Abstract
Background
Portal vein/superior mesenteric vein (PV/SMV) resection during distal pancreatectomy (DP) is often associated with technical difficulties due to the close anatomic relationship between pancreatic head and PV/SMV. In this paper, we present our operative technique and short-term outcomes of DP combined with venous resection (DP-VR) for left-sided pancreatic cancer (PC).
Methods
We reviewed 368 consecutive cases of DP for PC from January 2013 to December 2018 in our institution, and identified 41 patients (11.1%) who had undergone DP-VR. The remaining 327 DP patients (88.9%) were matched to DP-VR using propensity scores in the proportion of 1:2. Demographics, intraoperative details, postoperative complications and the pathological results were compared between the two groups.
Results
Out of the 41 DP-VR cases, in 14 (34.1%) venous resection with primary closure was performed, while the remaining 27 (65.9%) underwent end-to-end anastomosis without graft. A propensity-score-matched analysis revealed that DP-VR caused an increased risk of postoperative bleeding (17.1% vs. 3.7%, P = 0.016) and delayed gastric emptying (9.8% vs. 1.2%, P = 0.042) compared to standard DP. Overall morbidity (46.3% vs. 36.6%, P = 0.332), postoperative pancreatic fistula (31.7% vs. 26.8%, P = 0.672), R0 resection (58.5% vs. 67.1%, P = 0.223), 30-day reoperation (2.4% vs. 3.7%, P = 0.719), and 90-day mortality (0% vs. 2.5%, P = 0.550) were comparable between the two groups. In postoperative computed tomographic scans of 34 patients (82.9%) at a 90-day follow-up, PV/SMV stenosis was suggested in two patients (5.9%).
Conclusion
Despite the higher rates of postoperative bleeding, DP-VR was found to be a feasible and safe surgery with acceptable postoperative morbidity and mortality compared to standard DP for left-sided pancreatic cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radical resection with negative margin remains the only curative treatment for pancreatic cancer patients. However, only 15–20% of patients with localised tumours are suitable for curative resection [1], since tumours are frequently diagnosed at more advanced stages, and often appear with an involvement of adjacent structures due to delayed symptoms. Tumour infiltration of the portal vein/superior mesenteric vein (PV/SMV) occurs frequently in pancreatic neck cancer due to the close anatomical relationship between these vessels and the tumour. Early experience suggested that extended pancreatectomy with PV/SMV resection led to increased morbidity without any survival benefit [2, 3]. However, with improvements in correct preoperative assessment, perioperative care and surgical techniques, extended surgery with PV/SMV resection and reconstruction is currently an accepted practice in specialised centres.
Pancreaticoduodenectomy combined with venous resection (PD-VR) had been widely performed in selected PC patients with PV/SMV infiltration. Peng et al.’s newly published meta-analyses revealed that PD-VR was associated with a lower R0 resection rate (OR 0.64, 0.55–0.74, P < 0.0001) and a greater risk of certain specific complications that increased the mortality rate (OR 2.02, 1.46–2.79, P < 0.0001) [4]. Similarly, Giovinazzo et al.’s meta-analyses found that mixed pancreatectomies (PD, DP, total and subtotal pancreatectomies) with VR were associated with increased postoperative mortality (RD 0.01, 0.00–0.03, P = 0.02), higher rates of non-radical surgery (RD 0.09, 0.06–0.13, P < 0.001) and a worse 5-year survival rate (HR 3.18, 1.95–5.19, P < 0.001) [5].
Mixed pancreatectomies or PD with VR seemed to increase the risk of complications and mortality, and were related to poor survival. However, DP-VR was rarely performed due to the close anatomic relationship between pancreatic head and PV/SMV. Therefore, the present study aimed to focus on our operative technique and the short-term safety outcomes of DP-VR for left-sided cancer based on our single-centre experience.
Patients and methods
Patient selection
For this study, patients who consecutively underwent DP-VR for left-sided pancreatic cancer (PC) at the First Affiliated Hospital of Nanjing Medical University between January 2013 and December 2018 were identified. Perioperative data was retrospectively collected through a review of the medical records. The study was approved by the institution’s ethics committee.
DP-VR was indicated when tumour invasion to PV/SMV was suspected on a preoperative imaging study or during the operation. Artery invasion is not a contraindication for radical intent DP if sub-adventitial divestment of tumour-invading can be safely applied [6, 7], but distant metastasis was considered an absolute contraindication. Due to the standard oncological practices in China during the study period, no patients received neoadjuvant chemotherapy prior to surgery.
Surgical procedure
Kocher’s manoeuvre was performed as the first step of the procedure to facilitate the manual control of PV during the surgery. In cases of artery invasion by the tumour, the artery-first approach was used to evaluate the resectability before transection of the pancreas neck. The pancreas was frequently transected to the right of the portal vein, close to the gastroduodenal artery (GDA), to ensure a better chance of R0 at the pancreatic neck. If the margin was still positive, as suggested by the frozen section, total pancreatectomy (TP) or sacrifice of the GDA was considered if the celiac artery (CA) could be preserved. Whenever a major artery was transected (splenic artery or CA), a gradient double ligation technique was used, with the first ligation made a little loose to avoid endarterium transection injury, followed by a tight transfixation to fully control the artery stump.
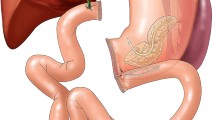
The major branches of PV/SMV (the gastrocolic trunk of Henle, the inferior mesenteric vein, and the coronary vein) and the uncinate process of the pancreas could be divided during the dissection to obtain control of PV/SMV and help reduce tension at the venous anastomosis (Fig. 1). However, the coronary vein had to be preserved for the venous return of the stomach if TP was intended to be performed after evaluation.
A tumour at the pancreatic neck with vein invasion could cause difficulty in PV/SMV resection because body-tail mobilisation and antegrade right-to-left resection was not applicable; therefore, a left-to-right dissection of the distal pancreas and spleen was adopted. The posterior resection plane was kept at the anterior surface of the left renal vein and left kidney while the Gerota’s fascia was resected, similar to the principle of radical antegrade modular pancreatosplenectomy (RAMPS). The left adrenal gland was resected if the tumour had invaded beyond the posterior surface of the pancreas. The left part of the Heidelberg triangle area, together with the left semi-circumference of arterial adventitia at the root of the CA and superior mesenteric artery (SMA), were also resected [8].
The main pancreatic duct at the proximal stump was always suture ligated with 5–0 Prolene, prior to which a retrograde insertion of trans-papillary stent for the main pancreatic duct could be utilised. The ligamentum teres hepatis or the greater omentum was routinely used to cover the pancreatic stump and reduce the risk of significant fistula.
Different styles of resection and reconstruction of PV/SMV were chosen, depending on the extent of tumour invasion. However, pancreatic neck/body tumours usually involve a smaller range of PV/SMV compared to when the disease arises in the head of the pancreas. In our case series, the length of the longest resected vein was four centimetres. A tension-free direct end-to-end anastomosis could usually be performed without graft interposition, relying on the sufficient mobilisation and limited resected length of PV/SMV. Moreover, interposition autologous or prosthetic grafts could be used to bridge the ends of the PV/SMV if the resected vein was too long (> 5 cm).
PV/SMV was anastomosed using a running suture with 5–0 Prolene. Unfractionated heparin was injected into the PV/SMV lumen before the final one or two stitches. Systematic heparin was not routinely used during the operations. In end-to-end anastomosis, after suturing of the anastomosis was completed, we first loosened the distal vascular forceps at the SMV end to allow full expansion of the venous lumen at the anastomosis, and then finished the suture knot-tying.
Definitions
Postoperative mortality was defined as death within 90 days following surgery for any reason. Postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE) and postpancreatectomy haemorrhage (PPH) were defined according to the criteria proposed by the International Study Group of Pancreatic Surgery (ISGPS) [9,10,11,12]. Postoperative complications were categorised according to the Clavien-Dindo classification [13]. The definition of resectability was based on the National Comprehensive Cancer Network’s (NCCN) guidelines [14]. The postoperative surveillance computed tomographic of each patient was evaluated at 30th and 90th day after surgery, and time period for PV/SMV patency was defined as 90 days. Classification of venous resections was also proposed by the ISGPS, including (1) type 1: partial venous excision with direct closure (venorrhaphy) by suture; (2) type 2: partial venous excision using a patch; (3) type 3: segmental resection with primary veno-venous anastomosis; (4) type 4: segmental resection with an interposed venous conduit and at least two anastomoses [15]. Circumferential margins, including anterior surface margin, posterior margin and transection margin at the pancreatic neck, were examined according to the ‘1 mm rule’ to define the R1/R0 status [16]. The American Joint Committee on Cancer (AJCC) 8th edition tumour, node, metastasis (TNM) system was utilised in the tumour staging.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation (s.d.) or median with an interquartile range. Accordingly, a Student t test or a Mann–Whitney U test was used to compare variable distribution between the two groups. Category variables were presented as the frequency (percentage) and were compared with the Chi-square statistic or the Fisher exact test where appropriate. Two-sided p values < 0.05 were used as the threshold for statistical significance. Statistical analyses were performed using the SPSS software version 24.0 (IBM, USA).
Results
The demographics, intraoperative details, postoperative complications and pathological outcomes of the propensity-score-matched cohorts are shown in Table 1. Postoperative complications including total morbidity, POPF, PPH, DGE, PV/SMV stenosis, chylous fistula, intra-abdominal infection, 30-day reoperation, and 90-day mortality were compared (Fig. 2). Results reveal that DP-VR had an increased risk of PPH (17.1% vs. 3.7%, P = 0.016) and DGE (9.8% vs. 1.2%, P = 0.042) compared to standard DP. However, overall morbidity (46.3% vs. 36.6%, P = 0.332), POPF (31.7% vs. 26.8%, P = 0.672), 30-day reoperation (2.4% vs. 3.7%, P = 0.719), 90-day mortality (0% vs. 2.5%, P = 0.550), and Clavien-Dindo ≥ 3 rate (17.1% vs. 13.4%, P = 0.588) were comparable between the two groups. There was no statistically difference between the two groups in R0 resection (58.5% vs. 67.1%, P = 0.233) similarly. No 90-day mortality occurred in the DP-VR group, although it was not possible to follow-up three cases.
In postoperative computed tomographic scans of 34 patients (82.9%, missing data for seven patients) at a 90-day follow-up, PV/SMV stenosis was suggested in two patients (5.9%). No collaterals developed in either case. Both patients with PV/SMV stenosis did not develop any symptoms.
More detailed operative and pathological information of DP-VR is summarised in Table 2. Partial venous resection with primary closure was performed in 14 patients (34.1%) (type 1), while segmental venous resection with end-to-end anastomosis was performed in the remaining 27 (65.9%) (type 3). Two cases (4.9%) were preoperatively classified as locally advanced due to SMA encasement (tumour contact > 180°). Sub-adventitial divestment of major arteries (CA, CHA, SMA) was performed in 13 patients (37.1%). Of the seven PPH cases (17.1%), one (2.4%) was early PPH (occurring < 24 h after index pancreatic resection) and six (14.6%) were late PPH. One patient (2.4%) underwent reoperation due to POPF and PPH on the 10th day after primary surgery. The overall R0 resection rate was 58.5%, while R1 margin was seen in 17 patients (41.5%)—12 (29.3%) with R1-1 mm margin and five (12.2%) with R1-direct. R1-1 mm margin included nine cases (22.0%) of pancreatic neck margin, nine cases (22.0%) of anterior surface margin, and seven cases (17.1%) of posterior margin. R1-direct margin included one case (2.4%) of pancreatic neck margin, three cases (7.3%) of anterior surface, and one case (2.4%) of posterior surface margin.
Discussion
In contrast to PD-VR, tension-free venous reconstruction is more challenging in DP-VR. The whole vein, from SMV to PV, is slanted across the surface of the uncinate process of the pancreas, finally entering the right rear hepatic portal. After resection of the body and tail of the pancreas, the attachment of the residual pancreatic head on the right side of the PV/SMV and extrusion of the uncinate process on the posterior side of the pancreas will both increase the anastomotic tension and possibility of anastomotic stenosis. Moreover, PV/SMV is easily restricted by the duodenum mesenteric vein and its branches, resulting in increased difficulty during the dissection and anastomosis, such as the inferior anterior and posterior pancreaticoduodenal veins and the first jejunal branch and its branches. However, there was no graft interposition in our study. From our experience, pancreatic neck/body tumours usually involve a smaller range of PV/SMV compared to the range when the disease arises in the head of the pancreas. The length of the longest resected vein was four centimetres in our study. The major branches of PV/SMV (i.e. the gastrocolic trunk of Henle, inferior mesenteric vein, and coronary vein) could be divided during the dissection to help reduce tension at the venous anastomosis. Furthermore, the combined uncinate process resection could effectively reduce the anastomotic tension. In addition, liver mobilisation and Cattell–Braasch manoeuvre (utility of mobilisation of the right colon and the root of the mesentery) [17] could also be performed in DP-VR to reduce the tension. Due to the aforementioned factors, a tension-free, direct, end-to-end anastomosis could typically be performed without graft interposition. However, if the resected vein is too long, interposition with autologous or prosthetic grafts could bridge the ends of the PV/SMV, or even lead to considering total pancreatectomy.
Due to more advanced disease, PD-VR seemed to have a higher risk of complications and mortality, and worse long-term survival than the standard procedure for PC (4, 5). However, better survival benefits were obtainable from PD-VR compared to palliative treatment.
Similarly, DP-VR also had an increased risk of PPH (17.1% vs. 3.7%, P = 0.016) compared to standard DP, both in our cohort and in the literature report (17.1% vs. 2.0–8.6%) [18,19,20,21,22,23]. However, in our study, DP-VR was associated with comparable rates of total morbidity (46.3% vs. 36.6%, P = 0.332), POPF (31.7% vs. 26.8%, P = 0.672), 30-day reoperation (2.4% vs. 3.7%, P = 0.719), and 90-day mortality (0% vs. 2.5%, P = 0.550) compared to standard DP, which is also in line with previously published studies on POPF (18.0–27.7%), reoperation rates (7.0–7.2%) and mortality rates (0–4.0%) with standard DP [18,19,20,21,22,23]. Further analysis found that six out of seven cases (85.7%) of PPH occurred in the late phase, and this, combined with POPF—indicating secondary haemorrhage resulting from frequent vascular skeletonization in our cohort combined with possible POPF/intra-abdominal infection—may explain the higher risk of PPH in the current case series. One PPH case that occurred in the early phase may be directly related to the surgical procedure. Two technical factors may be helpful in lowering the PPH rate in the early phase. Firstly, if the transection line is close to the GDA and the GDA needs to be mobilised, it is recommended that the branches of the GDA are secured via ligation instead of with a surgical energy device. The second technical point is the so-called gradient double ligation technique, which, in our experience, can lower the risk of pseudoaneurysm formation after surgery, especially in older patients with arteriosclerosis.
It is also worth mentioning that although the POPF rate after DP-VR seemed to be similar to that after standard DP, the technical challenges in pancreatic remnant closure increased, with POPF risk arising if the transection line shifted to the right side of the PV/SMV due to the neck/body location of the tumour. According to the newly published ISGPS consensus, there is no difference in the POPF rate after DP between the hand-sewn and stapler techniques [24]. However, the worry about the positive margin, particularly when PV/SMV was involved, may have limited the use of the stapling technique in DP for neck/body pancreatic cancer.
Another key finding was that PV/SMV stenosis occurred at a lower rate (5.9%, two out of 34 patients) in our study than previously reported (9.0–17.8%) [25,26,27]. Multiple factors may have contributed to this high patency rate: (i) good surgical techniques to avoid narrowing, kinking, axial rotation and excessive tension at the anastomosis; (ii) complete mobilisation of PV/SMV to avoid the use of interposition prosthetic graft; (iii) aggressive prophylactic anti-thrombosis therapy after surgery with 40–80 mg LMWH per day starting on POD1-3; and (iv) lower risk of venous thrombosis in Asians due to genetic disparity among ethnicities [28]. The two patients with PV/SMV stenosis did not develop any symptoms in our study. The research on hepatobiliary pancreatic surgery showed that percutaneous transhepatic stent placement for PV stenosis was effective, and the stent patency was satisfactory during the follow-up period [29,30,31]. However, from our practical experience, the majority of PV/SMV stenosis cases displayed no symptoms and often did not require interventions, except suffering from refractory symptoms due to portal hypertension, since this procedure is invasive and potentially dangerous in terms of the risk of vascular injury and bile leakage.
Real R0 resection with strict standards (including examining circumferential margins and the ‘1 mm rule’) is associated with improved long-term survival in pancreatic cancer patients [1]. The R1 resection rate in the current cohort was 17 (41.5%), with five cases of R1-direct and 12 cases of R1–1 mm, which is comparable with historical data from the literature [18, 23]. In our centre, in order to improve R0 resection after surgery, retroperitoneal lymph nodes and connective tissues (including the nerve plexus surrounding major arteries) were removed en-bloc with the tumour, in accordance with the oncologic principles proposed in the ‘RAMPS’ [32] and ‘TRIANGLE’ procedures [8]. Neoadjuvant therapy (NAT) can increase the chance of obtaining R0 resection after radical pancreatectomy; although the benefits of NAT in patients with borderline resectable pancreatic cancer (BRPC) have been confirmed in many studies [33,34,35], few oncological results after NAT in BRPC with isolated venous vascular involvement or with venous resection have been reported. The only reported study showed that surgery following NAT had a higher R0 resection rate and superior disease-free survival (DFS) [36]. High-quality research is warranted to confirm the value of NAT in VR patients, and our techniques of DP-VR should also be tested in NAT cases.
Few studies on minimally invasive DP (MIDP) with VR have been reported. Only Giulianotti et al. described one case of robot-assisted DP with PV resection [37]. However, given that standard MIDP has been performed increasingly with evidence of minimally invasive benefits, and some initial results showed that minimally invasive PD (MIPD) with VR could be performed with similar short- and long-term outcomes compared to open PD with vein resection, we believe that MIDP with VR would be safe and feasible for patients with pancreatic malignancy with vein invasion.
There are still several limitations in our current study. First, the fact that no patients received neoadjuvant therapy in the study may have influenced the oncological results. Second, there was no detailed pathological information regarding resected PV/SMV, such as whether the tumour invaded it, or the length of the tumour invasion. Lastly, it was not possible to radiographically follow up seven patients (17.1%). This may have caused substantial bias in the venous patency rate.
In conclusion, despite the higher rates of PPH, DP-VR was found to be a feasible and safe surgery with acceptable perioperative morbidity and mortality compared to standard DP for left-sided pancreatic cancer.
References
Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK et al (2019) Pancreatic adenocarcinoma, Version 1 2019. J Natl Compr Canc Netw 17(3):202–10
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK et al (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236(3):355–66 (discussion 66-8)
Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J et al (2005) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma–part 3: update on 5-year survival. J Gastrointest Surg 9(9):1191–204 (discussion 204-6)
Peng C, Zhou D, Meng L, Cao Y, Zhang H, Pan Z et al (2019) The value of combined vein resection in pancreaticoduodenectomy for pancreatic head carcinoma: a meta-analysis. BMC Surg 19(1):84
Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I (2016) Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 103(3):179–191
Miao Y, Jiang K, Cai B, Yin L, Dai C (2017) Artery divestment for artery involved pancreatic cancer: a retrospective study. Pancreatology 17(4):S25–S26
Cai B, Lu Z, Jiang K, Wu J, Gao W, Chen J et al (2018) AB081. P053 survival of unresectable pancreatic cancer patients after artery divestment combined pancreatectomy: a retrospective and propensity score-matched analysis. Ann Pancreat Cancer 1(1):AB081-AB
Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Muller-Stich B et al (2017) The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford) 19(11):1001–1007
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161(3):584–591
Panwar R, Pal S (2017) The International Study Group of Pancreatic Surgery definition of delayed gastric emptying and the effects of various surgical modifications on the occurrence of delayed gastric emptying after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int 16(4):353–363
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142(1):20–25
Hartwig W, Vollmer CM, Fingerhut A, Yeo CJ, Neoptolemos JP, Adham M et al (2014) Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 156(1):1–14
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
NCCN clinical practice guidelines in oncology - Pancretic adenocarcinoma. Version 1.2020 — November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA et al (2014) Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 155(6):977–988
The Royal College of Pathologists (2002) Standards and mini- mum datasets for reporting cancers. Minimum dataset for the histopathological reporting of pancreatic, ampulla of Vater and bile duct carcinoma. The Royal College of Pathologists, London
Fujisaki S, Tomita R, Fukuzawa M (2001) Utility of mobilization of the right colon and the root of the mesentery for avoiding vein grafting during reconstruction of the portal vein. J Am Coll Surg 193(5):576–578
de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F et al (2019) Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg 269(1):2–9
Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G et al (2011) Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 377(9776):1514–1522
Hassenpflug M, Hinz U, Strobel O, Volpert J, Knebel P, Diener MK et al (2016) Teres ligament patch reduces relevant morbidity after distal pancreatectomy (the DISCOVER Randomized Controlled Trial). Ann Surg 264(5):723–730
Kondo N, Uemura K, Nakagawa N, Okada K, Kuroda S, Sudo T et al (2019) A multicenter, randomized, controlled trial comparing reinforced staplers with bare staplers during distal pancreatectomy (HiSCO-07 Trial). Ann Surg Oncol 26(5):1519–1527
Van Buren G 2nd, Bloomston M, Schmidt CR, Behrman SW, Zyromski NJ, Ball CG et al (2017) A prospective randomized multicenter trial of distal pancreatectomy with and without routine intraperitoneal drainage. Ann Surg 266(3):421–31
van Hilst J, de Rooij T, Klompmaker S, Rawashdeh M, Aleotti F, Al-Sarireh B et al (2019) Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a Pan-European propensity score matched study. Ann Surg 269(1):10–17
Miao Y, Lu Z, Yeo CJ, Vollmer CM Jr, Fernandez-Del Castillo C, Ghaneh P et al (2020) Management of the pancreatic transection plane after left (distal) pancreatectomy: expert consensus guidelines by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 168(1):72–84
Dua MM, Tran TB, Klausner J, Hwa KJ, Poultsides GA, Norton JA et al (2015) Pancreatectomy with vein reconstruction: technique matters. HPB (Oxford) 17(9):824–831
Krepline AN, Christians KK, Duelge K, Mahmoud A, Ritch P, George B et al (2014) Patency rates of portal vein/superior mesenteric vein reconstruction after pancreatectomy for pancreatic cancer. J Gastrointest Surg 18(11):2016–2025
Sgroi MD, Narayan RR, Lane JS, Demirjian A, Kabutey NK, Fujitani RM et al (2015) Vascular reconstruction plays an important role in the treatment of pancreatic adenocarcinoma. J Vasc Surg 61(2):475–480
Huang SS, Liu Y, Jing ZC, Wang XJ, Mao YM (2016) Common genetic risk factors of venous thromboembolism in Western and Asian populations. Genet Mol Res 15(1):15017644
Hiyoshi M, Fujii Y, Kondo K, Imamura N, Nagano M, Ohuchida J (2015) Stent Placement for portal vein stenosis after pancreaticoduodenectomy. World J Surg 39(9):2315–2322
Kim KR, Ko GY, Sung KB, Yoon HK (2011) Percutaneous transhepatic stent placement in the management of portal venous stenosis after curative surgery for pancreatic and biliary neoplasms. AJR Am J Roentgenol 196(4):W446–W450
Zhou ZQ, Lee JH, Song KB, Hwang JW, Kim SC, Lee YJ et al (2014) Clinical usefulness of portal venous stent in hepatobiliary pancreatic cancers. ANZ J Surg 84(5):346–352
Strasberg SM, Drebin JA, Linehan D (2003) Radical antegrade modular pancreatosplenectomy. Surgery 133(5):521–527
Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH et al (2018) Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 268(2):215–222
Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC et al (2018) Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol 4(7):963–969
Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG et al (2018) Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 105(8):946–958
Lee JH, Kang CM, Bang SM, Choi JY, Seong JS, Hwang HK et al (2015) The Role of neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic cancer with isolated venous vascular involvement. Medicine (Baltimore) 94(31):e1233
Giulianotti PC, Addeo P, Buchs NC, Ayloo SM, Bianco FM (2011) Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 40(8):1264–1270
Funding
This study was funded by the National Natural Science Foundation of China (grant number 82072706/ 81871980); the National Science Foundation for Young Scientists of China (grant number 81703301).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, D., Wu, P., Zhang, K. et al. The short-term outcomes of distal pancreatectomy with portal vein/superior mesenteric vein resection. Langenbecks Arch Surg 407, 2161–2168 (2022). https://doi.org/10.1007/s00423-021-02382-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02382-8