Abstract
Aims
Adjuvant chemotherapy for resected rectal cancer is widely used. However, studies on adjuvant treatment following neoadjuvant chemoradiotherapy (CRT) and total mesorectal excision (TME) have yielded conflicting results. Recent studies have focused on adding oxaliplatin to both preoperative and postoperative therapy, making it difficult to assess the impact of adjuvant oxaliplatin alone. This study was aimed at determining the impact of (i) any adjuvant treatment and (ii) oxaliplatin-containing adjuvant treatment on disease-free survival in CRT-pretreated, R0-resected rectal cancer patients.
Method
Patients undergoing R0 TME following 5-fluorouracil (5FU)-only-based CRT between January 1, 2008, and December 31, 2010, were selected from a nationwide registry. After propensity score matching (PSM), comparison of disease-free survival (DFS) using Kaplan-Meier analysis and log-rank test was performed in (i) patients receiving no vs. any adjuvant treatment and (ii) patients treated with adjuvant 5FU/capecitabine without vs. with oxaliplatin.
Results
Out of 1497 patients, 520 matched pairs were generated for analysis of no vs. any adjuvant treatment. Mean DFS was significantly prolonged with adjuvant treatment (81.8 ± 2.06 vs. 70.1 ± 3.02 months, p < 0.001). One hundred forty-eight matched pairs were available for analysis of adjuvant therapy with or without oxaliplatin, showing no improvement in DFS in patients receiving oxaliplatin (76.9 ± 4.12 vs. 79.3 ± 4.44 months, p = 0.254). Local recurrence rate was not significantly different between groups in either analysis.
Conclusion
In this cohort of rectal cancer patients treated with neoadjuvant CRT and TME surgery under routine conditions, adjuvant chemotherapy significantly improved DFS. No benefit was observed for the addition of oxaliplatin to adjuvant chemotherapy in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant chemotherapy using 5-fluorouracil (5FU) and folinic acid (FA) is the recommended treatment standard for patients undergoing R0 resection for rectal cancer following neoadjuvant chemoradiotherapy (CRT) or short-course radiotherapy (RT) according to European Society of Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines. However, it is acknowledged at least in the ESMO guidelines that the level of evidence for this recommendation is lower than in colon cancer. In contrast to stage III colon cancer, for which postoperative, adjuvant chemotherapy only has been used since the early 1990s [1, 2], perioperative treatment has always been more complex for rectal cancer, as radiation had to be integrated as an additional component [3]. After preoperative CRT or RT had become standard of care in stage II and III rectal cancer following the landmark German and Dutch rectal cancer trials which demonstrated an improvement in local recurrence rate but not in survival [4–6], the role of postoperative chemotherapy was focused on in four randomized trials, two of which were prematurely closed due to poor accrual [7–10]. Although theoretically, as in colon cancer, adjuvant chemotherapy was expected to improve the distant recurrence rate and survival through the eradication of subclinical micrometastases, no survival benefit could be demonstrated in any of these trials.
Therefore, robust evidence for the use of any adjuvant therapy in pretreated rectal cancer is currently not available. However, recent studies have focused on the intensification of perioperative treatment in rectal cancer through the addition of oxaliplatin to 5FU or capecitabine. Theoretically, a similar benefit from adding oxaliplatin to postoperative 5FU-based therapy as demonstrated in colon cancer in the MOSAIC and NSABP-C07 trials [11–13] would be expected also in rectal cancer. However, with the exception of one study [14], available trials investigated the addition of oxaliplatin to both preoperative and postoperative treatment rather than adjuvant chemotherapy alone [15, 16], making it impossible to draw a conclusion regarding the use of the intensification of adjuvant chemotherapy in pretreated rectal cancer.
In light of all of these results, the role for postoperative chemotherapy in patients undergoing neoadjuvant CRT and surgery for stage II and stage III rectal cancer is currently not well defined. As adjuvant treatment is widely used in daily practice, conducting a randomized trial has proven difficult, and it is unlikely that a sufficiently powered trial of adjuvant treatment vs. observation in pretreated, resected rectal cancer will be completed in the future. In this situation of limited level 1 evidence, large observational registries can contribute valuable information as they usually comprise large numbers of subjects, although the limitations of retrospective analyses must be borne in mind, and appropriate methods must be used to reduce the bias inherent to them. We therefore assessed disease-free survival in patients undergoing total mesorectal excision (TME) for rectal cancer following 5FU-based CRT and either no adjuvant chemotherapy, adjuvant chemotherapy with 5FU or capecitabine only, or adjuvant chemotherapy with 5FU/capecitabine plus oxaliplatin under routine conditions from a large multicentric quality assurance database.
Patients and methods
Study design
We retrospectively reviewed data from the Quality Assurance in Rectal Cancer Surgery multicenter observational study. Since January 1, 2005, this registry has been prospectively collecting epidemiologic and treatment-related parameters as well as data on the early postoperative course and long-term follow-up of rectal cancer patients from more than 300 hospitals of all levels of care throughout Germany. Whereas the database was kept as a purely surgical registry in the early years, data on neoadjuvant and adjuvant chemotherapy or chemoradiation have been recorded since 2008. Data were collected by the institutions involved in patient care using a standardized questionnaire. Written informed consent was obtained from all patients whose data were collected.
Inclusion/exclusion criteria for retrospective data analysis
All rectal cancer patients documented in the Quality Assurance study database undergoing non-emergency TME surgery following conventionally fractioned neoadjuvant 5FU-based CRT between January 1, 2008, and December 31, 2010, with a documented R0 status at the end of the surgical procedure were included in the present retrospective data analysis. Patients with metastatic disease (UICC stage IV) and patients undergoing emergency surgery, local tumor excision without a formal rectal resection, or incomplete resection (R1 or R2) were excluded. Also, all patients undergoing upfront surgery without neoadjuvant treatment and patients undergoing neoadjuvant short-course RT (without chemotherapy) or CRT involving drugs other than 5FU/folinic acid were excluded.
Data analysis
Two separate analyses were performed. First, to investigate the role of any adjuvant treatment in the predefined patient cohort, two groups were formed (group A, no adjuvant treatment; group B, adjuvant treatment with 5FU or capecitabine with or without oxaliplatin). Since patients were not randomly assigned to either treatment group due to the retrospective nature of the analysis, propensity score matching (PSM) [17, 18] was used to determine the independent impact of adjuvant treatment on disease-free survival (DFS) taking into account age; sex; ASA score; cardiovascular, pulmonary, renal, and hepatic risk factors; tumor distance from the anal verge (<4 cm/4–7.9 cm/8–11.9 cm/12–16 cm); histopathologically determined T stage and lymph node involvement (pT and pN stages); tumor grading; number of examined lymph nodes; presence of anastomotic leakage; case load of the operating surgeon (1–9/10–19/>19 rectal cancer procedures per year); and histopathologically determined quality of the TME specimen according to the Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY) grading system (grades 1–3) [19] as possible confounding factors. First, logistic regression using these variables was performed to obtain the propensity score for each patient (defined as the probability to be assigned to group A or B as a result of the individual profile of these covariates). Then, patients in group A and B were matched according to the calculated propensity score using a k nearest neighbors (KNN) algorithm with a threshold of c ≤ 0.01. After matching, Kaplan-Meier analysis for DFS was performed and DFS was compared between groups A and B using the log-rank test. Additionally, the local recurrence rate between both groups was compared using the same methodology.
To investigate the impact of the addition of oxaliplatin to adjuvant 5FU-based therapy, the subset of patients receiving any adjuvant treatment was then subdivided into group B1 (adjuvant treatment using 5FU/folinic acid or capecitabine without oxaliplatin) and group B2 (adjuvant treatment using 5FU/folinic acid or capecitabine in combination with oxaliplatin) and the analysis was repeated for groups B1 and B2 taking into account the same potential confounders.
As no information was available in the database whether patients assigned to any given chemotherapy had actually completely received it, all patients in the matched cohort were contacted either directly or through their treating family doctors and/or oncologists. Then, DFS was again compared between groups A vs. B and groups B1 vs. B2 using Kaplan-Meier analysis and log-rank test, this time only taking into account patients who had received their chemotherapy with the full number of cycles without dose reductions greater than 25% at any treatment cycle.
Statistical analysis was done using the SPSS Version 21 software package (IBM Corporation, Armonk, NY, USA). DFS values are given as mean +/− standard deviation. A two-sided p value <0.05 was considered significant.
Results
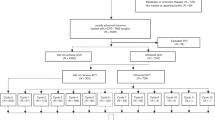
A total of 1497 patients undergoing rectal cancer surgery within the specified timeframe and matching the inclusion/exclusion criteria were identified in the Quality Assurance study database. Of these, 569 did not receive postoperative chemotherapy (group A), whereas 928 were given adjuvant chemotherapy (group B). Among patients who underwent adjuvant treatment, 768 patients received adjuvant 5FU or capecitabine only (group B1) and 160 patients received additional oxaliplatin with their adjuvant chemotherapy (group B2) (Fig. 1). Baseline characteristics of patients in each group are summarized in Tables 1 and 2. Median follow-up was 38 months in groups A and B. When factors influencing DFS in the unmatched cohort were calculated using Cox regression analysis, age, cardiovascular and renal comorbidity, ypT stage, ypN stage, tumor distance from the anal verge, number of lymph nodes examined, and TME MERCURY grade, and administration of adjuvant chemotherapy were all found to be significantly associated with DFS (Table 3).
The results of logistic regression analysis to identify factors associated with the probability of a given patient to receive adjuvant chemotherapy which were to be used to calculate the propensity score are displayed in Table 4. Propensity score matching yielded 520 patients in group A and 520 patients in group B for a total matched cohort of 1040 patients assessable for the impact of any adjuvant chemotherapy (5FU/capecitabine +/− oxaliplatin) vs. no adjuvant chemotherapy. The distribution of baseline characteristics in both groups (A and B) of the matched cohort was verified using Pearson’s correlation and Student’s t test. No significant differences between matched groups A and B were found. When the Cox regression was repeated in the matched cohort, only age, renal and pulmonary comorbidity, ypT stage, ypN stage, TME MERCURY grade, and administration of adjuvant chemotherapy showed significant association with DFS (Table 5).
Mean DFS in group B (adjuvant treatment) was significantly prolonged in comparison to group A (no adjuvant treatment) (group B, 81.8 ± 2.06 months; group A, 70.1 ± 3.02 months, p < 0.001). Kaplan-Meier curves for DFS for matched patients in groups A and B are displayed in Fig. 2. No difference in the local recurrence rate between groups A and B was found (p = 0.706; data not shown). Mean overall survival (OS) was 87.7 months in group B and 76.9 months in group A (p < 0.001).
After contacting all patients or their treating physicians for follow-up information, adequate information regarding treatment completeness was obtained from 316 of the 520 patients in group B (60.8%). Of these, 280 (88.6%) had received their adjuvant chemotherapy without treatment interruptions or discontinuations and without dose reductions >25% at any cycle, whereas in 36 patients (11.4%), dose reductions and/or treatment interruptions or discontinuations were recorded. When only the 280 patients from group B who had received their full adjuvant chemotherapy were included in the analysis, DFS was 88.7 ± 2.25 months, which was again significantly longer than DFS in the 520 patients without adjuvant chemotherapy (p < 0.001).
The results of the Cox regression analysis to identify factors influencing DFS among the 928 patients who received adjuvant chemotherapy are displayed in Table 6, showing ASA group, renal comorbidity, ypT stage, ypN stage, and tumor distance from the anal verge as significant factors. Results of the logistic regression to calculate factors to be included into propensity calculation are shown in Table 7.
Propensity score matching yielded a total matched cohort of 296 patients (148 receiving 5FU or capecitabine only (group B1) and 148 patients receiving additional oxaliplatin (group B2)). Again, baseline characteristics were well balanced between groups B1 and B2 after matching. Median follow-up was 48.7 months for groups B1 and B2. When the Cox regression was repeated in the matched cohort, only cardiovascular comorbidity and ypN stage showed significant association with DFS (Table 8).
No significant DFS difference between groups B1 and B2 was found (group B1, 79.3 ± 4.44 months; group B2, 76.9 ± 4.12 months, p = 0.456). DFS curves for matched patients in groups B1 and B2 are displayed in Fig. 3. Again, local recurrences occurred with similar frequency in groups B1 and B2 (p = 0.499; data not shown). Mean OS was 85.3 months in group B1 and 82.5 months in group B2 (p = 0.382).
After contacting all patients or their treating physicians for follow-up information, adequate information regarding treatment completeness was obtained from 76 of the 148 patients (51.4%) in group B1 and from 80 of the 148 patients (54.1%) in group B2. Of the patients for whom adequate information was obtained, 62 of 76 (81.6%) in group B1 and 76 of 80 (95.0%) in group B2 had received their adjuvant chemotherapy without treatment interruptions or discontinuations and without dose reductions >25% at any cycle, whereas in 14 patients (18.4%) and 4 patients (5.0%) in groups B1 and B2, respectively, dose reductions and/or treatment interruptions or discontinuations were recorded. When only the 76 patients from group B1 and the 80 patients from group B2 who had received their full adjuvant chemotherapy were included in the analysis, DFS was 86.2 ± 4.83 vs. 84.4 ± 5.49 months for groups B2 vs. B1, with no significant difference between groups (p = 0.790).
Discussion
The rationale for the current treatment standard of administering adjuvant chemotherapy with or without oxaliplatin to patients with UICC stage II and stage III rectal cancer mainly stems from extrapolation from colon cancer studies [1, 2] and from studies in rectal cancer patients treated before the introduction of neoadjuvant treatment [20–22]. In the era of neoadjuvant RT or CRT for rectal cancer, the use of adjuvant chemotherapy has been examined in four randomized trials, all of them failing to show a significant DFS or OS benefit but none of them able to change the current guideline standard. However, the four studies were not fully comparable in their methodology, limiting the validity of the evidence generated by them. Two of the studies were closed prematurely due to poor accrual [7, 8] and two [9, 10] used the same reduced-dose chemotherapy schedule that was part of preoperative CRT also as adjuvant treatment, potentially limiting its systemic efficacy. Finally, patients were included into two of the studies based on their clinical tumor stage prior to treatment (cTcN stage) [9, 10] whereas randomization was performed based on postoperative findings (ypTypN stage) in the other two [7, 8]. As a result of these non-constant methodologies, meta-analyses of these studies have also yielded varying results. In one meta-analysis of individual patient data [23], no benefit from the use of adjuvant chemotherapy with respect to DFS, OS, or distant recurrence rate was found; however, in a subgroup analysis, patients with rectal cancer located 10–15 cm from the anal verge had improved DFS and fewer distant recurrences if they received adjuvant treatment. A second meta-analysis found significantly improved DFS with the use of adjuvant chemotherapy in studies randomizing patients according to their postoperative tumor stage, whereas no benefit from adjuvant treatment was recorded from studies enrolling patients based on their clinical tumor stage before start of treatment [24]. Although the role for adjuvant chemotherapy in rectal cancer patients treated with neoadjuvant RT or CRT is therefore not clear, studies have mainly focused on the intensification of perioperative treatment through the addition of oxaliplatin in recent years. Two of the recently presented studies (CAO/ARO/AIO-04 and PETACC-6) [15, 16] have investigated the addition of oxaliplatin to both preoperative and postoperative treatment, making it difficult to draw a conclusion regarding the effect of postoperative oxaliplatin (when the drug is intended to eradicate residual micrometastatic disease rather than serve as a radiosensitizer) alone. Only the Korean phase II ADORE study [14] specifically addressed the issue of adding oxaliplatin to the adjuvant chemotherapy regimen in patients who had undergone preoperative 5FU-based CRT plus TME surgery and still had ypN+ or ypT3-4ypN0 disease. This study found a significantly improved DFS for the intensified regimen, a subgroup analysis indicating that the effect was limited to patients with lymph node-positive disease.
In this complicated field of available evidence and established standard practice, we believe that despite their retrospective nature, the results presented here can add valuable information to the debate. Similar to other retrospective studies [25, 26], our results indicate a substantial DFS benefit from adjuvant chemotherapy in patients undergoing neoadjuvant CRT and TME surgery for rectal cancer. This DFS benefit must be mainly attributed to a decrease in distant recurrences as the local recurrence rate was not different between the groups. Retrospective studies on the effect of adjuvant chemotherapy are frequently criticized for their proneness for selection bias (patients in good condition after surgery preferably being selected for adjuvant treatment, making it impossible to differentiate the effects of treatment and patient fitness on survival); however, this issue was addressed in our study by using propensity score matching taking into account all factors that may have influenced the clinician’s decision to recommend adjuvant treatment and for which data were available in our database. Even though it is a general problem of propensity score matching that only factors that are known to influence the target variable and for which data are available can be included in the calculation of the propensity score, and there may have been factors influencing the decision to administer adjuvant chemotherapy that we were not aware of or had no data for, baseline characteristics were well balanced after matching, creating a “pseudo-randomized” cohort for further analysis. Moreover, one of the strengths of our Quality Assurance registry is that it reflects the situation under routine clinical care conditions which may be different from the environment of a thoughtfully planned, closely supervised clinical study. Although the surgical quality was generally good with approximately 85% of the TME specimens being graded as MERCURY grade I by the pathologist and more than three quarters of patients having at least 12 lymph nodes examined, no quality assessment was performed regarding neoadjuvant CRT and no central pathology review was available. One might argue that this limits the validity of our data; however, as most rectal cancer patients in Germany are treated in community or district hospitals rather than academic cancer centers, these data may possibly provide a more accurate image of real-life patient care than a prospective study would yield.
As oxaliplatin is not standard of care as part of adjuvant treatment for rectal cancer in Germany, the matched patient cohort for the analysis of adjuvant therapy with or without oxaliplatin was relatively small. The addition of oxaliplatin to postoperative chemotherapy was not associated with a difference in DFS or OS in our study, thus not supporting the use of this drug in this setting. This is in contrast to the results from the phase 2 ADORE study [14], in which a significant benefit from an oxaliplatin-containing regimen compared to 5FU/folinic acid in terms of DFS was demonstrated. As no other trials are available that specifically investigated the use of postoperative oxaliplatin in addition to 5FU/folinic acid in pretreated rectal cancer, controversy regarding this issue is likely to persist. In the ADORE study, a subgroup analysis indicated that the beneficial effect of treatment intensification was limited to patients with lymph node-positive disease; however, given the small cohort size in our study that could be analyzed for the DFS impact of chemotherapy with vs. without oxaliplatin, we were not able to further subdivide this cohort to perform subgroup analyses and thus could not investigate a possible association of lymph node positivity with a treatment benefit from oxaliplatin.
Our study has several limitations. In addition to the lack of quality control for neoadjuvant CRT and histopathological assessment as well as the possibility that factors that were not included in the propensity score analysis may have impacted on the decision for or against adjuvant chemotherapy with or without oxaliplatin in a given patient, perhaps the most important one is that no reliable information was available in the database as to the completeness of the adjuvant chemotherapy protocols performed. To address this issue, we attempted to obtain this information by contacting all patients or their treating physicians at the time of data analysis. This was successful in 60.8% of the patients included into the analysis of no vs. any adjuvant treatment (group B) and in 51.4 and 54.1% of the patients in groups B1 and B2, respectively, that were analyzed for the DFS impact of adjuvant chemotherapy with vs. without oxaliplatin. Of the patients for whom this information was obtained, more than 80% in each group had received their prescribed chemotherapy fully and without major dose reductions. Moreover, the results of the DFS analysis were not different from the entire matched cohort when only patients who had received their full postoperative chemotherapy were included. Although a more complete follow-up would certainly be desirable, we believe that these figures justify the conclusion that our results obtained in the full matched cohort are valid. In relation to the randomized trials available, adherence to the prescribed adjuvant chemotherapy protocol was rather good in our study population. Only between 43% [10] and 74% [7] of patients completed the study protocol in the trials on adjuvant treatment vs. no adjuvant treatment, while only 68 and 53% of patients received the full scheduled dose of adjuvant therapy in the capecitabine-only and capecitabine + oxaliplatin arms of the PETACC-6 trial, respectively. As the patient’s ability to tolerate chemotherapy shortly [27] after major surgery will continue to be a major obstacle to the conduct of adjuvant chemotherapy trials, it is unlikely that a definitive answer to the question if and according to which protocol rectal cancer patients should receive adjuvant chemotherapy following preoperative CRT and TME surgery will be obtained from future studies. Given the better tolerance for chemotherapy in the preoperative compared to the postoperative setting as well as the considerable capacity of chemotherapy alone to downstage rectal cancer even without concurrent radiation [28], currently recruiting studies are focusing on the optimization of the treatment sequence, namely, the administration of all chemotherapy prior to surgery [29], and on a more selective use of radiation for patients who achieve insufficient downstaging of their cancers following chemotherapy alone [30].
Conclusion
In the field of conflicting evidence, and in the absence of reliable data from randomized trials, our results lend support to the position that patients undergoing neoadjuvant 5FU-based CRT and TME surgery should receive adjuvant chemotherapy. Regarding the use of adjuvant oxaliplatin in addition to a fluoropyrimidine, our analysis does not demonstrate any benefit from this treatment approach, although this must be interpreted with caution due to the limited cohort size, which does not permit to perform valid subgroup analyses.
References
Moertel CG, Fleming TR, Macdonald JS et al (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352–358
O'Connell MJ, Mailliard JA, Kahn MJ et al (1997) Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 15:246–250
Gastrointestinal Tumor Study Group (1985) Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 312:1465–1472
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Sauer R, Liersch T, Merkel S et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933
Kapiteijn E, Marijnen CA, Nagtegaal ID et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Breugom AJ, van Gijn W, Muller EW et al (2014) Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomised phase III trial. Ann Oncol 26:696–701
Glynne-Jones R, Counsell N, Quirke P et al (2014) Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 25:1356–1362
Sainato A, Nunzia CL, Valentina VV et al (2014) No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol 113:223–229
Bosset J, Calais G, Mineur L et al (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190
André T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Kuebler JP, Wieand HS, O'Connell MJ et al (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 25:2198–2204
Yothers G, O'Connell MJ, Allegra CJ et al (2011) Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 29:3768–3774
Hong YS, Nam B, Kim K et al (2014) Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 15:1245–1253
Schmoll HJ, Haustermans K, Price TJ et al. (2014) Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival results at interim analysis. J Clin Oncol 32(suppl; abstr 3501)
Rodel C, Liersch T, Fietkau R et al (2015) Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: results of the German CAO/ARO/AIO-04 randomized phase III trial. Lancet Oncol 16:979–989
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55
Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Di Renzo V (2009) Observational studies: propensity score analysis of non-randomized data. Int MS J 16:90–97
Hermanek P, Hohenberger W, Klimpfinger M, Köckerling F, Papadopoulos T (2003) The pathological assessment of mesorectal excision: implications for further treatment and quality management. Int J Colorect Dis 18:335–341
Fisher B, Wolmark N, Rockette H et al (1988) Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Nat Cancer Inst 80:21–29
Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S (2012) Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 3:CD004078
International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators (1995) Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 345:939–944
Breugom AJ, Swets M, Bosset J et al (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16:200–207
Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M (2015) Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol 41:713–723
Ahn DH, Wu C, Wei L et al (2015) The efficacy of adjuvant chemotherapy in patients with stage II/III resected rectal cancer treated with neoadjuvant chemoradiation therapy. Am J Clin Oncol. doi:10.1097/COC.0000000000000185
Petrelli F, Coinu A, Lonati V, Barni S (2015) A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorect Dis 30:447–457
Gresham G, Cheung WY, Speers C, Woods R, Kennecke H (2015) Time to adjuvant chemotherapy and survival outcomes among patients with stage 2 to 3 rectal cancer treated with preoperative chemoradiation. Clin Colorectal Cancer 14:41–45
Schrag D, Weiser MR, Goodman KA et al (2014) Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 32:513–518
Nilsson PJ, van Etten B, Hospers GAP et al (2013) Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 13:279
Chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery. https://clinicaltrials.gov/ct2/show/NCT01515787. Accessed 11 Oct 2015
Author contributions
BG conceptualized and designed the study, was involved in data collection and analysis, and wrote the manuscript. HP was involved in designing the study, data collection, interpretation, and analysis and helped to write the manuscript. FB and FP were involved in data collection, interpretation, and analysis. RO did the statistical analysis and was involved in data processing for presentation. KR, IG, HL, and CBr were involved in data collection and interpretation, as well as data extraction and processing from the Quality Assurance database. CBe helped to integrate the reviewer’s comments into the revised version and was involved in collecting additional data for manuscript revision as well as the processing of these data for presentation. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no financial and personal relationships with other people or organizations that could inappropriately influence (bias) their existing work. This includes, but is not limited to, employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Funding
There has been no external funding for the conduct of the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Garlipp, B., Ptok, H., Benedix, F. et al. Adjuvant treatment for resected rectal cancer: impact of standard and intensified postoperative chemotherapy on disease-free survival in patients undergoing preoperative chemoradiation—a propensity score-matched analysis of an observational database. Langenbecks Arch Surg 401, 1179–1190 (2016). https://doi.org/10.1007/s00423-016-1530-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1530-0