Abstract
Purpose
Parathyroidectomy to treat tertiary hyperparathyroidism (THPT) is now on a par with calcimimetic treatment. The effects of cinacalcet and parathyroidectomy on kidney transplant function remain controversial. The aim of this study was to evaluate kidney transplant function in THPT patients treated either by parathyroidectomy, cinacalcet, or not treated.
Methods
Between 2009 and 2019, 231 patients with functional grafts presenting THPT, defined either by calcaemia superior to 2.5 mmol/L with elevated PTH level or hypercalcaemia with non-adapted PTH level 1 year after kidney transplantation, were included. Hyperparathyroid patients treated by cinacalcet and parathyroidectomy were matched for age, sex, graft rank, and baseline eGFR with cinacalcet-only and untreated patients. Conditional logistic regression models were used to compare eGFR variations 1 year after parathyroidectomy between operated patients and matched controls. Five-year survivals were compared with the Mantel-Cox test.
Results
Eleven patients treated with parathyroidectomy and cinacalcet were matched with 16 patients treated by cinacalcet-only and 29 untreated patients. Demographic characteristics were comparable between groups. Estimated odds ratios for eGFR evolution in operated patients compared with cinacalcet-only and untreated patients were 0.92 [95%CI 0.83–1.02] and 0.99 [0.89–1.10] respectively, indicating no significant impairment of eGFR 1 year after surgery. Five-year allograft survival was not significantly impaired in operated patients.
Conclusions
Parathyroidectomy did not appear to substantially alter or improve graft function 1 year after surgery or 5-year allograft survival. It could be hypothesized that in addition to its known benefits, parathyroidectomy can be safely performed vis-à-vis graft function in tertiary hyperparathyroidism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease with altered glomerular filtration rate (GFR) is often complicated by an adapted increase of serum parathormone (PTH) concentration owing to several metabolic factors including hyperphosphatemia and low calcitriol synthesis [1]. Kidney transplantation restores glomerular filtration rate and proximal tubule function that can correct this secondary hyperparathyroidism (sHPT). However, patients with persisting sHPT may suffer from an autonomous and non-adapted secretion of PTH by parathyroid cells which defines tertiary hyperparathyroidism (THPT). THPT, often revealed by hypercalcaemia with non-adapted PTH concentration [2, 3], may persist in 25 to 50% of kidney recipients and is known to lead to mineral bone disorder such as osteoporosis or brown tumors [4,5,6]. Parathyroidectomy (PTX) is the historical and reference treatment for THPT which is now counterbalanced with calcimimetics (activating directly the calcium-sensing receptors in parathyroid cells) that are widely used in transplanted patients [7].
Although the benefit of PTX and calcimimetics on serum calcium in THPT is well established, their indications are not consensual [8] and their deleterious effects on kidney transplant function remain controversial [9,10,11,12,13,14,15]. However, this effect has only been evaluated in small or non-controlled cohorts. Moreover, the association of PTX and calcimimetics, used in the vast majority of operated patients, has not been well evaluated [15].
We recently published a meta-analysis comparing effects of calcimimetics and PTX on graft function in THPT patients [16]. We showed that neither calcimimetics nor PTX alter or improve graft function. However, these results are limited by a large heterogeneity (defined by Cochran’s Q, P < 0.01) between published studies and the lack of control groups. Heterogeneity was due to a non-consensual definition of THPT, to the variations of surgical indication and of mean time between transplantation and PTX.
The aim of this retrospective study was to evaluate the effects of surgical treatment (PTX) on kidney transplant function in THPT patients receiving a calcimimetic agent, cinacalcet, compared with cinacalcet alone and with untreated THPT patients. In order to limit bias we found in the meta-analysis, patients were matched to compare similar groups.
Methods
Population characteristics
Adult kidney transplant patients followed up at the Nantes University Hospital, France, between January 2002 and May 2018 were included (2377 patients). Data were extracted from the French DIVAT cohort (www.divat.fr, approved by the CNIL, n°914,184). The quality of the DIVAT data bank is validated by an annual cross-center audit. All participants gave informed consent.
In patients with functional grafts (glomerular filtration rate superior to 30 mL/min/1.73 m2), 302 presented persisting THPT 1 year after transplantation, i.e., elevated PTH level (> 65 pg/mL), serum calcium (SCa) level > 2.5 mmol/L or significant hypercalcaemia (> 2.6 mmol/L) with non-adapted PTH level (> 15 pg/mL), or underwent PTX for THPT during the first year after transplantation. Patients with histories of thyroid or parathyroid surgery before transplantation (n = 34), with cinacalcet treatment introduction more than 1 year after transplantation (n = 23) and with missing data on primary outcome (GFR 12 months after transplantation, n = 14) were excluded. The remaining 231 THPT patients were divided into 3 groups: (i) PTX patients treated by cinacalcet followed by PTX (“PTX + cinacalcet” group), (ii) patients treated with cinacalcet only (“cinacalcet-only” group), and (iii) patients presenting THPT with no treatment (“untreated THPT” group). In order to strengthen the comparability between these non-randomized groups, another selection between these patients was performed by matching untreated THPT and cinacalcet-only patients with PTX + cinacalcet patients according to sex, age (± 5 years), kidney graft rank, and baseline estimated GFR (eGFR) (± 10 mL/min/1.73m2). When possible, more than one unexposed patient per exposed patient (patients who underwent PTX) was enrolled (optimal matching).
Cinacalcet was administered before kidney transplantation or within 1 year after transplantation at 30 mg per day and was then adjusted on calcaemia.
Surgical procedures
Indications for PTX were patients displaying persistent THPT despite cinacalcet treatment, persistent high calcium levels threatening kidney grafts (> 2.7 mmol/L), or symptomatic THPT (osteoporosis, neuropsychologic troubles, mental status change, history of renal calculi).
All PTX were performed at the Nantes University Hospital by two trained endocrine surgeons who performed the same procedure: bilateral cervical exploration followed by subtotal PTX (3 glands excised). Otherwise, a less-than-sub-total approach was used with the excision of 1 or 2 glands in case of single adenoma (enlarged gland) or suspected asymmetric hyperplasia. No patient underwent total parathyroidectomy. Patients underwent intra-operative PTH measurement, surgical success being predicted by a 50% serum PTH decrease 15 min after gland excision, independently from the raw serum PTH concentration.
Biological measurements
Serum creatinine, SCa (uncorrected with albumin, normal values: 2.20–2.55 mmol/L), serum phosphate (normal values: 0.81–1.45 mmol/L), and serum PTH level (normal values: 15–65 pg/mL) were all measured in the biochemical laboratory of the Nantes University Hospital using enzymatic methods (serum creatinine, SCa, and serum phosphate) and immunoradiometric assays (serum PTH) yearly after kidney transplantation and PTX. Graft function was estimated by eGFR via the following MDRD equation: 186 × (creatinine (µmol/L) × 0.0113)−1.154 × age−0.203 × 0.742 (if female) × 1.21 (if African origin).
The evolutions of eGFR, SCa, and serum PTH levels were calculated between baseline (the last measured values before PTX in PTX + cinacalcet group, and the measure at the same time after kidney transplantation in the matched patients in cinacalcet-only and untreated THPT groups) and 1 year after PTX or the corresponding time in matched patients (Fig. 1).
Outcomes
The main outcome was the effect of PTX on the evolution of kidney transplant function 1 year after surgery in patients already treated by cinacalcet, compared with cinacalcet-only and untreated THPT.
Secondary outcomes were the evolution of THPT biological parameters (the 1-year variation of SCa and PTH levels 1 year after PTX compared with cinacalcet only and untreated THPT patients with the same definition of baseline as eGFR) and 5-year allograft survival, defined by a functional transplant in a patient being alive.
Statistics
Continuous and categorical variables were described as mean plus standard deviation and as count plus percentage respectively. We used one-way analysis of variance (ANOVA test) to compare means between the groups of interest with respect to the treatment for multiple comparisons; otherwise, a Mann–Whitney test was applied. For frequency distributions between groups, we used Chi-square test with appropriate degrees of freedom or Fisher’s exact test as appropriate. We did not perform any imputations. A p-value < 0.05 was deemed as statistically significant.
In order to estimate the associations between treatments and eGFR evolution, we built conditional logistic regression models to account for pairs of matched patients with the treatment group of interest (PTX and cinacalcet treatment) being the dependent variable to be explained by eGFR evolution between baseline and 1 year of follow-up. Models were adjusted according to the histories of cardiovascular events, diabetes, and dialysis duration before transplantation. Five-year allograft survival was compared between groups using a Mantel-Cox test. Analyses were performed using R version 4.0.1 in particular package survival for conditional logistic regression, tableone for Table 1 preparation and tidyverse for data manipulation and visualization.
Results
Population characteristics
Among the 231 THPT patients, 25 had PTX, 89 were treated by cinacalcet only, and 117 were untreated. Six patients had PTX but no treatment by cinacalcet and were excluded. After matching, four patients who underwent end-stage renal disease (ESRD) within the first year of the follow-up and returned in dialysis were withdrawn from the analysis of the main outcome with no available 1-year eGFR with their numbers comparable between groups (1 from the PTX-cinacalcet group, 1 from the cinacalcet-only group, and 2 untreated THPT patients). Eleven patients with PTX + cinacalcet were matched with 29 untreated THPT patients, and 10 PTX + cinacalcet patients were matched with 16 cinacalcet-only patients (Fig. 2) and were analyzed for the main outcome.
Sex ratio and mean age at the time of transplantation in the PTX + cinacalcet, cinacalcet-only, and untreated-THPT groups were not significantly different (Table 1). The main causes of ESRD were glomerulopathies (12 patients including 7 IgA nephropathies), autosomal dominant polycystic kidney disease (11 patients), and chronic hypertension (10 patients). The mean duration of dialysis before transplantation was significantly lower in the untreated THPT group compared with the PTX + cinacalcet and cinacalcet only groups (P = 0.01). The histories of cardiovascular diseases (coronaropathy and chronic heart failure), graft rank, and number of previous transplantations were not significantly different between groups. The mean cinacalcet dose at baseline in the PTX + cinacalcet group was significantly higher than in the cinacalcet-only group (P = 0.0098).
The presence of donor-specific HLA antibodies 1 year after transplantation was equivalent between groups. Two patients experienced acute cellular rejection needing steroid bolus (1 in the PTX + cinacalcet group and 1 in the untreated THPT group).
At the time of THPT diagnosis (12 months after transplantation), mean SCa, serum phosphorus, and PTH levels were not significantly different between groups.
PTX was performed at a mean of 3 years (range: 0.2–7.1) after kidney transplantation (Table 2). The indications were hypercalcaemia (n = 5), persisting THPT despite cinacalcet treatment (n = 4), or symptomatic (osteoporosis and cognitive impairment THPT, n = 2). Four patients underwent one-gland excision, 3 underwent two-gland excision, and sub-total PTX was performed in 4 patients. The histopathologic results revealed adenoma in 2 cases and hyperplastic glands in 9 cases. Only 1 patient presented postoperative complication: an alternate treatment with Levothyroxine was administered because surgery led to thyroid devascularization.
Effect of THPT treatment on kidney transplant function
Mean baseline eGFR values were extracted at 36 ± 28, 37 ± 24, and 29 ± 27 months after transplantation respectively in the PTX + cinacalcet, cinacalcet-only, and untreated tHPT groups (Table 1).
eGFR variations between baseline and 1 year after PTX (or the corresponding date in matched patients) were compared between PTX + cinacalcet, matched cinacalcet-only, and untreated THPT patients adjusted according to the histories of cardiovascular events, diabetes, and dialysis duration before transplantation.
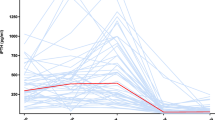
The mean baseline eGFR was not statistically different between the matched groups (Fig. 3). The estimated odds ratio for eGFR evolution between PTX + cinacalcet and untreated THPT patients was OR95%CI = 0.92 [0.83–1.02]. When the eGFR evolution of PTX + cinacalcet and cinacalcet-only groups was compared, the estimated odds ratio was OR95%CI = 0.99 [0.89–1.10].
Comparison of 1-year eGFR, serum calcium, and PTH level variations between PTX + cinacalcet patients and matched controls. Estimated odds ratio for 1-year eGFR, SCa, and PTH variations between PTX + cinacalcet group and matched untreated and cinacalcet-only patients were calculated, indicating no significant variation of these variables 1 year after surgery. PTX, parathyroidectomy; eGFR, estimated glomerular filtration rate; THPT, tertiary hyperparathyroidism
Effect of THPT treatment on SCa and PTH evolution
The 1-year biological evolution of THPT after PTX (the variation of SCa and serum PTH levels between baseline and 1 year after surgery) was compared with matched patients adjusted according to the histories of cardiovascular events, diabetes, and dialysis duration before transplantation (Fig. 3). Mean serum PTH decreased significantly after PTX (Table 1). The estimated odds ratios for SCa increase between PTX + cinacalcet and untreated THPT or cinacalcet-only were respectively OR95%CI = 0.79 [0.37–1.68] and OR95%CI = 0.68 [0.16–2.97]. The estimated odds ratio for serum PTH increase was 1.00 [0.97–1.01] when compared with the untreated patients, and 0.99 [0.99–1.00] when compared with the cinacalcet-only group.
Five-year allograft survival according to THPT treatment
Five-year allograft survival analysis was performed in matched patients with a follow-up of at least 5 years from baseline (n = 8/11, 9/16, 17/29 in the PTX + cinacalcet, cinacalcet-only, and untreated groups respectively) and the 4 patients excluded for the primary outcome analysis owing to ESRD (1 patient from the PTX + cinacalcet group (matched with 1 untreated patient), 1 cinacalcet patient, and 2 untreated THPT patients). A total of 9 PTX + cinacalcet, 10 cinacalcet-only, and 20 untreated THPT were included. The relevant characteristics of these patients are shown in Table 3. Patients in the untreated group tended to be older and have shorter dialysis time before transplantation. During this 5-year follow-up, cardiovascular events were noted in 2/9 PTX + cinacalcet patients (two instable ischemic cardiopathy, one of them leading to angioplasty), 1/10 cinacalcet-only patient (ischemic stroke), and 5/20 untreated patients (three cardiac decompensations due to conduction or rhythmic anomalies and two due to ischemic heart disease including one leading to cardiac arrest). Two out of nine patients in the PTX group displayed persistent THPT 1 year after PTX, and no recurrence was observed after this period. At 5 years, the lowest survival with functional graft rate was in the PTX + cinacalcet group (56%) without significant difference between groups (70% and 80% in the cinacalcet-only and untreated THPT groups respectively) (Fig. 4).
Five-year allograft survival according to THPT treatment. Year 0 corresponds to the time of PTX or the equivalent time from kidney transplantation in matched cinacalcet-only and untreated THPT patients (i.e., baseline). Allograft survivals between groups were compared using Mantel-Cox test. PTX, parathyroidectomy; THPT, tertiary hyperparathyroidism
Discussion
In this observational study, PTX in addition to cinacalcet treatment did not appear to significantly alter or improve graft function 1 year after surgery or 5-year allograft survival, compared with the THPT patients treated with cinacalcet only or untreated.
In 1997, Rostaing et al. suggested for the first time that PTX should alter graft function in PTX patients [17]. Numerous studies have since alternately shown permanent [9, 10, 18] or transitory [12, 19,20,21] impairment of graft function after PTX. However, these studies included limited numbers of patients and the only available control group was composed of transplanted patients without history of THPT which limits interpretation of the results. In the present study, we compared for the first time, patients who underwent PTX with matched patients treated with cinacalcet and untreated patients with THPT.
All of the patients in the PTX group were also treated with cinacalcet. Cinacalcet has been shown to be an effective treatment for THPT [7] and despite the absence of confirmed indication following kidney transplantation, is widely used in these patients [22]. Despite the fact that most THPT patients are currently treated by calcimimetics, including those who undergo PTX, the association of PTX and cinacalcet has not been well studied. In 2019, Finnerty et al. showed in a cohort of 133 patients including 33 PTX that the association of surgical and medical treatment could be beneficial for graft function when compared with cinacalcet alone [15]. However, these promising results were limited by the fact that the values were compared without uniformity concerning the time after kidney transplantation and that most but not all of the PTX patients were also treated with cinacalcet. In the present study, comparability between groups was obtained by matching patients for age, sex, graft rank, and baseline eGFR, which reinforces our conclusions. Patients in the PTX + cinacalcet group were treated with a higher mean cinacalcet daily dose than patients with cinacalcet-only, which is not surprising given that many of them underwent PTX because of cinacalcet failure despite increasing doses. However, graft function was compared at the same time after kidney transplantation in the PTX + cinacalcet patients and the matched cinacalcet and untreated patients, taking into account the evolution of eGFR after transplantation. The present study is the first to use such a comparison between patients to evaluate the effects of PTX on graft function.
We believe that another strength of the present study is the clear definition of THPT. The heterogeneity of results in the literature concerning the evolution of eGFR after PTX is in part due to significant variations in the definition of THPT. Indeed, the definition of THPT includes either hypercalcaemia [9, 10, 21, 23], high serum PTH level associated or not with elevated SCa [24,25,26], or no definition at all [13, 19, 20, 27, 28]. PTH secretion should progressively decrease after kidney transplantation: a first drop of 50% is observed within 3 to 6 months after transplantation [2] and PTH continues to decline progressively thereafter [29, 30]. The required time for diagnosis after transplantation varies from 6 months [24, 25] to 1 year [10]. High PTH level associated with hypercalcaemia, reported in numerous studies, is not sufficient to assure diagnosis: autonomous PTH secretion can be recognized by normal PTH level associated with elevated serum calcium, reflecting the absence of change in response to calcium level [31], although the “normal” PTH level in transplanted patients is not well known and the only means to properly assess the autonomous property of the parathyroid glands are dynamic tests [32]. According to the retrospective nature of the study, the best definition of THPT should be that of an inappropriate PTH level regarding serum calcium level, 6 or 12 months after transplantation. This definition, however, implies that all patients underwent PTX after kidney transplantation and present with functional transplant at the time of diagnosis, limiting our conclusions to this population of patients only. Previously, authors suggested that performing PTX for THPT before kidney transplantation could preserve the graft function [33].
PTX, performed in 0.6 to 5.6% of transplanted patients [3], has been shown to treat THPT efficiently with few complications [34] and increases bone mineral density [35, 36]. Despite these significant results, the surgical indications currently remain unclear [8]. PTX should be discussed in case of severe or persistent hypercalcaemia, severe osteopenia, or hyperparathyroidism-related symptoms (such as fatigue, pruritus, bone pain or fracture, peptic ulcer, mental status changes, or kidney stones) [37]. Surgery for THPT may be total PTX with auto-transplantation [10, 38], sub-total PTX [9, 10, 21, 24, 25], or removal of a single adenoma when identified [9, 21] since THPT may be due to four-gland hyperplasia, asymmetric hyperplasia, or sporadic adenoma [39]. The outcomes of the various surgical procedures have been poorly studied. Regarding graft function, Schlosser et al. suggested in 2007 that total PTX with auto-transplantation may endanger the graft more than sub-total PTX [10] which is supported by the results of two other studies [23, 40] with limited numbers of patients. These results are believed to be due to the rapid serum PTH drop after surgery which exists after all types of PTX and is known to decrease the glomerular filtration rate [41].
In our study, a more conservative treatment was performed, excising only 1 or 2 glands when asymmetrically enlarged glands were identified intraoperatively. Otherwise, subtotal PTX was performed with the excision of 3 glands. This approach significantly decreased post-operative serum PTH, which was lower than in the cinacalcet-only group, as it has been suggested by others [24]. The fact that post-operative serum PTH stayed above the upper limit, although in regard with normal SCa level, suggests participation of secondary hyperparathyroidism, and highlights the fact that “normal” PTH laboratory values should be taken with caution in the context of kidney transplantation.
The principal limitation of our study is the low number of included patients. Only 24 patients were operated for THPT between 2002 and 2018 in our institution which is, however, a tertiary referral center in endocrine surgery. This low number of patients reflects the reluctance to operate when cinacalcet can be proposed as an alternative for the treatment of THPT. However, the long-term effects need to be clarified and the metabolic benefits appear to be more moderate compared with surgery [24]. Only 11 PTX patients were finally included at baseline in order to focus on the comparability between groups which is lacking in the current literature. Obtaining a fairly good comparability involved to restrain the number of included patients.
Patients in the untreated THPT group had significantly shorter dialysis duration before transplantation compared with the other groups. This could mean that these patients are less likely to develop cardiovascular or metabolic conditions that will impair graft function or survival. However, comparisons for the main outcome were adjusted on this variable to limit this bias. Moreover, patients in this group may have milder THPT as higher phosphatemia and similar PTH values than the treated patients at the time of THPT diagnosis could suggest (12 months after transplantation), although the difference did not reach significance. However, these observations may enhance the absence of negative effect of PTX performed on patients with more severe THPT with comparable eGFR evolution 1 year after surgery and 5-year allograft survival.
Finally, bone disorders were not systematically assessed on the present cohort although it potentially provides an argument in the indication for surgery. Compared with cinacalcet, the effects of THPT treatments on bone disorders could be an important benefit for patients. The use of bone mineral density measurement alone in patients with chronic kidney disease being debated [42], its measurement in association with other markers of bone disorders, for example, the trabecular bone score [43], should be extensively studied with rigorous methodology and a precise definition of THPT.
Conclusion
In conclusion, PTX is rarely performed in case of THPT, limiting the possibilities of conducting studies on large cohorts. This observational retrospective study evaluating graft function in THPT patients operated with PTX and treated with cinacalcet matched with control patients treated with cinacalcet-only or untreated, focusing on a high comparability between patients, showed that PTX, associated with calcimimetics, did not appear to substantially alter graft function within 1 year of surgery or 5-year allograft survival.
Data availability
All data and materials are available and comply with field standards.
Code availability
All codes and software applications are available and comply with field standards.
References
Naveh-Many T, Volovelsky O (2020) Parathyroid cell proliferation in secondary hyperparathyroidism of chronic kidney disease. Int J Mol Sci 21(12):4332
Evenepoel P, Claes K, Kuypers D, Maes B, Bammens B, Vanrenterghem Y (2004) Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant 19(5):1281–1287
Evenepoel P (2013) Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol 33(2):191–203
Heaf J, Tvedegaard E, Kanstrup I-L, Fogh-Andersen N (2003) Hyperparathyroidism and long-term bone loss after renal transplantation. Clin Transplant 17(3):268–274
Heaf J, Tvedegaard E, Kanstrup I-L, Fogh-Andersen N (2000) Bone loss after renal transplantation: role of hyperparathyroidism, acidosis, cyclosporine and systemic disease. Clin Transplant 14(5):457–463
Kunzendorf U, Kramer BK, Arns W, Braun J, Grossmann J, Pietruck F et al (2007) Bone disease after renal transplantation. Nephrol Dial Transplant 23(2):450–458
Serra AL, Schwarz AA, Wick FH, Marti H-P, Wüthrich RP (2005) Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism. Nephrol Dial Transplant 20(7):1315–1319
Tang JA, Friedman J, Hwang MS, Salapatas AM, Bonzelaar LB, Friedman M (2017) Parathyroidectomy for tertiary hyperparathyroidism: a systematic review. Am J Otolaryngol sept 38(5):630–635
Evenepoel P, Claes K, Kuypers D, Maes B, Vanrenterghem Y (2005) Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant 20(8):1714–1720
Schlosser K, Endres N, Celik I, Fendrich V, Rothmund M, Fernández ED (2007) Surgical treatment of tertiary hyperparathyroidism: the choice of procedure matters! World J Surg 31(10):1947–1953
Kandil E, Florman S, Alabbas H, Abdullah O, McGee J, Noureldine S et al (2010) Exploring the effect of parathyroidectomy for tertiary hyperparathyroidism after kidney transplantation. Am J Med Sci 339(5):420–424
Parikh S, Nagaraja H, Agarwal A, Samavedi S, Visger SS, Nori U et al (2013) Impact of post-kidney transplant parathyroidectomy on allograft function. Clin Transplant 27(3):397–402
Tseng P-Y, Yang W-C, Yang C-Y, Tarng D-C (2015) Long-term outcomes of parathyroidectomy in kidney transplant recipients with persistent hyperparathyroidism. Kidney Blood Press Res 40(4):386–394
Chudzinski W, Wyrzykowska M, Nazarewski S, Durlik M, Galazka Z (2016) Does the parathyroidectomy endanger the transplanted kidney? Transplant Proc 48(5):1633–1636
Finnerty BM, Chan TW, Jones G et al (2019) parathyroidectomy versus cinacalcet in the management of tertiary hyperparathyroidism: surgery improves renal transplant allograft survival. Surgery 165(1):129–134
Frey S, Goronflot T, Kerleau C, Gourraud P-A, Caillard C, Hourmant M et al (2021) Parathyroidectomy versus cinacalcet: do we still not know the best option for graft function in kidney-transplanted patients? A meta-analysis Surgery 170(3):727–735
Rostaing L, Moreau-Gaudry X, Baron E, Cisterne JM, Monroziès-Bernadet P, Durand D (1997) Changes in blood pressure and renal function following subtotal parathyroidectomy in renal transplant patients presenting with persistent hypercalcemic hyperparathyroidism. Clin Nephrol 47(4):248–255
Lee PP, Schiffmann L, Offermann G, Beige J (2004) Effects of parathyroidectomy on renal allograft survival. Kidney Blood Press Res 27(3):191–196
Evenepoel P, Claes K, Kuypers DR, Debruyne F, Vanrenterghem Y (2007) Parathyroidectomy after successful kidney transplantation: a single centre study. Nephrol Dial Trans 22(6):1730–1737
Meng C, Martins P, Frazão J, Pestana M (2017) Parathyroidectomy in persistent post-transplantation hyperparathyroidism — single-center experience. Transplant Proc 49(4):795–798
Garcia A, Mazuecos A, Garcia T, González P, Ceballos M, Rivero M (2005) Effect of parathyroidectomy on renal graft function. Transplant Proc 37(3):1459–1461
Wüthrich RP, Martin D, Bilezikian JP (2007) The role of calcimimetics in the treatment of hyperparathyroidism. Eur J Clin Invest 37(12):915–922
Park JH, Kang S-W, Jeong JJ, Nam K-H, Chang HS, Chung WY et al (2011) Surgical treatment of tertiary hyperparathyroidism after renal transplantation: a 31-year experience in a single institution. Endoc J 58(10):827–833
Cruzado JM, Moreno P, Torregrosa JV, Taco O, Mast R, Gómez-Vaquero C et al (2016) A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol 27(8):2487–2494
Soliman AR, Maamoun HA, Soliman MA, Darwish H, Elbanna E (2016) Cinacalcet versus parathyroidectomy in the treatment of secondary hyperparathyroidism post renal transplantation. Rom J Intern Med 54(3):184–189
Rayes N (2008) Long-term results of subtotal vs total parathyroidectomy without autotransplantation in kidney transplant recipients. Arch Surg 143(8):756
Ivarsson KM, Akaberi S, Isaksson E, Reihnér E, Rylance R, Prütz KG et al (2015) The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant 30(12):2027–2033
Ferreira GF, de Montenegro FLM, Machado DJ, Ianhez LE, Nahas WC, David-Neto E (2011) Parathyroidectomy after kidney transplantation short-and long-term impact on renal function. Clinics 66(3):431–435
Bonarek H, Merville P, Bonarek M, Moreau K, Morel D, Aparicio M et al (1999) Reduced parathyroid functional mass after successful kidney transplantation. Kidney Int 56(2):642–649
Triponez F, Clark OH, Vanrenthergem Y, Evenepoel P (2008) Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg 248(1):18–30
Copley JB, Wüthrich RP (2011) Therapeutic management of post-kidney transplant hyperparathyroidism: managing post-kidney transplant HPT. Clin Transplant 25(1):24–39
Zhu X, Shan C, Zhu Q, Song L, Zhou Y, Liu J et al (2014) Clinical value of calcium load test in differential diagnosis of different types of hyperparathyroidism. Int J Clin Med 7(12):5445–5452
Jeon HJ, Kim YJ, Kwon HY, Koo TY, Baek SH, Kim HJ et al (2012) Impact of parathyroidectomy on allograft outcomes in kidney transplantation. Transpl Int 25(12):1248–1256
Dulfer RR, Franssen GJH, Hesselink DA, Hoorn EJ, van Eijck CHJ, van Ginhoven TM (2017) Systematic review of surgical and medical treatment for tertiary hyperparathyroidism: Surgical and medical treatment of tertiary hyperparathyroidism. Br J Surg 104(7):804–813
Chou F-F, Hsieh K-C, Chen Y-T, Lee C-T (2008) Parathyroidectomy followed by kidney transplantation can improve bone mineral density in patients with secondary hyperparathyroidism. Transplantation 86(4):554–557
Collaud S, Staub-Zähner T, Trombetti A, Clerici T, Marangon N, Binet I et al (2008) Increase in bone mineral density after successful parathyroidectomy for tertiary hyperparathyroidism after renal transplantation. World J Surg 32(8):1795–1801
Pitt SC, Panneerselvan R, Chen H, Sippel RS (2009) Tertiary hyperparathyroidism: is less than a subtotal resection ever appropriate? A study of long-term outcomes Surgery 146(6):1130–1137
Yamamoto T, Tominaga Y, Okada M, Hiramitsu T, Tsujita M, Goto N et al (2016) Characteristics of persistent hyperparathyroidism after renal transplantation. World J Surg 40(3):600–606
Kilgo MS, Pirsch JD, Warner TF, Starling JR (1998) Tertiary hyperparathyroidism after renal transplantation: surgical strategy. Surgery 124(4):677–684
Jäger MD, Kaaden S, Emmanouilidis N, Lück R, Beckmann JH, Güner Z et al (2011) Effect of incomplete parathyroidectomy preserving entire parathyroid glands on renal graft function. Arch Surg 146(6):704
Schwarz A, Rustien G, Merkel S, Radermacher J, Haller H (2006) Decreased renal transplant function after parathyroidectomy. Nephrol Dial Transplant 22(2):584–591
Pimentel A, Ureña-Torres P, Zillikens MC, Bover J, Cohen-Solal M (2017) Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 92(6):1343–1355
Naylor KL, Prior J, Garg AX, Berger C, Langsetmo L, Adachi JD et al (2016) Trabecular bone score and incident fragility fracture risk in adults with reduced kidney function. Clin J Am Soc Nephrol 11(11):2032–2040
Acknowledgements
DIVAT consortium: Gilles Blancho, Julien Branchereau, Diego Cantarovich, Agnès Chapelet, Jacques Dantal, Clément Deltombe, Lucile Figueres, Claire Garandeau, Magali Giral, Caroline Gourraud-Vercel, Maryvonne Hourmant, Georges Karam, Clarisse Kerleau, Aurélie Meurette, Simon Ville, Christine Kandell, Anne Moreau, Karine Renaudin, Anne Cesbron, Florent Delbos, Alexandre Walencik, Anne Devis, Lucile Amrouche, Dany Anglicheau, Olivier Aubert, Lynda Bererhi, Christophe Legendre, Alexandre Loupy, Frank Martinez, Rébecca Sberro-Soussan, Anne Scemla, Claire Tinel, Julien Zuber, Pascal Eschwege, Luc Frimat, Sophie Girerd, Jacques Hubert, Marc Ladriere, Emmanuelle Laurain, Louis Leblanc, Pierre Lecoanet, Jean-Louis Lemelle, Lionel Badet, Maria Brunet, Fanny Buron, Rémi Cahen, Sameh Daoud, Coralie Fournie, Arnaud Grégoire, Alice Koenig, Charlène Lévi, Emmanuel Morelon, Claire Pouteil-Noble, Thomas Rimmelé, Olivier Thaunat, Sylvie Delmas, Valérie Garrigue, Moglie Le Quintrec, Vincent Pernin, Jean-Emmanuel Serre.
Author information
Authors and Affiliations
Consortia
Contributions
Samuel Frey, Lucile Figueres, and Éric Mirallié participated in study conception, drafting of manuscript, and critical revision of manuscript. Thomas Goronflot, Matthieu Wargny, and Pierre-Antoine Gourraud made the statistical analysis and participated in the acquisition and interpretation of data. Clarisse Kerleau, Claire Blanchard, Cécile Caillard, and Maryvonne Hourmant participated in acquisition of data and critical revision of manuscript.
Corresponding author
Ethics declarations
Ethics approval
The French DIVAT cohort was approved by the CNIL, n°914184.
Consent to participate
All participants gave informed consent.
Consent for publication
All authors approved the last version of the manuscript and gave their consent for publication.
Conflict of interest
PA Gourraud is the founder (2008) (www.methodomics.com) and the co-founder of Wedata (2018) (www.wedata.science). He is consulting for major pharmaceuticals companies all dealt with through academic pipelines (AstraZeneca, Biogen, Boston Scientific, Cook, Edimark, Ellipses, Elsevier, Methodomics, Merck, Mérieux, Sanofi-Genzyme, WeData). He has no prescription activity neither drugs nor devices. The other authors have no conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frey, S., Goronflot, T., Blanchard, C. et al. Impact of parathyroidectomy on kidney graft function in post-transplant tertiary hyperparathyroidism: a comparative study. Langenbecks Arch Surg 407, 2489–2498 (2022). https://doi.org/10.1007/s00423-022-02555-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02555-z