Abstract
Purpose
Extended pancreatectomy aimed at R0 resection of pancreatic tumors with adjacent vessel and organ involvement may be the only option for cure. This study was done with an objective to analyze the short- and long-term outcomes of extended pancreatic resections.
Methods
All pancreatectomies performed between 2006 and 2015 were included. The pancreatectomies were classified as standard or extended, as per the International Study Group for Pancreatic Surgery. All surgical complications and terminologies were according to Clavien-Dindo classification and International Study Group for Pancreatic Surgery guidelines. Morbidity and mortality were primary outcomes and disease-free survival was a secondary outcome.
Results
Sixty-three extended and 620 standard pancreatectomies were performed. Major morbidity (Clavien grades III, IV and V) (37 vs. 29%, p = 0.21) and mortality (6 vs. 4%, p = 0.3) for extended pancreatectomies were comparable to those for standard pancreatectomies. Blood loss > 855 ml, need for blood transfusion, and tumor size were independent risk factors for morbidity, and the latter two for mortality. Standard pancreatectomies were associated with better 3-year disease-free survival than extended pancreatectomies (67 vs. 41%, p < 0.001). Extended pancreatectomies resulted in a significantly better median disease-free survival for non-pancreatic adenocarcinoma vs. pancreatic adenocarcinoma (33.3 vs. 9.5 months, p = 0.01).
Conclusion
Extended pancreatectomies resulted in similar peri-operative morbidity and mortality compared to standard pancreatectomies. Although the survival of patients undergoing these complex procedures is inferior to standard pancreatectomies, they should be undertaken not only in selected cases of pancreatic cancer but even more so in other complex pancreatic tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Experiments with extended resections date back to 1952, inspired by McDermott’s thought, “with very rare exceptions, carcinoma of the pancreas is a fatal disease” [1]. With a dismal resectability rate, plateaued at 20–30% (1983 to 2007), many have challenged the anatomical limits of resection for pancreatic ductal adenocarcinoma (PDAC) [2, 3]. Locally advanced tumors, which account for 30% of all pancreatic tumors, are associated with a sinister prognosis. However, the long-term survival of this group can be improved by offering them extended resections, but with an acceptable peri-operative morbidity and mortality [4]. Although extended pancreatectomies (EP) were initiated as early as 1972 by Fortner under the helm of “regional pancreatectomy,” it is the recent years that have seen an increasing acceptance for vascular resections, especially vein resections [2, 5]. With the International Study Group for Pancreatic Surgery (ISGPS) guidelines for EP published in 2014, many retrospective reviews have surfaced, implying that many have already pushed beyond standard resections under controlled settings, as surgery continues to be the only hope for cure [6,7,8,9]. We evaluated world literature in 2010 and now analyze our very own outcomes of EP as defined by the ISGPS to assess feasibility, safety, and benefit of these radical procedures [10, 11].
The primary objective of our study was to assess the peri-operative morbidity and mortality of EP compared to standard pancreatectomy (SP) and the secondary objective was to determine the long-term survival outcome following EP.
Patient and methods
Study design
A retrospective cohort analysis of a prospectively maintained database of pancreatic resections from January 2006 to August 2015 of the Hepato-Pancreato-Biliary Surgical Oncology Unit at Tata Memorial Centre (Mumbai, India) was performed. All patients undergoing EP and SP were included in the final analysis. Patients who were explored and were metastatic or inoperable due to locally advanced unresectable disease were excluded from the final analysis. All cases of EP were included as per the ISGPS definitions [11]. As per ISGPS consensus statement, EP is defined as resection of an adjacent organ or vasculature in addition to standard pancreatic resection [11].
Ethics
The data were collected prospectively during routine clinical practice, and accordingly, signed informed consent was taken from each patient before any surgical or clinical procedure. The study protocol conforms to the ethical guidelines of the “World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended in Fortaleza, Brazil, 2013 [12]. No dedicated approval was needed from the institutional review board.
Surgical considerations
Pre-operative
Treatment plan for all patients was decided upon in a multi-disciplinary clinic. With regard to pancreatic adenocarcinoma, the patients were evaluated using a pancreatic protocol CT scan along with imaging of the thorax. Based on resectability, they were categorized as resectable, borderline resectable, locally advanced unresectable, and metastatic. Resectable cases were treated with upfront resection. Borderline resectable cases were treated with neoadjuvant therapy after confirming tissue diagnosis and reassessed for resection. Pre-operative biliary drainage was performed where indicated [13].
Surgery
Staging laparoscopy was used selectively prior to curative resection in suspected pancreatic head adenocarcinoma patients with CA 19-9 > 100 U/ml without any concomitant obstructive jaundice or cholangitis and in all patients with suspected adenocarcinoma of the distal pancreas, irrespective of the CA 19-9 level. Pancreatoduodenectomies were routinely pylorus-preserving unless otherwise indicated, using appropriate approaches [14]. Alimentary continuity was maintained using a standardized duct-to-mucosa pancreato-jejunostomy, along with an end-to-side hepatico-jejunostomy and antecolic duodeno-jejunostomy [15]. Pancreatic stump closure was hand-sewn in distal pancreatectomies (DP). Spleen-preserving DP was performed in select situations. Peri-operative octreotide and prophylactic drains were used in all the cases. Intra-operative anticoagulation used in vascular resections was local use of heparinized saline. Primary end-to-end venous anastomosis was performed if the resected segment was < 4 cm. The threshold for using interposition graft was for vein resections > 4 cm to ensure a tension-free anastomosis. Vascular reconstructions were classified according to the ISGPS [16]. Nasojejunal tube was routinely placed intra-operatively for enteral nutrition. None of the colonic anastomoses were diverted.
Post-operative
Drain and serum amylases were sent as per unit protocol on post-operative days 3 and 7, respectively. All complications were defined according to the ISGPS criteria and were graded according to Clavien-Dindo [17,18,19,20]. Major morbidity referred to any morbidity which was grade 3 and above. Mortality included all-cause death up to 90 days from surgery. Margin-positivity (R1) was defined according to the Royal College of Pathologists guidelines [21]. All resected patients were included in survival calculation, irrespective of their histopathological correlation of vessel/adjacent organ involvement.
Statistical analysis
Statistical analysis was done using a statistical software package, SPSS v.21.0 (SPSS Inc., Chicago, IL, USA). The data were represented as median (range) or frequency (%) as appropriate. Disease-free survival (DFS) was calculated from date of surgery to date of recurrence or last follow-up. The Kaplan–Meier method was used to estimate survival using two-sided log-rank for group comparison. Association was analyzed using Chi-square test or Fisher’s exact test and Mann–Whitney U test for categorical and continuous variables, respectively. Factors contributing to peri-operative morbidity and mortality which were significant (p < 0.05) on univariate analysis were included in the multivariate model using logistic regression. p value < 0.05 was considered statistically significant for all comparisons.
Results
Patient characteristics and pre-operative details (Table 1)
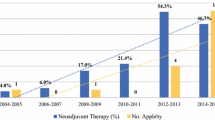
A total of 683 patients underwent pancreatectomies during this period. There were 63 EP and 620 SP. During the same period, curative intent surgery was attempted for 92 patients but resection abandoned due to various reasons (Fig. 1). Pre-operative characteristics of the EP and SP patients are shown in Table 1. Seventy-three percent (46/63) of EP were operated in the time span 2012–2015. Significantly more patients were subjected to pre-operative biliary drainage in SP group (64 vs. 40%, p < 0.001). A significantly higher proportion was treated with neoadjuvant therapy in EP vs. SP (11 vs. 3%, p = 0.002). Neoadjuvant regimen amongst EP included chemoradiation in four, chemotherapy followed by chemoradiation in two, and chemotherapy in one patient. Among SP group regimen used included chemotherapy and chemoradiation in ten patients each.
Flowchart showing consort diagram of all the patients subjected to surgery. *2 patients; total pancreatectomy in a patient with cardiac co-morbidity (1) and technically difficult surgery due to highly friable tissues (?post-radiation) (1). PNET pancreatic neuroendocrine tumor, SP standard pancreatectomy, EP extended pancreatectomy, NA not available, LAUR locally advanced unresectable
Peri-operative details (Tables 1 and 2)
The median duration of surgery was significantly longer in EP (510 vs. 430 min, p < 0.001). Duration of hospital stay (13 vs. 13 days, p = 0.69) and re-admission rates (16 vs. 10%, p = 0.16) were similar between the two groups. EP was associated with a significantly higher median blood loss (1500 vs. 800 ml, p < 0.001), the percentage of patients requiring blood transfusion (56 vs. 20%, p < 0.001), and a median number of units transfused (2 vs. 1, p < 0.001). Of 31 VR patients, 29 underwent vein-only, 1 underwent vein and artery, and another underwent artery-only resection, comprising 32 vascular repairs in this VR group. Of the five MVR+VR, four underwent vein-only resection while one patient underwent vein and artery resection, comprising six vascular repairs in this MVR+VR group. The vein resected included segments of the portal vein (PV), superior mesenteric vein (SMV), or their confluence while the arterial segments resected were those of the common hepatic artery (CHA). The median length of vessel resected was 2 cm (0.5–8 cm) while the median vessel-clamp time was 10 min (6–35 min). There were 38 vascular reconstructions in all, performed in 36 patients of the VR and MVR+VR groups. Majority of reconstructions were Type 3 (55%). The remaining were Type 1 (26%) and Type 4 (18%) repairs. Interposition graft was used in seven patients. Six of these were prosthetic grafts and one was autologous saphenous vein graft. One of these patients succumbed. Of the remaining five in whom prosthetic grafts were used, three developed post-operative collections requiring intervention. However, no patient developed a graft infection. Table 3 shows the adjacent organs and vessels resected in the EP group.
Histopathology (Table 1)
Majority of the EP were for PDAC (27 of 63, 43%). Remaining histologies included 14 adenocarcinomas of non-pancreatic origin (22%), 5 solid pseudopapillary epithelial neoplasms (SPEN) (8%), 5 pancreatic neuroendocrine tumor (8%), 4 sarcomas (6%), 3 renal cell carcinomas (5%), 3 cystic pancreatic neoplasms (5%), and 2 gastrointestinal stromal tumor (GIST) (3%). Non-pancreatic origin adenocarcinoma included 6 colonic, 5 common bile duct, and 3 ampulla of Vater primaries. Median tumor size was significantly larger for MVR vs. VR cases. The tumors in the EP group were significantly larger in size and were associated with a higher lympho-vascular invasion (LVI) and peri-neural invasion (PNI) compared to those in the SP group. R1 resections were significantly higher in the EP group compared to the SP group. There was one case of R2 resection in the EP group—a patient in whom a PV resection was done but a major arterial resection was also required, and the latter was deferred due to inter-aorto-caval node positivity on frozen section.
Complications (Table 1; Fig. 2)
The bar graph in Fig. 2 shows the individual complications following SP and EP. Major morbidity for EP vs. SP in the PDAC group was 48.1% (13/27) vs. 23.2% (16/69) (p = 0.01) and that for non-PDAC group was 27.8% (10/36) vs. 29.8% (164/551) (p = 0.8). A univariate logistic regression was carried out of factors which might contribute to morbidity and mortality considering age, sex, American Society of Anesthesiologists (ASA) grade, pre-operative serum bilirubin and albumin, pre-operative biliary drainage, neoadjuvant therapy, surgical blood loss, need for blood transfusion, tumor size and type of pancreatic resection as the predictor variables, and morbidity and peri-operative mortality as the outcome. Of these, factors entered into multivariate analysis included age, blood loss, need for blood transfusion, and tumor size, which revealed that blood loss > 855 ml, need for blood transfusion, and tumor size were found to contribute significantly to major morbidity, and need for blood transfusion and tumor size were found to contribute significantly to peri-operative mortality.
Peri-operative mortality and survival (Table 1; Fig. 3)
During the 90-day peri-operative period, 4 (6%) of the patients in the EP group and 23 (4%) of the SP group (p = 0.30) died. Mortality for EP vs. SP in the PDAC group was 14.8% (4/27) vs. 2.9% (2/69) (p = 0.03) and that in the non-PDAC group was 0% (0/36) vs. 3.8% (22/551) (p = 0.23). Of the four EP who died, three were > 60 years of age. Two patients died of disseminated intravascular coagulation (DIC)—one had undergone right colonic resection along with PV resection and polytetrafluoroethylene (PTFE) graft repair, i.e., Type 4 repair, and the other after Type 3 repair between hepatic artery proper and splenic artery. The third patient died of hepatic failure after a PV and CHA resection and the fourth patient died after discharge due to a cardiac event, 22 days after surgery. The median disease-free survival (DFS) for patients with PDAC was 17.2 months with a median follow-up of 18.5 months. Among the EP group, median DFS for the non-PDAC cohort was 33.3 vs. 9.5 months for the PDAC cohort (p = 0.01). In the EP group, 29 (46%) patients had disease progression, i.e., 5 (8%) loco-regional failure, and 22 (35%) distant relapse, predominantly in the liver (11 of 22, 50%). The 3-year DFS for EP was 41 vs. 67% for SP (p < 0.001). The percentage of patients who received adjuvant chemotherapy in the SP vs. EP was 47 vs. 54% (p = 0.29). The median survival of SP vs. EP for PDAC was 19.5 vs. 9.5 months (p = 0.06). The 3-year DFS of SP vs. EP for the non-PDAC group was 71 vs. 49.5% (p = 0.009).
Discussion
The purpose of this study was to examine the outcomes of EP, grouping together the cohorts of VR and MVR, as recently advised by the ISGPS [11]. Currently, there is a lack of robust evidence comparing SP vs. EP, excluding extended lymphadenectomies [5]. Conducting a randomized control trial regarding the former would entail ethical issues, thus rendering importance to a retrospective analysis. The most pertinent issue with EP is if they can be performed with an acceptable morbidity, mortality, and survival outcome.
In our study, EP was associated with significantly higher blood loss, number of units of blood transfused, need for blood transfusion, and duration of surgery. However, overall, EP had a similar hospital stay, peri-operative morbidity and mortality, and re-admission rates as SP. The peri-operative mortality and morbidity for pancreatic adenocarcinoma patients undergoing EP were significantly higher than those undergoing SP. Significantly higher proportion of patients undergoing EP had LVI and PNI as well as R1 resections. EP was associated with a significantly lower DFS when compared to SP overall, for the PDAC and non-PDAC groups. However, following EP, patients with non-PDAC histology expectedly enjoyed a significantly better DFS as compared to PDAC.
The estimated blood loss and need for blood transfusion were significantly higher for EP in our study, similar to the observations by Shoup et al., two studies by Hartwig et al., and Burdelski et al., respectively, with the latter three including all types of pancreatic resections [22,23,24,25]. The blood loss for VR in our series is higher when compared to standard pancreatic resections (1300 vs. 800 ml). Similar results with regard to blood loss have been observed in another large single-institution series [26]. However, others have shown that concomitant vascular resections are associated with similar blood loss and transfusion requirement [27, 28]. Burdelski et al. and Hartwig et al. noted significantly increased morbidity (69 vs. 37% and 36.6 vs. 25.3%, respectively) but comparable in-hospital mortality (7 vs. 4% and 6.9 vs. 3.5%, respectively) when compared to SP, while the recent study by Hartwig et al. noted increased in-hospital and 90-day mortality [23,24,25]. Bhayani et al. observed significantly higher morbidity and mortality in patients who underwent MVR when compared to SP [29]. It was noted that our patients who underwent MVR+VR had a higher blood loss than those who underwent either alone. There are studies which have described comparable morbidity, as seen in our study. However, of these, Nikfarjam et al. and Seeliger et al. studied only MVR, and Dar et al. studied only VR and extended lymph node dissections [8, 30, 31].
In the studies by Hartwig, Burdelski, and Klempnauer et al., 28–38% of patients with MVR had concomitant distant metastases, making them unsuitable for comparison [23, 25, 32]. However, the recent publication by Hartwig had a cohort more comparable to ours, though in larger numbers [24]. Kulemann et al. reported higher peri-operative morbidity and mortality in MVR as compared to VR, in patients undergoing PD, a feature not seen in our study [9]. Comparable to the study by Temple et al. where 11% of patients who underwent EP with colonic resections received neoadjuvant therapy, 11% of our EP received neoadjuvant therapy [7]. While the majority of our VR were for pancreatoduodenectomies, MVR comprised more distal pancreatectomies. Also, EP had significantly more classical than pylorus-preserving surgeries, not found to influence morbidity.
Most studies differed from ours in that they either focused on left-sided [22, 31, 33, 34] or right-sided resections [7,8,9, 30]. Only the studies by Sasson et al. [35] and, recently, Hartwig et al. [24] had a study population most similar to ours that included all pancreatectomies, MVR, and VR. These studies differed from our study in that they included only PDAC, a more homogenous histology. The former had comparable morbidity (35 vs. 38%) and mortality (2.7 vs. 1.7%) for extended resections [35]. The significantly worse peri-operative morbidity and mortality seen in our study for PDAC undergoing EP are similar to the worse peri-operative outcomes following EP documented by Hartwig et al. [24].
One of the limitations of our study is the varied histology included. This was essential as our focus was mainly morbidity and mortality encountered in EP irrespective of tumor heterogeneity. A comparable morbidity and mortality for EP in our study reflects on a growing experience with complex pancreatic resections. Although the Indian setting entails a low incidence of PDAC, we do encounter neuroendocrine, cystic neoplasms, etc. with advanced presentation. The latter histologies may benefit more from radical surgeries than the otherwise traditionally aggressive PDAC. This is reflected in the survival difference we see between PDAC and their non-PDAC counterparts (median DFS 9.5 vs. 33.3 months, p < 0.001). Although histology of tumors and their corresponding survival has been analyzed, this was not our primary end-point. PDAC had inferior survival, trending towards significance, when they underwent EP when compared to SP (median DFS 9.5 vs. 19.5 months, p = 0.06). The inferior survival difference contradicts the ISGPS consensus [11] but is similar to the observations by De Reuver et al. [36] and recently by Hartwig et al. [24]. This may be the result of aggressive biology of tumors in the EP group, as reflected by their significantly larger size, higher PNI, and LVI. The other possible explanation may be significantly higher R1 resections seen in EP, in our series. These factors also explain the median DFS of 9.5 months following EP performed for PDAC. A larger cohort of PDAC followed up for a longer period can reflect the true picture. Contrastingly, Ravikumar et al. reported comparable PNI/LVI in patients undergoing VR [6]. Microscopic vascular invasion was noted in only 39% (12 of 31) of our VR patients. With vascular invasion ranging from 3 to 80%, it often occurs that tumors thought to have invaded the porto-mesenteric vasculature intra-operatively are often found to have only inflammatory adhesions to the resected vein on pathology [37,38,39]. Contrastingly, 82% (22 of 27) of our MVR demonstrated microscopic invasion of the additionally resected organs. Our EP had significantly more R1 resections (11 of 63, 18%) compared to SP (38 of 620, 6%) similar to Konstantinidis et al. who reported that patients with R1 resection had a longer survival compared with those who had locally advanced unresectable cancers (14 vs. 11 months; p < 0.001) [40]. Although R+ resections in EP ranges considerably from 9 to 39% [11], ESPAC-1 had initially suggested it to be a negative predictor of survival, only to be re-questioned by Tseng et al. as well as by the ESPAC-3 trial [41,42,43]. A significant proportion of our EP (73%) were operated over the last 3 years (2012–2015), indicating a combination of our growing experience with SP over a decade, coupled with the changing trends world over with more evidence-based acceptance for MVR and VR [7, 9, 11, 44,45,46,47]. More than 50% (36/63) of the resections in the extended pancreatectomy group were vascular resections. This is an indication of experience available at a high-volume center. Performing a vein resection vs. avoiding one is also a matter of experience which is difficult to document objectively, and a number of vein resections can be avoided with an increasing experience.
Another limitation of this study is the retrospective nature over a long study period of 9 years and the limited sample size of each cohort and type of surgery (PD vs. DP). Also, the smaller numbers of PDAC might under-power the study to detect smaller differences in outcome. While larger accrual would have facilitated more accurate survival data analyses, more MVR and VR would have allowed estimation of whether a specific organ (e.g., colon) or vessel (artery or vein), specifically contributed to increased morbidity. The VR and MVR clubbed together as “extended pancreatectomies” makes it a heterogeneous group. This along with the existing literature, which has inclusion and exclusion criteria similar and dissimilar to our own, precludes easy comparison [11].
Our study, however, provides evidence favoring EP, a procedure often condemned by surgeons. These resections can be performed with morbidity, mortality, hospital stay, and re-admission rates comparable to SP and also provide an acceptable long-term survival for non-PDAC histologies. This series, the first from India, clearly highlights the technical feasibility of these complex procedures. The study also underlines the importance of performing these demanding resections after careful patient selection and inexperienced high-volume units which are able to manage these demanding and challenging resections with their attendant problems. Future studies should aim at a larger accrual with mature long-term data and quality of life assessment, to further justify these radical procedures.
Conclusion
Extended pancreatectomies as defined by the ISGPS result in a similar peri-operative morbidity and mortality compared to standard pancreatectomies. Although the survival of patients undergoing these complex procedures is inferior to standard pancreatectomies for pancreatic ductal adenocarcinoma, they should be undertaken not only in selected cases of pancreatic cancer but even more so in other complex pancreatic tumors.
References
McDermott WV (1952) A one-stage pancreatoduodenectomy with resection of the portal vein for carcinoma of the pancreas. Ann Surg 136(6):1012–1018. https://doi.org/10.1097/00000658-195212000-00015
Fortner JG (1984) Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Ann Surg 199(4):418–425. https://doi.org/10.1097/00000658-198404000-00008
Baxter NN, Whitson BA, Tuttle TM (2007) Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol 14(4):1320–1326. https://doi.org/10.1245/s10434-006-9249-8
Gillen S, Schuster T, Meyer Zum BC et al (2010) Pre-operative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 7:e1000267
Gurusamy KS, Kumar S, Davidson BR et al (2014) Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev 2:CD010244
Ravikumar R, Sabin C, Hilal MA et al (2014) Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 218:401–411
Temple SJ, Kim PTW, Serrano PE, Kagedan D, Cleary SP, Moulton CA, McGilvray ID, Gallinger S, Greig PD, Wei AC (2014) Combined pancreaticoduodenectomy and colon resection for locally advanced peri-ampullary tumours: analysis of peri-operative morbidity and mortality. HPB 16(9):797–800. https://doi.org/10.1111/hpb.12263
Dar FS, Bhatti AB, Dogar AW (2015) Is pancreaticoduodenectomy with vascular resection a safe procedure in developing country? Early outcomes and review of national literature. Int J Surg 21:8–13. https://doi.org/10.1016/j.ijsu.2015.06.073
Kulemann B, Hoeppner J, Wittel U (2015) Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastointest Surg 19(3):438–444. https://doi.org/10.1007/s11605-014-2725-8
Shrikhande SV, Barreto SG (2010) Extended pancreatic resections and lymphadenectomy: an appraisal of the current evidence. World J Gatrointest Surg 2(1):39–46. https://doi.org/10.4240/wjgs.v2.i2.39
Hartwig W, Vollmer CM, Fingerhut A, Yeo CJ, Neoptolemos JP, Adham M, Andrén-Sandberg A, Asbun HJ, Bassi C, Bockhorn M, Charnley R, Conlon KC, Dervenis C, Fernandez-Cruz L, Friess H, Gouma DJ, Imrie CW, Lillemoe KD, Milićević MN, Montorsi M, Shrikhande SV, Vashist YK, Izbicki JR, Büchler MW, International Study Group on Pancreatic Surgery (2014) Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 156(1):1–14. https://doi.org/10.1016/j.surg.2014.02.009
World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/. Accessed November 13, 2016
Jagannath P, Dhir V, Shrikhande S et al (2005) Effect of preoperative biliary stenting on immediate outcome after pancreaticoduodenectomy. Br J Surg 92:356–361
Shrikhande SV, Barreto SG, Bodhankar YD, Suradkar K, Shetty G, Hawaldar R, Goel M, Shukla PJ (2011) Superior mesenteric artery first combined with uncinate process approach versus uncinate process first approach in pancreatoduodenectomy: a comparative study evaluating perioperative outcomes. Langenbeck's Arch Surg 396(8):1205–1212. https://doi.org/10.1007/s00423-011-0824-5
Shrikhande SV, Barreto G, Shukla PJ (2008) Pancreatic fistula after pancreaticoduodenectomy: the impact of a standardized technique of pancreaticojejunostomy. Langenbeck's Arch Surg 393:87–91
Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR, International Study Group of Pancreatic Surgery (2014) Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 155(6):977–988. https://doi.org/10.1016/j.surg.2014.02.001
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 138:8–13
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142(1):20–25. https://doi.org/10.1016/j.surg.2007.02.001
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Dindo D, Clavien PA (2004) Classification of surgical complications—a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Campbell F, Foulis AK, Verbeke CS (2010) Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct. Royal college of Pathologists (May 2010). Available from https://www.rcpath.org/resourceLibrary/g091-draftpancreasdataset-nov16.html
Shoup M, Conlon KC, Klimstra D, Brennan MF (2003) Is extended resection for adenocarcinoma of the body or tail of the pancreas justified? J Gastrointest Surg 7(8):946–952. https://doi.org/10.1016/j.gassur.2003.08.004
Hartwig W, Hackert T, Hinz U et al (2009) Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg 250:81–87
Hartwig W, Gluth A, Hinz U et al (2016) Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br J Surg 103:1683–1694
Burdelski CM, Reeh M, Bogoevski D et al (2011) Multivisceral resections in pancreatic cancer: identification of risk factors. World J Surg 35:2756–2763
Jeong J, Choi DW, Choi SH, Heo JS, Jang KT (2015) Long-term outcome of portomesenteric vein invasion and prognostic factors in pancreas head adenocarcinoma. ANZ J Surg 85(4):264–269. https://doi.org/10.1111/ans.12502
Malleo G, Maggino L, Marchegiani G et al (2017) Pancreatectomy with venous resection for pT3 head adenocarcinoma: perioperative outcomes, recurrence pattern and prognostic implications of histologically confirmed vascular infiltration. Pancreatology 17:847–857
Banz VM, Croagh D, Coldham C et al (2012) Factors influencing outcome in patients undergoing portal vein resection for adenocarcinoma of the pancreas. Eur J Surg Oncol 38:72–79
Bhayani NH, Enomoto LM, James BC, Ortenzi G, Kaifi JT, Kimchi ET, Staveley-O'Carroll KF, Gusani NJ (2014) Multivisceral and extended resections during pancreatoduodenectomy increases morbidity and mortality. Surgery 155(3):567–574. https://doi.org/10.1016/j.surg.2013.12.020
Nikfarjam M, Sehmbey M, Kimchi ET et al (2009) Additional organ resection combined with pancreaticoduodenectomy does not increase postoperative morbidity and mortality. J Gastrointest Surg 13:915–921
Seeliger H, Christians S, Angele MK et al (2010) Risk factors for surgical complications in distal pancreatectomy. Am J Surg 200:311–317
Klempnauer J, Ridder GJ, Bektas H, Pichlmayr R (1996) Extended resections of ductal pancreatic cancer-impact on operative risk and prognosis. Oncology 53(1):47–53. https://doi.org/10.1159/000227534
Roch AM, Singh H, Turner AP et al (2015) Extended distal pancreatectomy for pancreatic adenocarcinoma with splenic vein thrombosis and/or adjacent organ invasion. Am J Surg 209:564–569
Kleef J, Diener MK, Z’graggen K et al (2007) Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg 245(4):573–582. https://doi.org/10.1097/01.sla.0000251438.43135.fb
Sasson AR, Hoffman JP, Ross EA et al (2002) En bloc resection for locally advanced cancer of the pancreas: is it worthwhile? J Gastrointest Surg 6(2):147–157. https://doi.org/10.1016/S1091-255X(01)00063-4
De Reuver PR, Mittal A, Neale M et al (2015) Extended pancreatoduodenectomy as defined by the International Study Group for Pancreatic Surgery is associated with worse survival but not with increased morbidity. Surgery 158:183–190
Schafer M, Mullhaupt B, Clavien PA (2002) Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg 236:137–148
Shibata C, Kobari M, Tsuchiya T et al (2001) Pancreatectomy combined with superior mesenteric-portal vein resection for adenocarcinoma in pancreas. World J Surg 25:1002–1005
Yoshimi F, Asato Y, Tanaka R et al (2003) Reconstruction of the portal vein and the splenic vein in pancreaticoduodenectomy for pancreatic cancer. Hepato-Gastroenterology 50:856–860
Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Wargo JA, Lillemoe KD, Ferrone CR (2013) Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 257(4):731–736. https://doi.org/10.1097/SLA.0b013e318263da2f
Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, Bassi C, Dervenis C, Fernandez-Cruz L, Lacaine F, Buckels J, Deakin M, Adab FA, Sutton R, Imrie C, Ihse I, Tihanyi T, Olah A, Pedrazzoli S, Spooner D, Kerr DJ, Friess H, Büchler MW, European Study Group for Pancreatic Cancer (2001) Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 234(6):758–768. https://doi.org/10.1097/00000658-200112000-00007
Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB (2004) Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 8(8):935–949. https://doi.org/10.1016/j.gassur.2004.09.046
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW, European Study Group for Pancreatic Cancer (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304(10):1073–1081. https://doi.org/10.1001/jama.2010.1275
Shrikhande SV, Barreto SG, Somashekhar BA et al (2013) Evolution of pancreatoduodenectomy in a tertiary cancer centre in India: improved results from service reconfiguration. Pancreatology 13(1):63–71. https://doi.org/10.1016/j.pan.2012.11.302
Bockhorn M, Burdelski C, Bogoevski D et al (2011) Arterial en bloc resection for pancreatic carcinoma. Br J Surg 98:86–92
Nentwich MF, Konig A, Izbicki JR (2014) Limits of surgery for pancreatic cancer. Rozhl Chir 93:445–449
Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, Weitz J (2011) Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 254(6):882–893. https://doi.org/10.1097/SLA.0b013e31823ac299
Author information
Authors and Affiliations
Contributions
Abhishek Mitra—acquisition of data, analysis, interpretation of data, drafting of manuscript, critical revision of manuscript; Esha Pai—acquisition of data, analysis, interpretation of data, drafting of manuscript; Priya Ranganathan—analysis and interpretation of data, critical revision of manuscript; Ashwin DeSouza—critical revision of manuscript; Mahesh Goel—critical revision of manuscript; Shailesh V. Shrikhande—study conception and design, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Abhishek Mitra and Esha Pai share first authorship.
Rights and permissions
About this article
Cite this article
Mitra, A., Pai, E., Dusane, R. et al. Extended pancreatectomy as defined by the ISGPS: useful in selected cases of pancreatic cancer but invaluable in other complex pancreatic tumors. Langenbecks Arch Surg 403, 203–212 (2018). https://doi.org/10.1007/s00423-018-1653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-018-1653-6