Abstract
Background
Centralisation of highly specialised medicine (HSM) has changed practice and outcome in pancreatic surgery (PS) also in Switzerland. Fewer hospitals are allowed to perform pancreatic surgery according to nationally defined cut-offs.
Objective
We aimed to examine trends in PS in Switzerland. First, to assess opinions and expected trends among Swiss pancreatic surgeons in regard of PS practice and second, to assess the evolution of PS performance in Switzerland by a nationwide retrospective analysis.
Methods
First, a 26-item survey among all surgeons who performed PS in 2016 in Switzerland was performed. Then, nationwide data from 1998 to 2018 from all hospitals performing PS was analysed including centre volume, perioperative morbidity and mortality, surgical indications and utilisation of minimally invasive pancreatic surgery (MIPS). The national cut-off for regulatory accredited volume centres (AVC) was ≥ 12. Additionally, an international benchmark definition for high volume (≥ 20 surgeries/year) was used.
Results
Among 25 surgeons from 15 centres (response rate 51%), the survey revealed agreement that centralisation is important to improve perioperative outcomes. Respondents agreed on a minimum case load per surgeon or centre. Within the nationwide database, 8534 pancreatic resections were identified. Most resections were performed for pancreatic ductal adenocarcinoma (58.9%). There was a significant trend towards centralisation of PS with fewer non-accredited volume centres (nAVC) (36 in 1998 and 17 in 2018, p < 0.001) and more AVC (2 in 1998 and 18 in 2018, p < 0.001). A significantly higher adjusted mortality after pancreatoduodenectomy (PD) was observed in low-volume compared to high-volume hospitals (OR 1.45 [95% CI 1.15–1.84], p = 0.002) and a similar trend compared among AVC and nAVC (OR 1.25 [95% CI 0.98–1.60], p = 0.072), while mortality after distal pancreatectomy (DP) was not influenced by centre volume.
Conclusions
Over the last two decades, centralisation of PS towards higher-volume centres was observed in Switzerland with a decrease of mortality after PD and low mortality after DP. Further centralisation is supported by most pancreatic surgeons. However, the ideal metric and outcome measures for the allocation of highly specialised medicine need further discussion to allow a fair and outcome-focused allocation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the late 1990s, the direct relationship of postoperative outcome and case volume for complex surgical procedures leading to lower mortality in high-volume centres was first described in the USA [1, 2]. This association has been further established since and also applies to European countries [3,4,5,6,7,8,9,10,11]. Hospital and surgeon volume has since been used as a measurable variable of centralisation aiming to improve patient outcomes. In abdominal surgery, minimal case numbers for procedures such as esophagectomy, rectal resection, hepatectomy or pancreatic surgery (PS) have been introduced in many countries to ensure high-quality care [12]. In 2008, centralisation based on minimal case numbers for complex abdominal surgery was introduced also in Switzerland. Specific criteria for highly specialised medicine (HSM) and definition of high-volume centres were set by cantonal authorities and only HSM institutions are reimbursed if they perform complex abdominal surgery including PS [13]. The increasing complexity of PS with extended vascular reconstructions and its interdisciplinary treatment including neoadjuvant treatment strategies make further centralisation appealing [14]. Simultaneously, abdominal surgeons are confronted with other evolving fields like minimally invasive pancreatic surgery (MIPS), highly specialised surgical training and health care cost spending pressure [15,16,17,18]. How this situation is currently perceived among Swiss pancreatic surgeons and whether the current centralisation strategy leads to better outcomes is largely unknown.
The first objective of this study was to explore opinions and centre characteristics of patients undergoing PS in Switzerland based on a survey among Swiss pancreatic surgeons. Secondly, we aimed to assess trends of PS regarding centre volume, mortality, surgical indications and utilisation of MIPS over two decades using nationwide data.
Materials and methods
As this study only used anonymised retrospective hospitalisation data, a waiver of consent was granted according to the cantonal ethics committee (BASEC-Nr. Req-2020–00,493).
Survey assessing current practice in pancreatic surgery in Switzerland
A self-administered 26-item survey was designed by the authors. During item generation, no Likert-type questions were used. For the pre-testing, a panel of 4 researchers and 3 surgeons provided feedback on understanding and meaning of items. During debriefing, the respondent’s input was integrated during two refinement rounds (Appendix 1). The survey was delivered in English and was anonymised.
Finally, the following sections were defined: (i) surgeon and hospital case load for assessing the annual volume, (ii) surgeon’s opinion on centralisation in PS, (iii) current practice including indications and (iv) use of MIPS, (v) further plans to implement or strengthen MIPS as well as (vi) training in PS. The voluntary, non-incentive survey was then sent via the web-based tool SurveyMonkey Inc. (San Mateo, CA, USA) from March to May 2020 with periodic electronic reminders.
Hospitals (n = 38) performing PS in 2016 were identified using data from the Federal Office for Public Health. Surgeons from the hepatopancreatobiliary team or the head of the surgical department if no such team existed were contacted in 2020 via email with the opportunity to name other surgeons within the same surgical department to participate in this survey. Identified surgeons subsequently received a link to access the survey. Email reminders were sent twice at intervals of 2 weeks. The survey was conducted over 3 months. Participants were able to review and change answers. The survey was considered complete if a minimum of 80% of questions were answered while also incomplete questionnaires were analysed. Analysis was based on individual respondents.
Nationwide data analysis on pancreatic resections
The Federal Office of Statistics (FSO, Neuchâtel, Switzerland) database is a mandatory reporting system that collects information on all hospitalisations > 24 h among Swiss hospitals. The database from 1998 until 2018 was queried. This database contains anonymised patient-level data including the main diagnosis responsible for hospitalisation and diagnoses for complications coded using the International Classification of Diseases (ICD-10 German modification) definitions. Procedures are coded by national Swiss surgical classification codes (CHOP), issued annually by the FSO for the classification of all medical interventions [19]. The database was searched for pancreatic interventions such as PD and DP by respective year-matched CHOP codes (Appendix). Minimally invasive or open procedures were also distinguished based on CHOP codes. Diagnoses were assessed with ICD main codes (Appendix). Cases were grouped as open, laparoscopic or robotic to assess trends over time. The definition of an AVC was ≥ 12 surgeries/year and based on the accreditation requirements by the Swiss government to promote centralisation [13]. The caseload per centre was calculated by the number of pancreatic resections per hospital. We used the ISGPS Evidence Map of Pancreatic Surgery to identify other volume cut-offs [20]. Analyses were repeated with the internationally acknowledged cut-off of ≥ 20 surgeries/year for high-volume centres to assess the impact of an internationally accepted and established benchmark [21]. In-hospital mortality was assessed for the two most common procedures PD and DP.
Statistical analysis
To assess the number of annual pancreatic procedures covered by the surgeons who participate in this survey, the annual number was compared to the estimation by the HSM body [13].
Respondents and non-respondents to the survey were classified in terms of the institution (university, regional, private hospital). Results from the survey were summarised as counts (%). The predetermined cut-off for “consensus” was set at 80%; otherwise, it was considered to be “non-consensus”.
Data from the FSO database were presented as median with interquartile range (IQR) and numbers with percentage were used to summarise continuous and categorical data, respectively. The Mann–Kendall test for monotonic analysis of trend was used for trend analyses for time series data in number of hospitals stratified by volume. Categorical variables were compared using Fisher’s exact test, continuous data by using the Wilcoxon rank-sum test. To assess the influence of hospital volume stratified by type of procedure, a logistic regression model was fit to the data with mortality being the dependent variable. Type of procedure, sex, age, nationality, insurance, Elixhauser Comorbidity Index, period of treatment (Q1 from 1998 to 2002, Q2 from 2003 to 2007, Q3 from 2008 to 2012 and Q4 from 2013 to 2018), diagnosis, surgical approach, readmission, reoperation, insufficiency of the pancreatic anastomosis, bleeding and centre volume were independent variables. The Elixhauser Comorbidity Index is a method for measuring patient comorbidity based on ICD-9-CM and ICD-10 diagnosis codes found in administrative data [22]. R version 3.5.1 was used for all database processing, statistical analyses and graphical representations for the nationwide data. Analysis for the survey data was done with GraphPad Prism.
Results
Survey assessing current practice in pancreatic surgery in Switzerland
Surgeon and hospital case load
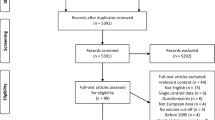
In 2020, a total of 59 surgeons from 38 institutions were identified and contacted. Ten did no longer perform PS and declined participation. Of the remaining 49 surgeons, 25 returned a complete survey (overall response rate 51%) (Fig. 1). Respondents covered 17 of 38 institutions (55%) including 15 out of 18 AVC HSM hospitals (83%).
Most respondents worked at university (9/25, 36%) or regional hospitals (12/25, 48%) while 4/25 (16%) worked at private hospitals. Among non-responders, 22/25 (88%) worked in nAVC non-HSM hospitals. All but one respondent (96%) presented their oncologic cases preoperatively at the multidisciplinary team meeting and all cases were discussed postoperatively.
Proposed criteria for centralisation of pancreatic surgery in Switzerland
All but one respondent agreed on a minimum number of resections per year as a criterion for PS centralisation. Two respondents added instructing activities and quality assessment (i.e. mortality rates) as further criteria and one respondent, the cumulative lifetime experience in resections, respectively (Fig. 2A). Alternative or complementary measures were suggested and included 5-year survival for patients with pancreatic ductal adenocarcinoma (PDAC) and lifetime surgeon experience.
Findings from a survey among surgeons. A The proposed annual case number per surgeon was agreed to be lower than for centres, 8–30 cases vs 10–50 cases, respectively. Surgeons working in regional hospitals suggested a lower minimum centre case load than private and university hospitals. B Rate estimation by surgeons grouped by pancreatic ductal adenocarcinoma, cystic lesions, chronic and acute pancreatitis. C Estimated use of minimally invasive surgery for laparoscopic DP and PD. PDAC, pancreatic ductal adenocarcinoma; DP, distal pancreatectomy; PD pancreatoduodenectomy
Indications for surgery
Twenty-three (92%) of the respondents answered that they operate mostly on malignant diseases. They named them the most common indication for resection and PDAC was the largest subgroup throughout the study period. The distribution of resections stratified by the most frequent diagnosis is shown in Fig. 2B. Cystic lesions were increasingly treated as the second most common indication for surgery, accounting for 10 to 45% of all resections depending on the centre.
Minimally invasive pancreatic surgery
Most respondents (96%) reported using MIPS, mostly laparoscopy, with up to a quarter using both laparoscopic and robotic approaches. Only a minority (28%) has introduced MIPS for PD (Fig. 2C). Fifteen centres (60%) performed most DP using MIPS, while open PD was the usual approach for pancreatic head tumours in every institution.
Plans to implement or strengthen MIPS
Fourteen respondents (58%) plan to further strengthen MIPS in their department. Of those who specified their plans, most (5/8) intend to strengthen their robotic surgery program. The reasons not to strengthen MIPS were that no additional value of MIPS for the patients is expected, the low case number in their institution and that MIPS can only be applied to highly selected cases.
Training in pancreatic surgery
Seven hospitals (44%), including three university hospitals, offer a structured training program for PS. Five institutions have a fellowship in HPB surgery and two a subspecialisation in abdominal surgery including PS. Fourteen of the respondents (56%) completed fellowships in PS or HPB surgery themselves. Among respondents, only a minority of resections were taught to other surgeons. Seventeen surgeons (68%) performed most or all resections themselves, six surgeons (24%) taught as many as they performed themselves, and two (8%) taught more than half of their procedures or some steps (e.g. gastrojejunostomy) of the operation.
Nationwide data analysis on pancreatic resections
Retrospective analyses of reported data of the FSO identified a total of 15,442 pancreatic interventions over 21 years. After exclusion of 82 pancreatic transplantations, 416 total pancreatectomies, 1491 not-otherwise specified partial pancreatectomies, 1243 pancreatic cystic drainages, 3648 other various interventions and 26 cases with no indicated primary diagnosis, we focused on 6408 PD and 2126 DP for subsequent analyses. The number of pancreatic resections increased from 124 in 1998 (105 PD, 19 DP) to 666 in 2018 (455 PD, 211 DP) (Fig. 3A). Details of the cohort stratified by AVC and high-volume centres are depicted in Table 1 and supplementary table 2. The majority of resections were performed for PDAC (58.9%), followed by carcinomas of the papilla of Vater or the duodenum (11.6%), intraductal papillary mucinous neoplasms (IPMN) (8.9%) and cholangiocellular carcinomas (4.5%) (Fig. 3A). DP was increasingly performed minimally invasive over time (0% in 1998 vs 61% in 2018) and more frequently at AVC (401 [27.8%] vs 109 [15.9%]). In contrast, the numbers of minimally invasive PD remained low among all hospitals in the national database (0% in 1998 vs 16% MIPS in 2018, Fig. 3B). Mortality was not significantly lower in AVC (for PD 5.6% vs 6.2% and for DP 1.3% vs 2.0%, Table 1, p = 0.156). There was a trend towards centralisation with fewer hospitals treating more patients and a subsequent increase of AVC (2 in 1998 and 18 in 2018, p < 0.001) while at the same time the number of nAVC was decreasing (36 in 1998 and 17 in 2018, p < 0.001) (Fig. 4A). At the same time, the number of operations performed at AVC increased, while the number of surgeries at nAVC decreased (p = 0.001) (Fig. 4B). After multivariable adjustment, treatment in nAVC versus AVC showed a trend towards higher mortality (OR 1.25 [95% CI 0.98–1.60], p = 0.072) for PD while a lower mortality was found for the most recent years (2013–2018) in comparison with early days of surgical data collection (OR 0.36 [95% CI 0.23–0.57], p < 0.001) (Fig. 5 and supplementary Table 1). There was no significant effect of centre volume or time period on mortality after DP. The characteristics of the cohorts using a volume cut-off of ≥ 20 cases per year to assess the impact of an internationally more accepted minimal case load are shown in supplemental table 2. Low-volume centres (LVC) had a higher mortality compared to high-volume centres (HVC) after PD (OR 1.45 [95% CI 1.15–1.84], p = 0.002) and again no significant differences were found after DP (supplemental table 3).
A Trends in treatment indications for pancreatic resections over two decades. B Trends of the utilisation of minimally invasive pancreatic surgery from 1998 to 2018 stratified by hospital volume AVC vs nAVC (AVC: ≥ 12 cases/year; nAVC: ≤ 12 cases/year). Ca, carcinoma; CCC, cholangiocellular carcinoma; IPMN, intraductal papillary mucinous neoplasm; PDAC, pancreatic ductal adenocarcinoma
Interestingly, the mean Elixhauser Comorbidity Index was higher in the cohort of patients treated in AVC (for PD 16.1 (SD 12.8) vs 11.5 (SD 10.3) p < 0.001 and for DP 10.5 (SD 11.5) vs 8.84 (SD 10.4) p < 0.001) and HVC (for PD 16.3 (SD 12.8) vs 12.5 (SD 11.1) p < 0.001 and for DP 10.8 (SD 11.6) vs 8.96 (SD 10.5) p < 0.001). Complications and reoperation rates were similarly higher in AVC and HVC compared to nAVC and LVC, but mortality rates were lower (Table 1 and supplementary Table 2).
Discussion
This study assessed surgeons’ perception of practice and centralisation of pancreatic surgery in Switzerland, while simultaneously retrospectively assessed PS practice on a comprehensive nationwide level. It reflects the trend indicator of practising pancreatic surgeons in Switzerland and extends the insight into the centralisation of pancreatic surgery in Switzerland over 21 years. The data from the survey supports the agreement on the necessity of a minimal number of annual resections with respect to volume per institution or surgeon. Despite the support for simple caseload metrics, it was pointed out that more sophisticated criteria for the assessment of highly specialised medicine should be introduced [23]. The nationwide analysis from 1998 to 2018 demonstrated an ongoing trend towards further centralisation and decreasing mortality over time as well as better outcomes for PD in AVC. When using a cut-off of ≥ 20 surgeries per year, the effect on lower mortality in these centres was even higher indicating a volume-mortality relationship.
Since Birkmeyer et al. found an inverse correlation between case volume and perioperative mortality, hospital and surgeon volume has become a focus of discussion around quality of health care [1, 2]. While there is strong agreement among survey participants that a minimal number of case volume per hospital should be in place, it was found difficult to define a precise number as an annual requirement. This is reflected by the high variety of minimal numbers in different European health care systems. Minimal numbers lie between 10 (Germany, Austria) and 100 (Denmark) [12]. Increasing case volume improves patient outcome, e.g. through surgeon’s and team experience. Performing a procedure more frequently accumulates experience over the years and thus results in better outcomes [24, 25]. Another contributing factor is that a hospital with a high caseload is better equipped and has multidisciplinary teams involved that are available at all times [26, 27]. In Switzerland and other countries with low annual numbers as requirement, this decision was mainly politically influenced. In Switzerland also by the decentralised federalist organisation of the health care system. Other factors relevant for accreditation are proof of qualified surgical staff, permanent available diagnostic and interventional radiologists as well as endoscopists, qualified intensive care, oncology service, interdisciplinary team meetings, research in the field, training of surgeons, audits and annual reporting. Some of the requirements are structural while others are ill-defined like research activities [13]. Case load remains the most simple and well-defined, easily measurable and comparable parameter.
A recent analysis assessing surgical volume-outcome metric in Switzerland was performed by Güller et al. in 2017 [28]. They evaluated outcomes of different major cancer resections in Switzerland including PS. Compared to centres with a volume > 20 patients/year, lower-volume centres performing PS had a significantly higher in-hospital mortality (5.4 vs 2.0%). In line with their risk-adjusted analysis, this resulted in a decreased odd of postoperative death in higher-volume centres by 68% (OR 0.32, 95% CI 0.11–0.89) [28]. In line with the nationwide findings in this current study including also non-cancer cases, mortality after PD and DP decreased over time and was lower in AVC. When using a stricter threshold, at least 20 cases per year, the better outcome for high-volume centres was confirmed. These findings are in line with results from other population-based studies [9, 14, 21]. Interestingly, AVC and HVC had higher complications rates such as bleeding or insufficiency of pancreatic anastomosis, while vice versa having decreased mortality rates, suggesting a higher success in the rescue of patients in AVC/HVC, and/or hypothetically more complex surgery (e.g. vascular reconstruction etc.).
While for PD, a change in mortality according to hospital volume was shown, the interpretation of this finding remains challenging, however, due to the many interacting factors. Influenced by the change of patient characteristics, getting sicker and presenting with more advanced diseases, treatment in some cases is extremely demanding. For example the surgical procedure for PDAC may differ from the treatment of benign pancreatic diseases due to the need for vascular reconstruction to achieve oncologic radicality. Beneficial or aggravating changes in the tissue caused by neoadjuvant chemotherapy or radiation have been reported [29, 30]. This will continue to challenge us even more in the future, as pancreatic cancer incidence is increasing with about half a million newly diagnosed cases worldwide each year [31]. The trend towards more PS, especially for malignant (PDAC) and premalignant (e.g. IPMN) conditions, is strongly perceived in the survey and supported by the population-based national data.
Certainly, required minimal case numbers for complex surgeries is a popular metric due to simplicity but should not replace a more holistic view on the topic [32]. The pressure of a minimal annual caseload per year might lead to wrong incentives with “pseudo-indications”. In addition, despite a significant centralisation over the last two decades, no improvement in postoperative mortality was found which might reflect that we already reached the bottom of outcome optimisation in open PD supporting high-quality oncologic surgery and perioperative management among those centres that currently perform PS. Wacker and Zwahlen recently confirmed in their analysis including the years 1998 to 2014 that sex and age-adjusted mortality after pancreatic resections remained unchanged over time [33].
Whether the implementation of MIPS also for PD might shift those numbers has to be further investigated. One might expect worse outcomes through MIPS at least temporarily due to the learning curve. As an example, the LEOPARD 2 trial was stopped early due to a fourfold increase in postoperative mortality after laparoscopic PD compared to an open approach. One reason was the early phase of the learning curve [34]. The current study showed that minimally invasive PD has only rarely been performed in Switzerland up to 2018. The robotic approach has meanwhile been adopted or is planned by some centres, hence will possibly become an important factor for outcome interpretation and more specific training programs in centres performing PS.
With the introduction of highly specialised medicine, specifications that must be met for hospitals have been introduced. Currently, there is limited comprehensive outcome data on a national level. The lack of nationwide data on quality metrics was highlighted by Wacker and Zwahlen already [33].
Unfortunately, the granularity of outcome data on a national level in Switzerland is scarce and does not allow to further assess reasons why outcomes after PD do not further improve. The now mandatory reporting obligation of all HSM centres performing PS will make adjustable outcome analysis possible but only after some years. Based on the then acquired insight including surgeon and centre factors associated with patient outcome, a further requirement for providing HSM should be specified. Only then, additional HSM inclusion criteria should be evaluated as patient-focused outcomes should become the mainstay of quality assessment.
Our study has certain limitations. First, the federal database only provided in-hospital mortality data without extension to 30 or 90 days postoperatively and did not report on postoperative complications in detail. Second, there was no use of standardised definitions of postoperative complications (e.g. fistula) available from the database and moreover standardised reporting of pancreas-specific complications was only introduced in 2005 with revisions in 2016 from the ISGPS [35,36,37,38], which is after the starting date of data collection for this study (1998) and a post hoc reconstruction of fistula grading is not feasible given missing information.
Furthermore, the findings of our survey mainly represent the situation in HSM institutions, while only a few surgeons outside these hospitals actively participated despite repeated invitations to participate and the overall response rate was 51%. This might reflect the dissatisfaction of those surgeons with the current HSM allocation and the consequent unwillingness to participate in this study. Even more important, this can cause selection bias in this study as we were mainly able to collect data from pancreatic surgeons who would benefit from further centralisation. However, strict mandates to perform PS within HSM institutions will limit future PS to hospitals that were covered by this survey, making this survey even more relevant. Lastly, we focused on centre volume but there are many other factors that are required for HSM institutions and patient outcome. Systematic reviews of the relationship between outcomes and volumes suggest that for some services at least (for example complex surgery) there is a relationship between the frequency with which the surgeon performs a procedure and quality [39]. There is a strong clinical consensus that higher volumes lead to better patient outcome but, in some cases, there is limited evidence to support this consensus and there remains little evidence on specific volume thresholds [40, 41]. However, potential biases cannot be excluded, for example as the total number of procedures of a centre does not necessarily reflect the number of individual surgeons or that the experience of the interdisciplinary team is not evaluated. Furthermore, a third-party data wrangler (paid by taxpayers/hospitals) should be introduced to produce high-quality, non-biased data. To ascertain quality in the long term in a health care system is very difficult and hopefully the recommendations from the “outcome4medicine” conference will give some answers how to properly assess the impact of any medical intervention from different and broader perspectives [42].
Conclusion
Over the past two decades, centralisation of PS towards higher-volume centres was observed in Switzerland. While the volume of pancreatic resections increased considerably, a trend towards decreasing mortality was found for PD while DP remained with a low mortality. Future regulation of PS should be based on nationwide valid, high-quality data acquisition, favourably done by qualified data managers from third parties. Analysis including long-term quality indicators such as procedure-specific adjusted perioperative morbidity/mortality, which should ideally take oncologic, and patient-reported outcome measurements (PROMs) into account. This is a complex undertaking in which public and personal health can only be assured by utilising an array of rigorous science-based outcome measures.
Data availability
The Swiss Federal Statistical Office’s (BFS, Neuchatel, Switzerland) databases covering the mandatory, nationwide reporting of all stationary hospitalisations (≥ 24 h) in Swiss hospitals starting from 1998 was used for the current analyses. These databases contain anonymised patient-level data including the main diagnosis responsible for hospitalisation and up to 49 secondary diagnoses for comorbidities and complications coded via International Classification of Diseases (ICD-10 German modification) definitions. Procedures are coded by national Swiss surgical classification codes (CHOP), issued annually by the BFS classifying all medical interventions. The national database covering hospitalisations is available from the BFS upon signature of a research and data protection agreement for a fee of 712 Swiss francs. All other data used in this study are freely available under the given links. All codes used for filtering, analyses and graphics are available from the first author upon reasonable request.
Code availability
Not applicable.
Abbreviations
- AVC:
-
Accredited volume centre
- CHOP:
-
Swiss classification of operations
- DP:
-
Distal pancreatectomy
- FSO:
-
Federal Statistical Office of Switzerland
- HSM:
-
Highly specialised medicine
- HVC:
-
High-volume centre
- ICD:
-
International Classification of Diseases
- IPMN:
-
Intraductal papillary mucinous neoplasms
- IQR:
-
Interquartile range
- LVC:
-
Low-volume centre
- MIPS:
-
Minimally invasive pancreatic surgery
- nAVC:
-
Non-accredited volume centre
- PD:
-
Pancreatoduodenectomy
- PROMs:
-
Patient-reported outcome measures
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PS:
-
Pancreatic surgery
References
Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I et al (2002) Hospital volume and surgical mortality in the United States. N Engl J Med 346(15):1128–1137. https://doi.org/10.1056/NEJMsa012337
Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES (1999) Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 125(3):250–256. https://doi.org/10.1016/S0039-6060(99)70234-5
Krautz C, Nimptsch U, Weber GF, Mansky T, Grützmann R (2018) Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann Surg 267(3):411–417. https://doi.org/10.1097/sla.0000000000002248
Ghadban T, Reeh M, Bockhorn M, Grotelueschen R, Bachmann K, Grupp K et al (2019) Decentralized colorectal cancer care in Germany over the last decade is associated with high in-hospital morbidity and mortality. Cancer Manag Res 11:2101–2107. https://doi.org/10.2147/cmar.S197865
Filmann N, Walter D, Schadde E, Bruns C, Keck T, Lang H et al (2019) Mortality after liver surgery in Germany. Br J Surg 106(11):1523–1529. https://doi.org/10.1002/bjs.11236
de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH et al (2012) Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg 99(3):404–410. https://doi.org/10.1002/bjs.8664
Sheetz KH, Dimick JB, Nathan H (2019) Centralization of high-risk cancer surgery within existing hospital systems. J Clin Oncol 37(34):3234–3242. https://doi.org/10.1200/jco.18.02035
van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM et al (2005) Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 242(6):781–788. https://doi.org/10.1097/01.sla.0000188462.00249.36 (discussion 8-90)
Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA (2011) Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg 98(4):485–494. https://doi.org/10.1002/bjs.7413
Polonski A, Izbicki JR, Uzunoglu FG (2019) Centralization of pancreatic surgery in Europe. J Gastrointest Surg Off J Soc Surg Alimentary Tract 23(10):2081–2092. https://doi.org/10.1007/s11605-019-04215-y
Ahola R, Sand J, Laukkarinen J (2020) Centralization of pancreatic surgery improves results: review. Scand J Surg 109(1):4–10. https://doi.org/10.1177/1457496919900411
Vonlanthen R, Lodge P, Barkun JS, Farges O, Rogiers X, Soreide K et al (2018) Toward a consensus on centralization in surgery. Ann Surg 268(5):712–724. https://doi.org/10.1097/sla.0000000000002965
HSM-Fachorgan. Komplexe hochspezialisierte Viszeralchirurgie. Erläuternder Bericht für die Leistungszuteilung.: Konferenz der kantonalen Gesundheitsdirektorinnen und ‑direktoren (GDK); 2019.
Dudekula A, Munigala S, Zureikat AH, Yadav D (2016) Operative trends for pancreatic diseases in the USA: analysis of the nationwide inpatient sample from 1998–2011. J Gastrointest Surg Off J Soc Surg Alimentary Tract 20(4):803–811. https://doi.org/10.1007/s11605-015-3067-x
Liu R, Wakabayashi G, Palanivelu C, Tsung A, Yang K, Goh BKP et al (2019) International consensus statement on robotic pancreatic surgery. Hepatobiliary Surg Nutr 8(4):345–360. https://doi.org/10.21037/hbsn.2019.07.08
Cheng Y, Briarava M, Lai M, Wang X, Tu B, Cheng N et al (2017) Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev 12(9):CD012257. https://doi.org/10.1002/14651858.CD012257.pub2.
Lyu Y, Cheng Y, Wang B, Zhao S, Chen L.(2019) Peritoneal drainage or no drainage after pancreaticoduodenectomy and/or distal pancreatectomy: a meta-analysis and systematic review. Surg Endosc. https://doi.org/10.1007/s00464-019-07293-w.
Maggino L, Malleo G, Salvia R, Bassi C, Vollmer CM Jr (2019) Defining the practice of distal pancreatectomy around the world. HPB (Oxford) 21(10):1277–1287. https://doi.org/10.1016/j.hpb.2019.02.016
Bundesamt für Statistik (2020) Swiss surgical classification codes (CHOP). https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/nomenklaturen/medkk.html. Accessed 12 Oct 2021
Probst P, Hüttner FJ, Meydan Ö, Abu Hilal M, Adham M, Barreto SG et al (2021) Evidence map of pancreatic surgery-a living systematic review with meta-analyses by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 170(5):1517–1524. https://doi.org/10.1016/j.surg.2021.04.023
Kovoor JG, Ma N, Tivey DR, Vandepeer M, Jacobsen JHW, Scarfe A et al (2022) In-hospital survival after pancreatoduodenectomy is greater in high-volume hospitals versus lower-volume hospitals: a meta-analysis. ANZ J Surg 92(1–2):77–85. https://doi.org/10.1111/ans.17293
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36(1):8–27. https://doi.org/10.1097/00005650-199801000-00004
Baum P, Lenzi J, Diers J, Rust C, Eichhorn ME, Taber S et al (2022) Risk-adjusted mortality rates as a quality proxy outperform volume in surgical oncology-a new perspective on hospital centralization using national population-based data. J Clin Oncol 40(10):1041–1050. https://doi.org/10.1200/jco.21.01488
Casciani F, Trudeau MT, Asbun HJ, Ball CG, Bassi C, Behrman SW, et al. (2020) Surgeon experience contributes to improved outcomes in pancreatoduodenectomies at high risk for fistula development. Surgery. https://doi.org/10.1016/j.surg.2020.11.022.
Krautz C, Haase E, Elshafei M, Saeger HD, Distler M, Grützmann R et al (2019) The impact of surgical experience and frequency of practice on perioperative outcomes in pancreatic surgery. BMC Surg 19(1):108. https://doi.org/10.1186/s12893-019-0577-6
Strobel O, Neoptolemos J, Jäger D, Büchler MW (2019) Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 16(1):11–26. https://doi.org/10.1038/s41571-018-0112-1
El Amrani M, Clement G, Lenne X, Farges O, Delpero JR, Theis D et al (2018) Failure-to-rescue in patients undergoing pancreatectomy: is hospital volume a standard for quality improvement programs? Nationwide Analysis of 12,333 Patients. Ann Surg 268(5):799–807. https://doi.org/10.1097/sla.0000000000002945
Güller U, Warschkow R, Ackermann CJ, Schmied B, Cerny T, Ess S (2017) Lower hospital volume is associated with higher mortality after oesophageal, gastric, pancreatic and rectal cancer resection. Swiss Med Wkly. 147:w14473. https://doi.org/10.4414/smw.2017.14473
Cools KS, Sanoff HK, Kim HJ, Yeh JJ, Stitzenberg KB (2018) Impact of neoadjuvant therapy on postoperative outcomes after pancreaticoduodenectomy. J Surg Oncol 118(3):455–462. https://doi.org/10.1002/jso.25183
Marchegiani G, Andrianello S, Nessi C, Sandini M, Maggino L, Malleo G et al (2018) Neoadjuvant therapy versus upfront resection for pancreatic cancer: the actual spectrum and clinical burden of postoperative complications. Ann Surg Oncol 25(3):626–637. https://doi.org/10.1245/s10434-017-6281-9
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Sánchez-Velázquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N et al (2019) Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg 270(2):211–218. https://doi.org/10.1097/sla.0000000000003223
Wacker J, Zwahlen M (2019) Uncertain progress in Swiss perioperative mortality 1998–2014 for 22 operation groups. Swiss Med Wkly 149:w20034. https://doi.org/10.4414/smw.2019.20034
van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG et al (2019) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 4(3):199–207. https://doi.org/10.1016/s2468-1253(19)30004-4
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1):8–13. https://doi.org/10.1016/j.surg.2005.05.001
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M et al (2017) The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161(3):584–591. https://doi.org/10.1016/j.surg.2016.11.014
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142(1):20–25. https://doi.org/10.1016/j.surg.2007.02.001
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142(5):761–768. https://doi.org/10.1016/j.surg.2007.05.005
Halm EA, Lee C, Chassin MR (2002) Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 137(6):511–520. https://doi.org/10.7326/0003-4819-137-6-200209170-00012
Glanville J, Paisley S (2010) Identifying economic evaluations for health technology assessment. Int J Technol Assess Health Care 26(4):436–440. https://doi.org/10.1017/s0266462310000991
Or Z, Renaud T. Is there a relationship between volume of activity and quality of care in France. http://www.irdes.fr/EspaceAnglais/Publications//Qes149.pdf. Accessed 15 Jan 2022
Universtiy Hospital Zurich (2022). Available from: https://www.outcome4medicine.ch/
Acknowledgements
We thank Christian Oberkofler and Philipp Müller, both Department of Surgery and Transplantation, University Hospital Zurich, for their help with the design and distribution of the survey.
Funding
This study was supported by the Swiss Pancreas Foundation.
Author information
Authors and Affiliations
Contributions
Study conception and design: Christoph Kuemmerli, Marcel Schneider, Mathias Worni, Martin Bolli and Dominique Birrer. Acquisition of data: Marcel Schneider, Christoph Kuemmerli and Dominique Birrer. Analysis and interpretation of data: all authors. Drafting of manuscript: Christoph Kuemmerli and Dominique Birrer. Critical revision of manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. According to the cantonal ethics committee (BASEC-Nr. Req-2020–00493) all criteria were met, and the waiver of consent granted.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuemmerli, C., Schneider, M.A., Joliat, GR. et al. Trends in pancreatic surgery in Switzerland: a survey and nationwide analysis over two decades. Langenbecks Arch Surg 407, 3423–3435 (2022). https://doi.org/10.1007/s00423-022-02679-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02679-2