Abstract
Purpose
Postoperative infectious complications have a negative impact on survival outcomes in patients with gastric cancer. It is recently reported that preoperative chemotherapy may eliminate this negative impact. This study aimed to confirm whether preoperative chemotherapy can eliminate the negative impact of postoperative infectious complications (IC) on survival outcomes and elucidate the association between postoperative infectious complications and recurrence patterns.
Methods
We retrospectively reviewed data of 86 patients who received preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by R0 gastrectomy at the Kitasato University between 2006 and 2016. Patients who developed grade II or higher infectious complications during hospitalization were grouped into the IC group, while others were grouped into the non-IC (NIC) group. Survival outcomes and recurrence patterns were analyzed between the two groups.
Results
Infectious complications with Clavien-Dindo classification of grade II or higher were found in 12 patients (14.0%, IC group). The median observational period was 61 months. Overall survival and progression-free survival were similar in the IC and NIC groups. Recurrence occurred in 39 patients. The proportions of peritoneal and lymph node recurrences were not significantly different between the two groups. However, the proportion of distant metastasis in the IC group was significantly higher than that in NIC group (3/4 [75%] vs. 9/35 [17%], p = 0.04).
Conclusions
Pathological stage after neoadjuvant therapy plays a stronger role in recurrence than postoperative complications. Lymph node and peritoneal metastasis may be suppressed by preoperative chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide [1]. Surgical resection remains the primary treatment for resectable gastric cancer.
The negative impact of postoperative infectious complications (ICs) on survival outcomes is reported in patients with gastric cancer [2, 3], colorectal cancer [4, 5], and esophageal cancer [6, 7].
In Japan, based on the results of clinical trials [8, 9], surgical resection and postoperative chemotherapy are regarded as the standard treatment for pStage II/III gastric cancers. However, perioperative chemotherapy using fluorouracil plus leucovorin, oxaliplatin, and docetaxel (FLOT) demonstrated better survival rates than other chemotherapy regimens [10], and became the standard treatment for resectable advanced gastric cancer in Western countries. The Japan Clinical Oncology Group (JCOG) is currently conducting phase III trial to demonstrate the effect of perioperative chemotherapy against current standard treatment for postoperative chemotherapy (JCOG1509) [11].
Two retrospective studies have demonstrated that treatment with preoperative chemotherapy eliminates the negative impact of postoperative ICs on the survival outcomes in patients with gastric cancer [12, 13]. However, recurrence patterns have not been analyzed in association with postoperative ICs after preoperative chemotherapy.
Therefore, we aimed to confirm the elimination effect of preoperative chemotherapy on the negative impact of postoperative ICs, and to clarify the association between postoperative ICs and recurrence pattern in patients with gastric cancer who underwent preoperative chemotherapy followed by surgical resection.
Methods
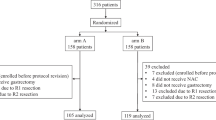
A total of 103 patients received preoperative chemotherapy plus gastrectomy between October 2006 and April 2016 at the Kitasato University. Of these, 86 who underwent R0 surgical resection were enrolled in this study. Of the 86 patients, 54 received preoperative chemotherapy as neoadjuvant chemotherapy (NAC). The reasons for administering NAC were as follows: type 4 or large type 3 (n = 32), bulky N (one lymph node larger than 3 cm or two lymph nodes larger than 1.5 cm along the celiac, splenic, common, or proper hepatic arteries; n = 12), esophageal invasion (n = 5), duodenal invasion (n = 2), and cT3/4 and cN+ (n = 3). NAC for type 4 or large type 3 or bulky N was administered as part of a clinical trial [14, 15]. The other patients received NAC as a practical treatment after providing written informed consent. Of the 86 patients, 32 initially had unresectable advanced gastric cancer that transformed to resectable cancer after administering chemotherapy. The reasons for unresectable cancer were as follows: paraaortic lymph node metastasis (n = 12), extra-regional lymph node metastasis (n = 3), peritoneal dissemination (n = 6), peritoneal lavage cytology positive (n = 4), liver metastasis (n = 4), and pancreatic head invasion (n = 3) (Fig. 1).
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the Kitasato University School of Medicine, Japan. All study participants provided written informed consent for personal and medical data collection prior to enrollment in the study.
Chemotherapy and surgery
All the included patients received a DCS (docetaxel/cisplatin/S-1) regimen as preoperative chemotherapy. In the DCS regimen, docetaxel (40 mg/m2) and cisplatin (60 mg/m2) were administered on day 1, while S-1 (40 mg/m2) was administered orally twice daily for 2 weeks, followed by a 2-week rest [16]. In the NAC setting, four cycles of DCS were administered in the patients; while in the conversion setting, a maximum of six cycles of DCS were administered in the patients. Patients without disease progression were then administered a combination of docetaxel and S-1. The decision to perform resection was made by experienced medical oncologists, radiologists, and surgeons at a multidisciplinary meeting [17].
In terms of surgical resection, distal gastrectomy or total gastrectomy with D2 lymphadenectomy was performed for R0 resection. When the tumor invaded adjacent organs, concomitant resection was performed to achieve R0 resection. Paraaortic node dissection was performed in patients with visible paraaortic nodes in computed tomography (CT) images after preoperative chemotherapy, while it was not performed in those without visible paraaortic nodes after preoperative chemotherapy. Splenectomy was performed with D2 dissection to achieve R0 resection, if necessary.
The pathological response was graded according to the Japanese classification of gastric carcinoma, third English edition [18], as follows: grade 1a, viable tumor cells occupy > two-thirds of the tumor area; grade 1b, > one-third but < two-thirds; grade 2, < one-third; grade 3, no viable tumor cells. In this study, the pathological response was defined as grade 1b to grade 3.
Postoperative adjuvant chemotherapy with S-1 was administered according to response to preoperative chemotherapy and postoperative performance status.
Postoperative complications
Postoperative complications were defined according to the Clavien–Dindo classification [19, 20]. Patients who developed grade II or higher ICs during hospitalization were grouped into the IC group; rest others were grouped into the NIC group. Those who developed grade III or higher ICs during hospitalization were grouped into the IC (III) group; rest others were grouped into the NIC (III) group. The ICs were defined as pancreatic fistula, anastomotic leakage, duodenal stump leakage, abdominal abscess, and pneumonia.
Statistics
Progression-free survival (PFS) was measured from the date of chemotherapy initiation in patients who received preoperative chemotherapy to the first date of disease progression and was censored on the last follow-up for a progression-free surviving patient. The causes of deaths were confirmed from hospital medical records. Patients who died from causes other than gastric cancer were regarded as censored at the time of death.
Student’s t test was used to analyze continuous variables, and the chi-square test or Fisher’s exact test was used to analyze categorical variables. Patient survival was calculated using the Kaplan–Meier method. Univariate analyses of prognostic factors for overall survival (OS) and PFS were performed using log-rank tests. Factors with p < 0.05 in univariate analyses were subjected to multivariate analysis using the Cox proportional-hazards model to identify independent prognostic factors. All calculations were performed using JMP Pro Version 14.0 (SAS Institute Inc., Cary, NC, USA). p < 0.05 was considered statistically significant.
Results
Patient demographics
Eighty-six patients were included in the current study. ICs with Clavien-Dindo grade II or higher were found in 12 patients (14.0%, IC group). Table 1 shows the patient demographics. Age, gender, American Society of Anesthesiologists (ASA) status, rate of bulky N/no.16 lymph node (LN) metastasis, rate of macroscopic large type 3/type 4, number of DCS cycles, and cStage were not significantly different between the IC and NIC groups. A middle location was more frequent in the IC than in NIC group (7/12 [58.3%] vs. 20/74 [27.0%]), while a lower location was more frequent in the NIC than in IC group (19/74 [25.7%] vs. 0/12 [0%]).
Table 2 shows comparison of intra- and postoperative factors between the IC and NIC groups. Operative time and estimated blood loss were not significantly different between the IC and NIC groups. Proximal gastrectomy plus lower esophagectomy with gastric tube reconstruction was performed in one patient with esophageal invasion of 5 cm. Pancreaticoduodenectomy was performed in one patient with metastatic lymph nodes invading the head of the pancreas. Splenectomy was not associated with ICs in this cohort. The highest postoperative C-reactive protein (CRP) level in the IC group was significantly higher than that in NIC group (18.7 ± 8.9 mg/dL vs. 9.6 ± 4.4 mg/dL, p < 0.001).
Postoperative complications
Table 3 shows the details of postoperative complications. Details of postoperative ICs were as follows: pancreatic fistula (n = 7), abdominal abscess (n = 1), duodenal stump fistula (n = 2), and pneumonia (n = 2). No postoperative mortality was recorded during the study period.
Survival analysis
The median observational period was 61 months. OS was better in the IC group than in NIC group, with 5-year OS rates of 91.7% and 53.2%, respectively; however, this difference was statistically insignificant (p = 0.17) (Fig. 2a). When we set the cutoff for IC as Clavien–Dindo grade III, OS was also better in the IC (III) group than in NIC (III) group, with 5-year OS rates of 86% and 57%, respectively; but, this difference was also statistically insignificant (p = 0.43) (Supplementary Fig. 1a). In the univariate analysis, ypT and ypN showed p < 0.05. Grade II or higher inflammatory complications were not selected as potential prognostic factors for OS. Multivariate Cox analysis showed that ypT3/4 (hazard ratio [HR] 3.49, 95% confidence interval [CI] 1.31–12.11, p = 0.010) and ypN1/2/3 (HR 2.97, 95% CI 1.33–7.58, p = 0.007) were significant independent risk factors for OS (Table 4).
PFS was better in the IC group than in NIC group with a 5-year PFS rate of 75.0% and 49.3%, respectively; however, this difference was statistically insignificant (p = 0.26) (Fig. 2b). When we set the cutoff for IC as Clavien–Dindo grade III, PFS was also better in the IC (III) group than in NIC (III) group with 5-year PFS rates of 71% and 52%, respectively; but, this difference was statistically insignificant (p = 0.29) (Supplementary Fig. 1b). In the univariate analysis, gender, tumor location, ypT, and ypN showed p < 0.05. Grade II or higher inflammatory complications were not selected as potential prognostic factors for PFS. Multivariate Cox analysis showed that upper tumor location (HR 2.24, 95% CI 1.17–4.39, p = 0.015), ypT3/4 (HR 3.39, 95% CI 1.27–11.78, p = 0.012), and ypN1/2/3 (HR 2.36, 95% CI 1.12–5.49, p = 0.023) were significant independent risk factors for PFS (Table 5).
Recurrence pattern
Cancer recurrence was found in 39 patients (35 in the NIC group and 4 in the IC group). When we set the cutoff value for IC as Clavien–Dindo grade III, cancer recurrence was found in 37 and two patients in the NIC (III) and IC (III) group, respectively. The recurrence patterns are listed in Table 6 and Supplementary Table 1. The peritoneum was the most frequent site for recurrence. The proportions of each recurrent site were compared between the IC and NIC groups, and between the IC (III) and NIC (III) groups. The proportions of peritoneal and lymph node recurrence were not significantly different between these groups (Fig. 3a, b; Supplementary Fig. 2a, 2b). However, the proportion of distant metastasis in the IC group was significantly higher than that in NIC group (3/4 [75%] vs. 9/35 [26%], p = 0.04) (Fig. 3c); and it was also higher in the IC (III) group than in NIC (III) group (2/2 [100%] vs. 10/37 [27%], p = 0.089), but without statistical significance (Supplementary Fig. 2c).
Comparison of the proportion of metastasis at each site between the IC and NIC groups. IC group includes patients with grade II or higher infectious complications, while NIC group includes those without grade II or higher infectious complications. PER peritoneal dissemination, LNM lymph-node metastasis, DM distant metastasis
Discussion
The current study did not demonstrate that postoperative IC had a negative impact on OS and PFS in patients with gastric cancer who underwent DCS chemotherapy followed by surgical resection. However, we demonstrated that the upper location is an independent poor prognostic factor for PFS. Additionally, the proportion of distant metastasis was significantly higher in the IC group than in NIC group.
First, postoperative IC had no negative impact on the OS and PFS in patients with gastric cancer who underwent preoperative DCS chemotherapy followed by surgical resection. This finding is consistent with that in previous reports [12, 13]. Inflammatory responses to severe postoperative complications are associated with host immunosuppression [21, 22]. Thus, due to immunosuppression caused by ICs, residual tumor cells may become clinically evident to be diagnosed as cancer recurrence. Preoperative chemotherapy has the potential to eradicate micrometastatic cancer cells. Additionally, according to Zingoni et al., many chemotherapy-induced stress pathways modulate the expression of NK cell-activating and inhibitory ligands, and increase the immunogenicity of tumor cells [23]. These mechanisms may be involved in the prophylactic effects of preoperative chemotherapy even in cases with postoperative ICs.
OS and PFS were better in the IC group than in NIC group. However, these differences in survival failed to reach statistical significance due to low patient numbers. This difference may be attributed to the difference in the incidence of type 4 cancer. We previously reported that administration of neoadjuvant chemotherapy with DCS for type 4 gastric cancer is not as effective as that for other types of gastric cancer [15]. The proportion of macroscopic type 4 was higher in the NIC group than in IC group. This may be one of the reasons for the better OS and PFS in the IC group than in NIC group.
Second, the upper location of the tumor was selected as one of the independent poor prognostic factors for PFS. The upper third gastric cancer is reported to have worse prognosis than that in middle or lower third gastric cancer [24, 25]. The current study also demonstrated worst prognosis in patients with gastric cancer in the upper third location independent of the status of the well-known prognostic factors—pT and pN. Wang et al. speculated that as the cardia and fundus are incompletely covered by the visceral peritoneum, the upper third gastric cancer may increasingly tend to infiltrate the serosa and show peritoneal metastasis than distal gastric cancer [25]. The exact reason for this remains unclear, but cases with the upper third gastric cancer should be carefully followed even after preoperative chemotherapy.
Third, the proportion of distant metastasis was significantly higher in the IC group than in NIC group, indicating that preoperative chemotherapy cannot completely suppress formation of micrometastases. This raises a question: What if preoperative chemotherapy destroyed most of the micrometastases in the lymph nodes and peritoneum, but less frequently distant micrometastatic cells? When complications occur, distant micrometastatic cells can proliferate, and lead to recurrence of distant metastases. When no complications occur, distant micrometastatic cancer cells that survive after preoperative chemotherapy may become more immunogenic, and thus, can finally be destroyed by the host immune system. This may explain why the proportion of distant metastasis was significantly higher in the IC group than in NIC group. Moreover, the ACTS-GC trial demonstrated that adjuvant chemotherapy with S-1 significantly suppressed recurrence in the lymph nodes and peritoneum, but not the hematogenous recurrence [9]. The detailed mechanism is unknown, but eliminating the negative impact of postoperative complications may act more strongly on lymph node and peritoneal metastases.
The cutoff for IC using Clavien–Dindo grade II or higher does not seem to really make sense, as the grade II may include clinically irrelevant complications. Due to the small sample size, ICs with Grade III or higher occurred only in 7 out of the 86 patients. The same analysis was performed with Grade III as the cutoff, but the survival curves were similar. The recurrence pattern in which only the distal metastasis was not suppressed in the IC group was also similar, except that no statistically significant difference was observed when cutoff for IC was set to grade III. However, there were only two recurrences in the group with grade III or higher complications, and the use of a cutoff for IC using Clavien–Dindo grade III or higher showed no improvement in the statistical analysis. Additionally, small infections such as urinary tract infections were not included in the ICs. Moreover, in a previous study [13], complications of grade II or higher were defined as cutoff values. Therefore, it is considered relevant to set a cutoff value for IC to be Clavien–Dindo grade II.
The rate of pancreatic fistula of 8.1% was relatively higher than that of other complications in the current study. Of the 7 patients who developed pancreatic fistula, three underwent splenectomy. Pancreatic fistula was significantly more common in splenectomy than in spleen preservation in a randomized controlled trial [26]. Additionally, one patient in the present study had pancreatic head invasion of the tumor, and one had bulky N2 lymph node (one larger than 3 cm or two larger than 1.5 cm along the celiac, splenic, common hepatic, or proper hepatic arteries) that was difficult to dissect without injuring the pancreas. This may be the reason for the relatively high rate of pancreatic fistulas in the current study. Moreover, the rate of pancreatic fistula in the previous NAC studies was reported to be 7.7–14.3% [27,28,29]. In contrast, the rate of pancreatic fistula in the current study was not very high.
We analyzed the impact of IC on survival and recurrence patterns in 146 patients with cStage II or III gastric cancer without preoperative chemotherapy. However, the negative impact of ICs widely known to be certain was not detected for OS and PFS in these patients. The rate of distant metastases, on the other hand, showed no significant difference between the IC and NIC groups (data not shown). Thus, all we can say is that pathological stage after neoadjuvant therapy plays a stronger role in recurrence than postoperative complications.
There are some limitations to this study. First, it was conducted through a retrospective analysis at a single institution, and thus, may have several biases. Moreover, the number of included patients was relatively small. When inflammatory responses are associated with host immunosuppression, there should be some differences in the postoperative highest CRP levels between patients with recurrence and those without recurrence in the IC group. However, no difference was found between the two groups (data not shown), possibly due to the small number of included patients. Additionally, the small sample size is the biggest weakness of this study. However, the sample sizes in previous studies [12, 13] were 115 and 101, and thus, were inadequate. Thus, a prospective, multi-institutional study with an adequate number of patients is required to validate the study findings. Second, due to a long study period, the treatment strategy including the extent of lymph node dissection and decision to perform splenectomy was inconsistent throughout the study period.
In conclusion, pathological stage after neoadjuvant therapy plays a stronger role in recurrence than postoperative complications. Lymph node and peritoneal metastasis may be suppressed by preoperative chemotherapy.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M (2013) Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 20:1575–1583. https://doi.org/10.1245/s10434-012-2720-9
Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, Aikou S, Watanabe R, Kosuga T, Yamaguchi T (2014) Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 21:891–898. https://doi.org/10.1245/s10434-013-3384-9
Bell SW, Walker KG, Rickard MJFX, Sinclair G, Dent OF, Chapuis PH, Bokey EL (2003) Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg 90:1261–1266. https://doi.org/10.1002/bjs.4219
Law WL, Choi HK, Lee YM, Ho JWC, Seto CL (2007) Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg 11:8–15. https://doi.org/10.1007/s11605-006-0049-z
Booka E, Takeuchi H, Nishi T, Matsuda S, Kaburagi T, Fukuda K, Nakamura R, Takahashi T, Wada N, Kawakubo H, Omori T, Kitagawa Y (2015) The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Med (United States) 94:e1369. https://doi.org/10.1097/MD.0000000000001369
Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N, Kitagawa Y (2017) Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: exploratory analysis of JCOG9907. Ann Surg 265:1152–1157. https://doi.org/10.1097/SLA.0000000000001828
Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, Kaji M, Okitsu H, Nomura T, Matsui T, Yoshikawa T, Matsuyama J, Yamada M, Ito S, Takeuchi M, Fujii M (2019) Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol 37:1296–1304. https://doi.org/10.1200/JCO.18.01138
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820. https://doi.org/10.1056/NEJMoa072252
Al-Batran SE, Homann N, Pauligk C et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a ra. Lancet 393:1948–1957. https://doi.org/10.1016/S0140-6736(18)32557-1
Tokunaga M, Mizusawa J, Machida N, Fukagawa T, Katai H, Nishida Y, Yabusaki H, Ito S, Sano T, Sasako M, Boku N, Yoshikawa T, Katayama H, Fukuda H, Terashima M (2017) Phase III trial to evaluate the efficacy of neoadjuvant chemotherapy with S-1 plus oxaliplatin followed by D2 gastrectomy with adjuvant S-1 in locally advanced gastric cancer: Japan Clinical Oncology Group study JCOG1509 (NAGISA trial). J Clin Oncol 35:TPS4134–TPS4134. https://doi.org/10.1200/JCO.2017.35.15_suppl.TPS4134
Hayashi M, Yoshikawa T, Yura M, Otsuki S, Yamagata Y, Morita S, Katai H, Nishida T (2019) Does neoadjuvant chemotherapy cancel out the negative survival impact induced by surgical complications after gastrectomy? Gastric Cancer 22:1274–1284. https://doi.org/10.1007/s10120-019-00957-5
Eto K, Hiki N, Kumagai K, Shoji Y, Tsuda Y, Kano Y, Yasufuku I, Okumura Y, Tsujiura M, Ida S, Nunobe S, Ohashi M, Sano T, Yamaguchi T (2018) Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer 21:703–709. https://doi.org/10.1007/s10120-017-0781-y
Hosoda K, Azuma M, Katada C, Moriya H, Mieno H, Ishido K, Ema A, Ushiku H, Wada T, Washio M, Watanabe A, Higuchi K, Tanabe S, Koizumi W, Watanabe M, Yamashita K (2018) A phase II study of neoadjuvant chemotherapy with docetaxel, cisplatin, and S-1, followed by gastrectomy with D2 lymph node dissection for high-risk advanced gastric cancer: results of the KDOG1001 trial. Gastric Cancer 22:598–606. https://doi.org/10.1007/s10120-018-0884-0
Hosoda K, Katada C, Ishido K, Niihara M, Ushiku H, Sakuraya M, Washio M, Wada T, Watanabe A, Harada H, Sato T, Tajima H, Kaizu T, Kosaka Y, Kato H, Sengoku N, Tanaka K, Naito T, Kumamoto Y, Sangai T, Tanabe S, Koizumi W, Yamashita K, Hiki N (2020) Neoadjuvant chemotherapy plus surgery for high-risk advanced gastric cancer: long-term results of KDOG1001 trial. Langenbeck's Arch Surg 405:777–785. https://doi.org/10.1007/s00423-020-01924-w
Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K, Nakatani K, Naruke A, Katada C (2012) A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol 69:407–413. https://doi.org/10.1007/s00280-011-1701-1
Mieno H, Yamashita K, Hosoda K et al (2017) Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surg Today 47. https://doi.org/10.1007/s00595-017-1512-z
Sano T, Kodera Y (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112. https://doi.org/10.1007/s10120-011-0041-5
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12:223–226. https://doi.org/10.1097/MCO.0b013e32832a7902
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444. https://doi.org/10.1038/nature07205
Zingoni A, Fionda C, Borrelli C, Cippitelli M, Santoni A, Soriani A (2017) Natural killer cell response to chemotherapy-stressed cancer cells: role in tumor immunosurveillance. Front Immunol:8. https://doi.org/10.3389/fimmu.2017.01194
Ma X, Zhang C, Wang C, Miao W, Zhou W, An J, Qiao W, Li M, Lai M, Yu P (2020) Comparison of clinicopathologic profiles and prognosis of gastric cancer in the upper, middle and lower third of the stomach: a retrospective cohort study. Medicine (Baltimore) 99:e21261. https://doi.org/10.1097/MD.0000000000021261
Wang X, Liu F, Li Y, Tang S, Zhang Y, Chen Y, Khan SA (2019) Comparison on clinicopathological features, treatments and prognosis between proximal gastric cancer and distal gastric cancer: a national cancer data base analysis. J Cancer 10:3145–3153. https://doi.org/10.7150/jca.30371
Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K, Stomach Cancer Study Group of the Japan Clinical Oncology Group (2017) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg 265:277–283. https://doi.org/10.1097/SLA.0000000000001814
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M (2014) Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 101:653–660. https://doi.org/10.1002/bjs.9484
Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A, on behalf of the Gastric Cancer Surgical Study Group of the Japan Clinical Oncology Group (2013) Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 107:741–745. https://doi.org/10.1002/jso.23301
Terashima M, Iwasaki Y, Mizusawa J et al (2019) Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer 22:1044–1052. https://doi.org/10.1007/s10120-019-00941-z
Author information
Authors and Affiliations
Contributions
Study conception and design: Naoki Hiki, Keishi Yamashita; Preoperative chemotherapy: Chikatoshi Katada, Kenji Ishido; Surgical resection: Kei Hosoda, Hideki Ushiku, Hiroki Harada, Mikiko Sakuraya, Ippeita Araki, Marie Washio, Keishi Yamashita; Acquisition of the data: Hideki Ushiku, Hiroki Harada; Analysis and interpretation of data: Hideki Ushiku, Kei Hosoda, Keishi Yamashita, Naoki Hiki; Manuscript draft preparation: Kei Hosoda, Hideki Ushiku, Naoki Hiki; Critical revision: Masahiro Niihara, Keishi Yamashita, Naoki Hiki
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of the Kitasato University School of Medicine and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Fig. 1
Kaplan-Meier curves for (a) Overall Survival and (b) Progression-free survival between the IC (III) and NIC (III) groups. IC (III) group includes patients with grade III or higher infectious complications, while NIC (III) group includes those without grade III or higher infectious complications (PPTX 49 kb)
Supplementary Fig. 2
Comparison of the proportion of metastasis at each site between the IC (III) and NIC (III) groups. IC (III) group includes patients with grade III or higher infectious complications, while NIC (III) group includes those without grade III or higher infectious complications. PER peritoneal dissemination, LNM lymph-node metastasis, DM distant metastasis (PPTX 81 kb)
ESM 3
(XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Hosoda, K., Ushiku, H., Katada, C. et al. Preoperative chemotherapy could modify recurrence patterns through postoperative complications in patients with gastric cancer. Langenbecks Arch Surg 406, 1045–1055 (2021). https://doi.org/10.1007/s00423-021-02153-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02153-5