Abstract
Purpose
Chyle leak resulting from thoracic duct (TD) injury poses significant morbidity and mortality challenges. We assessed the feasibility of using near-infrared (NIR) indocyanine green (ICG) imaging for intraoperative fluorescence TD lymphography during minimal access esophagectomy (MAE) in a semiprone position with inguinal nodal injection of ICG dye.
Methods
Ninety-nine patients with esophageal or gastroesophageal junctional cancer undergoing MAE received inguinal node injections of 2.5 mg ICG dye (total 5 mg) under sonographic guidance during anesthesia induction. Stryker’s 1688 AIM HD system was used in 76 cases, Karl Storz OPAL 1 S in 20, and in three cases the Karl Storz Rubina.
Results
In 93 patients (94%), the TD was clearly delineated along its entire length; it was not visualized in 6 patients (6%). Fluorescence guidance facilitated TD ligation in 16 cases, while 3 cases required clipping of duct tributaries for oncological considerations. Twenty-eight patients exhibited minor duct variations. Fluorescence was sustained throughout surgery (median observation time 60 min post-injection; range 30–330). No patient experienced any chyle leak within 30 days post-surgery and no adverse reactions to ICG was evident.
Conclusions
Intraoperative fluorescence TD lymphography using ICG during MAE in a semiprone position with inguinal nodal injection proved safe, feasible, and effective, allowing clear visualization of the TD in almost all cases. This approach aids safe ligation and reduces chyle leak risk. It offers real-time imaging of TD anatomy and variations, providing valuable feedback to surgeons for managing TD injuries during MAE procedures and represents an excellent educational tool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophagectomy is one of the mainstay treatment in the curative management of esophageal cancer. It is a complex procedure with high morbidity and mortality [1, 2]. Thoracic duct (TD) injury is rare complication following esophagectomy but carries a major morbidity and difficult to treat [3, 4]. It increases hospital stay and negatively impacts survival [4].
Some form of intraoperative image guidance of the TD is essential for a safer surgery [5]. Nowadays, most esophageal cancer cases are treated by neoadjuvant chemoradiotherapy, and thoracic dissection may be rendered more difficult due to therapy-related fibrosis, further increasing the risk of TD injury. A clear intraoperative recognition of the TD is essential to prevent its injury [6]. Most chyle injuries occur because of failure to identify the TD. Many tools have been put forward to better identify the TD intraoperatively. However, intraoperative identification of the TD course or leakage site is often challenging.
Although oral administration of heavy cream before surgery is sometimes performed to visualize the TD, it can be difficult to administer [7,8,9]. In addition, preoperative anatomical information is challenging to directly transfer to the intraoperative situation. Lymphangiography and lymphoscintigraphy, though available preoperatively to identify the site of TD damage, do not permit precise localization of the injured site [6]. Moreover, the complete TD anatomy, its tributaries, and uncommon course cannot be sufficiently addressed by lymphangiography and lymphoscintigraphy. Also, both tools are difficult to transfer to the operative table.
The intraoperative use of indocyanine green (ICG) with near-infrared (NIR) fluorescence is an emerging technology used in many surgical areas to visualize surgical anatomy, perfusion, and tissue function with high spatial resolution [10]. The intraoperative use of NIR-ICG fluorescence imaging in the literature has also been used in surgery to identify chyle leaks after esophagectomy, lung surgery, and neck dissection [6]. In addition, fluorescence imaging also guides safer lymphadenectomy. In the limited case reports, ICG dye was injected either into the small bowel mesentery or subcutaneously in the inguinal region [11,12,13,14]. There are a few small series of injection in the inguinal nodes [15,16,17]. None of them has reported the utility of NIR-ICG in real-time intraoperatively.
We report our experience herein with NIR-ICG fluorescence imaging to identify the TD intraoperatively and define its utility in patients undergoing minimal access transthoracic esophagectomy (MAE) in a semi-prone position.
Patients and methods
The study is a retrospective analysis of a prospectively maintained database carried out at the Department of Surgical Oncology at a tertiary cancer center in South India. Between October 2020 and till January 2023, 203 patients were operated at our institute. Among which 123 were operated by single surgical team, and 102 procedures were transthoracic esophagectomies (TTE). In the current study, 99 patients with resectable esophageal and gastroesophageal junctional (GEJ) cancer who underwent curative intent MAE were included. Patients aged between 18 and 75 years, who provided written informed consent, were eligible for the study. Patients allergic to iodine, those who did not undergo TTE, who did not consent, and those who were pregnant were excluded. The study was approved by the Medical Ethical Committee of the institute.
Surgical technique
Patients with carcinoma of the middle or lower third esophagus or GEJ underwent thoraco-laparoscopic assisted esophagectomy. Surgical technique has been standardized and consists of thoracoscopic mobilization of the esophagus with a standard infrahilar lymphadenectomy in a left lateral semi-prone position with dual lung ventilation. The patient is then placed in the supine position. Standard laparoscopic ports are placed. The gastro-hepatic ligament is divided and a conventional D2 lymphadenectomy is done barring infrapyloric and splenic hilar nodes. Lastly, the greater curvature is divided, preserving the gastroepiploic arcade.
After esophagectomy, a 3-cm-wide gastric conduit for the anastomosis is made using linear staplers buttressed with interrupted seromuscular polydioxanone sutures or hand sewn with the interrupted 2–0 polydioxanone sutures. The right gastroepiploic vessels are preserved. The short gastrics, left gastroepiploic, and left gastric vessels are ligated and divided at their origin with a vessel sealer. The gastric conduit, thus fashioned, is placed along the anterior chest wall to assess its length. Then, the gastric conduit is brought up to the neck through the posterior mediastinum; a doubled stapled side-to-side anastomosis is done using 75- and 100-mm GIA staplers using the modified Collard technique. Most of the patients with squamous cell carcinoma underwent thorax first approach, but a few patients with adenocarcinoma of GEJ underwent abdomen first approach to rule out metastatic disease.
ICG protocol
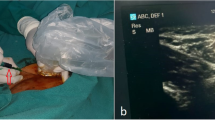
ICG is available as a 25-mg powder (Aurogreen; Aurolabs, Madurai, India) and is reconstituted in 10 ml of water, with each ml of the solution containing 2.5 mg of dye. ICG is injected in the inguinal nodes bilaterally (each side 1 ml, total 5 mg) under sonographic guidance before the start of surgery at the time of induction of anesthesia. For the last 6 months, we have been injecting ICG just before thoracoscopy.
To perform ICG lymphography, we selected inguinal lymph nodes as they are superficial, easily accessible, consistently present, and relatively larger in size and easy to visualize under ultrasound.
We systematically conduct sonographic evaluation of the inguinal region, specifically the femoral triangle, with the patient in a supine position and the thigh slightly abducted. It is crucial to have a deep understanding of the region’s anatomy. The lymph nodes are positioned superficially to the fascia lata. A typical lymph node exhibits an oval shape, regular contours, a hilum with blood flow, and a central hyper-echogenic medulla surrounded by a thin and uniformly hypo-echogenic cortex. A successful injection is indicated by the enlargement of the nodal size, and the central hyper-echogenic medulla loses its normal echotexture, becoming hypoechoic.
System description
Fluorescence TD lymphography was performed in majority using the Stryker 1688 High Definition (HD) advanced imaging modalities (AIM) 4 K platform (Stryker Endoscopy, Kalamazoo, MI). It offers 4 K resolution images with HD ICG imaging. The system consists of an infrared fluorescence (IRF)-enabled LED light source, light cable, camera control unit (CCU), 1688 AIM Camera head coupler, 0° and 30° 10 mm telescopes, and a 32″ monitor with 4 K display. A button on the camera head is used to toggle from visible light to near-infrared images. The surgeon can easily switch between normal mode (white light) and fluorescence mode (near infrared) at any time during the surgical procedure. The Stryker system facilitates the procedure in real time using an overlay mode, where the NIR image is fused with the white light image using artificial intelligence. It also permits storage of high quality 1080 p video in an internal hard disc which is easily retrievable.
Karl Storz OPAL 1S HD system (KARL STORZ SE & Co. KG, Tuttlingen, Germany) employs the Image 1S software. It utilizes a dedicated laser-free LED (Xenon light source) for optical illumination and contrast enhancement, combined with IMAGE1 S™ CLARA and CHROMA technologies. The system offers a foot switch to toggle between white light and ICG modes. It is important to note that this system does not include an overlay mode.
Karl Storz Image1S™ RUBINA HD system (KARL STORZ SE & Co. KG, Tuttlingen, Germany) comes with an integrated 4 K, 3D, and NIR-ICG imaging technology. The integrated technology provides increased resolution, excellent image quality in white light and NIR/ICG modes, and a natural color rendition. The camera chip enables 4 K white light and 4 K NIR/ICG modes. NIR/ICG offers three modes: the overlay mode, the intensity map mode, and the monochromatic mode incorporated in one system by the OPAL1 NIR/ICG technology. It offers storage of high-definition videos in internal hard disc AIDA.
Intraoperative identification rates of TD and its anatomical variation in real time were the primary outcomes of the study.
Results
Table 1 depicts patient characteristics, including demographic, operative, and postoperative parameters. The predominant histology was squamous cell carcinoma (n = 79). Among the patients, 83 received neoadjuvant chemoradiotherapy, 11 received neoadjuvant chemotherapy, and 4 underwent salvage esophagectomy after a failed definitive chemoradiotherapy. All patients underwent MAE with two-field nodal dissection.
No complications due to ICG injection were observed, either at the injection site or systemically. The median time taken to observe the TD from the injection time was 60 min (range 30–330). Seventy-six cases were performed using Stryker’s 1688 AIM HD system, three under Karl Storz Rubina, and 20 under Karl Storz OPAL 1 S. The TD was clearly delineated brightly along its entire length in 93 patients (94%) and was not seen in six patients. Real-time lymphography was performed in 73 cases using the overlay mode (available only in Stryker’s 1688 AIM HD and Karl Storz Rubina systems), where real-time NIR-ICG guidance was obtained by superimposing fluorescent on white light images. Minor variations in TD were noted in 28 patients (28%). The most common variation observed was saccular dilatation in 14 patients. Other variations noticed were duplication of a part of the duct in four, fusiform dilatation in three, plexiform variation in five, and loop formation and accessory duct joining the main duct in one (more than one anomaly seen in few patients).
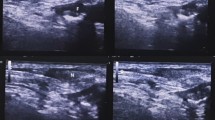
Normal fluorescent TD appeared bright green and located between the azygous vein and aorta (Fig. 1). Figure 2 shows normal TD in white light, with the Karl Storz OPAL 1S HD system and Rubina system. NIR-ICG guidance facilitated real-time recognition and clipping of the TD and its tributaries, ensuring a safe surgery (Fig. 3). In 16 cases, the TD was ligated to ensure resection-free margins, while in the three cases, the main TD was preserved, and only tributaries were clipped and ligated to achieve complete lymph node dissection. The significant usefulness of real-time NIR-ICG guidance was observed in patients undergoing salvage esophagectomy or post-thoracic radiation, where extensive thoracic fibrosis blurs the dissection planes (Fig. 4). All patients were followed up for 30 days after surgery, and no patient had chyle leak or adverse reactions related to ICG.
Normal fluorescent thoracic duct lymphography in a patient undergoing minimal access esophagectomy in a semi-prone position. Panel A displays the white light thoracoscopic image, where the TD is not visible. Panels B to D reveal the normal fluorescent thoracic duct lymphogram: B in overlay mode, C in fluorescent mode, and D in SPY Env mode
A Thoracoscopic white light image of the thoracic duct. Note that the TD is visible as a faint tubular structure. B NIR-ICG image of the thoracic duct corresponding to the image in Panel A (Karl Storz OPAL 1S HD system). C Real-time dissection of the thoracic duct in an integrated 4 K and 3D system (Karl Storz Rubina) in overlay mode. D Supra-azygous part of the thoracic duct in the Stryker 1688 HD AIM system
Discussion
We have demonstrated the safety and effectiveness of fluorescence imaging of the TD during MAE using NIR-ICG. The overall visualization rate in our series was > 93%. NIR-ICG facilitates safer dissection, even in salvage surgeries, by enabling specific ligation of TD tributaries under real-time ICG guidance.
ICG is a small hydrophilic tricarbocyanine dye that exhibits fluorescence when activated by NIR light (760 to 780 nm), delivered by a dedicated near-infrared optical system [18]. ICG has various surgical applications, including assessing flap vascularity, evaluating anastomotic perfusion, recognizing anatomical structures (such as blood vessels, biliary vessels, and lymphatic vessels), and detecting lymphatic drainage for sentinel node biopsy [19].
Studies by Ashitate et al. [20] and Steffey and Mayhew [21] have reported real-time imaging of TD anatomy in animal models using NIR fluorescence imaging with ICG. Numerous case reports and series have successfully identified the TD by injecting ICG into subcutaneous tissue, mesentery, dorsum of the foot, and intranodally [11,12,13,14,15,16,17, 19, 22]. However, intranodal injection has shown the most consistent TD visualization, even after repeat and failed surgeries [6]. Ultrasound-guided inguinal node injection is a safe and feasible method to obtain TD visualization, especially for real-time lymphography [6, 10, 19]. In our series, six patients did not show TD. These cases occurred during the initial phase of implementing the technique at our institute. This approach is safe and easy to learn, but we want to be self-critical and believe in these cases the dye was not successfully injected into the nodes. In most cases, clear and fast visualization was possible with NIR-ICG, as demonstrated in 93% of our series.
Recently, large series have reported on the utility of fluorescence guidance to delineate the TD during MAE using intranodal injection [16, 17], mesenteric injection [23], and subcutaneous inguinal injection [24, 25]. However, none of these studies has reported on real-time fluorescence guidance for TD identification intraoperatively.
In our series, we used intraoperative NIR-ICG fluorescence imaging to provide real-time visualization of the TD. In white light, the TD appears faintly as a thin tubular structure on the dorsal aspect during surgery, making it easily missed and putting it at high risk of injury. However, NIR-ICG imaging highlights TD in fluorescence, enabling effective recognition, preservation, and real-time dissection during MAE. The real utility of real-time NIR-ICG guidance becomes evident in salvage esophagectomy, post-thoracic radiation field, and re-exploration cases, where extensive thoracic fibrosis blurs the surgical planes, making TD recognition challenging. Fluorescence guidance ensures safe and comfortable dissection, providing constant feedback to the surgeon without the need for additional instruments or interruptions in the surgical flow. Additionally, in cases requiring resection of the TD for oncological radicality, fluorescence imaging aids in easy ligation of the stumps and checking for any chyle leakage. Moreover, NIR-ICG helps preserve the TD when only tributaries need to be resected and ligated close to the main TD for oncological reasons.
We have used different systems in our study. Karl Storz’s Rubina and Stryker’s 1688 AIM HD systems are highly regarded and preferred for surgical use. They excel in comparison to Karl Storz Opal 1S due to their dedicated overlay mode, which allows real-time visualization by superimposing ICG images onto white light. Both systems offer 4 K resolution, with Rubina also providing 3D image display for an enhanced surgical experience. However, comparing Stryker’s 1688 and Rubina is challenging, as they are both considered equally excellent based on our experience. Every institute has a different system, and a comparative study is simply not possible between the various available systems as the technical composition will vary between each system and companies for obvious reasons will not conduct such trials.
The variation in the TD, as far as we know from our experience, current knowledge, and literature, is not influenced by the delivery of neoadjuvant treatment; it is actually an anatomical feature. However, we do acknowledge that in cases of salvage and neoadjuvant chemoradiotherapy, identifying variations can be challenging due to thoracic fibrosis but especially in those cases, the utility of NIR-ICG is unquestionable as it clearly helps to perform safer dissection in these scenarios.
We observed that the key to successful imaging of the TD is proper ultrasonographic identification of the inguinal node, image-confirmed intranodal ICG injection with node expansion, and loss of central hypoechoic region. An advantage of intranodal injection is that it provides a constant supply of ICG for fluorescence, lasting for the entire duration of surgery, enabling real-time fluorescence surgery. Our experience suggests that ultrasound-guided intranodal injection is easy to perform, fast (5–10 min), and can be performed by surgeons themselves. In six cases, we encountered difficulties visualizing the duct because the dye had not been properly injected into the nodes. Once the dye is within the nodes, it consistently remains within the lymphatic system and, consequently, within the TD. The absence of TD visibility implies that the dye was likely injected outside the node, a common occurrence, especially when administered by someone inexperienced or in early phase of learning.
Conclusion
Intraoperative recognition of TD anatomy stands as the most effective method for avoiding TD injury and subsequent chylothorax. Real-time esophageal dissection under fluorescence guidance enables safer esophageal dissection but also provides constant anatomic feedback to the surgeon during surgery by offering continuous visualization. The technique can be performed without any time delay and can be easily reproducible.
Data Availability
The data is available with the corresponding author and can be shared upon a suitable request.
References
Rindani R, Martin CJ, Cox MR (1999) Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust N Z J Surg 69(3):187–194. https://doi.org/10.1046/j.1440-1622.1999.01520.x
Hulscher JB, Tijssen JG, Obertop H, van Lanschot JJ (2001) Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 72(1):306–313. https://doi.org/10.1016/s0003-4975(00)02570-4
Schild HH, Strassburg CP, Welz A, Kalff J (2013) Treatment options in patients with chylothorax. Dtsch Arztebl Int 110(48):819–826. https://doi.org/10.3238/arztebl.2013.0819
Brinkmann S, Schroeder W, Junggeburth K, Gutschow CA, Bludau M, Hoelscher AH, Leers JM (2016) Incidence and management of chylothorax after Ivor Lewis esophagectomy for cancer of the esophagus. J Thorac Cardiovasc Surg 151(5):1398–1404. https://doi.org/10.1016/j.jtcvs.2016.01.030
Stange S, Sziklavari Z (2021) Modern treatment options for postoperative chylothorax: a systematic review. Pneumologie 75(6):439–446. https://doi.org/10.1055/a-1172-7288
Nusrath S, Thammineedi SR, Saksena AR, Patnaik SC, Reddy P, Usofi Z, Kumar S (2022) Thoracic duct lymphography by near-infrared indocyanine green fluorescence imaging in thoracic surgery. A review Indian J Surg Oncol 13(2):415–420. https://doi.org/10.1007/s13193-022-01493-y
Shen Y, Feng M, Khan MA, Wang H, Tan L, Wang Q (2014) A simple method minimizes chylothorax after minimally invasive esophagectomy. J Am Coll Surg 218(1):108–112. https://doi.org/10.1016/j.jamcollsurg.2013.09.014
Shackcloth MJ, Poullis M, Lu J, Page RD (2001) Preventing of chylothorax after oesophagectomy by routine pre-operative administration of oral cream. Eur J Cardiothorac Surg 20(5):1035–1036. https://doi.org/10.1016/s1010-7940(01)00928-9
Du ZS, Li XY, Luo HS, Wu SX, Zheng CP, Li ZY, Fu JH (2019) Preoperative administration of olive oil reduces chylothorax after minimally invasive esophagectomy. Ann Thorac Surg 107(5):1540–1543. https://doi.org/10.1016/j.athoracsur.2018.10.053
Thammineedi SR, Patnaik SC, Shuka S, Nusrath S (2021) Fluorescence-guided cancer surgery-a new paradigm. J Surg Oncol 123(8):1679–1698. https://doi.org/10.1002/jso.26469
Matsutani T, Hirakata A, Nomura T, Hagiwara N, Matsuda A, Yoshida H, Uchida E (2014) Transabdominal approach for chylorrhea after esophagectomy by using fluorescence navigation with indocyanine green. Case Rep Surg 2014:464017. https://doi.org/10.1155/2014/464017
Kamiya K, Unno N, Konno H (2009) Intraoperative indocyanine green fluorescence lymphography, a novel imaging technique to detect a chyle fistula after an esophagectomy: report of a case. Surg Today 39(5):421–424. https://doi.org/10.1007/s00595-008-3852-1
Chang TI, Chen YS, Huang SC (2014) Intraoperative indocyanine green fluorescence lymphography to detect chylous leakage sites after congenital heart surgery. J Thorac Cardiovasc Surg 148(2):739–740. https://doi.org/10.1016/j.jtcvs.2014.03.021
Kaburagi T, Takeuchi H, Oyama T, Nakamura R, Takahashi T, Wada N, Saikawa Y, Kamiya S, Tanaka M, Wada T, Kitagawa Y (2013) Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy: Report of a case. Surg Today 43(2):206–210. https://doi.org/10.1007/s00595-012-0391-6
Bassi M, Vannucci J, Venuta F, De Giacomo T (2020) Effectiveness of indocyanine green fluorescence for the identification of thoracic duct in recurrent idiopathic chylothorax. Interact Cardiovasc Thorac Surg 31(2):284. https://doi.org/10.1093/icvts/ivaa080
Vecchiato M, Martino A, Sponza M, Uzzau A, Ziccarelli A, Marchesi F, Petri R (2020) Thoracic duct identification with indocyanine green fluorescence during minimally invasive esophagectomy with patient in prone position. Dis Esophagus 33(12). https://doi.org/10.1093/dote/doaa030
Varshney VK, Nayar R, Soni SC, Selvakumar B, Garg PK, Varshney P, Khera PS (2022) Intra-nodal indocyanine green injection to delineate thoracic duct during minimally invasive esophagectomy. J Gastrointest Surg 26(8):1559–1565. https://doi.org/10.1007/s11605-022-05341-w
Benson RC, Kues HA (1978) Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol 23(1):159–163. https://doi.org/10.1088/0031-9155/23/1/017
Thammineedi SR, Patnaik SC, Shuka S, Nusrath S (2022) Indocyanine green fluorescent thoracic duct lymphography by inguinal nodal injection approach for identifying thoracic duct and chyle leak: a case report. Indian J Surg. https://doi.org/10.1007/s12262-022-03619-6
Ashitate Y, Tanaka E, Stockdale A, Choi HS, Frangioni JV (2011) Near-infrared fluorescence imaging of thoracic duct anatomy and function in open surgery and video-assisted thoracic surgery. J Thorac Cardiovasc Surg 142(1):31-8.e1–2. https://doi.org/10.1016/j.jtcvs.2011.03.004
Steffey MA, Mayhew PD (2018) Use of direct near-infrared fluorescent lymphography for thoracoscopic thoracic duct identification in 15 dogs with chylothorax. Vet Surg 47(2):267–276. https://doi.org/10.1111/vsu.12740
Chakedis J, Shirley LA, Terando AM, Skoracki R, Phay JE (2018) Identification of the thoracic duct using indocyanine green during cervical lymphadenectomy. Ann Surg Oncol 25(12):3711–3717. https://doi.org/10.1245/s10434-018-6690-4
Barnes TG, MacGregor T, Sgromo B, Maynard ND, Gillies RS (2022) Near infra-red fluorescence identification of the thoracic duct to prevent chyle leaks during oesophagectomy. Surg Endosc 36(7):5319–5325. https://doi.org/10.1007/s00464-021-08912-1
Tokumaru S, Kitazawa M, Nakamura S, Koyama M, Soejima Y (2022) Intraoperative visualization of morphological patterns of the thoracic duct by subcutaneous inguinal injection of indocyanine green in esophagectomy for esophageal cancer. Ann Gastroenterol Surg 6(6):873–879. https://doi.org/10.1002/ags3.12594
Coratti F, Barbato G, Cianchi F (2021) Thoracic duct identification with indocyanine green fluorescence: a simplified method. Dis Esophagus 34(3). https://doi.org/10.1093/dote/doaa130 doaa130
Author information
Authors and Affiliations
Contributions
SR—acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript. SP—acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript. SS—study conception and design, drafting of manuscript, critical revision of manuscript. PR—drafting of manuscript, critical revision of manuscript. YV—interpretation of data, drafting of manuscript, critical revision of manuscript. SN—study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thammineedi, S.R., Patnaik, S.C., Reddy, P. et al. Impact of fluorescent thoracic duct lymphography via intranodal approach in minimal access esophageal cancer surgery. Langenbecks Arch Surg 408, 426 (2023). https://doi.org/10.1007/s00423-023-03162-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03162-2