Abstract
Purpose
NPWT has been tried in many surgical fields, including colorectal, thoracic, vascular, and non-healing wounds, for the prevention of SSI. However, its efficacy in the prevention of SSI-grade IV closed abdominal wounds is yet to be explored.
Methods
All patients with grade IV abdominal wounds were included in the study. They were randomized into the conventional arm and the VAC arm after confirming the diagnosis intra-operatively. The sheath was closed, and the skin was laid open in the postoperative period. In the VAC arm, the NPWT dressing was applied on postoperative day (POD)-1 and removed on POD-5. In the conventional arm, only regular dressing was done postoperatively. The skin was closed with a delayed primary intention on POD-5 in both arms. The sutures were removed after 7 to 10 days of skin closure.
Results
The rate of SSI (10% in the VAC arm vs. 37.5% in the conventional arm, p-value = 0.004) was significantly lower in the VAC arm, as were the rates of seroma formation (2.4% in the VAC arm vs. 20% in the conventional arm, p = 0.014) and wound dehiscence (7.3% vs. 30%, p = 0.011). The conventional arm had a significant delay in skin closure beyond POD5 due to an increased rate of SSI, which also led to a prolonged hospital stay (5 days in the VAC arm vs. 6.5 days in the conventional arm, p-value = 0.005).
Conclusion
The VAC dressing can be used routinely in grade IV closed abdominal wounds to reduce the risk of SSI and wound dehiscence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rate of global surgical site infection (SSI) burden varies in different studies and is reported to range from 2.5 to 41.9% [1, 2]. In India, it is shown to be consistently higher, i.e., 23 to 38%, despite implementing many guidelines. SSI rates are highest in the case of abdominal surgeries when compared to other system surgeries [3], especially in emergency cases [4], with the worst being the dirty abdominal wounds (grade IV), which are reported as high up to 60% [5]. Prevention of SSI is the most challenging and crucial part of patient care in the postoperative period. Few interventions have been found to reduce SSI rates beyond pre-operative antibiotic prophylaxis and aseptic technique. SSIs are associated with a prolonged hospital stay, prolonged ICU stay, higher hospital readmission rates, and higher morbidity and mortality rates than patients without SSI [6]. This also causes a significant economic burden to the patient [7], along with the loss of disability-adjusted life years (DALYs) [8]. The recent development of novel Negative Pressure Wound Therapy (NPWT) and its contribution to SSI prevention is revolutionary and has drawn attention worldwide for a few decades. It has been proposed that NPWT on closed surgical incisions may reduce infection in high-risk wounds and also avoid the morbidity of an open abdominal wound [9].
Open wound management has traditionally been used to treat contaminated and dirty wounds with a secondary or delayed primary intention. Compared to typical open wound management with wet-to-dry dressing changes, NPWT for open wounds is related to improved patient comfort and faster healing [10]. Due to the high risk of SSI in these two categories of wounds, the efficacy of NPWT has not been explored much in closed abdomen wounds.
This prompted us to do this study in India, where the SSI rate is higher than in many other countries, and to explore the efficacy of NPWT in preventing SSI in dirty abdominal wounds.
Objectives
The study’s primary objective was to compare the rate of SSI between NPWT-assisted skin closure and delayed primary closure in class IV abdominal wounds. The secondary objectives were to compare the postoperative wound complications, length of hospital stay, and skin closure after POD 5 between both arms.
Methods
Patients who presented to the emergency department (ED) of All India Institute of Medical Sciences, Bhubaneswar, with features of peritonitis, were initially resuscitated with intravenous fluids. Broad-spectrum antibiotics (3rd generation Cephalosporin), proton pump inhibitors, and injectable analgesics were started immediately. The diagnosis of peritonitis was based on clinical and/or radiological (USG or X-ray chest) investigations, following which consent for participation in the study was obtained in the emergency department before shifting the patient to the operation room.
All patients older than 18 years included in this study were undergoing emergency abdominal surgery with a midline incision and were diagnosed with grade IV abdominal wounds after opening the abdomen. Patients who were pregnant, undergoing laparoscopy surgeries, elective abdominal surgeries, or allergic to adhesive dressing materials participated in another study, and those who did not give consent to participate were excluded. Patients with peritonitis undergoing re-exploration for complications of previous surgery were also excluded.
The trial was ethically approved on 15th July 2019 by the institutional ethical committee with the ref. no. IEC/AIIMS BBSR/ PG Thesis/2019-20/58. The study was registered prospectively with the Clinical Trials Registry – India (CTRI) on 23/09/2019 with the CTRI registration number – CTRI/2019/09/021388 (https://ctri.nic.in/Clinicaltrials/showallp.php?mid1=36514&EncHid=10300.19720&userName=Dr%20Pradeep%20Kumar%20Singh) [11].
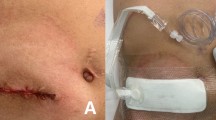
The recruitment of the first patient was done in November 2019, and the recruitment ended in June 2021. In a similar previous study [12], with SSI rates of 32% and 8.3% in the control and intervention groups, respectively, p-value = 0.043, power = 80%, and alpha error = 5%, the sample size was calculated to be 88, 44 patients in each arm. The eligible patients were allocated randomly to both groups through a computer-generated sequence using a simple randomization online tool. The random numbers were generated by using online tools. The allocation sequence is concealed from the operating surgeon by using the sequentially numbered opaque closed envelope technique (SNOCE). The random number was disclosed to the surgeon intra-operatively once the grade IV abdominal wound was confirmed after opening the abdomen. Part preparation was done on the table before the procedure. All cases were performed with the patient in the supine position. The abdominal skin was painted from nipple to mid-thigh before the incision with a 10% aqueous povidone-iodine solution. After opening the abdomen, the peritoneal fluid sample was sent for culture and sensitivity. The cause of peritonitis was noted, and then around a 6-L normal saline lavage was given. After treating the underlying disease, the drain was placed as decided by the surgeon based on the requirements, and closure of the rectus sheath was done using polydioxanone suture (PDS) loop no. 1 suture in a continuous fashion with full-thickness bites. After the closure of the rectus, the skin was left open. The time duration of surgery was measured as the interval of minutes between the time of incision and closure of the skin, and the intra-operative blood loss was also noted in milliliters. The patient was extubated and shifted to the surgery ward. For those who could not get extubated, the patient was shifted to an ICU ventilator bed. On postoperative day 1, the vacuum-assisted closure (VAC) dressing was applied with intermittent setting and a negative pressure of −125 mmHg. The VAC dressing was checked regularly for any leakage. The treating surgeon removed the VAC dressing on POD 5 and assessed the feasibility of delayed primary wound closure. Delayed primary closure of the skin was done on POD 5 in the absence of any signs of SSI (Figs. 1, 2, 3, 4, and 5).
In the conventional arm, daily dressing was done using sterile gauge pieces and normal saline. Delayed primary closure of the skin was done on POD 5 in the absence of SSI. The postoperative wound assessment was done by the treating surgeon, and SSI was diagnosed based on local signs.
The Centers for Disease Control and Prevention(CDC) defined SSI as “a surgical site infection that occurs after surgery in the part of the body where the surgery took place.” It is classified as superficial, deep, or organ- or space-incisional SSI [13].
The patients were discharged on POD 5 after skin closure if they were fit hemodynamically and taking an oral diet. If there are any signs of SSI or other surgical site-related complications, the length of hospital stay is prolonged, and the complications are managed by the hospital. The patient is being followed up until POD 30 in OPD (outpatient door). The midline wound sutures were removed after 7 to 10 days of skin closure (on POD 12 to 15) on the outpatient door (OPD) follow-up visit. The primary outcome of the study was to see the rate of SSI, and the secondary outcomes were to see the difference in postoperative wound complications, length of hospital stays, and skin closure after POD 5 between both arms.
NPWT dressing
This NPWT system is manufactured by Meditech Devices Pvt. Ltd. and was fully sponsored by the hospital. This was available freely for the patients, and the machine was reusable. This system of wound dressing contains wound dressing materials (foam, gauze), wound adjunct (protective adhesive transparent barrier, gauge piece), canister, and connecting tubes (one tube has a porous adhesive end that will be attached to the wound site, and another tube is connected to the NPWT machine canister). Both tubes will be interconnected for the negative suction to act. Each device has a specific design and manufacturer’s instructions for use that should be reviewed. After removing the prior dressing very carefully to avoid pain and bleeding, the wound is cleaned with normal saline, and wound size and depth are assessed. With proper measurement, foam dressing material is cut to the appropriate size and placed over the wound without extending onto the surrounding skin. After that, take the protective adhesive transparent barrier and place it over the sponge and surrounding skin. This is typically made of polyurethane. This dressing is thin and creates an airtight seal around the wound. Cut a hole into the protective adhesive transparent barrier about the size of a quarter (2.5 cm) in the middle of the wound site. Attach the wound side tube at this hole site and connect it to the other tube, which is connected to the machine cannister, and be sure the tube clamps are open. Turn on power to the vacuum device, set the prescribed pressure settings, and confirm that the dressing and foam shrink down. The canister attached to the pump collects the drainage and stores it. The pressure is maintained at − 125 mmHg, with the interrupted setting turned off for 30 min every 4 h. This dressing will be removed on POD5.
Results
A total of 88 patients were included in this study, out of 168 patients. A total of 81 patients were analyzed after 5 deaths and 2 lost to follow-up due to the COVID-19 pandemic during the study period, which included 41 in the VAC arm and 40 in the conventional arm.
Continuous variables are represented as the mean (standard deviation) unless otherwise specified.
The skew-continuous variable is represented as the median (interquartile range).
Nominal variables are expressed in frequency (proportion) Fig. 6.
Clinicopathological characteristics
Pre-operative baseline characteristics of the patients between the groups were compared in Table 1. The mean age of the participants in this study was 39.59 ± 15.15 years in the VAC group and 41 ± 16.55 years in the conventional arm.
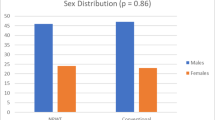
In the VAC arm, out of 41, 35 (85%) were male, and 6 (15%) were female. And in the conventional arm, 32 (80%) out of 40 were male, and 8 (20%) were female, which was evenly distributed between the two groups.
The other baseline characteristics showed no significant difference between the groups. Two patients in the conventional arm had an active tuberculosis infection. 1 in the VAC arm and 2 in the conventional arm had malignancies.
Operative characteristics
The median blood loss in the VAC arm was 150 ml ± 100 ml, and that in the conventional arm was 120 ml ± 60 ml, without making any significant difference in outcome between the two groups (Fig. 9). A total of 30 patients had to make a stoma based on intra-operative findings, including 15 (36.5%) in the VAC arm and 15 (37.5%) in the conventional arm. Table 2 summarizes the intra-operative parameters.
Postoperative outcomes
In contrast to the conventional arm, there was a statistically significant shorter length of postoperative hospital stay in the VAC arm (5 ± 1 days vs. 6.5 ± 5 days, respectively) with a p-value of 0.005 (Figs. 7 and 8).
Out of 81, we were able to close the skin in 64 patients on POD 5 (79%). 38 patients from the VAC arm (92.6%) and 26 from the conventional arm (65%) delayed primary closure, which was successful. The difference was statistically significant, with a p-value of 0.003. Table 3 shows the differences in postoperative parameters between both arms.
There was no statistically significant difference between the groups in terms of passing first flatus in postoperative periods, POD to allow oral liquids, POD of drain removal, POD ambulation, and rate of paralytic ileus. This shows the effectiveness of VAC, which does not affect the postoperative hospital stay or recovery, as the patient can ambulate while carrying the machine and can carry out his or her routine activities.
Wound complications
The most common wound complication in this study was found to be SSI, which was present in 19 patients out of 81 (23.4%). This shows a greater rate of SSI in the conventional arm as compared to the VAC arm, with a statistically significant difference (4 in the VAC arm vs. 15 in the conventional arm, p-value = 0.004) (Fig. 9).
This study also concluded that VAC also prevents seroma formation, which was evident by the significant difference in the rate of seroma formation in both arms with a p-value of 0.014 (2.4% in the VAC arm vs. 20% in the conventional arm). Table 4 shows the differences in wound complications between both arms.
Added to this, there was a statistically significant smaller number of wound dehiscence in the VAC arm (3 out of 41 in the VAC arm vs. 12 out of 40 in the conventional arm) with a p-value of 0.011. Overall wound dehiscence was 18.5%. Out of 15 wound dehiscence, four patients were managed with VAC re-application in the conventional arm. There was only one reported case of hematoma in this study in the conventional arm.
A total of 5 out of 81 (6%) patients had to undergo re-exploration, three in the VAC arm and two in the conventional arm, but without any statistically significant difference.
Skin closure was not possible in a total of 11 patients; nine of them had burst abdomens, and two were expired. Nine patients with burst abdomens were managed with secondary intention healing, consisting of two (5%) patients in the VAC arm and seven (16%) patients in the conventional arm.
Discussion
The overall rate of SSI in this study was 23.4% (19 patients out of 81), which was also the most common wound complication in this study. This was found to be much higher than the SSI rate in clean and clean-contaminated surgeries, which is in the range of 6 to 15% [3, 14, 15]. These high rates were attributable to the cases being taken exclusively from the emergency setting, and only class IV wounds were included [16,17,18,19]. Similarly, a high rate of SSI was also found in a study on patients who underwent emergency appendicectomy and had an SSI rate of 22.12% [20]. Allegranzi et al., in their review of SSI rates in developing countries, found 38.8% of SSI in contaminated and dirty wounds [21]. Similarly, in a study of emergency colorectal surgery published by Watanabe et al., the incisional SSI rate was 32.1%, and they concluded that the rates were greatly influenced by the degree of contamination of the wound [22].
In this study, we can conclude that NPWT significantly reduces SSI, which was evident by the difference in the rate of SSI between the two groups (4 out of 41 in the VAC arm, 15 out of 40 in the conventional arm, p-value = 0.004). A NEPTUNE study by Sami et al. in 2015 showed a significant decrease in the SSI rate with NPWT in colorectal surgeries. Also, there is a decrease in cost associated with SSI [23]. Similarly, another prospective randomized pilot study showed that contaminated and dirty surgical wounds benefit from closed NPWT with significantly faster healing rates [24]. There is much other published literature reporting that NPWT decreases wound complications in closed abdominal incisions. On the contrary, there are other studies too, which showed that NPWT does not make any difference in rates of SSI significantly [25].
Out of 81 cases, 15 developed wound dehiscence in this study: 3 in the VAC arm (7.3%) and 12 in the conventional arm (30%), which was statistically significant with p value of 0.011. The overall rate was 18.5%. Hegazy et al. noted 12.4% of burst abdomen in emergency abdominal midline laparotomies and concluded that wound infections were the most common risk factor for its development [26]. Similarly, another prospective study was done on 50 wound dehiscences by Ramneesh et al. and found that 88% were associated with contaminated or dirty laparotomy wounds [27]. Some studies also concluded the opposite of our result. A study by Sahani et al. noted wound dehiscence in 6% of cases, and another study by Bonds et al. showed a similar incidence of wound dehiscence in elective and emergency settings [28]. Eleven cases of partial wound dehiscence were managed with a sterile dressing daily until healthy granulation tissue covered the defect, followed by skin closure. Total wound dehiscence occurred in four patients. They managed with Bogota bag repair of the abdominal wall, daily sterile dressing, and a high-protein diet. Out of these four patients with total wound dehiscence, two patients managed with split skin grafting (SSG) of the midline wound once the healthy granulation tissue covered the defect. The other two were managed with daily dressing with NS with the secondary intention of healing without skin grafting.
Overall, in most of the patients in the VAC arm, we were able to close the skin on POD 5 (92.6% in the VAC arm vs. 65% in the conventional arm, p-value = 0.003). The mean POD on which skin closure was done in the VAC arm was 5.39 ± 3.12. This was significantly shorter than the conventional arm, where the patient had skin closure on a mean POD of 6 ± 2.08. A similar study was conducted in 2019, which included Grade II–IV wounds, and shows there is no significant difference in skin closure on NPWT [29].
The median postoperative hospital stay was 5 ± 1 days in the VAC arm and 6.5 ± 5 days in the conventional arm (p-value = 0.005). One previous study in groin wounds showed a similar result of shorter hospital stays with NPWT and without any difference in readmission or reoperation for SSI or mortality between the two groups [30].
The rate of seroma formation after skin closure was significantly decreased in the VAC arm, which was only 2.4%, against 20% in the conventional arm. NPWT has been used on many different types of traumatic and non-traumatic wounds. The study by Pachowsky et al. in patients who underwent total hip arthroplasty showed a decrease in the postoperative seroma formation in the wound [31]. Previously, a similar result was shown in an animal study by Suh et al. (2011), which demonstrated the use of NPWT in wounds with dead space significantly reduces seroma formation, thereby reducing SSI and further wound-related complications [32]. Also, similar results were concluded from human studies, where seroma and hematoma formation were significantly reduced with the use of NPWT [33]. The effect of NPWT has also been tried in breast surgeries, which shows a significant decrease in wound seroma formation and wound necrosis [34].
Reiping et al., in their prospective study of 3809 patients, concluded that the creation of an ostomy is a risk factor for future SSI, similar to how the presence of a drainage tube also contributes to developing SSI after elective colorectal surgeries [35]. A similar result was also found in this study, where the presence of stoma caused a significant increase in the SSI rate. Out of thirty patients with a stoma, thirteen developed SSI (43.3%), and only seven developed SSI out of 51 patients without a stoma (13.7%), with a p-value of 0.03.
In this study, appendicular perforation was the most common cause of peritonitis in patients undergoing laparotomy, contributing to 32% of cases. This was followed by peptic perforation (26%) and traumatic bowel perforation (16%). In one study by Bali et al., gastroduodenal perforation due to acid peptic disease accounted for 45% of cases, and 16% of cases due to appendicular perforation were the second most common cause [36]. In a study by Gebremedhn et al., trauma was the commonest cause of emergency laparotomies [37], and in a study by Sahani et al., ileal perforation was the commonest (28%), followed by gastric (20%), duodenal (20%), jejunal (10%), and appendicular (10%) [38]. Overall, appendicular perforation and peptic perforation contributed the most to peritonitis in this study, accounting for approximately 60%.
The major limitations of the study were the patients’ poor compliance with the machine, as it had to be carried by the patient all along. The machine had continuous noise and an alarm that disturbed the sleep of the patient. The observer bias could not be excluded in this study as the SSI was diagnosed by the treating surgeon. Two patients were lost to follow-up due to the COVID-19 pandemic during the study period.
Conclusion
SSIs are one of the most important causes of healthcare-associated infections, causing considerable morbidity and mortality, particularly in grossly contaminated surgeries. This study concludes that NPWT-assisted delayed primary skin closure results in a significant reduction in the rate of SSI as compared to conventional delayed primary skin closure in grossly contaminated emergency surgeries. There is also a significant reduction in wound dehiscence and length of postoperative hospital stay in the NPWT group.
Data availability
Data that support the findings of this research is available with the corresponding author.
References
World Health Organization (2018) Global guidelines for the prevention of surgical site infection, 2nd edn. Geneva, Licence: CC BY-NC-SA 3.0 IGO
Asaad AM, Badr SA (2016) Surgical site infections in developing countries: current burden and future challenges. Clin Microbiol 5:e136 https://doi.org/10.4172/2327-5073.1000e136
Mukagendaneza MJ, Munyaneza E, Muhawenayo E, Nyirasebura D, Abahuje E, Nyirigira J et al (2019) Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg 13(1):4–11
Singh M, Agarwal R, Singh R (2020) A prospective study on pattern of superficial surgical site infections in patients undergoing emergency laparotomy for perforation peritonitis. Int Surg J 7(6):1893
Thombare D, Joshi D (2019) A study of incidence and risk factors in post-operative abdominal wound infection in tertiary care centre. MVP J Med Sci 6(1):8–14
Kirkland K, Briggs J, Trivette S, Wilkinson W, Sexton D (1999) The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 20(11):725–730. https://doi.org/10.1086/501572
Jenks PJ, Laurent M, McQuarry S, Watkins R (2014) Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect [Internet] 86(1):24–33. https://doi.org/10.1016/j.jhin.2013.09.012
Koek MBG, Van Kooi TII, Stigter FCA, De Boer PT, De Gier B, Hopmans TEM et al (2019) The burden of surgical site infections in the Netherlands : cost analyses and disability-adjusted life years. J Hosp Infect [Internet] 103(3):293–302. https://doi.org/10.1016/j.jhin.2019.07.010
Scalise A, Calamita R, Tartaglione C, Pierangeli M, Bolletta E, Gioacchini M, Gesuita R, Di Benedetto G (2016) Improving wound healing and preventing surgical site complications of closed surgical incisions: a possible role of Incisional Negative Pressure Wound Therapy. A systematic review of the literature. Int Wound J 13:1260–1281. https://doi.org/10.1111/iwj.12492
Gupta N, Verma R, Dhiman RK, Rajsekhar K, Prinja S (2020) Cost-effectiveness analysis and decision modelling: a tutorial for clinicians. J Clin Exp Hepatol 10(2):177–184. https://doi.org/10.1016/j.jceh.2019.11.001
O’Leary DP, Peirce C, Anglim B, Burton M, Concannon E, Carter M, Hickey K, Coffey JC (2017) Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: the P.I.C.O. trial. Ann Surg 265(6):1082–1086. https://doi.org/10.1097/SLA.0000000000002098
Borchardt RA, Tzizik D (2018) Update on surgical site infections: the new CDC guidelines. JAAPA 31(4):52–54 https://doi.org/10.1097/01.JAA.0000531052.82007.42
Niekerk, (Magnus) JM van et al (2019) Risk factor identification and contribution to surgery-specific surgical site infection
Curcio D, Cane A, Fernández F, Correa J (2019) Surgical site infection in elective clean and clean-contaminated surgeries in developing countries. Int J Infect Dis [Internet] 80:34–45. https://doi.org/10.1016/j.ijid.2018.12.013
Young PY, Khadaroo RG (2014) Surgical site infections. Surg Clin NA [Internet] 94(6):1245–64. https://doi.org/10.1016/j.suc.2014.08.008
Miima SE, Oliech JS, Ndaguatha PLW, Opot EN (2016) Incidence and risk factors for surgical site infection following emergency laparotomy at Kenyatta National Hospital. East Afr Med J 93(8):345–347
Allegranzi B, Aiken AM, Zeynep Kubilay N, Nthumba P, Barasa J, Okumu G et al (2018) A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before–after, cohort study. Lancet Infect Dis 18(5):507–515
Alkaaki A, Al-Radi OO, Khoja A, Alnawawi A, Alnawawi A, Maghrabi A et al (2019) Surgical site infection following abdominal surgery: a prospective cohort study. Can J Surg 62(2):111–117
Peralta Vargas CE, López A, Díaz Gil JR, Rodríguez Montoya RM, Angulo Guzmán WR (2004) Surgical wound infection in appendectomized patients in the surgical service of Hospital III Essalud-Chimbote. Rev Gastroenterol del Peru organo Of la Soc Gastroenterol del Peru 24(1):43–49
Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L et al (2011) The burden of endemic health-care-associated infection in developing countries : systematic review and meta-analysis. Lancet [Internet] 377(9761):228–41. https://doi.org/10.1016/S0140-6736(10)61458-4
Watanabe M, Suzuki H, Nomura S, Maejima K, Chihara N, Komine O et al (2014) Risk factors for surgical site infection in emergency colorectal surgery: a retrospective analysis. Surg Infect (Larchmt) 15(3):256–261
Chadi SA, Vogt KN, Knowles S, Murphy PB, Van Koughnett JA, Brackstone M et al (2015) Negative pressure wound therapy used to decrease surgical nosocomial events in colorectal resections ( NEPTUNE ): study protocol for a randomized controlled trial. Trials [Internet] 1–6. https://doi.org/10.1186/s13063-015-0817-8
Frazee R, Manning A, Abernathy S, Isbell C, Isbell T, Kurek S et al (2018) Open vs closed negative pressure wound therapy for contaminated and dirty surgical wounds: a prospective randomized comparison. J Am Coll Surg [Internet] https://doi.org/10.1016/j.jamcollsurg.2017.12.008
Howerton R, Mogal H, Dodson R (2016) Brief title : negative pressure for laparotomy incision SC. J Am Coll Surg [Internet] (2017). https://doi.org/10.1016/j.jamcollsurg.2016.12.028
Hegazy TO, Soliman SS (2020) Abdominal wall dehiscence in emergency midline laparotomy: Incidence and risk factors. The Egyptian Journal of Surgery 39(2):489–497. https://doi.org/10.4103/ejs.ejs_7_20
Ramneesh G, Sheerin S, Surinder S, Bir S (2014) A prospective study of predictors for post laparotomy abdominal wound dehiscence. J Clin Diagn Res. 8(1):80–83. https://doi.org/10.7860/JCDR/2014/7348.3921
Bonds AM, Novick TK, Dietert JB, Araghizadeh FY, Olson CH (2013) Incisional negative pressure wound therapy significantly reduces surgical site infection in open colorectal surgery. Dis Colon Rectum 56(12):1403–1408
Ota H, Danno K, Ohta K, Matsumura T, Komori T, Okamura S et al (2020) Efficacy of negative pressure wound therapy followed by delayed primary closure for abdominal wounds in patients with lower gastrointestinal perforations: multicenter prospective study. J Anus Rectum Colon 4(3):114–121
Lee K, Murphy PB, Ingves MV, Duncan A, DeRose G, Dubois L et al (2017) A randomized clinical trial of negative pressure wound therapy for high-risk groin wounds in lower extremity revascularization. J Vasc Surg [Internet] 66(6):1814–9. https://doi.org/10.1016/j.jvs.2017.06.084
Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz-Drost S, Schlechtweg P et al (2012) Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 36(4):719–722
Suh H, Lee AY, Park EJ, Hong JP (2016) Negative pressure wound therapy on closed surgical wounds with dead space animal study using a swine model. Ann Plast Surg 76(6):717–722
Itani HE (2015) Reviewing the benefits and harm of NPWT in the management of closed surgical incisions. Br J Community Nurs 20(June):S28-34
Cagney D, Simmons L, O’Leary DP, Corrigan M, Kelly L, O’Sullivan MJ et al (2020) The efficacy of prophylactic negative pressure wound therapy for closed incisions in breast surgery: a systematic review and meta-analysis. World J Surg [Internet] 44(5):1526–37. https://doi.org/10.1007/s00268-019-05335-x
Tang R, Chen HH, Wang YL, Changchien CR, Chen JS, Hsu KC et al (2001) Risk factors for surgical site infection after elective resection of the colon and rectum: a single-centre prospective study of 2,809 consecutive patients. Ann Surg 234(2):181–189
Bali RS, Verma S, Agarwal PN, Singh R, Talwar N (2014) Perforation peritonitis and the developing world. ISRN Surg 2014:1–4
Gebremedhn EG, Agegnehu AF, Anderson BB. Outcome assessment of emergency laparotomies and associated factors in a low resource setting. A case series. Ann Med Surg [Internet] 36:178–84. https://doi.org/10.1016/j.amsu.2018.09.029
Sahani IS, Dhupia R, Kothari A, Rajput M, Gupta A (2017) Study of bacterial flora and their antibiotic sensitivity in peritonitis of various causes. Int Surg J 4(12):3999
Acknowledgements
I would like to express my sincere gratitude to the Institutional Ethical Clearance team of the institute for approving the study. I am thankful to all my teachers and colleagues for supporting me to complete the study.
Author information
Authors and Affiliations
Contributions
Dr. P K Singh, Dr. M K Sethi, Dr. T S Mishra, Dr. P Kumar, Dr. S M Ali, Dr. P K Sasmal, and Dr. S S Mishra participated in the study conception and design. Dr. P K Singh and Dr. M K Sethi participated in the analysis and interpretation of data. Dr. P K Singh, Dr. M K Sethi, Dr. T S Mishra, Dr. P Kumar, Dr. S M Ali, Dr. P K Sasmal, and Dr. S S Mishra drafted the manuscript. Dr. P K Singh, Dr. M K Sethi, Dr. T S Mishra, Dr. P Kumar, Dr. S M Ali, Dr. P K Sasmal, and Dr. S S Mishra participated in the critical revision of the manuscript.
Corresponding author
Ethics declarations
Declarations
We wish to draw the attention of the editor to the following facts: There are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors, and the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, concerning intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property. The article has not been submitted to any other journal for publication.
We understand that the corresponding author is the sole contact for the editorial process (including the editorial manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions, and final approval.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, P.K., Sethi, M.K., Mishra, T.S. et al. Comparison of surgical site infection (SSI) between negative pressure wound therapy (NPWT) assisted delayed primary closure and conventional delayed primary closure in grossly contaminated emergency abdominal surgeries: a randomized controlled trial. Langenbecks Arch Surg 409, 19 (2024). https://doi.org/10.1007/s00423-023-03202-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03202-x