Abstract
Aim
Laparoscopic surgery is widely used for small gastric gastrointestinal stromal tumors (GISTs) (≤ 5 cm) but remains a controversial approach for larger gastric GISTs (> 5 cm). This study aims to compare short- and long-term outcomes of laparoscopic resection in comparison with open resection for gastric GISTs measuring over 5 cm.
Method
All patients receiving surgery for gastric GIST > 5 cm between 2000 and 2021 in a single tertiary hospital were included. Data were collected from prospectively maintained records. Kaplan–Meier method and log rank test were used to compare survival outcomes.
Results
Among 108 included patients, 59 patients had minimally invasive (MI) surgery (54.6%) whereas 49 patients had open surgery (46.4%). The rate of overall postoperative morbidity was 14.8% and the median length was significantly shorter in the MI group [4 (range 2–30) vs. 7 (range 4–33) days; P = 0.007]. The overall R0 resection rate was 98.2% and the rate of tumor rupture was 13%, not different between the two groups. Recurrence occurred in 24% of the whole population without any difference between groups (20.3% vs. 28.7%, p = 0.31). Minimally invasive surgery was not found as a negative prognostic disease-free survival factor.
Conclusion
Laparoscopic surgery could be a safe and feasible alternative to open surgery in large gastric GIST, bringing the benefits of minimally invasive surgery without compromising oncologic results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal tumours of the digestive tract. Within the past decade, emerging targeted therapies and improvement of minimally invasive surgery have revolutionized its management and prognosis.
Usually sporadic, their reported incidence ranges between 4 and 20 cases/million inhabitants/year, with a sex ratio of one and a median age at diagnosis of 60 years-old. They develop mostly from the stomach (55%) and small intestine, more rarely from the rectum, oesophagus or mesentery. Pathologically, the diagnosis relies on morphology and immunohistochemistry: GISTs derive from Cajal cells (type of interstitial cell responsible of the contraction of the intestinal smooth muscle), and harbour activating mutations of genes encoding tyrosine kinase receptors KIT or PDGFRA [1,2,3].
The most common symptoms leading to diagnosis are gastrointestinal bleeding and abdominal pain, but around 20% are incidentally discovered [4]. The key exam for staging is the CT-scanner. The prognosis mainly relies on the risk of relapse, and risk factors for the latest are tumour size, mitotic index (expressed as the number of mitoses on a total area of 5 mm2), non-gastric site and tumour rupture.
For localised gastric GISTs, the gold standard treatment is a complete monobloc R0 resection without spillage of tumour contents [2, 3]. Limited macroscopic margins are considered sufficient, and lymph node dissection does not improve survival or decrease recurrence (lymph node metastases risk less than 1%) whereas an incomplete resection considerably worsens the prognosis. Thus, wedge resection is the procedure of choice, and if not feasible, a segmental resection is adequate to achieve a complete surgical excision. Total gastrectomy is rarely necessary, except for voluminous cardial tumours [2, 3, 5]. Besides, overall survival for high-risk tumours has been widely improved by adjuvant tyrosine kinase inhibitor treatments such as imatinib.
For small gastric GISTs (< 5 cm), laparoscopic resection has been proven to be associated with less postoperative morbidity and equivalent oncological prognosis, as being stated in recent reviews and metanalyses [6,7,8,9,10], as well as in United States’ National Comprehensive Cancer Network and Spanish guidelines [11, 12]. Even though for now, laparoscopy is not recommended in gastric GISTs superior to 10 cm due to concerns of inferior oncological outcomes [11]. Considering the supposedly higher risk of tumour spillage, guidelines are lacking concerning the abdominal wall access type to intermediate tumours measuring between 5 and 10 cm [11, 12]. However, widen access to laparoscopy, development of technical skills and its demonstrated post-operative benefit in other surgical pathologies should make us reconsider the role of minimally invasive surgery in the management of large GISTs. According to demographic studies, GISTs ≥ 5 cm represent up to 50% of the cases, as the global incidence of this tumour type continues to rise, making their management a key issue [4].
Several international retrospective studies are available on the subject, suggesting improved post-operative outcomes such as an earlier resumption of diet and shorter length of hospital stay as well as equivalent oncological safety without increasing R1 resection [13,14,15,16,17,18]. However, most of them concerned asian populations and included only a small number of patients. Extensive knowledge of the clinical impact of larger tumour is needed in order to provide optimal oncological care.
The aim of our large retrospective study was to compare short- and long-term outcomes of laparoscopic resection with open resection for gastric GISTs measuring over 5 cm.
Patients and methods
Population
This was a retrospective, single center study of patients undergoing surgery for large gastric GIST > 5 cm at European George Pompidou Hospital in Paris between 2000 and 2021. All patients signed a consent form before surgery, and this study was approved by our Institutional Review Board.
Study design
Retrospective comparison of patients undergoing surgery for gastric GISTs measuring over 5 cm. Patients were assigned to two groups: patients having a surgical treatment by laparotomy (Open group), and patients having surgical treatment by laparoscopy / minimally invasive surgery (MI group). The diagnosis of gastric GIST was confirmed preoperatively by the histological analysis performed using endoscopy, or post operatively when suspected on the CT scanner by the histological analysis of the resected specimen.
Follow-up
Patients were followed up at 3–6 months to 1-year intervals. The time to recurrence was defined as the time of the first documented appearance of tumour after complete resection based on clinical or radiological examination.
Definitions
Tumour rupture was defined as any tumour spillage or fracture, laceration of the tumour capsule with or without macroscopic spillage piecemeal resection and incisional biopsy occurring either before or at the time of the operation.
Estimation of recurrence risk was performed using Armed Forces Institute of Pathology (AFIP) according Miettinen et al. [19], and Modified NIH classification system according Joensuu [20].
Overall survival (OS) was defined as the time from surgery to death, whatever the cause, and disease-free survival (DFS) was defined as the time from surgery to recurrence.
Endpoints and collected data
The primary endpoint was the postoperative morbidity of patients undergoing laparoscopic resection for large gastric GIST. Secondary endpoints were evaluation of factors influencing DFS, risk factors for conversion to laparotomy.
The recorded study parameters included preoperative data (age, gender, ASA score, history of chronical disease, history of abdominal surgery), data concerning tumor characteristics (diagnostic circumstances, size, location, margins, mitotic count, genetics.), pre-operative chemotherapy, surgical data (type of resection, rate of concomitant abdominal resection), post-operative morbidity (according Dindo classification [21]), histological data, and follow up (the adjuvant therapy rate, OS and DFS).
Inclusion criteria
Patients included in the study had to meet all of the following inclusion criteria: histologically proven gastric GIST, tumour size over 5 cm diameter on histological examination. Patients having gastric GIST measuring less than 5 cm on histological examination, metastatic GIST or other type of gastric tumor were excluded from the study.
Statistic
Quantitative variables are presented as median (range) and are compared using the Wilcoxon’s test, while qualitative variables are presented as count (percentage) and are compared using the Chi square or Fisher’s exact test as appropriate. A p value < 0.05 was considered significant. OS and DFS were estimated using Kaplan–Meier curves. Prognostic factors for DFS were evaluated using the log-rank test in univariate analysis. Analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Population study
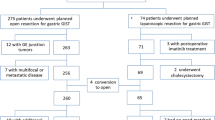
During the study period, 108 patients had a surgery for gastric GIST > 5 cm among which 59 had laparoscopy (54.6%, MI group) while 49 had open surgery one (46.4%, Open group). The patient’s ASA scores were 1 and 2 in 79.6% of cases. Tumors were incidentally diagnosed in 26.8% (n = 29). Most of the tumors were located in the greater curve of the stomach (50%) (Fig. 1). Tyrosine-kinase inhibitor (TKI) was given in 12% of our population in a neoadjuvant setting. The characteristics of the overall population are summarized in Table 1.
Surgical data
A wedge resection was performed in 68.5% of all cases but more often performed in the MI group [81.3% vs. 53%; p = 0.001]. Concomitant abdominal resection was necessary for 29.6% of cases, principally due to distal pancreatectomy (n = 12) and cholecystectomy (n = 8). Tumors were significantly smaller in the MI group [8.3 (SD 4.1) vs. 13 (SD 5.6) cm; p < 0.001].
Short-term outcomes
There was no post-operative. The rate of postoperative morbidity was 14.8%, including a 3.7% of major complication (grade III–IV) (Table 2). The median length of stay was 6 days in the overall population (range 2–33) and was significantly shorter in the MI group [4 (range 2–30) vs. 7 (range 4–33) days; p = 0.007]. Tyrosine-kinase inhibitor (TKI) was given in 39.8% in an adjuvant setting.
Histological data
All the resected tumors were positive for CD117 or DOG1 immunostaining. KIT mutations were found in 62% of the entire population whereas PDGFRA mutation occurred in 12.9%, equally balanced between the 2 groups.
The R0 resection rate was 98.2% and the rate of tumoral rupture was 13%, not different between the two groups. The median mitotic rate was 4 (range 0–125).
Follow-up
Median of follow-up in the whole population was 95.5 months (range 1-237 months). Recurrence occurred in 24% of the whole population without any difference between groups (20.3% vs. 28.7%, p = 0.31). According to AFIP and NIH risk stratification, the Open group was found to have more high-risk patients (p < 0.001) (Table 2).
Factors affecting DFS
Joensuu classification, Miettinen classification, Tumor size > 10 cm, tumour rupture and mitosis count were found to be prognostic factor for DFS (Figs. 2, 3 and 4). Minimally invasive surgery did not negatively impact the 5-year DFS (72.8% vs. 59.4%, p = 0.07). Compared with no rupture, the presence of tumoral rupture was associated with a hazard ratio (HR) of 6.84 (95% CI 2.83–16.5; p < 0.0001). In the same way, compared with a low mitotic rate (≤ 5/mm2), the HR with a high mitotic rate (> 5/mm2) was 5.14 (95% CI 2.13–12.3; p = 0.0003).
Risk factors for conversion to laparotomy
In the MI group, rate of conversion to open surgery was 32.6% (n = 16). Necessity of an anatomical resection and a concomitant abdominal resection were associated with the need of conversion, whereas tumor size > 10 cm or location of the tumor were not in univariate analysis (Table 3).
Discussion
In this present series, including 108 consecutive cases of large gastric GISTs, we found that laparoscopic surgery could be an alternative to open surgery, bringing the benefits of minimally invasive surgery without compromising oncologic results.
Commonly accepted definition of a large gastric GIST was tumour greater than 5 cm. In the present series, laparoscopic resection was performed in 54.6% of all cases. In Lin et al. series, including 66 patients with gastric GISTs of 5–8 cm, laparoscopic surgery has been performed in 36 patients (54.5%) with 94% of wedge resection [18]. Wedge resection was the key point of GIST surgery and was achieved in 81.3% of our minimally invasive procedures.
In our series, postoperative complications tended to decrease after laparoscopy compared to open surgery, even if not significant (5% vs. 18.3%, p = 0.07, respectively) whereas length of stay was significantly shorter in the MI group [4 vs. 7 days, P = 0.007, respectively]. Interestingly, the postoperative results of the CLASS01 RCT, comparing laparoscopy with open distal gastrectomy for patients with advanced gastric cancer, did not show differences in surgical morbidity but a decreased length of stay after laparoscopic surgery [22].
Other principles of GIST surgery included: complete R0 resection, and no spillage. In the present series, R0 resection was achieved in 100% in the MI group and 96% in the Open group (not significant). In the largest multicentric series comparing laparoscopic and open surgery for gastric GISTs, Piessen et al. showed 94% of R0 resection, equally balanced in the 2 groups with majority of small GISTs [10]. In the last ESMO guidelines, given the risk of tumour rupture, and also the risk of relapse, experts clearly discouraged the use of laparoscopy for patients having large tumors [3]. This specific recommendation was principally based on the international Joensuu et al. studies, where tumoral rupture was strongly associated with poor outcomes but the impact of the surgical approach was not really discussed [20, 23, 24]. Here, we showed that minimally invasive surgery was not associated with tumoral spillage since tumoral rupture rate was 10.1% in the MI group and 16.3% in the open group (p = NS). Even high, this rate was in accordance with published data, as in the Norwegian prospective sarcoma database which reported 9% of tumoral rupture, including small and large gastric tumours [25].
Disease-free survival analysis confirmed that mitotic index and tumoral rupture were important prognostic factors whereas surgical approach was not. In the Lin et al. studies, the oncological outcomes were similar between the laparoscopic group and open group and mean hospital stay was shorter in the laparoscopic group, as in our studies [26]. Recently, using the National Cancer Database (2010–2016) to assess outcomes of 1298 patients harboring GISTS ≥ 10 cm, Gevorkian et al. confirmed that minimally invasive surgery did not compromise long-term survival [16]. The 2 international commonly used classifications for risk stratification (NIH and AFIP) categorise two key prognostic variables, tumour size and mitosis count. Including exclusively large tumours, our data showed that inside this subgroup, we could identify patients with better prognosis, and the interest of these classifications is still preserved. Another clinically useful prognostic information could also be given by the GIST mutational status, since KIT exon 11 mutations were known to be most sensitive to imatinib, whereas the PDGFRA mutation is considered imatinib-resistant [27]. These mutational informations, principally guiding adjuvant treatment were not found to be prognostic factors in our study, probably due to the sample size of our population.
Conversion to open surgery occurred in nearly 1/3 of our laparoscopic procedures. This high rate was principally in relationship with the impossibility of performing a wedge procedure and the necessity of an extended resection to nearby organs. Even if not significant, there was a trend toward the association between the necessity of a conversion and a very large tumour > 10 cm (p = 0.007). In the Khoo et al. studies, only one conversion of 23 (4.3%) laparoscopic procedures for large gastric GIST occurred but the rate of conversion was of 10% (6/59) in patients having GIST < 5 cm [15]. In the recent Van den burg et al. studies, use of TKI in a neoadjuvant setting showed decreased tumour size of 36% and a less extensive surgery was possible for 51% of all patients [28]. In our studies, use of neoadjuvant TKI was performed in 12% which is higher than in the US registries but should always be proposed when an extended surgery is planned [29]. Indeed, more important than the size is the location of the tumour. According to the location of the tumour, Hsiao et al. dichotomised the accessibility of the gastric GISTs and classified as easy-to-access and difficult-to-access [30]. Performing a wedge resection in the greater curve for a large GIST is finally easier than performing a resection of a smaller one in the lesser curve. Different criteria must be integrated when a laparoscopic procedure is proposed for a GIST including the location of the tumour, the type of surgery planned, the need of a concomitant abdominal operation and the size of the tumour but the size by itself should not be an exclusion criterion.
Our present studies suffered from several limitations. First, this study was a monocentric retrospective study but is, to our knowledge, the largest single-center experience of consecutive large gastric GIST treated by laparoscopy. Additionally, this study comprised a long time period of inclusion (2000–2021) that could have influenced the choice of surgical procedure in relationship with the development of laparoscopic or even robotic surgery during the last years and also explained the low rate of neoadjuvant TKI use. Data concerning quality of life are lacking which could be of interest when comparing surgical options. Further studies preferably in the form of prospective randomized controlled trials in larger patient cohorts are needed to determine whether minimally invasive surgery is non inferior to open surgery to treat large gastric GISTs.
Conclusion
Laparoscopic approach can be safely performed in selected patients with large gastric GISTs at high-volume specialized institutions. Size of the tumor is an important criterion to take into account when laparoscopy is proposed but should not be an exclusion criterion by itself. In addition, laparoscopic approach for large gastric GIST is not associated with worst DFS in our study.
Data availability
No datasets were generated or analysed during the current study.
References
Blay JY, Kang YK, Nishida T, von Mehren M (2021) Gastrointestinal stromal tumours. Nat Rev Dis Primers 7(1):22. https://doi.org/10.1038/s41572-021-00254-5, March 18, 2021
Landi B, Blay JY, Bonvalot S, Brasseur M, Coindre JM, Emile JF, Hautefeuille V, Honore C, Lartigau E, Mantion G, Pracht M, Le Cesne A, Ducreux M, Bouche O (2019) Gastrointestinal stromal tumours (GISTs): French Intergroup Clinical Practice Guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO) Thésaurus National de Cancérologie Digestive (TNCD). Dig Liver Dis 51(9):1223–1231, https://doi.org/10.1016/j.dld.2019.07.006, August 3, 2019
Casali PG, Abecassis N, Bauer S et al (2022) Gastrointestinal stromal tumours: ESMO-EUROCAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 33:20–33. https://doi.org/10.1016/j.annonc.2021.09.005. September 21, 2021
Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR (2016) Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 40:39–46. https://doi.org/10.1016/j.canep.2015.10.031. November 24, 2015
Mazer L, Worth P, Visser B (2021) Minimally invasive options for gastrointestinal stromal tumors of the stomach. Surg Endosc 35(3):1324–1330. https://doi.org/10.1007/s00464-020-07510-x, March 27, 2020
Goh BK, Chow PK, Chok AY, Chan WH, Chung YF, Ong HS, Wong WK (2010) Impact of the introduction of laparoscopic wedge resection as a surgical option for suspected small/medium-sized gastrointestinal stromal tumors of the stomach on perioperative and oncologic outcomes. World J Surg. 34(8):1847-52, https://doi.org/10.1007/s00464-020-07510-x, August 2010
Liang JW, Zheng ZC, Zhang JJ, Zhang T, Zhao Y, Yang W, Liu YQ (2013) Laparoscopic versus open gastric resections for gastric gastrointestinal stromal tumors: a meta-analysis. Surg Laparosc Endosc Percutan Tech 4378–387. https://doi.org/10.1097/SLE.0b013e31828e3e9d, August 2013
Koh YX, Chok AY, Zheng HL et al (2013) A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol 20:3549–3560. https://doi.org/10.1097/SLE.0b013e31828e3e9d, June 21, 2013
Chen QL, Pan Y, Cai JQ, Wu D, Chen K, Mou YP (2014) Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: an updated systematic review and meta-analysis. World J Surg Oncol, 12:206, https://doi.org/10.1186/1477-7819-12-206, July 14, 2014
Piessen G, Lefèvre JH, Cabau M, Duhamel A, Behal H, Perniceni T, Mabrut JY, Regimbeau JM, Bonvalot S, Tiberio GA, Mathonnet M, Regenet N, Guillaud A, Glehen O, Mariani P, Denost Q, Maggiori L, Benhaim L, Manceau G, Mutter D, Bail JP, Meunier B, Porcheron J, Mariette C, Brigand C, AFC and the FREGAT working group (2015) Laparoscopic Versus Open Surgery for Gastric Gastrointestinal Stromal Tumors: What Is the Impact on Postoperative Outcome and Oncologic Results? Ann Surg, 262(5):831-9; discussion 829 – 40, https://doi.org/10.1097/SLA.0000000000001488, November 2015
Serrano C, Martín-Broto J, Asencio-Pascual JM et al (2023) 2023 GEIS guidelines for gastrointestinal stromal tumors. Therapeutic Adv Med Oncol 15. https://doi.org/10.1177/17588359231192388
Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, ChoiH, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, Fisher GA, Fletcher CD, Gronchi A, Hohenberger P, Hughes M, Joensuu H, Judson I, Le Cesne A, Maki RG, Morse M, Pappo AS, Pisters PW, Raut CP, Reichardt P, Tyler DS, Van den Abbeele AD, von Mehren M, Wayne JD, Zalcberg J, Force NT (2007) NCCNTask Force report: management of patients with gastrointestinalstromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5(Suppl 2):S1–29
Crocker AB, Vega EA, Kutlu OC, Salehi O, Mellado S, Li M, Kozyreva O, Conrad C (2022) Is minimally invasive surgery for large gastric GIST actually safe? A comparative analysis of short- and long-term outcomes. Surg Endosc 36(9):6975–6983, https://doi.org/10.1007/s00464-022-09066-4, March 21, 2022
Yu M, Wang DC, Wei J, Lei YH, Fu ZJ, Yang YH (2021) Meta-Analysis on the Efficacy and Safety of Laparoscopic Surgery for Large Gastric Gastrointestinal Stromal Tumors. Am Surg 87(3):450–457, https://doi.org/10.1007/s00464-022-09066-4, October 7, 2020
Khoo CY, Goh BKP, Eng AKH, Chan WH, Teo MCC, Chung AYF, Ong HS, Wong WK (2017) Laparoscopic wedge resection for suspected large (≥ 5 cm) gastric gastrointestinal stromal tumors. Surg Endosc 31(5):2271–2279. https://doi.org/10.1007/s00464-016-5229-7, September 8, 2016
Gevorkian J, Le E, Alvarado L, Davis B, Tyroch A, Chiba S, Konstantinidis IT (2022) Trends and outcomes of minimally invasive surgery for gastrointestinal stromal tumors (GIST). Surg Endosc 36(9):6841–6850. https://doi.org/10.1007/s00464-022-09014-2, January 19, 2022
Kasetsermwiriya W, Nagai E, Nakata K, Nagayoshi Y, Shimizu S, Tanaka M (2014) Laparoscopic surgery for gastric gastrointestinal stromal tumor is feasible irrespective of tumor size. J Laparoendosc Adv Surg Tech A 24(3):123-9, https://doi.org/10.1089/lap.2013.0433, March 2014
Lin J, Huang C, Zheng C, Li P, Xie J, Wang J, Lu J (2014) Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison. Surg Endosc 28(9):2577-83, https://doi.org/10.1007/s00464-014-3506-x, May 23, 2014
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83. https://doi.org/10.1053/j.semdp.2006.09.001. May 2006
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39(10):1411–1419. https://doi.org/10.1016/j.humpath.2008.06.025. October 2008
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.aeAugust 2004
Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Yu J, Zheng C, Liu F, Li Z, Zhao G, Zhang J, Chen P, Li G (2022) Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg 157(1):9–17, https://doi.org/10.1001/jamasurg.2021.5104, January 1, 2022
Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P (2012) Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 13(3):265–274 https://doi.org/10.1016/S1470-2045(11)70299-6, December 6, 2011
Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, Switaj T, Michej W, Wroński M, Głuszek S, Kroc J, Nasierowska-Guttmejer A, Joensuu H (2011) Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 37(10):890-6, https://doi.org/10.1016/j.ejso.2011.06.005, July 7, 2011
Hølmebakk T, Hompland I, Bjerkehagen B, Stoldt S, Bruland ØS, Hall KS, Boye K (2018) May Recurrence-free Survival after Resection of gastric gastrointestinal stromal tumors classified according to a strict definition of Tumor rupture: a Population-based study. Ann Surg Oncol
Lin SC, Yen HH, Lee PC, Lai IR (2023) Oncological outcomes of large gastrointestinal stromal tumors treated by laparoscopic resection. Surg Endosc 37(3):2021–2028. https://doi.org/10.1007/s00464-022-09693-x, October 25, 2022
Joensuu H, Rutkowski P, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Braconi C, Bordoni A, Magnusson MK, Sufliarsky J, Federico M, Jonasson JG, Hostein I, Bringuier PP, Emile JF (2015) KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 33(6):634–642 Epub 2015 Jan 20. PMID: 25605837
Van der Burg SJC, van de Wal D, Roets E et al (2023) Neoadjuvant Imatinib in locally advanced gastrointestinal stromal tumors (GISTs) is effective and safe: results from a prospective single-center study with 108 patients. Ann Surg Oncol 30:8660–8668. https://doi.org/10.1245/s10434-023-14346-x. Epub 2023 Oct 9
Crocker AB, Vega EA, Kutlu OC et al (2022) Is minimally invasive surgery for large gastric GIST actually safe? A comparative analysis of short- and long-term outcomes. Surg Endosc 36:6975–6983. https://doi.org/10.1007/s00464-022-09066-4Epub 2022 Mar 21
Hsiao CY, Yang CY, Lai IR, Chen CN, Lin MT (2015) Laparoscopic resection for large gastric gastrointestinal stromal tumor (GIST): intermediate follow-up results. Surg Endosc 29(4):868 – 73, https://doi.org/10.1007/s00464-014-3742-0, July 2023, 2014
Funding
none.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AM and MB. The first draft of the manuscript was written by AM, MB, LR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosures
Antoine Mariani, Melinda Bajul, Lionel Rebibo, Chloé Broudin, Widad Lahlou, Gabriel Rahmi, Mehdi Karoui have no conflicts of interest or financial ties to disclose. Julien Taieb has received honoraria as a speaker and/or in an advisory role from Roche, Genentech, Lilly, Servier, Sanofi, Celgene, Shire, Amgen, Sirtex, Merck Serono, and MSD. Aziz Zaanan has received honoraria as a speaker and/or in an advisory role from Amgen, Lilly, Merck, Roche, Sanofi, Servier, Baxter, MSD, Pierre Fabre, Astra Zeneca.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mariani, A., Bajul, M., Rebibo, L. et al. Is laparoscopic approach as treatment of large gastric GIST acceptable?. Langenbecks Arch Surg 409, 231 (2024). https://doi.org/10.1007/s00423-024-03415-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03415-8