated and pressurised third-order mesenteric resistance arteries using sharp microelectrodes (100–160 MΩ) filled with 0.2 % propidium iodide in 0.5 mmol L−1 KCl for identification of impaled cells. Records were amplified with an Axoclamp 200A (Molecular Devices, USA) and recorded using pClamp Software (v.10; Molecular Devices, USA). Criteria for successful impalements were an abrupt signal deflection upon cell impalement and withdrawal, with return to baseline potentials with less than 10 mV offset upon removal of the electrode. Membrane potentials were determined upon withdrawal of the electrode in each concentration of SKA-31.
Assessment of ascending vasodilation in cremaster muscle arterioles in vivo
Ascending vasodilation was studied in second- or third-order cremaster muscle arterioles of wild-type, Cx40Tg and Cx40T202STg mice anaesthetized intraperitoneally (1 mg kg−1 medetomidine and 10 mg kg−1 midazolam, Pfizer Australia Pty Ltd, NSW, Australia; and 0.1 mg kg−1 fentanyl, Mayne Pharma Ltd, Victoria, Australia) and continuously infused via a jugular vein catheter (medetomidine 0.02 mg h−1; midazolam 0.2 mg h−1; fentanyl 0.002 mg h−1). Body temperature was maintained at ~37 °C by a heating pad and mice were intubated with a polyethylene cannula to facilitate breathing. The right cremaster muscle was spread over a coverslip to enable visualisation of arterioles and superfused (3 ml min−1) with Krebs solution (in mmol L−1: NaCl, 118.4; KCl, 3.8; NaHCO3, 25; KH2PO4, 1.2; CaCl2·2H2O, 2.5; and MgSO4·7H2O, 1.2), gassed with 5 % CO2 in N2, pH 7.4, at 34 °C.
L-NAME and indomethacin (both 10 μmol L−1, Sigma-Aldrich, St Louis, MO, USA) were present in all experiments to inhibit the production of nitric oxide and prostaglandins, respectively, and isolate responses due to EDH [21]. Nitric oxide and prostaglandins contribute to the local, but not the ascending, mechanical response, because they do not affect membrane potential [41].
Ascending vasodilations were studied after local stimulation with acetylcholine (ACh, 1 mol L−1, Sigma-Aldrich, St Louis, MO, USA) via ionophoresis from a micropipette (tip diameter 1 μm; 500 nA; 0.5- and 1-s duration), positioned adjacent to the vessel surface; a retaining current (−200 nA) was applied to prevent continuous release of ACh from the pipette. In order to standardise the delivery of ACh to the vessel surface across genotypes, a small area of skeletal muscle was removed to expose the vessel surface. Our previous studies have shown that this procedure did not affect local or ascending ACh-induced dilations [41].
Changes in diameter were measured at the site of ACh application (0 mm) and then at 0.5, 1, 1.5 and 2 mm upstream without movement of the ionophoretic pipette (see [41]). Only vessels without intervening side-branches were studied. D Max was determined at the end of the experiment at each measurement site by superfusion of Krebs solution containing sodium nitroprusside (SNP, 30 μmol L−1; Univar, Ingleburn, NSW, Australia), ACh (30 μmol L−1) and adenosine (30 μmol L−1; Merck, Kilsyth, Victoria, Australia). Mice were killed at the end of the experiments by cervical dislocation under anaesthesia.
The amplitude of ACh-induced dilation at the local site and at sites up to 2 mm upstream was normalised to the dilator capacity at each site as 100 × (D ACh − D 0)/(D Max − D 0), where D ACh is the diameter after application of ACh and D 0 represents the resting diameter at that site.
Statistical methods
Data are presented as mean ± standard error of the mean for the number of animals given in figure legends. Exponential regression and sigmoidal concentration-response curves were fitted to the mean values obtained for each genotype and curve parameters were calculated by Graph Pad Prism 4. Statistical comparisons were made using one-way or two-way ANOVA, followed by Bonferroni tests for multiple comparisons, where n represents the number of mice.
Results
Expression of Cx40T202S in the endothelium causes blood pressure to be elevated at night
Mean arterial pressure (MAP) was continuously recorded in wild-type, Cx40Tg, Cx40T202STg and Cx40T202STg-LacI mice using radiotelemetry. In all genotypes tested, MAP was found to be higher at night than in the day (Fig. 1a), consistent with murine nocturnal behaviour. The MAP of each mouse was therefore determined for day and night time periods and the average for each genotype was calculated over five successive days (Fig. 1b, c). During the day, there was no significant difference amongst the MAPs of the four genotypes (Fig. 1b). However, at night, the MAP of Cx40T202STg mice was significantly higher than wild-type, Cx40Tg and Cx40T202STg-LacI mice (Fig. 1c). The absence of any comparable increase in blood pressure of Cx40Tg and Cx40T202STg-LacI mice demonstrated that the effect seen in Cx40T202STg mice was not simply due to over expression of Cx40 protein or an effect caused by random transgene integration, respectively. No difference in heart rate (Fig. 1d, e) or average locomotor activity (Fig. 1f, g) was observed amongst the genotypes either during the day or night.
Expression of Cx40T202S in the endothelium elevates night time blood pressure. a The mean arterial blood pressure (MAP) of all mouse strains was higher at night than during the day (means for 5 days and 5 nights). b During daylight hours, there was no significant difference in MAP amongst the genotypes. c During the night, the MAP of Cx40T202STg mice was significantly elevated when compared to wild-type, Cx40Tg and Cx40T202STg-LacI mice. d, e Heart rate was equivalent for all genotypes during both day and night. f, g Day time and night time activity was similar for all genotypes. D, day time. N, night time. BPM, beats per minute. CPM, mouse position changes per minute. n = 7 wild-type mice, n = 8 Cx40Tg mice, n = 8 Cx40T202STg mice, n = 4 Cx40T202STg-LacI mice. *P < 0.05 compared to wild-type. †P < 0.05 compared to Cx40Tg. ‡P < 0.05 compared to Cx40T202STg-LacI
To determine whether the effect seen in Cx40T202STg mice was specific to endothelial cells in arteries, and not the interaction between endothelial and renin secreting cells in the kidney, renin was also measured. Plasma renin concentration was not significantly different amongst wild-type, Cx40Tg and Cx40T202STg mice (wild-type: 262 ± 20 ng angiotensin I mL−1 h−1; Cx40Tg: 300 ± 36 ng angiotensin I ml−1 h−1; Cx40T202STg: 282 ± 36 ng angiotensin I ml−1 h−1).
Expression of Cx40T202S in the endothelium augments the blood pressure response to locomotor activity
Since mice are more active at night than during the day, we hypothesised that the higher blood pressure seen at night in Cx40T202STg mice was due to an increased response to activity. When the MAP and activity of a wild-type mouse were plotted over a 24-h period, elevations in MAP corresponded clearly to periods of elevated activity (Fig. 2a). In the absence of any activity, the average MAP of Cx40T202STg mice was significantly higher than that of wild-type or Cx40Tg mice (Fig. 2b), while there was no significant alteration in either heart rate or pulse pressure (Fig. 2c, d). These data demonstrated an activity-independent elevation of MAP in Cx40T202STg mice which did not result from alteration of cardiac function.
Expression of Cx40T202S in the endothelium augments the blood pressure response to locomotor activity. a Measurement of MAP over 24 h revealed periods of locomotor activity that corresponded to periods of elevated blood pressure in a wild-type mouse. b The average MAP for each mouse was calculated at zero activity and Cx40T202STg mice were found to have significantly higher MAP than wild-type and Cx40Tg mice. c, d No significant difference was found for heart rate (c) or pulse pressure (d) amongst the genotypes. e Average change in MAP corresponding to each level of activity revealed a relationship that could be fitted by a one phase exponential association. The plateau pressure in Cx40T202STg mice was significantly higher than wild-type while the plateau pressure in Cx40Tg mice was significantly lower. Pale lines represent the average ± SEM change in MAP that occurred in each genotype at each activity level. Summary statistics for the curve fit are listed in Table 1. CPM, mouse position changes per minute. n = 7 wild-type mice, n = 8 Cx40Tg mice, n = 8 Cx40T202STg mice. *P < 0.05 compared to wild-type
In order to study the effect of physical activity on MAP, in isolation from any activity-independent effect, we calculated the change in MAP with each increased level of activity above zero activity for each mouse and the corresponding average for each genotype (Fig. 2e). In all three genotypes, the average change in MAP from their respective blood pressure baseline in the absence of activity (Fig. 2b) was fitted by a one-phase exponential regression curve (Fig. 2e; Table 1). From these curves, the maximum blood pressure in response to activity was significantly higher in Cx40T202STg mice and significantly lower in Cx40Tg mice, compared to wild-type mice (Fig. 2e, Table 1). These maxima presumably result from the impact of adaptive mechanisms, such as the baroreflex, which serve to regulate blood pressure within physiological ranges that would preclude peripheral organ damage [32].
Thus, expression of Cx40T202S in the endothelium elevates blood pressure during inactivity and this is further augmented during activity.
Expression of Cx40T202S in the endothelium does not enhance pressor responses to L-NAME or phenylephrine
Treatment of wild-type, Cx40Tg and Cx40T202STg mice with the NOS inhibitor L-NAME caused a significant elevation in day time and night time MAP that was not different amongst the genotypes (Table 2), indicating that nitric oxide bioavailability was not altered in either Cx40Tg or Cx40T202STg mice. Although there was a trend for the blood pressure of Cx40T202STg mice to be higher than that of the other two genotypes, this was no longer significant under these pressor conditions (Fig. 3a). No difference in heart rate or activity was observed amongst the genotypes during L-NAME treatment, either during the day or night (Fig. 3b, c; Table 2). The significant decrease found in heart rate at night in all three genotypes during L-NAME treatment, relative to baseline (Table 2), suggested that central homeostatic mechanisms that regulate blood pressure were intact in all three genotypes.
Expression of Cx40T202S in the endothelium does not augment the pressor effects of L-NAME or phenylephrine. a During L-NAME treatment, the MAP did not vary amongst wild-type, Cx40Tg or Cx40T202STg mice during day or night. b No difference in heart rate or activity (c) was seen amongst the genotypes during L-NAME treatment during day or night (n = 7 wild-type, n = 8 Cx40Tg, n = 8 Cx40T202STg). d–f Injection of phenylephrine caused a rapid increase in MAP of individual wild-type (d), Cx40Tg (e), and Cx40T202STg (f) mice which then decayed over 2 to 3 h. Injection of vehicle had no effect. g Responses observed in groups of wild-type, Cx40Tg and Cx40T202STg mice were averaged and the change in MAP over time was fitted by an exponential decay model (n = 4 wild-type mice, n = 5 Cx40Tg mice, n = 4 Cx40T202STg mice). MAP returned to a significantly lower baseline in Cx40T202STg mice when compared to wild-type and Cx40Tg mice. *P < 0.05 compared to wild-type. †P < 0.05 compared to Cx40Tg. Pale lines in g represent the average ± SEM change in MAP that occurred in each genotype at each time point after injection. Summary statistics for the curve fit are listed in Table 2
Phenylephrine, given intraperitoneally, caused a rapid increase in MAP of wild-type, Cx40Tg and Cx40T202STg mice that declined over a period of 1 to 3 h, while injection of vehicle had no substantial effect (Fig. 3d–f). To examine whether sensitivity to phenylephrine was altered by Cx40T202S expression, the change in blood pressure caused by phenylephrine was analysed over 4 h following administration. No significant differences were observed in peak blood pressure response, increase in MAP or peak heart rate amongst wild-type, Cx40Tg and Cx40T202STg mice (Table 3). However, when the decline in blood pressure was fitted to a one-phase exponential decay model, it was found to be significantly faster in Cx40T202STg mice and returned to a significantly lower baseline when compared to wild-type and Cx40Tg mice (Fig. 3g; Table 3). Heart rate did not vary amongst the genotypes after return to baseline (Table 3).
Expression of Cx40T202S in the endothelium reduces activation of KCa channels by SKA-31
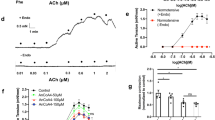
The observation of an activity-dependent increase in the blood pressure of Cx40T202STg mice might indicate impairment to peripheral vasodilatory function. In order to test whether the KCa channels which underpin EDH [3] were altered, we measured hyperpolarisation and vasodilation in response to the positive allosteric modulator of SKCa and IKCa channels [35] in pressurised small mesenteric arteries in vitro. Data showed that there was a significant concentration-dependent decrease in the amplitude of hyperpolarisation evoked by SKA-31 in smooth muscle cells of arteries of Cx40T202STg, compared to wild-type mice (Fig. 4a, b; P < 0.05; two-way ANOVA; wild-type: n = 5 mice, Cx40T202STg: n = 5 mice). The sensitivity to SKA-31 of vasodilation was also significantly reduced in arteries from Cx40T202STg compared to wild-type mice (Fig. 4c; P < 0.05; two-way ANOVA; wild-type: n = 12 mice; Cx40T202STg: n = 11 mice). The ability of the reduced hyperpolarisation in Cx40T202STg mice to produce the same maximal vasodilatory response as that in wild-type mice is consistent with our previous studies which showed that linearity between the electrical and mechanical responses is restricted to a narrow voltage window [41].
Expression of Cx40T202S in the endothelium reduces hyperpolarisation and vasodilation to SKA-31. Hyperpolarising responses to SKA-31 were significantly reduced in small mesenteric arteries of Cx40T202STg relative to wild-type mice. a Representative traces and b group data; n = 5 wild-type mice, n = 5 Cx40T202STg mice; *P < 0.05 from wild-type; two-way ANOVA. c SKA-31 caused a concentration-dependent vasodilation which was significantly less sensitive in Cx40T202STg than wild-type arteries (n = 12 wild-type, n = 11 Cx40T202STg mice; *P < 0.05 from wild-type; two-way ANOVA)
Block of SKCa channels with UCL1684 (1 μm L−1) significantly shifted the concentration-response curve to SKA-31 to the right in arteries from wild-type (P < 0.05; two-way ANOVA), but not Cx40T202STg mice (Fig. 5a, b). Addition of the IKCa blocker, TRAM-34 (1 μm L−1) to UCL1684 significantly reduced the amplitude of dilation in arteries from both wild-type and Cx40T202STg mice (Fig. 5c; two-way ANOVA). There was no difference between the genotypes in resting diameter, D Max or vascular tone (Table 4).
Expression of Cx40T202S in the endothelium reduces the SKCa component of vasodilation to SKA-31. a, b Vasodilation in response to SKA-31 was significantly reduced by block of SKCa channels (UCL1684; 1 μm L−1) in wild-type arteries, but not in Cx40T202STg arteries. c The additional block of IKCa channels (TRAM-34; 1 μm L−1) reduced dilation to SKA-31 to a similar extent in both wild-type and Cx40T202STg arteries (n = 5 wild-type mice, n = 5 Cx40T202STg mice; *P < 0.05 from wild-type; two-way ANOVA)
Expression of Cx40T202S in the endothelium reduces ascending vasodilation to ACh
A decrease in the local generation of EDH, which could result from a deficit in the activation of KCa channels [7, 10, 11], could affect ascending vasodilation, a response that contributes to the increased blood flow to muscles during activity and depends on electrical coupling via endothelial Cx40 [3, 9, 23].
We therefore measured vasodilation over 2 mm in vivo in cremaster muscle arterioles of wild-type, Cx40Tg and Cx40T202STg mice, following focal stimulation of EDH with ACh (1 s pulse). However, no significant attenuation in ascending vasodilation was found amongst the genotypes over the experimental window of 2 mm (Fig. 6a). It was therefore necessary to reduce the local stimulus (0.5 s pulse) in order to visualise the decay phase of ascending vasodilations, as we have done previously [41]. Although vasodilation at the local site did not differ significantly between the two stimuli (Fig. 6a cf. Fig. 6b), the decay of ascending vasodilation with distance was significantly greater in Cx40T202STg arterioles compared to that in arterioles of wild-type and Cx40Tg mice (Fig. 6b; P < 0.05; two-way ANOVA). Vascular tone was significantly greater in both Cx40T202STg and Cx40Tg mice than in wild-type mice, while D Max did not vary significantly amongst the genotypes (Table 5) or with distance from the local stimulation site.
Expression of Cx40T202S in the endothelium reduces ascending vasodilation. a Focal application of ACh (1 s) to cremaster muscle arterioles of wild-type, Cx40Tg, and Cx40T202STg mice caused ascending vasodilation that did not differ amongst the genotypes (n = 7 wild-type, n = 5 Cx40Tg, n = 8 Cx40T202STg). b Reduction in the local ACh stimulus (0.5 s) revealed a significantly greater attenuation in ascending vasodilation in Cx40T202STg mice, relative to Cx40Tg mice (n = 6 wild-type, n = 5 Cx40Tg, n = 5 Cx40T202STg). *P < 0.05 compared to wild-type; two-way ANOVA with Bonferroni post tests
Discussion
The findings of this study demonstrate that expression of a chemical mutant form of Cx40, Cx40T202S, specifically in the endothelium of mice significantly elevates nocturnal blood pressure, in the absence of any perturbation to cardiac activity. Importantly, this effect was specific to the mutant endothelial Cx40T202S transgene and not to any “position” effect caused by random insertion of the transgene into the genome, as blood pressure was normal in Cx40T202STg-LacI mice, in which transgene expression was inhibited [30]. Dysregulation of blood pressure was also not due to overexpression of endothelial Cx40 protein, since mice overexpressing wild-type Cx40 exhibited normal blood pressure. Detailed analyses of blood pressure and activity levels over the full day/night period showed that blood pressure was significantly elevated at rest and augmented by locomotor activity more than in wild-type mice. In contrast, pressor responses to L-NAME or phenylephrine were unchanged, although the blood pressure rebound following phenylephrine exposure was larger and faster in Cx40T202STg mice. While these studies indicated that bioavailability of nitric oxide was not altered, the mutant endothelial Cx40T202S transgene reduced the sensitivity of hyperpolarisation and vasodilation to the IKCa and SKCa channel modulator, SKA-31, in mesenteric arteries through a deficit in the SKCa component and attenuated ascending vasodilation following local ACh stimulation in skeletal muscle arterioles. Together, these data suggest that the increased blood pressure in Cx40T202STg mice might be in part related to a deficit in peripheral vasodilatory mechanisms mediated by hyperpolarisation through SKCa channels.
The in-depth analysis of blood pressure in this study demonstrates that the presence of the chemical mutant Cx40 amongst wild-type Cx40 in the endothelium is sufficient to disrupt arterial function and increase blood pressure. This finding is in contrast to conclusions made regarding the TIE2-Cre-Cx40fl/fl mice [23, 39], in which Cx40 is selectively deleted from the endothelium but blood pressure remains normal. However, it should be noted that in the latter study, blood pressure measurements were only recorded during the day time and the effect of activity was not analysed [39]. Under daytime conditions, we also did not see any significant increase in blood pressure in our endothelial-specific Cx40 model in the present study using radiotelemetry or in our previous study using tail cuff plethysmography [5].
In contrast to locomotor activity, pressor responses caused by phenylephrine or NOS inhibition were similar in Cx40T202STg mice to those in wild-type mice. Thus, the presence of the mutant Cx40 in the endothelium of Cx40T202STg mice affects tolerance to some cardiovascular stressors, such as activity, but not others. One explanation could be that the adaptive mechanisms possessed by the cardiovascular system are sufficient to buffer the blood pressure increase induced by these latter pressors. Indeed, the significant decrease in heart rate found at night time during L-NAME treatment in both Cx40T202STg and wild-type mice suggests that central homeostatic mechanisms are intact, irrespective of the endothelial Cx40 mutation. In addition, the absence of any significant difference in the pressor response to L-NAME between Cx40T202STg mice and wild-type mice provides evidence that the Cx40T202S mutant does not interfere with the nitric oxide pathway.
Interestingly, in Cx40T202STg mice, the decline in blood pressure following phenylephrine injection was faster and settled at a lower baseline than in wild-type mice and in mice overexpressing wild-type Cx40. The exponential decline in blood pressure that occurs after phenylephrine injection may in part reflect the activity of RGS2 (regulator of G-protein signalling 2) which can attenuate G protein signalling through most physiological vasoconstrictor receptors [19]. Thus, when RGS2 knockout mice are injected with phenylephrine, the decline in blood pressure is much slower than in wild-type mice [19]. Our data may therefore suggest that the adaptive response to some pressor-inducing substances, such as phenylephrine, is enhanced in Cx40T202STg mice.
An activity-dependent increase in the blood pressure of Cx40T202STg mice might result from impairment to peripheral vasodilatory function attributable to EDH. However, we did not expect that EDH would be impaired since Cx40 is not essential for the local endothelial hyperpolarisation or vasodilation in response to ACh [2, 20]. Moreover, the electrical passage of EDH from the endothelial cells to the smooth muscle cells would be expected to be intact through gap junctions containing Cx40T202S [5]. Nevertheless, genetic deletion of the SKCa and IKCa channels underpinning EDH [7, 10, 11] produces an activity-dependent hypertension of similar magnitude to that described here [3]. We therefore tested whether activation of these KCa channels was impaired in pressurised mesenteric arteries of Cx40T202STg mice, through use of the selective modulator, SKA-31 [35]. Data showed that the sensitivity of both the SKA-induced hyperpolarisation and vasodilation was reduced in arteries from Cx40T202STg mice compared to wild-type mice, and this resulted from reduction in activation of SKCa channels. Interestingly, in rat mesenteric arteries, SKCa channels have been localised in close association with endothelial Cx40 [29, 34], suggestive of a direct functional interaction. However, a comprehensive study is required in order to determine whether the reduction in SKCa function is due to a specific physical interaction with the mutant Cx40 channel or to a physiological interaction which may be modified through the blockade of chemical coupling by Cx40T202S.
Impairment to the activation of SKCa channels would be expected to impact on ascending vasodilation in skeletal muscle arterioles, since this response requires hyperpolarising current through both SKCa and IKCa channels [36]. In contrast, the subsequent spread of the hyperpolarisation upstream through endothelial Cx40 gap junctions, which is also essential to ascending vasodilation [9, 12, 13, 23], would not be expected to be impaired in Cx40T202STg mice, since electrical conductance of the mutant Cx40T202S was intact [5]. Any deficit in SKCa channel activity would therefore reduce ascending vasodilation by attenuating the amplitude of the local endothelial hyperpolarisation, rather than conduction of the hyperpolarisation along the endothelium. We therefore assessed the in vivo conduction of vasodilation in skeletal muscle arterioles and found that this response was attenuated in Cx40T202STg, compared to that in wild-type mice. This attenuation was specific to the Cx40T202S mutation, since conduction of vasodilation was not altered in arterioles of mice overexpressing native Cx40. Moreover, the attenuation in ascending vasodilation did not result from the increased tone of Cx40T202STg arterioles relative to wild-type mice, since arterioles from Cx40Tg mice exhibited a comparable increase in tone in the absence of other vasodilators.
Gap junctions are widely expressed in the heart and vasculature. In the heart, gap junctions play an essential role in synchronisation of the cardiac rhythm and, in line with this role, human carriers of Cx40 mutants are predisposed to atrial fibrillation. In the vasculature, endothelial cells are highly coupled by gap junctions, not only to each other but also to the overlying smooth muscle; connections which are essential for the spread of electrical responses both radially and longitudinally within the vascular wall. Our current studies provide new data that chemical impairment of endothelial Cx40 function contributes directly to the regulation of blood pressure. We also show that the Cx40T202S mutant reduces peripheral vasodilator capacity due to impairment to the activation of SKCa channels. To our knowledge, this is the first report of a functional link between endothelial Cx40 and SKCa channels. Although a reduction in peripheral vasodilator capacity could contribute to the increased blood pressure of the Cx40T202STg mice, so also could the loss of myoendothelial feedback which results in elevated myogenic tone, as we have reported previously [5]. Interestingly, the effects on blood pressure seen here resulted from the introduction of Cx40 molecules, with a single point mutation which suppressed chemical but not electrical conductance, into the endogenous Cx40 population in the endothelium. Since the process of ascending vasodilation relies critically on electrical conduction along the endothelium via Cx40 gap junctions [9, 23], it would be expected that Cx40 mutations which also attenuate electrical conductance, could have an even greater effect on ascending vasodilation and cardiovascular function than the one described here. Thus, heterozygous, human carriers of single point Cx40 mutations, which can act as dominant negatives, could be predisposed to the pathophysiological consequences of arterial dysfunction, in addition to their documented cardiac problems.
References
Bai D (2014) Atrial fibrillation-linked GJA5/connexin40 mutants impaired gap junctions via different mechanisms. FEBS Lett 588:1238–1243
Boettcher M, de Wit C (2011) Distinct endothelium-derived hyperpolarizing factors emerge in vitro and in vivo and are mediated in part via connexin 40-dependent myoendothelial coupling. Hypertension 57:802–808
Brähler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, Kacik M, Hasenau A-L, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Köhler R (2009) Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119:2323–2332
Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE (2006) Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol 290:R1324–1329
Chaston DJ, Baillie BK, Grayson TH, Courjaret RJ, Heisler JM, Lau KA, Machaca K, Nicholson BJ, Ashton A, Matthaei KI, Hill CE (2013) Polymorphism in endothelial connexin40 enhances sensitivity to intraluminal pressure and increases arterial stiffness. Arterioscler, Thromb, Vasc Biol 33:962–970
Chaytor AT, Evans WH, Griffith TM (1997) Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol 503(Pt 1):99–110
Coleman HA, Tare M, Parkington HC (2001) K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol 531:359–373
Cronin CA, Gluba W, Scrable H (2001) The lac operator-repressor system is functional in the mouse. Genes Dev 15:1506–1517
de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U (2000) Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86:649–655
Edwards G, Gardener MJ, Feletou M, Brady G, Vanhoutte PM, Weston AH (1999) Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br J Pharmacol 128:1064–1070
Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, Hoyer J, Kohler R (2003) Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol 138:594–601
Emerson GG, Segal SS (2000) Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86:94–100
Emerson GG, Segal SS (2001) Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol 280:H160–167
Firouzi M, Kok B, Spiering W, Busjahn A, Bezzina C, Ruijter J, Koeleman B, Schipper M, Groenewegen W, Jongsma H, de Leeuw P (2006) Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens 24:325–330
Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, Romanovsky AA (2011) Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci 31:1721–1733
Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, Tesson F, Klein GJ, Yee R, Skanes AC, Guiraudon GM, Ebihara L, Bai D (2006) Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med 354:2677–2688
Griffith TM, Chaytor AT, Edwards DH (2004) The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol Res 49:551–564
Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE (2006) Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol- Heart Circ Physiol 291:H2047–H2056
Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ (2003) Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest 111:445–452
Howitt L, Chaston DJ, Sandow SL, Matthaei KI, Edwards FR, Hill CE (2013) Spreading vasodilatation in the murine microcirculation: attenuation by oxidative stress-induced change in electromechanical coupling. J Physiol 591:2157–2173
Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE (2013) Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res 98:449–457
Isakson BE, Best AK, Duling BR (2008) Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol 294:H2898–2904
Jobs A, Schmidt K, Schmidt VJ, Lubkemeier I, van Veen TAB, Kurtz A, Willecke K, de Wit C (2012) Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension 60:1422–1429
Johnston CI, Mendelsohn F, Casley D (1971) Evaluation of renin and angiotensin assays and their clinical application. Med J Aust 1:126–128
Kansui Y, Garland CJ, Dora KA (2008) Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium 44:135–146
Kopkan L, Hess A, Huskova Z, Cervenka L, Navar LG, Majid DS (2010) High-salt intake enhances superoxide activity in eNOS knockout mice leading to the development of salt sensitivity. Am J Physiol Renal Physiol 299:F656–663
Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ (2005) Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium 37:311–320
Makita N, Seki A, Sumitomo N, Chkourko H, Fukuhara S, Watanabe H, Shimizu W, Bezzina CR, Hasdemir C, Mugishima H, Makiyama T, Baruteau A, Baron E, Horie M, Hagiwara N, Wilde AA, Probst V, Le Marec H, Roden DM, Mochizuki N, Schott JJ, Delmar M (2012) A connexin40 mutation associated with a malignant variant of progressive familial heart block type I. Circ Arrhythm Electrophysiol 5:163–172
Mather S, Dora KA, Sandow SL, Winter P, Garland CJ (2005) Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res 97:399–407
Morton SK, Chaston DJ, Baillie BK, Hill CE, Matthaei KI (2014) Regulation of endothelial-specific transgene expression by the LacI repressor protein in vivo. PLoS One 9:e95980
Neild TO (1989) Measurement of arteriole diameter changes by analysis of television images. Blood Vessels 26:48–52
Nobrega AC, O'Leary D, Silva BM, Marongiu E, Piepoli MF, Crisafulli A (2014) Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int 478965:9
Ryan A, Scrable H (2004) Visualization of the dynamics of gene expression in the living mouse. Mol Imaging 3:33–42
Sandow SL, Neylon CB, Chen MX, Garland CJ (2006) Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat 209:689–698
Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Kohler R, Wulff H (2009) Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 75:281–295
Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Kohler R (2006) Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res 99:537–544
Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG (2012) Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol 302:C1226–1242
VanderBrink BA, Sellitto C, Saba S, Link MS, Zhu W, Homoud MK, Estes NA 3rd, Paul DL, Wang PJ (2000) Connexin40-deficient mice exhibit atrioventricular nodal and infra-Hisian conduction abnormalities. J Cardiovasc Electrophysiol 11:1270–1276
Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A (2010) Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int 78:762–768
Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL (1995) Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol 488(Pt 3):721–728
Wölfle SE, Chaston DJ, Goto K, Sandow SL, Edwards FR, Hill CE (2011) Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation. J Physiol 589:2607–2623
Yang Y-Q, Liu X, Zhang X-L, Wang X-H, Tan H-W, Shi H-F, Jiang W-F, Fang W-Y (2010) Novel connexin40 missense mutations in patients with familial atrial fibrillation. Europace 12:1421–1427
Yeh H-I, Rothery S, Dupont E, Coppen SR, Severs NJ (1998) Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res 83:1248–1263
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia [grant number 471421] and Heart Foundation of Australia [grant number G12C 6361].
Conflict of Interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rebecca E. Haddock and Lauren Howitt contributed equally.
Rights and permissions
About this article
Cite this article
Chaston, D.J., Haddock, R.E., Howitt, L. et al. Perturbation of chemical coupling by an endothelial Cx40 mutant attenuates endothelium-dependent vasodilation by KCa channels and elevates blood pressure in mice. Pflugers Arch - Eur J Physiol 467, 1997–2009 (2015). https://doi.org/10.1007/s00424-014-1640-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1640-x