Abstract
Purpose
Indications for total pancreatectomy (TP) have increased, including for diffuse main duct intrapapillary mucinous neoplasms of the pancreas and malignancy; therefore, the need persists for surgeons to develop appropriate endocrine post-operative management strategies. The brittle diabetes after TP differs from type 1/2 diabetes in that patients have absolute deficiency of insulin and functional glucagon. This makes glucose management challenging, complicates recovery, and predisposes to hospital readmissions. This article aims to define the disease, describe the cause for its occurrence, review the anatomy of the endocrine pancreas, and explain how this condition differs from diabetes mellitus in the setting of post-operative management. The morbidity and mortality of post-TP endocrine insufficiency and practical treatment strategies are systematically reviewed from the literature. Finally, an evidence-based treatment algorithm is created for the practicing pancreatic surgeon and their care team of endocrinologists to aid in managing these complex patients.
Methods
A PubMed, Science Citation Index/Social sciences Citation Index, and Cochrane Evidence-Based Medicine database search was undertaken along with extensive backward search of the references of published articles to identify studies evaluating endocrine morbidity and treatment after TP and to establish an evidence-based treatment strategy.
Results
Indications for TP and the etiology of pancreatogenic diabetes are reviewed. After TP, ~80% patients develop hypoglycemic episodes and 40% experience severe hypoglycemia, resulting in 0–8% mortality and 25–45% morbidity. Referral to a nutritionist and endocrinologist for patient education before surgery followed by surgical reevaluation to determine if the patient has the appropriate understanding, support, and resources preoperatively has significantly reduced morbidity and mortality. The use of modern recombinant long-acting insulin analogues, continuous subcutaneous insulin infusion, and glucagon rescue therapy has greatly improved management in the modern era and constitute the current standard of care. A simple immediate post-operative algorithm was constructed.
Conclusion
Successful perioperative surgical management of total pancreatectomy and resulting pancreatogenic diabetes is critical to achieve acceptable post-operative outcomes, and we review the pertinent literature and provide a simple, evidence-based algorithm for immediate post-resection glycemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical indications for total pancreatectomy (TP) have expanded over recent years. Though the radicality of resection for many pancreatic tumors has decreased, there has been an extension of resection criteria for pancreatic malignancies and metastatic disease, in addition to a greater understanding of the natural history of premalignant neoplasms, including diffuse main duct intraductal mucinous neoplasms of the pancreas (IPMN) [1,2,3,4,5]. The brittle diabetes that results from total pancreatectomy is a source of significant morbidity and mortality and a leading cause of post-operative hospital readmissions. The estimated healthcare cost of a patient with brittle diabetes in the USA is $1500 per year compared with $564 per year for patients with non-pancreatogenic diabetes [6]. These are likely underestimated figures, though the proportional cost increase of brittle diabetes over type 1 or 2 diabetes highlights that these patients consume both increased time and healthcare resources. We found a need for a standardized, simple, and easy to follow algorithm to manage patient blood glucose after TP. This article aims to define the disease, describe the cause for its occurrence, review the anatomy of the endocrine pancreas, and explain how this condition differs from diabetes mellitus in the setting of post-operative management. The morbidity and mortality of post-TP endocrine insufficiency and practical treatment strategies are systematically reviewed from the literature. Finally, an evidence-based treatment algorithm is created for the practicing pancreatic surgeon and their care team of endocrinologists to aid in managing these complex patients.
Materials and methods
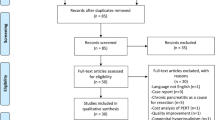
A systematic review was performed, in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement, to identify articles published that addressed post-pancreatectomy diabetes. An electronic literature search was performed of all publications from January 1, 1997 to January 1, 2017 to identify published data on pancreatogenic diabetes after total pancreatectomy. Databases searched were PubMed, Science Citation Index/Social sciences Citation Index, and Cochrane Evidence-Based Medicine. Terms used in the search were “total AND pancreatectomy AND diabetes.” The search strategy within PubMed was further enhanced to retrieve citations identified as systematic reviews, meta-analyses, reviews of clinical trials, evidence-based medicine, consensus development conferences, guidelines, and citations to articles from journals specializing in review studies of value to clinicians to identify all articles related to this topic that address the endocrine morbidity of TP and treatment strategies. Backward search of the references of published articles to identify studies evaluating endocrine morbidity and treatment after TP and to establish an evidence-based treatment strategy were added. Studies were included if they described physiological function of the pancreas, brittle diabetes following pancreatectomy, morbidity, and mortality of post-pancreatectomy diabetes or management of post-pancreatectomy diabetes. All series satisfying these criteria were included regardless of the size of the study population. Case reports, editorials, and unpublished data from conference abstracts were excluded. The initial search identified 588 articles. Applying the filters described resulted in the exclusion of 254 articles.
Results
Definition and causes of brittle diabetes
In the 1930s, Chicago physician R.T. Woodyatt introduced the concept of brittle diabetes as excessive fluctuations of blood sugar that could not be explained by the patient or by physician errors [7, 8]. Endocrine insufficiency secondary to TP can result in wide, fast, unpredictable, and inexplicable swings in blood glucose concentration, often resulting in ketoacidosis or hypoglycemic coma. These swings occur despite constancy in insulin injections, exercise, and diet [9]. These patients may require multiple or prolonged hospitalizations which disrupt daily life and cause mental and financial burden on the family and the healthcare system [7]. The diabetic state induced by total pancreatectomy is characterized by complete insulin deficiency (as confirmed by the absence of C-peptide in the serum), pancreatic polypeptide deficiency, and an absence of functional glucagon. Because the apancreatic state also results in a defect in gluconeogenesis secondary to hypoglucagonemia, daily insulin requirements in these patients are typically lower than in type 1 or type 2 diabetics [10, 11]. These patients also demonstrate increased insulin sensitivity secondary to increased expression of peripheral insulin receptors and enhanced plasma clearance of insulin. Hence, the therapeutic window to maintain euglycemia is narrowed, resulting in frequent episodes of mild to severe post-prandial hypoglycemia following insulin administration [12].
Whereas patients with diabetes mellitus will often be chronically hyperglycemic, patients with brittle diabetes on insulin who are chronically hypoglycemic have been shown to initiate upregulation of cerebral endothelial glucose transporters [13]. Due to enhanced brain glucose uptake, counter-regulatory hormones are not secreted leading to systemic episodes of diabetic hypoglycemic unawareness in pancreatectomized individuals [14, 15].
Pertinent anatomy of pancreas
The mature pancreas is composed of morphologically and functionally distinct endocrine and exocrine components. The endocrine pancreas functions in a “checks and balances” system, the details of which are pertinent to the surgeon planning a TP and briefly reviewed.
Endocrine pancreas
The endocrine cells form aggregates scattered throughout the exocrine pancreas in the form of islets of Langerhans. The normal human adult pancreas contains about one million islets of Langerhans, constituting 2–3% of the gland’s volume. A typical islet is composed of ~5000 endocrine cells. The islets contain five endocrine cell types—beta cells (β), glucagon-producing alpha cells (α), somatostatin-producing delta cells (δ), ghrelin-producing gamma cells (γ), and pancreatic polypeptide-producing PP cells [16].
Insulin
Insulin is a 51-amino acid peptide synthesized and secreted by the pancreatic beta cells throughout the pancreas. Insulin has a number of effects on glucose metabolism including inhibition of glucagon, glycogenolysis, and gluconeogenesis; increased glucose transport into fat and muscle via GLUT4; increased glycolysis in fat and muscle; and stimulation of glycogen synthesis [17,18,19]. Even partial pancreatectomy can decrease insulin secretion, leading to post-operative diabetes; however, in the setting of a total pancreatectomy, exogenous insulin treatment is necessary to control inevitable hyperglycemia.
Glucagon
Glucagon is a 29-amino acid peptide synthesized from proglucagon in alpha cells located predominantly in the body and tail of the pancreas [20]. The function of glucagon is to increase blood glucose concentration via stimulation of hepatic gluconeogenesis and glycogenolysis, in addition to stimulation of insulin and somatostatin secretion [17, 21]. Glucagon decreases pancreatic juice volume, protein, amylase, and bicarbonate content. It has a complex role in positive and negative feedback loops to regulate glucose homeostasis, which is primarily why pancreatogenic diabetes is so labile/brittle after TP.
Somatostatin
Somatostatin is present in δ cells of the islets, the small intestine, and in nerve terminals [22]. It is a potent inhibitor of insulin, glucagon, and PP secretion. Secretion of somatostatin is inhibited by insulin [23, 24]. Therefore, in the apancreatic state, this endocrine regulatory hormone is also unable to control glucose homeostasis.
Indications for TP
Due to long-term metabolic complications, difficulty in managing brittle diabetes, and the ensuing reduced quality of life, enthusiasm for TP has significantly waned over the last few decades [25]. However, advances in insulin formulations and modern pancreatic enzyme preparations have allowed sufficient control of endocrine and exocrine pancreatic insufficiency enabling a reasonable quality of life and decreased long-term morbidity. This, combined with improvement in the safety of pancreatic surgery, has decreased the morbidity and mortality of the procedure and reintroduced interest in TP in the treatment of pancreatic diseases [1, 26].
Premalignant lesions
Distinct non-invasive precursor lesions which can give rise to invasive carcinoma of the pancreas include pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasms (IPMNs) [27]. Patients with widespread and multifocal advanced pancreatic intraepithelial neoplasia may have a field defect in the gland that carry increased risk of progression to pancreatic adenocarcinoma. In highly selected patients, especially with a significant family history, prophylactic TP may be considered to avert the development of pancreatic carcinoma [28].
In addition, early studies of mucinous cystic lesions of the pancreas identified that approximately 30 to 40% of patients harbored an invasive malignancy at diagnosis. The remainder of patients in that report had intraductal micropapillary changes with atypia, dysplasia, or carcinoma in situ. The changes were multifocal and occurred throughout the gland; thus, it was defined that intraductal papillary mucinous neoplasms (IPMNs) were felt to represent a global disorder of the ductal epithelium and not just a localized defect [29]. In more modern series including updates to the original Sendai criteria, main duct IPMN, in particular, has been found to have significant risk of occult malignancy and malignant transformation. For patients with diffuse main duct IPMN along the length of the gland, current consensus from the International Association of Pancreatology IPMN working group and the European Study Group on Cystic Tumours of the Pancreas stipulate that TP may be selectively employed, particularly in high-risk IPMN with severe dysplasia at the surgical margin [30, 31]. For these high-risk lesions in few and limited highly selected individuals, TP is recommended until additional biomarkers reflective of the biology of disease can be identified [26, 31]. Similarly, for diffuse branch-duct IPMN with high-risk features or malignancy in multiple lesions spread throughout the gland, TP may be considered, though usually, each cyst may be individually considered for resection as an independent neoplasm [26, 31].
Familial pancreatic cancer
Familial pancreatic cancer implies that two or more first degree relatives experienced pancreatic cancer [32] and accounts for 3–10% of all cases of pancreatic cancer [33]. In familial disease, the risk of pancreatic cancer increases with the number of relatives affected. With one, two, and three affected family members, the risk of invasive disease is 4.6, 6.4, and 32 times for at-risk individuals [34]. Several pancreatic cancer hotspots have been identified, including both in high-penetrance genes such as BRCA2, STK11, p16/CDKN2, and PALB2 and in low-penetrance genes such as the ABO blood group locus [35]. Though a very rare indication, and only in highly selected individuals from these hereditary pancreatic cancer families, total pancreatectomy may be considered to eliminate or decrease the risk of life threatening malignancy where there is widespread and multifocal neoplasia throughout the gland [36, 37].
R0 resection
For patients with localized pancreatic adenocarcinoma, positive resection margins are a negative prognostic feature [38,39,40,41]. Completion pancreatectomy has been shown to improve survival in isolated pancreatic neck margin positive patients and has been recommended if necessary to achieve R0 resection after pancreaticoduodenecomy [42]. Similarly, for main duct IPMN with high-grade dysplasia at the margin, completion pancreatectomy or further resection to a negative margin may be considered. In many cases, simply extending the resection may avoid the need for total pancreatectomy and achieve negative margins, though in cases where arterial resection is necessary, total pancreatectomy may be performed to reduce the risk of pancreatic fistula induced pseudoaneurysm of a fresh vascular anastomosis in high-risk patients [37]. Furthermore, in highly selected patients with pancreas only metastases, most often from renal cell carcinoma, total pancreatectomy may be indicated after multidisciplinary discussion [43, 44].
Pancreatic fistula
Pancreatic fistula is a known complication after partial pancreatectomy [45,46,47,48]. Techniques of pancreatico-enteric reconstruction have been extensively studied and have reduced but not eliminated the risk of pancreatic leak. Prognostic factors for pancreatic anastomosis failure are well known including small pancreatic duct, soft gland texture, surgical technique, and extent of resection [49,50,51,52,53]. Though the majority of pancreatic fistulae will heal with appropriate drainage and clinical management, surgical intervention is occasionally necessary i.e., in type C fistulas. In instances when the patient condition or tissues are not amenable to creating a new anastomosis, or in the situation of peritonitis or bleeding, the safest procedure to eliminate pancreatic fistula may be completion pancreatectomy.
Chronic pancreatitis
Pain is the most common indication for surgery in patients with chronic pancreatitis, usually when it is recalcitrant to management with analgesics and pancreatic enzymes [54]. Although not very well understood [55], compartment syndrome, which is probably caused by elevated pressure in the main pancreatic duct, might be the origin of the pain [56,57,58]. Total pancreatectomy remains controversial as treatment for chronic pancreatitis but has a role in patients with severe pain resistant to other medical and surgical procedures and for significant intraductal obstruction not amenable to pancreatic surgical drainage procedures [56,57,58,59].
Neuroendocrine tumors
Pancreatic neuroendocrine tumors are rare neoplasms, and represent around 1–2% of all pancreatic tumors with an incidence of approximately 1/100,000 population. Surgical treatment for pancreatic neuroendocrine tumors varies according to site and size of tumor, if it is single or multiple, if it is benign or malignant, and if it is associated with multiple endocrine neoplasia (MEN) type 1 [60,61,62,63]. TP has a role in surgical management of recurrent, multicentric, and locally advanced neuroendocrine tumors [64, 65].
Nesidioblastosis
Nesidioblastosis is a condition that is characterized by hypertrophy and hyperplasia of the islets of Langerhans. It can result in persistent hyperinsulinemia with hypoglycemia and is the leading cause of hyperinsulinemic hypoglycemia in childhood, whereas in adults, it represents 0.5–5% of cases [66, 67]. Early diagnosis of this condition in infants is essential because it may lead to cerebral palsy, impaired mental development, epilepsy, or other forms of irreversible brain damage [68, 69]. Recent associations with bariatric surgery have also been identified. Near total pancreatectomy (95% resection) should be considered early when this condition is resistant to standard medical management and often results in difficult to control diabetes [70, 71].
Morbidity and mortality of post-pancreatectomy diabetes
Currently, elective TP leads to perioperative morbidity and mortality comparable to that of the other pancreatic resection procedures and without the major morbidity of a pancreato-enteric anastomosis. Despite limitations caused by the ensuing insulin-dependent diabetes, the overall quality of life is acceptable, and the limitations do not justify avoiding TP in patients in whom the complete removal of the pancreas would be beneficial [25, 26, 72].
The diabetes post-TP is characterized by frequent hypoglycemic episodes and severe malabsorption owing to exocrine insufficiency [25, 73,74,75,76,77]. Other metabolic consequences include osteoporosis, osteopathy, and hepatic dysfunction [25, 74].
Mortality rates vary from 0 to 8% and morbidity rates vary from 25 to 45% [25, 42, 73, 78,79,80].(Table 1). Pancreatogenic diabetes remains a major source of morbidity. It leads to frequent episodes of hypoglycemia and or ketoacidosis that can be difficult to control [74]. In a study from the Mayo Clinic, 79% of TP patients reported hypoglycemic episodes, 41% experienced severe hypoglycemia, and 4% developed DKA [82]. Barbier and colleagues reported that 40% of TP patients experienced loss of consciousness owing to hypoglycemia, and had a median of 10 episodes per month [79]. Overall mortality and morbidity of TP, as well as brittle diabetes-specific mortality and morbidity rates, are summarized in Table 1. Hospital readmission rates up to 16% due to of complications of pancreatogenic diabetes can be expected after TP [25].
HbA1c levels can be used as an indication of glycemic control over the previous 60 days for patients after TP [83]. Elevated levels of 6.7–11% have been reported [25, 74, 77, 78, 84, 85] though recent studies reflect lower HbA1c levels believed to be due to advances in diabetes management [82]. This is reflected in a recent study by Roberts and colleagues who reported almost no difference in diabetes-specific outcomes or control as assessed by a diabetes-specific questionnaire [86].
Weight loss is common after TP in 40 to 85% patients. With an average weight loss of 7.5 kg [25, 73, 74, 78,79,80, 82]. Quality of life (QoL) does remain an issue after TP. Though Muller et al. reported comparable global health status of patients after TP compared to a control group undergoing pancreaticoduodenectomy using a robust QoL questionnaire [25], Barbier et al. reported a global health quality of life score of 64 (0–100) after TP [79]. In general, with appropriate glucose management strategies, QoL after TP may be comparable to QoL after partial pancreatectomy [87].
Management
Management is based on patient education, glycemic control, and close collaboration between the surgeon, endocrinologist, and other key healthcare providers including outpatient clinic nurses and dieticians.
Education
The first priority in regard to treatment is to ensure that the patients have sufficient education in the management of their diabetes [88]. A thorough practitioner explanation and demonstrated patient comprehension of the physiology of pancreatogenic diabetes is critical. Preoperatively, patients should be evaluated by an endocrinologist and taught how to self-administer insulin and to perform blood glucose monitoring. We feel that inabilities to perform these tasks, or a lack of understanding on the part of the patient and/or the family, are contraindications for recommending or performing TP. It is critical to identify patients preoperatively who are incapable or non-compliant, for any reason, of having an ability to monitor and maintain glycemic control. Patients are educated regarding hypoglycemia and the use of glucose tablets. Data supports improved outcomes with follow-up for outpatient diabetes education with their dietician and endocrinologist [89]. An important consideration in some difficult to control cases, however, is that long-term avoidance of hypoglycemic episodes is a primary concern, and both patient and endocrinologist expectations of glucose set-points may need to be liberalized on an individual basis for patient safety and quality of life, though at the possible risk of added secondary long-term hyperglycemic complications. Furthermore, in addition to education on glycemic control, the importance of appropriate caloric intake is important to review with the patient, including recommendations for a mixture of short- and long-acting carbohydrates, adequate protein intake based on their weight, and small, but frequent meals.
Insulin therapy
Insulin can be delivered in secondary diabetes by means of insulin pumps/continuous subcutaneous insulin infusion (CSII) or multiple daily insulin injections (MDII) [90]. Unfortunately, all large diabetes clinical trials studying glycemic control have excluded patients with pancreatogenic/type 3c diabetes. In the absence of generally accepted guidelines for the management of these patients, it is has been consistently recommended, including by the PancreasFest Recommendation Conference, that insulin dosing guidelines for type 1 diabetes mellitus be followed [91, 92]. After pancreatectomy, continuous insulin infusion at 0.1 U/kg/h should begin along with dextrose fluids (D5NS to provide 150 g/24 h), requirements thereafter calculated, and then 60–80% replaced in the subsequent days by one half basal (most commonly glargine insulin) and one half immediate release insulin formulations (most commonly aspart or lispro insulin). It is imperative to administer the basal insulin dose 1–2 h before discontinuation of insulin infusion. Basal doses are then increased by 2 U q3d until fasting levels are appropriate. If fasting glucose is <70 mg/dl, then the basal dose is decreased by 4 U. If glucose is 70–130, then the same dose is continued. Blood sugars are checked a minimum of four times a day before each meal and at bedtime. The glycemic goals for hospitalized patients are 140–180 mg/dl, preprandial <140 mg/dl and maximum blood sugar <180 mg/dl [93, 94]. Insulin treatment with a basal formulation of glargine has markedly improved glycemic control in these patients. This recombinant, long-acting formulation has become increasingly available and allows a slow and steady absorption profile that limits peaks and troughs in insulin levels, thereby enabling most patients to achieve acceptable glycemic control [26]. When discontinuing intravenous insulin, this type of transition protocol is associated with less surgical morbidity/mortality and lower costs of care [95]. In our experience and based on a combination of the 2009 and 2017 American Diabetes Association guidelines, this simple and effective post-operative treatment algorithm for patients after TP is efficient and effective [93, 94]. It should be mentioned that these protocols are based on data from type 1 diabetes patients, and not only will less insulin likely be necessary to achieve the same glycemic set-points in apancreatic patients, but extra care and consideration for acceptance of permissive hyperglycemia may be acceptable to repeated episodes of hypoglycemia in this patient population on a per-patient basis.
Jethwa et al. reported that TP patients required a median starting insulin dose of 34 U (range 8–88 U) daily and median dose of 46 U (range, 8–88 U) after optimization [77]. In the Mayo clinic series, daily mean insulin requirement of 32 U daily was reported (range, 2–66 U) [73]. Data regarding the number of hypoglycemic episodes and predicted daily insulin requirements after total pancreatectomy are summarized in Table 2.
Patients may be transitioned to insulin pumps/CSII for optimization of glucose control and improvement in life style. CSII is the “gold standard” of basal insulin replacement and has the unique ability to deliver insulin in a continuous mode mimicking the endogenous insulin production of the pancreas. The rate of basal insulin by CSII can be delivered according to the patient’s needs. This approach is successful because CSII infuses reliably even at low rates of 0.05–0.1 U/h. An insulin to carbohydrate ratio and a correction factor (sensitivity factor) is used to individualize a patient’s insulin requirements based on the number of carbohydrates one consumes per meal while on the pump [96]. This outpatient strategy is a logical extension of the perioperative continuous post-operative blood glucose monitoring randomized controlled trial performed using an “artificial pancreas” that showed excellent glycemic control after pancreatic resection [97].
In most patients on CSII, mean blood glucose concentrations and glycated hemoglobin percentages are either slightly lower or similar to multiple insulin injections. However, hypoglycemia is markedly less frequent than during intensive injection therapy. Ketoacidosis occurs at the same rate. Nocturnal glycemic control is improved with insulin pumps, and automatic basal rate changes help to minimize the prebreakfast blood glucose increase (the “dawn phenomenon”) often seen with injection therapy [98].
Papygyri et al. reported the experience with use of continuous subcutaneous insulin infusion in 112 type 1 diabetic patients followed for 7 years and previously treated with multiple daily insulin injections (MDII). HbA1c decreased by 0.6–0.9% [99]. Several other studies have shown that CSII results in better glycemic control than multiple injections, with an improvement of HBA1c of 0.2–0.4%, with a decrease in hypoglycemic events [98, 100,101,102,103,104,105]. Hirsch et al. evaluated CSII of insulin aspartame versus MDII of insulin aspartame/insulin glargine in type 1 diabetic patients previously treated with CSII [106]. They found that CSII therapy resulted in lower glycemic exposure without increased risk of hypoglycemia, as compared with MDI. The main complication reported with CSII, mainly at the start of the treatment, is hypoglycemic episodes. Specific patient training and fine adjustment of insulin infusion doses are often sufficient to minimize this complication [99].
Real-time continuous glucose monitors are also being used for better glucose control. They check interstitial glucose levels every 5 min and provide 288 blood glucose readings daily along with graphs to give patients a better sense of their sugars. This may enable them to adjust their insulin requirements and also prevent the frequency and the severity of hypoglycemia. In a randomized trial of 322 adults and children with type 1 diabetes and baseline A1C level ≥ 7.0%, the Juvenile Diabetes Research Foundation (JDRF) Continuous Glucose Monitoring Study Group reported that real-time continuous monitors substantially improved A1C levels without increasing the frequency of hypoglycemia in adult’s ≥25 years of age [107].
It should be considered, however, that the role of CSII and insulin pumps specifically after TP is not well studied, and much of the data is from the type 1 diabetes literature. These patients clearly have less of a risk of hypoglycemia compared to TP patients, and thus, the data available to the surgeon and endocrinologist in using this strategy after TP is useful to extrapolate from, but remains limited.
Hypoglycemia
As mentioned, there remains the risk of hypoglycemia in these patients. Asymptomatic hypoglycemia (<70 mg/dl) should be treated with carbohydrate ingestion and adjustment of the insulin regimen [108]. Symptomatic patients should treat themselves with 15–20 g of a fast acting carbohydrate (fruit juice, glucose tablet, or hard candy), and severe hypoglycemia may require administration of glucagon (0.5–1 mg). If needed, it is typically administered intramuscularly, though a recent trial demonstrated similar efficacy in rescue with both intranasal and intramuscular glucagon [109]. That glucagon treatment is sometimes required and may need to be administered by someone other than the patient underscores the importance of education and assessment of both comprehension and home support in evaluating the patient for suitability for TP. Though glucagon replacement therapy has been attempted in small numbers of apancreatic patients [110, 111], with adequate education, insulin adjustment based on carbohydrate/total caloric ratios, and the more recent formulations and use of long-acting basal insulin, in our experience, it is rarely needed and certainly not routinely.
Islet cell transplantation
Islet cell transplantation (ICT) is a promising procedure for treatment of diabetic patients with brittle diabetes. The indications include hypoglycemic unawareness (i.e., onset not felt with glucose level ≤54 mg/dl) and/or metabolic lability (i.e., two or more severe hypoglycemic or ketoacidosis episodes requiring third party assistance within 1 year) and progressive chronic complications despite intensive insulin treatment [112]. Since 1999, allogenic islet transplantation data from North America, Europe, and Australia were submitted to the Collaborative Islet Transplantation Registry (CITR). Twenty-five US/Canadian medical institutions, two European centers, and an Australian center participated. The study reported that 71% of these patients were insulin independent at 1 year and 52% remained insulin independent at 2 years. Islet transplantation markedly reduced the occurrence of severe hypoglycemic events from more than 60% before transplant to <10% after 5 years. Islet transplantation also substantially improved HbA1c levels [113, 114]. In addition, in the recently reported phase 3 trial of islet cell allotransplantation by the Clinical Islet Transplantation (CIT) Consortium for patients with type 1 diabetes, the primary endpoint of achieving HgA1c levels of ≤7% was achieved in ~88% of patients, as was the goal of decreasing severe hypoglycemic events [115]. Insulin independence at 1 year was 52 and 42% at 2 years in this study, further supporting both previous trials and continued research of this intervention for this indication.
However, in addition to limited resources and availability, there are several limitations to ICT including the need for several donors for sufficient islet yield, the risks of lifelong immune suppression, and the progressive loss of islet function over time [116]. β cell regeneration from naїve pancreas and β cell generation from embryonic stem cells or induced pluripotent stem cells are potential approaches to manage diabetes after TP on the horizon. [117].
It is critical to note, however, that islet cell transplantation after surgery is primarily intended after TP only for highly selected patients with chronic pancreatitis, which is even more selective for allotransplantation in this disease, which in some institutions is only 1% of all considered cases [118, 119]. The issue with autotransplantation after resection of malignant or diffuse mucinous tumors is the potential seeding of malignant or premalignant cells. Thus, most centers consider this an absolute contraindication to autotransplantation [120], though a recent series of 31 patients with malignant pancreatic/ampullary disease have undergone the procedure [121]. In regard to islet allotransplantation, one of the main concerns is the obligatory immunosuppression and the risk of inducing malignancy [122], which is only further compounded in the setting of pancreatic neoplasia, and thus almost universally avoided therein. This limitation of immunosuppression is shared whether the allotransplant is of islets or the gland. An advantage of whole gland allotransplant theoretically is concomitant correction of exocrine insufficiency; however, data is limited comparing islet to gland transplantation after TP in terms of glycemic control, though graft survival rates of >75% can be achieved in this setting [123].
Conclusions
Oncologic indications for total pancreatectomy have increased. We found that there was a paucity of information on endocrine management of TP in this setting, particularly in the surgical literature, and that there was a need for a straightforward and easy to follow algorithm for surgeons to manage patients post-operatively after TP. This study is limited by a lack of randomized controlled trials, systematic reviews, or large cohort studies to address the endocrine needs of patients after TP in the literature. As a result, the review is limited to case series, observational studies, and extrapolation of data from type 1 and 2 diabetes; thus, the level of evidence of the available data is low, limiting the strength of our recommendations. This limitation spotlights the critical need for a systematic review of this nature; therefore, the available data was comprehensively summarized for the practicing pancreatic surgeon. The causes of brittle diabetes after TP were reviewed as they pertain to the pertinent anatomy and physiology of the endocrine pancreas, and the significance of the ensuing morbidity and mortality was highlighted. Strategies to reduce hyper- and hypoglycemic episodes depend greatly on patient education, and patients unable to comprehend treatment algorithms or to be compliant with outpatient visits and blood glucose management strategies are not recommended for TP. Close outpatient follow-up with endocrine nurses and dieticians have improved outcomes, as have personalized insulin therapies, especially with continuous subcutaneous insulin injections and real-time continuous glucose monitoring. A practical post-operative treatment algorithm based on the limited available data has been devised and is currently employed at our center. Future strategies may include more widespread use of islet transplantation (in non-neoplastic settings) or beta cell generation from stem cells.
References
Murphy MM, Knaus WJ 2nd, Ng SC, Hill JS, McPhee JT, Shah SA, Tseng JF (2009) Total pancreatectomy: a national study. HPB (Oxford) Sep;11(6):476-82. doi: 10.1111/j.1477-2574.2009.00076.x.
Blanchet MC, Andreelli F, Scoazec JY, Le Borgne J, Ozoux P, De Calan L et al (2002) Total pancreatectomy for mucinous pancreatic tumor. Ann Chir 127(6):439–448
Sauvanet A (2008) Intraductal papillary mucinous neoplasms of the pancreas: indication, extent, and results of surgery. Surg Oncol Clin N Am 17(3):587–606 ix
Yamaguchi K, Konomi H, Kobayashi K, Ogura Y, Sonoda Y, Kawamoto M et al (2005) Total pancreatectomy for intraductal papillary-mucinous tumor of the pancreas: reappraisal of total pancreatectomy. Hepato-Gastroenterology 52(65):1585–1590
Zerbi A, Ortolano E, Balzano G, Borri A, Beneduce AA, Di Carlo V (2008) Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol 15(4):1161–1168. doi:10.1245/s10434-007-9782-0
Willens D, Cripps R, Wolff K, Rothman R (2011) Interdisciplinary team care for diabetic patients by primary care physicians, advanced practice nurses, and clinical pharmacists. Clin Diabetes 29(2):60–68
Tattersall R, Gregory R, Selby C, Kerr D, Heller S (1991) Course of brittle diabetes: 12 year follow up. BMJ 302(6787):1240–1243
Tattersall RB (1997) Brittle diabetes revisited: the third Arnold Bloom Memorial Lecture. Diabet Med 14(2):99–110. doi:10.1002/(SICI)1096-9136(199702)14:2<99::AID-DIA320>3.0.CO;2-I
Pickup JC, Home PD, Bilous RW, Keen H, Alberti KG (1981) Management of severely brittle diabetes by continuous subcutaneous and intramuscular insulin infusions: evidence for a defect in subcutaneous insulin absorption. Br Med J (Clin Res Ed) 282(6261):347–350
Muller WA, Brennan MF, Tan MH, Aoki TT (1974) Studies of glucagon secretion in pancreatectomized patients. Diabetes 23(6):512–516
Vigili de Kreutzenberg S, Maifreni L, Lisato G, Riccio A, Trevisan R, Tiengo A et al (1990) Glucose turnover and recycling in diabetes secondary to total pancreatectomy: effect of glucagon infusion. J Clin Endocrinol Metab 70(4):1023–1029
Nosadini R, del Prato S, Tiengo A, Duner E, Toffolo G, Cobelli C et al (1982) Insulin sensitivity, binding, and kinetics in pancreatogenic and type I diabetes. Diabetes 31(4 Pt 1):346–355
Boyle PJ (1997) Alteration in brain glucose metabolism induced by hypoglycaemia in man. Diabetologia 40(Suppl 2):S69–S74
Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV (1988) Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 37(7):901–907
Horie H, Matsuyama T, Namba M, Itoh H, Nonaka K, Tarui S et al (1984) Responses of catecholamines and other counterregulatory hormones to insulin-induced hypoglycemia in totally pancreatectomized patients. J Clin Endocrinol Metab 59(6):1193–1196
Bonner-Weir S (1991) Anatomy of islet of Langerhans. In: Samols E (ed) The endocrine pancreas. Raven Press
Klover PJ, Mooney RA (2004) Hepatocytes: critical for glucose homeostasis. Int J Biochem Cell Biol 36(5):753–758
Ramnanan CJ, Edgerton DS, Rivera N, Irimia-Dominguez J, Farmer B, Neal DW et al (2010) Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 59(6):1302–1311. doi:10.2337/db09-1625
Kahn BB (1996) Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes 45(11):1644–1654
Slezak LA, Andersen DK (2001) Pancreatic resection: effects on glucose metabolism. World J Surg 25(4):452–460. doi:10.1007/s002680020337
Reichlin S (1983) Somatostatin (second of two parts). N Engl J Med 309(25):1556–1563. doi:10.1056/nejm198312223092506
Beglinger C, Drewe J (1999) Somatostatin and octreotide: physiological background and pharmacological application. Digestion 60(Suppl 2):2–8 doi:51474
McCann SM, Krulich L, Negro-Vilar A, Ojeda SR, Vijayan E (1980) Regulation and function of panhibin (somatostatin). Adv Biochem Psychopharmacol 22:131–143
Reichlin S (1983) Somatostatin. N Engl J Med 309(24):1495–1501. doi:10.1056/nejm198312153092406
Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U et al (2007) Is there still a role for total pancreatectomy? Ann Surg 246(6):966–974 discussion 74-5
Heidt DG, Burant C, Simeone DM (2007) Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg 11(2):209–216. doi:10.1007/s11605-006-0025-7
Hruban RH, Goggins M, Parsons J, Kern SE (2000) Progression model for pancreatic cancer. Clin Cancer Res 6(8):2969–2972
Kekis PB, Friess H, Kleeff J, Buchler MW (2001) Timing and extent of surgical intervention in patients from hereditary pancreatic cancer kindreds. Pancreatology 1(5):525–530
Sarr MG, Kendrick ML, Nagorney DM, Thompson GB, Farley DR, Farnell MB (2001) Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. Surg Clin North Am 81(3):497–509
Del Chiaro M, Verbeke C, Salvia R, Kloppel G, Werner J, McKay C et al (2013) European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 45(9):703–711. doi:10.1016/j.dld.2013.01.010
Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY et al (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197. doi:10.1016/j.pan.2012.04.004
Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF et al (2006) Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomark Prev 15(4):704–710. doi:10.1158/1055-9965.EPI-05-0734
(2011) and hereditary syndromes: screening strategy for high-risk individuals. J Gastroenterol 46(11):1249–1259. doi:10.1007/s00535-011-0457-z
Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ et al (2004) Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 64(7):2634–2638
Klein AP (2012) Genetic susceptibility to pancreatic cancer. Mol Carcinog 51(1):14–24. doi:10.1002/mc.20855
Brentnall TA (2005) Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options in Oncol 6(5):437–445
Kulu Y, Schmied BM, Werner J, Muselli P, Buchler MW, Schmidt J (2009) Total pancreatectomy for pancreatic cancer: indications and operative technique. HPB 11(6):469–475. doi:10.1111/j.1477-2574.2009.00085.x
Hartel M, Wente MN, Di Sebastiano P, Friess H, Buchler MW (2004) The role of extended resection in pancreatic adenocarcinoma: is there good evidence-based justification? Pancreatology 4(6):561–566
Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S (2006) Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg 10(4):511–518
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H et al (2006) Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 13(8):1035–1046. doi:10.1245/ASO.2006.08.011
Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW (2004) Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 91(5):586–594. doi:10.1002/bjs.4484
Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q et al (2007) Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery 142(4):572–578 discussion 8-80
Konstantinidis IT, Dursun A, Zheng H, Wargo JA, Thayer SP, Fernandez-del Castillo C et al (2010) Metastatic tumors in the pancreas in the modern era. J Am Coll Surg 211(6):749–753. doi:10.1016/j.jamcollsurg.2010.08.017
Reddy S, Edil BH, Cameron JL, Pawlik TM, Herman JM, Gilson MM et al (2008) Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann Surg Oncol 15(11):3199–3206. doi:10.1245/s10434-008-0140-7
Buchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’Graggen K (2003) Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 138(12):1310–1314; discussion 5. doi:10.1001/archsurg.138.12.1310
Welsch T, Frommhold K, Hinz U, Weigand MA, Kleeff J, Friess H et al (2008) Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery 143(1):20–28
Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J et al (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10(9):1199–1210 discussion 210-1
Z’Graggen K, Uhl W, Friess H, Buchler MW (2002) How to do a safe pancreatic anastomosis. J Hepato-Biliary-Pancreat Surg 9(6):733–737. doi:10.1007/s005340200101
Bartoli FG, Arnone GB, Ravera G, Bachi V (1991) Pancreatic fistula and relative mortality in malignant disease after pancreaticoduodenectomy. Review and statistical meta-analysis regarding 15 years of literature. Anticancer Res 11(5):1831–1848
Buchler MW, Kleeff J, Friess H (2007) Surgical treatment of pancreatic cancer. J Am Coll Surg 205(4 Suppl):S81–S86
Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD (2004) Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg 8(8):951–959
Muscari F, Suc B, Kirzin S, Hay JM, Fourtanier G, Fingerhut A et al (2006) Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery 139(5):591–598
van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ (1997) Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 185(1):18–24
Singh VV, Toskes PP (2003) Medical therapy for chronic pancreatitis pain. Curr Gastroenterol Rep 5(2):110–116
Madsen P, Winkler K (1982) The intraductal pancreatic pressure in chronic obstructive pancreatitis. Scand J Gastroenterol 17(4):553–554
Bradley EL III (1982) Pancreatic duct pressure in chronic pancreatitis. Am J Surg 144(3):313–316
Karanjia ND, Widdison AL, Leung F, Alvarez C, Lutrin FJ, Reber HA (1994) Compartment syndrome in experimental chronic obstructive pancreatitis: effect of decompressing the main pancreatic duct. Br J Surg 81(2):259–264
Sakorafas GH, Farnell MB, Farley DR, Rowland CM, Sarr MG (2000) Long-term results after surgery for chronic pancreatitis. Int J Pancreatol 27(2):131–142. doi:10.1385/ijgc:27:2:131
Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB et al (2012) Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg 214(4):409–424; discussion 24-6. doi:10.1016/j.jamcollsurg.2011.12.040
Bilimoria KY, Tomlinson JS, Merkow RP, Stewart AK, Ko CY, Talamonti MS et al (2007) Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J Gastrointest Surg 11(11):1460–1467; discussion 7-9. doi:10.1007/s11605-007-0263-3
Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF et al (2007) Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol 25(35):5609–5615. doi:10.1200/jco.2007.12.9809
Franko J, Feng W, Yip L, Genovese E, Moser AJ (2010) Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg 14(3):541–548. doi:10.1007/s11605-009-1115-0
Oberg K, Eriksson B (2005) Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 19(5):753–781. doi:10.1016/j.bpg.2005.06.002
Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT (2003) Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg 138(8):859–866. doi:10.1001/archsurg.138.8.859
Thompson GB, Service FJ, van Heerden JA, Carney JA, Charboneau JW, O’Brien PC et al (1993) Reoperative insulinomas, 1927 to 1992: an institutional experience. Surgery 114(6):1196–1204 discussion 205-6
Gould VE, Chejfec G, Shah K, Paloyan E, Lawrence AM (1984) Adult nesidiodysplasia. Semin Diagn Pathol 1(1):43–53
Walmsley D, Matheson NA, Ewen S, Himsworth RL, Bevan JS (1995) Nesidioblastosis in an elderly patient. Diabet Med 12(6):542–545
Bjerke HS, Kelly RE Jr, Geffner ME, Fonkalsrud EW (1990) Surgical management of islet cell dysmaturation syndrome in young children. Surg Gynecol Obstet 171(4):321–325
Kramer JL, Bell MJ, DeSchryver K, Bower RJ, Ternberg JL, White NH (1982) Clinical and histologic indications for extensive pancreatic resection in nesidioblastosis. Am J Surg 143(1):116–119
Langer JC, Filler RM, Wesson DE, Sherwood G, Cutz E (1984) Surgical management of persistent neonatal hypoglycemia due to islet cell dysplasia. J Pediatr Surg 19(6):786–792
Moazam F, Rodgers BM, Talbert JL, Rosenbloom AL (1982) Near-total pancreatectomy in persistent infantile hypoglycemia. Arch Surg 117(9):1151–1154
Del Chiaro M, Zerbi A, Capurso G, Zamboni G, Maisonneuve P, Presciuttini S et al (2010) Familial pancreatic cancer in Italy. Risk assessment, screening programs and clinical approach: a position paper from the Italian Registry. Dig Liver Dis 42(9):597–605
Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG et al (2005) Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg 9(8):1059–1066; discussion 66-7. doi:10.1016/j.gassur.2005.05.014
Dresler CM, Fortner JG, McDermott K, Bajorunas DR (1991) Metabolic consequences of (regional) total pancreatectomy. Ann Surg 214(2):131–140
MacLeod KM, Hepburn DA, Frier BM (1993) Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet Med 10(3):238–245
Warren KW, Poulantzas JK, Kune GA (1966) Life after total pancreatectomy for chronic pancreatitis: clinical study of eight cases. Ann Surg 164(5):830–834
Jethwa P, Sodergren M, Lala A, Webber J, Buckels JA, Bramhall SR et al (2006) Diabetic control after total pancreatectomy. Dig Liver Dis 38(6):415–419. doi:10.1016/j.dld.2006.01.022
Crippa S, Tamburrino D, Partelli S, Salvia R, Germenia S, Bassi C et al (2011) Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery 149(1):79–86. doi:10.1016/j.surg.2010.04.007
Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P et al (2013) Impact of total pancreatectomy: short- and long-term assessment. HPB. doi:10.1111/hpb.12054
Casadei R, Ricci C, Monari F, Laterza M, Rega D, D’Ambra M et al (2010) Clinical outcome of patients who underwent total pancreatectomy. Pancreas 39(4):546–547. doi:10.1097/MPA.0b013e3181c2dcd3
Watanabe Y, Ohtsuka T, Matsunaga T, Kimura H, Tamura K, Ideno N et al (2015) Long-term outcomes after total pancreatectomy: special reference to survivors’ living conditions and quality of life. World J Surg 39(5):1231–1239. doi:10.1007/s00268-015-2948-1
Parsaik AK, Murad MH, Sathananthan A, Moorthy V, Erwin PJ, Chari S et al (2010) Metabolic and target organ outcomes after total pancreatectomy: Mayo Clinic experience and meta-analysis of the literature. Clin Endocrinol 73(6):723–731. doi:10.1111/j.1365-2265.2010.03860.x
Jaleel A, Halvatsiotis P, Williamson B, Juhasz P, Martin S, Nair KS (2005) Identification of Amadori-modified plasma proteins in type 2 diabetes and the effect of short-term intensive insulin treatment. Diabetes Care 28(3):645–652
Kiviluoto T, Schroder T, Karonen SL, Kuusi T, Lempinen M, Taskinen MR (1985) Glycemic control and serum lipoproteins after total pancreatectomy. Ann Clin Res 17(3):110–115
Linehan IP, Lambert MA, Brown DC, Kurtz AB, Cotton PB, Russell RC (1988) Total pancreatectomy for chronic pancreatitis. Gut 29(3):358–365
Roberts KJ, Blanco G, Webber J, Marudanayagam R, Sutcliffe RP, Muiesan P et al (2014) How severe is diabetes after total pancreatectomy? A case-matched analysis. HPB (Oxford) 16(9):814–821. doi:10.1111/hpb.12203
Epelboym I, Winner M, DiNorcia J, Lee MK, Lee JA, Schrope B et al (2014) Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J Surg Res 187(1):189–196. doi:10.1016/j.jss.2013.10.004
Vantyghem MC1, Press M (2006) Management strategies for brittle diabetes. Ann Endocrinol (Paris) Sep;67(4):287-96
Joanne Dintzis, Sherita Golden (2011) Post-pancreatectomy diabetes. In: Johns Hopkins Diabetes Guide 2012: Treatment and Management of Diabetes (Johns Hopkins Medicine)Christopher D. Saudek; Rita Rastogi Kalyani; Frederick L. Brancati Published by Jones & Bartlett Learning (2011)
Bolli GB, Andreoli AM, Lucidi P (2011) Optimizing the replacement of basal insulin in type 1 diabetes mellitus: no longer an elusive goal in the post-NPH era. Diabetes Technol Ther 13(Suppl 1):S43–S52. doi:10.1089/dia.2011.0039
Rickels MR, Bellin M, Toledo FG, Robertson RP, Andersen DK, Chari ST et al (2013) Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology 13(4):336–342. doi:10.1016/j.pan.2013.05.002
Ewald N, Hardt PD (2013) Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol 19(42):7276–7281. doi:10.3748/wjg.v19.i42.7276
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB et al (2009) American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 32(6):1119–1131. doi:10.2337/dc09-9029
American Diabetes A (2017) 14. Diabetes care in the hospital. Diabetes Care 40(Suppl 1):S120–S1S7. doi:10.2337/dc17-S017
Schmeltz LR, DeSantis AJ, Thiyagarajan V, Schmidt K, O’Shea-Mahler E, Johnson D et al (2007) Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care 30(4):823–828. doi:10.2337/dc06-2184
Walsh J, Roberts R, Bailey T (2010) Guidelines for insulin dosing in continuous subcutaneous insulin infusion using new formulas from a retrospective study of individuals with optimal glucose levels. J Diabetes Sci Technol 4(5):1174–1181
Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Maeda H, Yatabe T et al (2009) Continuous postoperative blood glucose monitoring and control by artificial pancreas in patients having pancreatic resection: a prospective randomized clinical trial. Arch Surg 144(10):933–937. doi:10.1001/archsurg.2009.176
Pickup J, Keen H (2002) Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 25(3):593–598
Papargyri P, Ojeda Rodriguez S, Corrales Hernandez JJ, Mories Alvarez MT, Recio Cordova JM, Delgado Gomez M et al (2013) An observational 7-year study of continuous subcutaneous insulin infusion for the treatment of type 1 diabetes mellitus. Endocrinol Nutr. doi:10.1016/j.endonu.2013.09.003
Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R (2003) Insulin pump therapy: a meta-analysis. Diabetes Care 26(4):1079–1087
Helve E, Laatikainen L, Merenmies L, Koivisto VA (1987) Continuous insulin infusion therapy and retinopathy in patients with type I diabetes. Acta Endocrinol 115(3):313–319
(1995) Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care. 18(3):361–376
Mecklenburg RS, Benson JW Jr, Becker NM, Brazel PL, Fredlund PN, Metz RJ et al (1982) Clinical use of the insulin infusion pump in 100 patients with type I diabetes. N Engl J Med 307(9):513–518. doi:10.1056/NEJM198208263070901
Haakens K, Hanssen KF, Dahl-Jorgensen K, Vaaler S, Aagenaes O, Mosand R (1990) Continuous subcutaneous insulin infusion (CSII), multiple injections (MI) and conventional insulin therapy (CT) in self-selecting insulin-dependent diabetic patients. A comparison of metabolic control, acute complications and patient preferences. J Intern Med 228(5):457–464
Linkeschova R, Raoul M, Bott U, Berger M, Spraul M (2002) Less severe hypoglycaemia, better metabolic control, and improved quality of life in type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy; an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med 19(9):746–751
Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P et al (2005) Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 28(3):533–538
Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R et al (2008) Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 359(14):1464–1476. doi:10.1056/NEJMoa0805017
Cryer PE (2009) Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia 52(1):35–37. doi:10.1007/s00125-008-1205-7
Rickels MR, Ruedy KJ, Foster NC, Piche CA, Dulude H, Sherr JL et al (2016) Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care 39(2):264–270. doi:10.2337/dc15-1498
Bajorunas DR, Fortner JG, Jaspan J, Sherwin RS (1986) Total pancreatectomy increases the metabolic response to glucagon in humans. J Clin Endocrinol Metab 63(2):439–446. doi:10.1210/jcem-63-2-439
Tanjoh K, Tomita R, Mera K, Hayashi N (2002) Metabolic modulation by concomitant administration of insulin and glucagon in pancreatectomy patients. Hepato-Gastroenterology 49(44):538–543
Bertuzzi F, Secchi A, Di Carlo V (2004) Islet transplantation in type 1 diabetic patients. Transplant Proc 36(3):603–604. doi:10.1016/j.transproceed.2004.02.046
Alejandro R, Barton FB, Hering BJ, Wease S (2008) 2008 update from the Collaborative Islet Transplant Registry. Transplantation 86(12):1783–1788. doi:10.1097/TP.0b013e3181913f6a
Matsumoto S (2010) Islet cell transplantation for type 1 diabetes. J Diabetes 2(1):16–22. doi:10.1111/j.1753-0407.2009.00048.x
Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD et al (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 39(7):1230–1240. doi:10.2337/dc15-1988
Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP et al (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355(13):1318–1330. doi:10.1056/NEJMoa061267
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. doi:10.1016/j.cell.2007.11.019
Bellin MD, Gelrud A, Arreaza-Rubin G, Dunn TB, Humar A, Morgan KA et al (2015) Total pancreatectomy with islet autotransplantation: summary of an NIDDK workshop. Ann Surg 261(1):21–29. doi:10.1097/SLA.0000000000001059
Tekin Z, Garfinkel MR, Chon WJ, Schenck L, Golab K, Savari O et al (2016) Outcomes of pancreatic islet allotransplantation using the Edmonton Protocol at the University of Chicago. Transpl Direct 2(10):e105. doi:10.1097/TXD.0000000000000609
Dudeja V, Beilman GJ, Vickers SM (2013) Total pancreatectomy with islet autotransplantation in patients with malignancy: are we there yet? Ann Surg 258(2):219–220. doi:10.1097/SLA.0b013e31829c4a1b
Balzano G, Maffi P, Nano R, Mercalli A, Melzi R, Aleotti F et al (2016) Autologous islet transplantation in patients requiring pancreatectomy: a broader spectrum of indications beyond chronic pancreatitis. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg 16(6):1812–1826. doi:10.1111/ajt.13656
Ryan EA, Paty BW, Senior PA, Shapiro AM (2004) Risks and side effects of islet transplantation. Curr Diab Rep 4(4):304–309
Gruessner RW, Sutherland DE, Dunn DL, Najarian JS, Jie T, Hering BJ et al (2004) Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg 198(4):559–567; discussion 68-9. doi:10.1016/j.jamcollsurg.2003.11.024
Acknowledgements
The authors would like to acknowledge Daphne O’Reilly MD and Maria Ferrera MD for their contributions to this project. Dr. Maker is supported by the NIH/NCI K08CA190855.
Author information
Authors and Affiliations
Consortia
Contributions
Study conception and design—AVM; acquisition of data—RS, AVM, and VB; analysis and interpretation of data—RS, AVM, and VB; drafting of manuscript—RS and AVM; critical revision of manuscript—RS, AVM, and VB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Maker, A.V., Sheikh, R., Bhagia, V. et al. Perioperative management of endocrine insufficiency after total pancreatectomy for neoplasia. Langenbecks Arch Surg 402, 873–883 (2017). https://doi.org/10.1007/s00423-017-1603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-017-1603-8