Abstract
Background
Alpha-fetoprotein (AFP)-producing gastric cancer (AFPGC) is reported to have biologically aggressive features and poor prognosis. A relatively large number of patients with AFPGC have achieved a long-term prognosis after surgery in our institution. This study aimed to clarify the clinical features of and re-evaluate the long-term outcomes of AFPGC.
Methods
This analysis involved 465 patients who underwent surgery for gastric cancer (GC) at our institute between 1996 and 2020. The clinical features and long-term outcomes of the 24 patients with AFPGC were assessed. The differences in clinicopathological characteristics between AFPGC and non-AFPGC patients were statistically analyzed.
Results
In patients with AFPGC, the median preoperative serum AFP level was 232 ng/mL. Tumor invasion of AFPGC was classified and clinical characteristics of AFPGC patients were as follows: nodal metastasis, simultaneous liver metastasis, with malignant cells in ascites, lymphatic, and venous involvement. Postoperative surveillance revealed adjuvant therapy in fourteen, recurrence in eight, and four patients died of GC. The 3- and 5-year overall survival (OS) rates were 85.2% and 75.7% in AFPGC patients and 79.6% and 77.7% in non-AFPGC patients, respectively. The log-rank test identified no significant difference in OS between AFPGC and non-AFPGC patients. Tumor depth, nodal, and venous involvement showed significant differences between AFPGC and non-AFPGC patients.
Conclusions
AFPGC has aggressive biological features, but long-term prognosis after surgery does not seem to be as poor as claimed in previous studies. Therefore, it may be important to detect and start treatment early when surgery is feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common malignancy in the world [1]. The mortality rate of GC accounts for one-fifth of all tumors [2]. Alpha-fetoprotein-producing gastric cancer (AFPGC) was first reported in 1970 by Bourrille et al. [3] as a rare type of gastric cancer (GC) and is more common in Asian countries, such as Japan and China [4, 5]. Alpha-fetoprotein (AFP) is a glycoprotein that is normally synthesized in the fetal liver and yolk sac during gestation [6]. While AFP is a useful tumor marker of hepatocellular carcinomas or yolk sac tumors, several studies have shown the existence of other types of malignant tumors that produce AFP, including GC [7, 8].
Generally, AFPGC is considered to have poor prognosis. However, a relatively large number of patients with AFPGC have achieved long-term prognosis at our institution. Therefore, we were interested in determining further on the prognosis of patients with AFPGC. In this study, we aimed to primarily understand the clinical characteristics and prognosis of AFPGC using a case series study and then to re-evaluate the long-term prognosis of AFPGC using a retrospective cohort study.
Material and methods
Study population
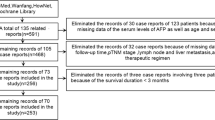
From a prospectively collected database maintained at the Department of Gastroenterological Surgery, Toranomon Hospital, Tokyo, Japan, 1189 patients who underwent surgery for GC between 1996 and 2020 and whose pathological data were available were identified. Of them, 465 patients (460 patients whose preoperative serous AFP values were available and 5 patients whose immunostaining showed positivity) were enrolled in this study. The remaining 724 patients were removed from this study because the unavailability of their preoperative AFP values could cause statistical bias (Fig. 1). Although the definition of AFPGC has not been clearly established, AFPGC was defined, in the present study, as gastric cancers whose preoperative AFP values were above the threshold (20.0 ng/mL) or whose immunohistochemistry showed positivity for AFP (Fig. 1). This study was performed in accordance with the Declaration of Helsinki and the ethical guidelines for clinical studies in Japan with the approval of the Institutional Review Board (No. 2349).
Clinicopathological factors
Detailed clinicopathological factors were retrieved from hospital records. Tumor staging was determined according to the TNM classification of the Union for International Cancer Control [9]. Macroscopic classification was based on the Borrmann classification (types I–V) [10]. Early stage superficial GC is categorized as type 0. The tumor infiltrative pattern (INF) was defined in the Japanese Classification of Gastric Carcinoma, which categorizes GC as expansive growth type (INFa), intermediate growth type (INFb), and infiltrative type (INFc) [11]. In our institute, patients are regularly screened for recurrence with esophagogastroduodenoscopy, computed tomography, ultrasonography, and tumor markers for at least 5 years after surgery, when possible. Relapse-free survival (RFS) was calculated from the day of surgery to the day of relapse or most recent follow-up. Overall survival (OS) was calculated from the day of surgery to the day of death or last follow-up.
Statistical analyses
Statistical analysis was performed using the JMP software (ver. 12.2.0; SAS Institute, Inc., Cary, NC, USA) and R 4.2.1 (https://cran.r-project.org/). Continuous values are expressed as median (range). Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. The propensity score weighting approach, inverse probability of treatment weighting (IPTW), was also utilized to reduce potential bias between AFPGC and non-AFPGC. This process created a weighted cohort of patients that was well balanced and appropriate for direct comparison. As our data contained several missing values, the final IPTW was calculated as an average of that generated in each imputed dataset. The propensity score model included the following covariates: patient age, sex, maximum tumor diameter, nodal involvement, lymphatic involvement, venous involvement, simultaneous liver metastasis, simultaneous peritoneal metastasis, T classification, and final tumor stage. Survival analysis using the Kaplan-Meier method and the log-rank test was also conducted in the IPTW sample. Categorical variables are expressed as percentages (%) and were compared using Fisher’s exact test. Statistical significance was set at p < 0.05.
Results
Case series study of patients with AFPGC
The clinicopathological characteristics of the 24 AFPGC patients are presented in Table 1. The median age was 85.5 years. The median serum AFP level was 232 ng/mL. The main tumors were located in the upper (7/24), middle (9/24), and lower parts (8/24) of the stomach. The tumors were found mainly at the anterior wall (3/24), posterior wall (3/24), lesser curvature (12/24), and greater curvature (5/19) in three, three, 12, and five patients. One patient had a circular lesion. Only two patients (Patients 22 and 24) had received neoadjuvant therapies. Lymphatic and venous involvement were found in 14 (58.3%) and 19 (79.1%) patients, respectively. The median tumor size was 45 mm.
Table 2 shows the postoperative therapies and long-term outcomes of the involved patients. Tumor invasion was classified as the following: T1b (4/24, 16.7%), T2 (5/24, 20.8%), T3 (10/24, 41.7%), T4a (4/24, 16.7%), and T4b (1/24, 4.2%). Seventeen patients (70.8%) had nodal metastasis (N1 in seven patients and N2 in ten patients). The final tumor staging was classified into IA in two (8.3%), IIA in eight (33.3%), IIB in five (20.8%), IIIA in five (20.8%), IIIB in one (4.2%), and IV in three (12.5%) patients. One patient with simultaneous liver metastasis, one with simultaneous peritoneal metastasis and one with malignant cells for cytodiagnosis of ascites were identified. Postoperative adjuvant chemotherapy was administered in 14 patients.
Patients with stage IA (Patients 10 and 17) showed no recurrence without any additional treatment. Of the eight patients with stage IIA disease (Patients 2, 7, 8, 9, 18, 19, 20, and 23), five patients (Patients 2, 7, 18, 19, and 23) showed no recurrence without additional treatment. Two patients with stage IIA (Patients 9 and 20) achieved relatively long survivals (107 and 137 months, respectively) after the administration of S-1. The other patient with stage IIA (Patient 8) attained 119-month survival regardless of the existence of liver recurrence, which was treated with radiofrequency ablation. Of the five patients with stage IIB (Patients 1, 3, 14, 16, and 21), two patients (Patients 1 and 16) were alive with the change in chemotherapy regimen, although two patients (Patients 3 and 21) died of liver recurrence. Of the five patients with stage IIIA (Patients 5, 6, 11, 12, and 22), two patients (Patients 6 and 12) died of tumor recurrence. Nonetheless, one patient with stage IIIB (Patient 15) achieved a 55-month RFS with the administration of S-1.
Study population with GC
Clinicopathological features of the global population in which surgery for GC was performed are shown in Table 3. The median observation term was 25.8 (0.0–276) months. Tumor invasion was categorized as T1a in 116 (24.9%) patients, T1b in 80 (17.2 %), T2 in 41 (8.8 %), T3 in 59 (12.7 %), T4a in 66 (14.2 %), T4b in 20 (4.3 %), and unknown in 83 (17.8 %) patients. Nodal involvement was observed in 127 (27.3%) patients. The final tumor staging was classified as I in 190 (0.8%), IIA in 48 (10.3%), IIB in 42 (9.0%), III in 54 (11.6%), IV in 51 (11.0%), and unknown in 80 (17.2%) patients. Lymphatic and venous involvement were found in 177 (38.1%) and 185 (39.8%) patients, respectively. Twenty-four patients (5.2%) were diagnosed with AFPGC. Simultaneous liver and peritoneal metastases were found in 7 (1.5%) and 27 (5.8%) patients, respectively. Among the total population, 56 (16.0%) patients died of tumor recurrence. Of these, 6 (1.7%) died of liver recurrence.
Comparison of long-term survival between AFPGC and non-AFPGC
The prognostic determinants of GC were investigated using the log-rank test for the entire cohort of 465 patients (Table 4). The results showed no significant difference in OS between AFPGC and without AFPGC (P = 0.600) (Figs. 1A and 2A). The 3- and 5-year survival rates were 85.2% and 75.7% in AFPGC patients and 79.6% and 77.7% in non-AFPGC patients, respectively. Even after IPTW adjustment, no statistically significance was identified in OS between AFPGC and without AFPGC (P = 0.844) (Fig. 2B).
Comparison of clinicopathological features between AFPGC and non-AFPGC
Table 5 shows the results of the comparison of clinicopathological factors between the 24 patients with AFPGC and 441 with non-AFPGC. In summary, tumor depth (P =0.019), nodal (P < 0.001), and venous involvement (P < 0.001) were significantly different between AFPGC and non-AFPGC patients.
Discussion
In the present study, we successfully summarized the clinicopathological characteristics and long-term postoperative outcomes of 24 patients who underwent surgery for AFPGC. This case series included a variety of information on each patient’s preoperative and postoperative follow-up periods would be extremely rare and valuable. Our analysis suggests that the long-term prognosis of AFPGC patients might not be as poor as that of non-AFPGC patients, as reported by recent researches. Furthermore, AFPGC tended to be associated with more lymphovascular invasion than non-AFPGC, and recurrence of AFPGC was characterized by a higher frequency of liver metastases, which is similar to the results of previous reports.
AFPGC has been reported to be associated with aggressive biological characteristics and poor prognosis compared with non-AFPGC [12] [13]. High proliferative activity, weak apoptosis, and rich neovascularization are currently reported to be at the root of the aggressive features of AFPGC [14]. Liu et al. reported a 5-year OS rate of only 28.0%, while that of non-AFPGC patients was 38.0% [12]. Previous studies focusing on the long-term outcomes of AFPGC have identified vascular invasiveness, liver metastasis, and nodal involvement as evidence of the aggressive nature of AFPGC [4] [15]. An interesting recent study also supported the idea of aggressiveness of AFPGC from a molecular biological point of view [16]. The present study is consistent with previous studies in that we also observed significant differences in tumor depth, nodal involvement, and lymphovascular involvement between AFPGC and non-AFPGC. An important result of this study is that AFPGC patients presented a satisfactory prognosis, with no significant difference compared to non-AFPGC patients. This indicates that the factors proving the aggressiveness of AFPGC (tumor depth, nodal involvement, and lymphovascular involvement) may not have negative effects on the long-term outcomes of AFPGC. Moreover, the favorable prognosis of AFPGC might be due, in part, to the fact that some cases of liver metastasis of AFPGC are relatively controllable by surgical resection [17]. Therefore, it is necessary to investigate the differences between the present and previous studies on the poor prognosis of AFPGC. According to Hirajima et al., multivariate analysis revealed that AFP positivity was not an independent prognostic factor [18]. The previous study also showed that the prognosis of AFPGC was similar to that of non-AFPGC without simultaneous liver metastasis and that liver metastasis was the only prognostic factor in AFPGC [18]. In our cohort, there was no significant difference in the incidence of simultaneous liver metastasis between AFPGC and non-AFPGC (P = 0.671), indicating that our study population was analyzed in a situation where the impact of simultaneous liver metastasis was statistically negligible. Thus, our study may indicate the validity of the study conducted by Hirajima et al. [18].
This study population was limited to patients whose preoperative blood AFP values were available. Thus, it can be noticed that high preoperative blood AFP levels would not necessarily mean positive immunostaining for AFP or that pathological study may later identify AFPGC even if preoperative blood AFP values are within the normal range (Fig. 1). Considering the characteristics of heterogeneity within a tumor, AFP-producing cells can sometimes form nodules and be hidden in some parts of the tumor. Therefore, the positivity for AFP could be partly influenced by how to cut the specimen. This may indicate that the definition of AFPGC in possible multicenter studies should be based not only on pathological findings, but also on the trends of blood AFP levels and clinical background.
Our study has some limitations. First, because this study was designed retrospectively, there was no unified follow-up strategy. It should be noted that some of the enrolled patients were followed-up for a relatively small observation period, while others were meticulously followed-up for a long period. Second, due to the single institutional analysis, this study included a relatively small number of patients mainly due to the low frequency of AFPGC (with approximately 1.2–15% of GC) [9]. A different result might be obtained if a larger sample size is involved. Third, there may have been a selection bias, since this study was limited to operable patients. In addition, not all gastric specimens have been routinely immunostained for AFP at our institution, since it was performed only when AFPGC was suspected by pathologists based on preoperative serum AFP levels and pathological findings. Therefore, the possibility of AFPGC misdiagnosis should be considered. Further multicenter studies with larger sample sizes are needed before definitive conclusions can be drawn, providing guidelines for optimizing therapeutic strategies for AFPGC.
In conclusion, although AFPGC may have aggressive features, such as tumor depth, nodal involvement, and lymphovascular involvement, the long-term prognosis of patients who underwent surgery does not seem to be as poor as previously reported. In AFPGC, early detection and initiation of therapeutic intervention at the stage when surgery is feasible may be important.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Sun W, Liu Y, Shou D et al (2015) AFP (alpha fetoprotein): who are you in gastrology? Cancer letters 357:43–46
Bourreille J, Metayer P, Sauger F et al (1970) Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med 78:1277–1278
Chun H, Kwon SJ (2011) Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J Gastric Cancer 11:23–30
Kong XX, Li XL, Tian Y et al (2021) The clinicopathological characteristics of alpha-fetoprotein-producing adenocarcinoma of the gastrointestinal tract-a single-center retrospective study. Front Oncol 11:635537
Bergstrand CG, Czar B (1956) Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest 8:174
Nishimura H, Okamoto Y, Takahashi M et al (1976) Occurrence of alpha-fetoprotein, Regan isoenzyme, and variant alkaline phosphatase in the serum of a patient with gastric cancer. Gastroenterology 71:497–499
Masuzawa M, Lee PK, Kamada T et al (1977) Carcinoembryonic antigen, alpha-fetoprotein and carcinoplacental alkaline phosphatase in gastric carcinoma metastatic to the liver. Cancer 39:1175–1180
Brierley J, Gospodarowicz M, Wittekind C (2017) Digestive system tumours. In: TNM Classification of Malignant Tumours, 8th edn. Wiley-Blackwell, Oxford, pp 83–90
Borrmann R, Henke F (1926) Lubarsch O. Handbuch spez pathol anat und histo
Japanese Gastric Cancer A (2011) Japanese classification of gastric carcinoma. Gastric Cancer 14:101–112
Liu X, Cheng Y, Sheng W et al (2010) Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol 102:249–255
Chang YC, Nagasue N, Kohno H et al (1990) Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol 85:1480–1485
Koide N, Nishio A, Igarashi J et al (1999) Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol 94:1658–1663
Wang D, Li C, Xu Y et al (2015) Clinicopathological characteristics and prognosis of alpha-fetoprotein positive gastric cancer in Chinese patients. Int J Clin Exp Pathol 8:6345–6355
Sun W, Liu B, Chen J et al (2017) Novel characteristics of alpha-fetoprotein (AFP)-producing gastric cancer. Oncotarget 8:101944–101951
Ohkura Y, Shinohara H, Haruta S et al (2015) Hepatectomy offers superior survival compared with non-surgical treatment for ≤ 3 metastatic tumors with diameters < 3 cm from gastric cancer: a retrospective study. World J Surg 39:2757–2763
Hirajima S, Komatsu S, Ichikawa D et al (2013) Liver metastasis is the only independent prognostic factor in AFP-producing gastric cancer. World J Gastroenterol 19:6055–6061
Funding
Miho Akabane received a research grant from the Okinaka Memorial Institute for Medical Diseases.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by Miho Akabane. The first draft of the manuscript was written by Miho Akabane, and all the authors commented on the previous versions of the manuscript. Akikazu Yago helped to draft the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of the Toranomon Hospital. The study procedures adhered to the tenets of the Declaration of Helsinki.
Consent to participate
The requirement for individual informed consent was waived due to the retrospective nature of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akabane, M., Yago, A., Haruta, S. et al. Re-evaluation of the prognosis of alpha-fetoprotein-producing gastric cancer from a single center: a case series study. Langenbecks Arch Surg 408, 66 (2023). https://doi.org/10.1007/s00423-023-02817-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02817-4