Abstract
Purpose
The purpose of this study is to evaluate the long-term survival outcomes of KDOG1001 trial after a minimum follow-up of 3 years.
Methods
Patients with bulky N2 lymph nodes, linitis plastica (type 4), or large ulcero-invasive-type tumors (type 3) received up to four 28-day cycles of DCS neoadjuvant chemotherapy (docetaxel at 40 mg/m2, cisplatin at 60 mg/m2 on day 1, and S-1 at 40 mg/m2 twice daily for 2 weeks) followed by gastrectomy with D2 lymphadenectomy plus adjuvant S-1 therapy for 1 year. The final preplanned analysis of long-term outcomes including overall survival and relapse-free survival was conducted after minimum follow-up of 3 years. This trial is registered with the University Hospital Medical Information Network Clinical Trials Registry, number UMIN 000003642, and has been completed.
Results
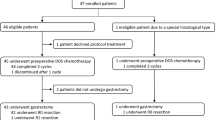
From May 2010 through January 2017, 40 patients were enrolled. All included patients underwent neoadjuvant chemotherapy with DCS followed by gastrectomy with D2 lymphadenectomy, and 32 (80%) completed adjuvant S-1 therapy for 1 year. After a median follow-up for surviving patients of 68 months at the last follow-up in January 2020, 3-year overall survival rate was 77.5% (95% confidence interval 62.1–87.9%), while 3-year relapse-free survival rate was 62.5% (95% confidence interval 46.8–76.0%).
Conclusion
Neoadjuvant chemotherapy with 4 cycles of DCS followed by D2 gastrectomy plus adjuvant S-1 was associated with relatively good long-term oncologic outcomes for patients with the high-risk gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical resection remains mainstay of curative treatment for patients with gastric cancer. However, particularly in those with high risk of recurrence such as those with bulky lymph nodes along the celiac, splenic, common hepatic, or proper hepatic arteries (bulky N2 lymph nodes); linitis plastica (type 4); or large ulcero-invasive-type (type 3), the prognosis remains poor even when the tumor can be curatively resected [1,2,3]. Those with high risk of recurrence have been the target of clinical trials. The Japan Clinical Oncology Group (JCOG) has conducted a phase II trial (JCOG0405 [3]) to evaluate the safety and efficacy of neoadjuvant cisplatin and S-1 (CS) chemotherapy followed by gastrectomy with extended lymphadenectomy for gastric cancer with extended lymph node metastasis including bulky N2 lymph nodes. This trial showed good feasibility, with an excellent clinical response rate of 64.7% and a 3-year overall survival (OS) rate of 58.8%. The JCOG also conducted a phase II trial (JCOG0210 [1]) to evaluate the safety and efficacy of neoadjuvant CS chemotherapy followed by gastrectomy with D2 node dissection for gastric cancer with a type 4 or large type 3 tumor. The study exhibited good feasibility with a 3-year OS rate of 24.5%. Based on these results, the next phase III study (JCOG0501) was conducted.
Docetaxel-containing regimens have been considered worthy of evaluating in patients with gastric cancer with high risk of recurrence because the addition of docetaxel to cisplatin and 5-fluorouracil was shown to improve the survival outcomes of patients with unresectable or recurrent gastric cancer [4, 5].
KDOG1001 trial was a single-institutional, phase II study designed to evaluate the safety and efficacy of docetaxel, cisplatin, and S-1 as a neoadjuvant chemotherapy (DCS NAC) for gastric cancer with bulky N2 lymph nodes, type 4, or large type 3. In the primary analysis, the R0 resection rate was 90% (36/40, 90% confidence interval (CI) 79.5–95.4%). Common grade 3 or grade 4 adverse events during DCS NAC were leukocytopenia (27.5%), neutropenia (55.0%), and hyponatremia (22.5%). The most common grade 3 or grade 4 surgical morbidity was pancreatic fistula (12.5%). The pathological response rate was 57.5% (23/40) [6]. Accordingly, we concluded that DCS NAC therapy was feasible and showed an enough R0 resection rate. We now report the survival analysis from KDOG1001 trial done after a minimum follow-up of 3 years.
Methods
KDOG1001 was conducted as a prospective, single-institutional, phase II trial at the Kitasato University, Sagamihara, Japan. All enrolled patients provided written informed consent. The study protocol was approved by The Kitasato University School of Medicine Research Ethics Committee and was conducted in accordance with the Declaration of Helsinki as well as the Japanese Ethical Guidelines for Clinical Studies. This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (http://www.umin.ac.jp/ctr/) as UMIN 000003642. The study design has been described previously [6]. The main eligibility criteria were an age of 20–75 years and histologically proven clinically resectable gastric adenocarcinoma with bulky N2 lymph nodes (one larger than 3 cm or two larger than 1.5 cm along the celiac, splenic, common, or proper hepatic arteries), type 4, or large type 3 measuring ≥ 8 cm. An Eastern Cooperative Oncology Group performance status of 0 or 1 and no prior chemotherapy, radiotherapy, or surgery was also required.
CY1 was defined by a positive result for carcinoma cells on the peritoneal cytology test, whereas CY0 was defined by a negative result for carcinoma cells on the peritoneal cytology test. Initially, the absence of peritoneal dissemination and the absence of carcinoma cells on the peritoneal cytology test (CY0) were confirmed via staging laparoscopy before entry into the study. However, patient accrual was so poor that we amended the study protocol at 2013 to include patients with CY1. The absence of peritoneal dissemination was confirmed by staging laparoscopy for all the patients before entry into the study.
Treatment
The treatment involved the following three steps. First, neoadjuvant chemotherapy was administered. Neoadjuvant chemotherapy consisted of an infusion of docetaxel (40 mg/m2) and cisplatin (60 mg/m2) on day 1, and S-1 (40 mg/m2) administered orally twice daily for 2 weeks on days 1–14, followed by a 2-week rest. After the first and second cycles of DCS NAC, efficacy was evaluated based on CT findings and tumor marker levels. If the tumor obviously progressed but remained resectable after the first cycle of chemotherapy or if the tumor remained stable without a marginal response after the second cycle of chemotherapy, surgical resection was performed. Otherwise, 4 cycles of the DCS therapy were given to the patients.
Second, surgical resection was performed between 15 and 42 days after the last administration of S-1. As a first step in surgical resection, intraperitoneal washing cytology specimens were examined. If the cytology findings were negative, R0 resection was attempted via total or distal gastrectomy with D2 lymphadenectomy, as defined by the Japanese gastric cancer treatment guidelines [7]. If R0 resection was considered impossible and if there was clinical significance such as control of bleeding or removal of stenosis, R1 or 2 resections would be performed. Even then, the data of those patients would be used to analyze the outcomes.
Third, adjuvant chemotherapy was administered. Adjuvant chemotherapy with S-1 was started within 42 days after surgery when R0 resection was achieved pathologically. Adjuvant chemotherapy consisted of 4 weeks of oral administration of S-1 at a dosage of 40 mg/m2 twice daily followed by a 2-week rest during the first year after surgery. If S-1 therapy was not started within 3 months after surgery for any reason, the protocol treatment was terminated. The protocol treatment was completed when a patient had received one or more cycles of DCS NAC, had undergone R0 resection via gastrectomy with lymphadenectomy, and had received postoperative chemotherapy. After completion of the protocol, no further treatment was given until tumor recurrence. Detailed treatment protocol was previously reported [6].
Outcomes
The primary endpoint was R0 resection rate. The secondary endpoints were 3-year survival rate, completion rate of the protocol treatment, pathological response rate of DCS NAC, and adverse events. Overall survival (OS) was defined as the time from the date of surgery to the date of death from any cause and was censored on the last contact for a surviving patient. Relapse-free survival (RFS) was defined as the time from the date of surgery to the first date of relapse and/or death from any cause and was censored on the last contact for a relapse-free surviving patient. The pathological response was graded by pathologists according to the Japanese classification of gastric carcinoma, third English edition [8]: grade 1a, viable tumor cells occupy more than two-thirds of the tumorous area; grade 1b, more than one-third but less than two-thirds; grade 2, less than one-third; and grade 3, no viable tumor cells. In this study, the pathological response (responder) was defined as grade 1b to grade 3 responses.
Statistical analysis
The R0 resection rates in the JCOG0210 and the JCOG0405 were 63% and 82%, respectively, and the efficacy of DCS therapy was expected to be superior to that of CS therapy. We set the expected R0 resection rate to be 85% and the threshold R0 resection rate to be 65%. The sample size was calculated to be 40 cases with one-sided testing at the 5% significance level with power of 90%. The cutoff date for this long-term analysis was January 1, 2020. OS and RFS were calculated by the Kaplan–Meier method for all eligible patients. Univariate analyses of prognostic factors for OS and RFS were performed using log-rank tests. Multivariate analyses were performed using Cox proportional hazards model to identify independent prognostic factors. All statistical analyses were performed using JMP Pro Version 14.0 (SAS Institute Inc., Cary, NC, USA).
Results
From May 2010 through January 2017, 40 patients were enrolled. The baseline characteristics were shown in Table 1. Clinically, 17.5% of patients had bulky N2 lymph nodes, 45.0% had a type 4 tumor, and 40% had a large type 3 tumor with 2.5% having both a large type 3 tumor and bulky N2 lymph nodes. Four patients had positive washing cytology test (CY1).
At the cutoff date in January 2020, the median follow-up for the OS analysis was 68 months (range 36–110 months). There were 18 deaths. All the causes of death were progressive disease. The 3-year OS rate was 77.5% (95% CI 62.1–87.9%) and the 5-year OS rate was 69.4% (95% CI 53.4%–81.8%) (Fig. 1a). The 3-year RFS rate was 62.5% (95% CI 46.8–76.0%) and the 5-year RFS rate was 54.0% (95% CI 38.3–68.9%) (Fig. 1b). Nineteen patients developed cancer recurrence. The most frequent site of recurrence was peritoneum (n = 13), followed by lymph nodes (n = 2), hematogenous (n = 3), and local recurrence (n = 1). Subgroup analysis was performed between bulky N2 lymph nodes, type 4, and large type 3. The 3-year OS and 3-year RFS rates in bulky N2 lymph nodes were 83.3% and 66.7%, respectively. The 3-year OS and 3-year RFS rates in type 4 were 66.7% and 50.0%, respectively. The 3-year OS and 3-year RFS rates in large type 3 were 87.5% and 75.0%, respectively (Fig. 2a, b). Patients with type 4 tumor had significantly worse OS (P < 0.001) and RFS (P = 0.015) than those with non-type 4 tumor (Fig. 2c, d).
Kaplan–Meier curves of overall survival (a) and relapse-free survival (b) stratified by the subgroups. Kaplan–Meier curves of overall survival (c) and relapse-free survival (d) with log-rank test between type 4 and non-type 4. OS overall survival, RFS relapse-free survival, BN bulky N2 lymph nodes, T4 type 4, LT3 large type 3
Adverse events associated with preoperative chemotherapy and surgical complications were reported previously [6]. Grade 3 or 4 toxicity during DCS NAC was experienced by 27 patients (67.5%). Grade 3 or 4 surgical complications occurred in 9 patients (22.5%). No treatment-related deaths occurred during protocol treatment.
Of the 4 patients with CY1, 2 had type 4 tumor and 2 had large type 3 tumor. Three patients converted to CY0 after DCS NAC. The 3-year OS rate was 25%. Only one patient with persistent positive cytology test had a large type 3 tumor and survived over 3 years.
Univariate analysis revealed that pathological response of grade 1b or more (responder) had better RFS and OS than non-responder and that 4 cycles of DCS tended to have better OS and RFS than 3 or less cycles of DCS (Tables 2 and 3). Univariate analysis results as well as multivariate Cox proportional hazards model confirmed that the cancer type, specifically type 4 were an independent predictive factor for poor prognosis in OS (HR 12.62, 95% CI 2.60–79.70; P = 0.001) and RFS (HR 3.74, 95% CI 1.09–13.22; P = 0.036) (Tables 2 and 3).
Discussion
The final preplanned analysis of the KDOG1001 trial revealed that 4 cycles of DCS NAC followed by gastrectomy with D2 lymphadenectomy plus adjuvant S-1 therapy showed good survival outcomes for patients with resectable high-risk advanced gastric cancer with bulky N2 lymph nodes, type4, or large type 3.
Comparisons of JCOG studies of NAC for resectable high-risk advanced gastric cancer are summarized in Table 4. In the JCOG0405 trial, patients with bulky N2 lymph nodes or para-aortic metastatic lymph nodes had a good 3-year OS rate of 58.8% [3]. To further improve the long-term oncologic outcomes, JCOG1002 trial which tested the efficacy of 2 cycles of DCS NAC was conducted. However, the results did not demonstrate the significant improvement of the treatment outcomes. Not only the clinical response dropped below the expected rate with the response rate of 57.7%, but there was no significant improvement in the OS with 3-year OS rate of 62.7% [9, 10]. Moreover, when we look at the cases with only bulky N2 lymph nodes, no improvement of OS was found with the 5-year OS rate of 57.1% compared with that of 68% in the JCOG0405 trial.
Although included patients was as small as 6, this current study found that patients with bulky N2 lymph nodes had relatively good 3-year OS rate of 83.3% and 5-year OS rate of 83.3% with two recurrences in the liver and the lung. Of 6 patients, 5 received 4 cycles of DCS NAC. Aoyama et al. reported that the rate of pathological response, defined as a complete response or < 10% residual cancer remaining, in 4 cycles of DCS was 18.8% which is better than that of 12.1% in 2 cycles of DCS [11]. This cutoff point of 10% is reported to better predict survival outcomes [12]. In the JCOG1002 trial, most frequent site of recurrence was reported to be lymph nodes. Four cycles of DCS NAC might have improved survival than 2 cycles of DCS NAC by eliminating cancer cells in metastatic lymph nodes.
We have conducted univariate and multivariate prognostic analyses for the purpose of detecting factors that made the prognosis of this single-arm trial better than other trials. Patients with good pathological response of grade 1b or more had relatively better survival rate than those without good pathological response. This good pathological response was seen in only 33.3% (4/12) of the patients receiving 2 cycles of DCS NAC; by contrast, it was seen in 69.5% (16/23) of the patients receiving 4 cycles of DCS NAC [6]. Tables 2 and 3 show that patients with 4 cycles of DCS tended to have better survival rate than those with 3 or less cycles of DCS. In addition, the pathological response of 58% in this current study is the best among studies listed in Table 4. Therefore, another reason why the prognosis was better in this single-arm study may possibly be high dose intensity of chemotherapy.
Another possible reason why the OS was better than the preceding trials is that the proportion of patients with CY1 is 10% and relatively low. The proportion of patients with CY1 in the JCOG0210 and the JCOG0501 trials which included patients with type 4 or large type 3 tumors was 30% (diagnosed after NAC) and 21%, respectively. CY1 has been regarded as a worse prognostic factor in patients with gastric cancer [13, 14]. In this current study, we initially excluded patients with CY1 confirmed via staging laparoscopy. However, patient accrual was so poor that we amended the study protocol to include patients with CY1. In fact, the 3-year OS rate of the patients with CY1 is as poor as 25%. If patients with CY1 had been involved from the start of this study, the prognostic outcomes would have been much worse than the obtained results.
There is still room for improvement for the treatment of type 4 gastric cancer. The JCOG has conducted clinical trials on type 4 and large type 3 gastric cancer as the same entity. In the JCOG0501 trial, adding NAC CS did not demonstrate the improvement of survival outcomes as compared with the standard treatment of adjuvant S1 chemotherapy alone [15]. In this current study, significant difference was found in the survival outcomes between type 4 and large type 3. Most of the type 4 tumor was thought to be categorized as the genomically stable (GS) subtype in The Cancer Genome Atlas (TCGA) project [16]. This subtype is reported to be resistant to 5-FU [17]. On the other hand, large type 3 tumor may include the chromosomal instability (CIN) subtype in TCGA which is reported to be most benefitted from adjuvant chemotherapy [17]. Considering these molecular differences, optimal therapy for type 4 and large type 3 gastric cancer may need to be separately developed with consideration of target molecules.
JCOG1013 failed to demonstrate the survival benefit of DCS over CS in the metastatic setting [18]. However, this current study indicated that, when used in NAC setting for non-type 4 resectable advanced gastric cancer, 4 cycles of DCS NAC had the possibility to improve survival outcomes. Because of the significantly worse survival of type 4 gastric cancer, this cancer should be treated with different treatment strategy. On the other hand, for resectable high-risk gastric cancer with bulky N2 and large type 3, 4 cycles of DCS NAC would be evaluated in a future phase III trial.
In Europe, all patients with high-risk gastric cancer receive perioperative therapy since more than 10 years ago when MAGIC-Trial demonstrated a survival benefit of perioperative chemotherapy with ECF [19]. Even if cancer biology in Asian gastric cancer patients seems to differ from Caucasian patients, our result belatedly demonstrated the benefit of perioperative chemotherapy even in Asian high-risk gastric cancer.
Current standard treatment of resectable high-risk advanced gastric cancer with bulky N2 lymph nodes, type 4, or large type 3 is upfront gastrectomy with D2 lymphadenectomy followed by adjuvant chemotherapy in the Japanese gastric cancer treatment guidelines [20]. In Japan, the standard regimens of adjuvant chemotherapy are regarded as S-1 for pathological stage II and docetaxel and S-1 (DS) for stage III based on the results of ACTS-GC trial [21, 22] and START-II trial [23], respectively. The control group of future phase III trial may possibly be upfront gastrectomy with D2 lymphadenectomy followed by adjuvant chemotherapy with S-1 or DS.
The inclusion criteria for this trial were quite strict. FLOT4 trial in Europe [19] and PRODIGY trial in Korea [24] provided evidence for better survival in perioperative chemotherapy for resectable advanced gastric cancer. The inclusion criteria for these trials were wider than those for this current trial. On the other hand, resectable advanced gastric cancer except for type 4 and large type 3 is reported to have relatively good survival outcomes [25, 26]. In Japan, JCOG1509 trial which evaluates perioperative chemotherapy using S-1 and oxaliplatin is underway for resectable advanced gastric cancer except for bulky N2, type 4, and large type 3. Until the results of JCOG1509 will be available, upfront D2 gastrectomy followed by adjuvant chemotherapy would be recommended to Japanese patients who do not meet the inclusion criteria but are diagnosed with advanced gastric cancer.
This study has some important limitations. First, the present trial had a single-arm, phase II design and was conducted at a single institution. Second, the accrual period was so long that not only NAC regimen has become obsolete but also chemotherapy regimens after cancer progression changed in the study period. We initially planned to include 10 patients per year. However, because of the unexpectedly declining incidence of gastric cancer as well as screening program for gastric cancer in Japan, not so many patients were diagnosed as having the high-risk gastric cancer with adequate organ function in the recruitment period. That is the reason for the poor recruitment of 5.7 patients per year. Cisplatin is being replaced by oxaliplatin, both of which are classified as platinum-based anticancer agent. Although cisplatin has renal toxicity, its prevention has been well established. Moreover, the anticancer effect of cisplatin has been well confirmed for a long period of time. On the other hand, oxaliplatin, which is well known to have less renal toxicity, has peripheral neurotoxicity. If oxaliplatin was used multiple times, peripheral neuropathy with numbness and tingling may frequently occur and may take a long time to be alleviated, which may worsen quality of life of the patients. Therefore, DCS regimen, along with FLOT and DOS, is still a target for the development of treatments for high-risk gastric cancer, which would provide relevance of this current study.
In conclusion, our updated analysis showed that 4 cycles of DCS NAC followed by D2 gastrectomy and postoperative S-1 therapy for resectable high-risk advanced gastric cancer were associated with relatively good long-term oncologic outcomes except for type 4. For resectable high-risk gastric cancer with bulky N2 and large type 3, 4 cycles of DCS NAC would be evaluated in a future phase III trial.
References
Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A, on behalf of the Gastric Cancer Surgical Study Group of the Japan Clinical Oncology Group (2013) Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 107:741–745. https://doi.org/10.1002/jso.23301
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima N (2009) Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 96:1015–1022. https://doi.org/10.1002/bjs.6665
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, on behalf of the Stomach Cancer Study Group of the Japan Clinical Oncology Group (2014) Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 101:653–660. https://doi.org/10.1002/bjs.9484
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V25 study group. J Clin Oncol 24:4991–4997. https://doi.org/10.1200/JCO.2006.06.8429
Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K, Nakatani K, Naruke A, Katada C (2012) A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol 69:407–413. https://doi.org/10.1007/s00280-011-1701-1
Hosoda K, Azuma M, Katada C, Moriya H, Mieno H, Ishido K, Ema A, Ushiku H, Wada T, Washio M, Watanabe A, Higuchi K, Tanabe S, Koizumi W, Watanabe M, Yamashita K (2018) A phase II study of neoadjuvant chemotherapy with docetaxel, cisplatin, and S-1, followed by gastrectomy with D2 lymph node dissection for high-risk advanced gastric cancer: results of the KDOG1001 trial. Gastric Cancer 22:598–606. https://doi.org/10.1007/s10120-018-0884-0
Sano T, Kodera Y (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123. https://doi.org/10.1007/s10120-011-0042-4
Sano T, Kodera Y (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112. https://doi.org/10.1007/s10120-011-0041-5
Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M (2017) A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer 20:322–331. https://doi.org/10.1007/s10120-016-0619-z
Takahari D, Ito S, Mizusawa J et al (2019) Long-term outcomes of preoperative docetaxel with cisplatin plus S-1 therapy for gastric cancer with extensive nodal metastasis (JCOG1002). Gastric Cancer 23:293–299. https://doi.org/10.1007/s10120-019-01007-w
Aoyama T, Nishikawa K, Fujitani K, Tanabe K, Ito S, Matsui T, Miki A, Nemoto H, Sakamaki K, Fukunaga T, Kimura Y, Hirabayashi N, Yoshikawa T (2017) Early results of a randomized two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of cisplatin/S-1 and docetaxel/cisplatin/S-1 as neoadjuvant chemotherapy for locally advanced gastric cancer. Ann Oncol 28:1876–1881. https://doi.org/10.1093/annonc/mdx236
Nakamura K, Kuwata T, Shimoda T, Mizusawa J, Katayama H, Kushima R, Taniguchi H, Sano T, Sasako M, Fukuda H (2015) Determination of the optimal cutoff percentage of residual tumors to define the pathological response rate for gastric cancer treated with preoperative therapy (JCOG1004-A). Gastric Cancer 18:597–604. https://doi.org/10.1007/s10120-014-0401-z
Hosoda K, Yamashita K, Katada N, Moriya H, Mieno H, Sakuramoto S, Kikuchi S, Watanabe M (2015) Preoperative tumor size is a critical prognostic factor for patients with Borrmann type III gastric cancer. Surg Today 45:68–77. https://doi.org/10.1007/s00595-014-1060-8
De Andrade JP, Mezhir JJ (2014) The critical role of peritoneal cytology in the staging of gastric cancer: an evidence-based review. J Surg Oncol 110:291–297. https://doi.org/10.1002/jso.23632
Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito Y, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Gotoh M, Takagane A, Yabusaki H, Hirabayashi N, Sano T, Sasako M (2018) Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer: Japan Clinical Oncology Group study (JCOG0501). J Clin Oncol 36:4046. https://doi.org/10.1200/JCO.2018.36.15_suppl.4046
Bass AJ, Thorsson V, Shmulevich I et al (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209. https://doi.org/10.1038/nature13480
Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, Cheong JH, Jeong W, Cho JY, Kim J, Chae J, Lee J, Kang WK, Kim S, Noh SH, Ajani JA, Lee JS (2017) Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas project. Clin Cancer Res 23:4441–4449. https://doi.org/10.1158/1078-0432.CCR-16-2211
Yamada Y, Boku N, Mizusawa J et al (2019) Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol 4:501–510. https://doi.org/10.1016/S2468-1253(19)30083-4
Al-Batran SE, Homann N, Pauligk C et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a ra. Lancet 393:1948–1957. https://doi.org/10.1016/S0140-6736(18)32557-1
Kodera Y, Sano T (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20:1–19. https://doi.org/10.1007/s10120-016-0622-4
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387–4393. https://doi.org/10.1200/JCO.2011.36.5908
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820. https://doi.org/10.1056/NEJMoa072252
Yoshida K, Kodera Y, Kochi M et al (2019) Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol 37:1296–1304. https://doi.org/10.1200/JCO.18.01138
Kang Y-K, Yook JH, Park Y-K, Kim YW, Kim J, Ryu MH, Rha SY, Chung IJ, Kim IH, Oh SC, Yoo CH, Choi JH, Zang DY, Kim G, Lee Y, Noh SH (2019) Phase III randomized study of neoadjuvant chemotherapy (CT) with docetaxel(D), oxaliplatin(O) and S-1(S) (DOS) followed by surgery and adjuvant S-1, vs surgery and adjuvant S-1, for resectable advanced gastric cancer (GC) (PRODIGY). Ann Oncol 30:v876–v877. https://doi.org/10.1093/annonc/mdz394.032
Yamashita K, Ema A, Hosoda K, Mieno H, Moriya H, Katada N, Watanabe M (2017) Macroscopic appearance of Type IV and giant Type III is a high risk for a poor prognosis in pathological stage II/III advanced gastric cancer with postoperative adjuvant chemotherapy. World J Gastrointest Oncol 9. https://doi.org/10.4251/wjgo.v9.i4.166
Hosoda K, Watanabe M, Yamashita K (2019) Re-emerging role of macroscopic appearance in treatment strategy for gastric cancer. Ann Gastroenterol Surg 3:122–129
Author information
Authors and Affiliations
Contributions
Satoshi Tanabe, Wasaburo Koizumi, and Keishi Yamashita are responsible for the study conception and design; Kei Hosoda, Chikatoshi Katada, Kenji Ishido, Hideki Ushiku, Hiroki Harada, Mikiko Sakuraya, Marie Washio Takuya Wada, Akinori Watanabe Satoshi Tanabe, Wasaburo Koizumi, and Keishi Yamashita for the acquisition of data; Kei Hosoda, Keishi Yamashita, and Naoki Hiki for the analysis and interpretation of data; Kei Hosoda and Naoki Hiki for the drafting; and Masahiro Niihara, Takeo Sato, Hiroshi Tajima, Takashi Kaizu, Yoshimasa Kosaka, Hiroshi Kato, Norihiko Sengoku, Kiyoshi Tanaka, Takeshi Naito, Yusuke Kumamoto, Takafumi Sangai, Satoshi Tanabe, Wasaburo Koizumi, Keishi Yamashita, and Naoki Hiki for the critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosoda, K., Katada, C., Ishido, K. et al. Neoadjuvant chemotherapy plus surgery for high-risk advanced gastric cancer: long-term results of KDOG1001 trial. Langenbecks Arch Surg 405, 777–785 (2020). https://doi.org/10.1007/s00423-020-01924-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-020-01924-w