Abstract
Background
Islet transplantation has become a valuable therapy for patients with diabetes mellitus type 1.However, only selected patients with exhausted insulin therapy characterized by instable metabolic control and repeated severe hypoglycemia are transplant candidates. This strict indication is mainly due to the requirement for lifelong immunosuppression and the critical shortage for donor organs. Therefore, numerous research activities address these issues in order to provide beta cell replacement therapy to a broader cohort of patients with diabetes.
Methods
The encapsulation of pancreatic islets within mainly alginate-based macro- or microcapsules withvarious physical configurations may allow protecting the islet graft without the need for immunosuppressive agents and moreover expanding the donor pool to animal tissue and novel insulin-producing cells. Despite major advances in encapsulation technology, a significant translation into clinical application is not evident. There are still issues that need to be resolved associated with graft oxygenation, immunprotection, inflammatory response, material biocompatibility, and transplantation site to list some of them.
Conclusion
The recent advances in xenotransplantation and particularly in the field of stem cell-derived beta cells have generated a renewed scientific interest in encapsulation. This review aims to provide an overview on current encapsulation technologies as a treatment modality in cell replacement therapy for type 1 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Islet transplantation has evolved into a viable treatment option for a subset of patients with type 1 diabetes [1–4]. Improvements in islet isolation techniques and more tailored immunosuppressive therapies and anti-inflammatory regimen have positively impacted on clinical outcome [5]. However, in spite of these achievements, there remain a number of factors that hamper a more widespread utilization of this therapeutic modality (Fig. 1). The main limitation to advancement in islet transplantation is access to a sufficient number of high-quality donor pancreata [6, 7]. Secondly, in successful islet transplantation, partial or total loss of the islet graft still occurs within the early post transplantation phase, which is mainly attributed to hypoxia and inflammation. Instant blood-mediated inflammatory reaction (IBMIR) represents thrombo-inflammatory injury elicited upon pancreatic islet transplantation, thereby dramatically affecting transplant survival and function [8]. Thirdly, effective strategies to minimize immune response to the transplant and recurrence of autoimmunity are essential to move this field forward [9].
Limiting factors in clinical islet transplantation. The main obstacles in current islet transplantation are the lack of human cadaveric donor organs, the chronic need for immunosuppression, insufficient revascularization of islet graft resulting in chronic hypoxia, and a gradual loss of functional islet mass due to an inadequate microenvironment
Encapsulation of islets prior to transplantation could potentially address some of those issues. Moreover, these barrier-creating methodologies might pave the way for safe application of alternative cell sources such as xenogeneic tissue or novel insulin-producing cells to approach the critical factor of human organ shortage.
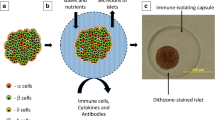
Cell encapsulation is an old concept that was introduced almost 60 years ago. In a first review article on this topic 30 years ago, David W. Scharp et al. collected the knowledge and research strategies at that time and pioneered the field of “immuno-isolation” as an approach to protect encapsulated pancreatic islets from autoimmunity and allo- or xenograft rejection [10]. The fundamental strategy of encapsulation is to create a selectively permeable barrier that allows free diffusion of oxygen, nutrients, and effector molecules, while preventing the migration of immune cells and cytotoxic molecules that could potentially harm the cells. The most obvious application for this concept is the field of islet transplantation.
This review aims to provide an overview of strategies in islet encapsulation, critical issues for achieving the level of a clinical therapy, current preclinical and clinical trials, and future perspectives in this tantalizing field of diabetes research.
Structural approaches and materials
The three key aspects that need to be considered for the development of an implantable bioartificial pancreas device are the material for encapsulation, the dimension of the capsule, and the implantation site. In general, encapsulation devices can be classified in macroscale, microscale, and nanoscale devices that can be implanted in either intravascular or extravascular sites in the body.
Macroencapsulation
Macroencapsulation systems combine the total transplanted cell volume in a single, defined container that can be easily transplanted and retrieved. The main challenge for macrodevices is a sufficient input of oxygen and nutrients and free output of effector hormones.
Extravascular devices
Extra-vascular macroencapsulation devices have been developed since the early 1950s. These devices were associated with only minor implantation complications but most animal trials were compromised by the insufficient oxygen and nutrients diffusion into the device and to the cells [11]. However, in these early studies, important measures for any further encapsulation research such as membrane biocompatibility, host cell membrane overgrowth, delays in immune rejection of encapsulated tissues, and prevention of allograft rejection were defined [11–16]. Following the development of hollow fiber technology for renal dialysis, hollow fibers became the target of inserting islets inside to use as diffusion devices [17–19]. Based on this system, first encapsulated islet transplants were successfully performed in rodents and introduced in a non-curative preclinical trial. This study demonstrated the recovery of viable and functional human islets after several weeks of implanted islet allografts [20, 21]. However, the low packing density of the device reduced the clinical interest; due to the large volume of encapsulated islets hollow fibers required for a curative clinical trial.
Another approach to develop a macroencapsulation system for extravascular implantation is planar devices, which consist of encapsulated islets immobilized in flat sheets fastened to make a sealed chamber. This configuration is believed to provide better stability than fiber systems and improve oxygen diffusion to the cell-containing slabs. The most prominent device of this kind was designed by Baxter Healthcare in the early 1990s. Promising efficacy data were published in rodent models [22, 23]. The critical issue of oxygenation was resolved by induction of a robust capillary ingrowth in the outer cover of the polyester shell though the results were not quite reproducible in large animal models. Several research groups including pharmaceutical industry are investigating different modifications to create a clinically relevant device [24]. This system is known as the Theracyte device (sold by Baxter® to Theracyte®) and is currently used in the first preclinical and clinical trials for the use of human embryonic stem cell-derived islets.
As outlined above, one of the major challenges in macroencapsulation is the engineering of a device that provides an ideal oxygen environment to the cells but is of an appropriate clinically applicable scale. Many of the approaches found that the major acute cause of encapsulated islet death was hypoxia [9, 25]. While vascularization of the implant may improve oxygen gradients within the device, the time required for the formation of a fully functional vascular bed is too long to maintain islet viability. Since 2005, the biotechnology start-up Beta O2© has been exploring methods to provide direct oxygen delivery to the encapsulated islets; their most successful devices to date being oxygen supplemented via an oxygen chamber that is refilled daily through peripheral connections [26–28]. These Beta O2© studies have been successful in rodents and more recently in large animals [28, 29]. The first individual patient trial for this device showed persistent islet graft function in the chamber for 10 months with regulated insulin secretion and preserved islet morphology without immunosuppression [30]. Expanded trials in patients are planned for the near future.
Intravascular devices
Intravascular devices consist of hollow fibers or tubes filled with encapsulated islets that are attached to the recipient’s vascular system. While these systems have advantages compared to extravascular devices in regards to oxygen and nutrient supply, they have the tendency for thrombosis of the fibers and bleeding complications. The most popular intravascular device was developed by WR Grace Company in a research venture with Biohybrid© [31–33]. Their so called “Hockey Puck” device, which was perfused by arterial blood through tubing around, was very successful in curative large animal models for islet allo- and xenotransplantation. Unfortunately, disastrous bleeding complications in the recipient animals (disconnection of the carotid artery cannulae to the device, which resulted in exsanguination of the diabetes cured canine recipient) stopped this promising device configuration from further development and clinical exploration.
Microencapsulation
In islet microencapsulation, islets are immobilized in alginate, agarose gel, or other biocompatible hydrogels inside microcapsules and implanted into the recipient. The most common implantation site is the peritoneal cavity [34]. Numerous studies on islet microencapsulation have been published and reviewed on various rodent diabetes models [35, 36]. However, this approach has shown limitations in achieving significance within large animals and human clinical trials [37–43]. The standard islet encapsulating alginate microcapsules are produced by dropwise addition of sodium alginate, mixed with islets, into a bath of CaCl2 or BaCl2, which rapidly crosslinks to form a hydrogel capsule containing the islet. Standard alginate microcapsules are 500–1000 microns in diameter. Compared to macroencapsulation devices, those microspheres are mechanically more stable and have a better surface area to volume ratio and a superior immunological profile [41]. However, technical challenges with this approach include empty (islet-free) capsules, incomplete encapsulation of larger islets, capsule instability in vivo, and fibrotic overgrowth [24, 44]. This encapsulation method for islets generates a significant increase in the total volume of the transplant required to achieve a clinically relevant dose. Therefore, transplant sites are limited basically to the peritoneal cavity. Multiple methods have been developed to reduce capsule size. Those approaches are also driven by the observation that hypoxic injury and apoptosis occur in a relevant percentage of microencapsulated islets following transplantation [44].
Overall, hundreds of publications with multiple successes have been produced in rodent studies using microencapsulated islets. Successful large animal and clinical trials are limited. Very recently, Living Cell Technologies© has been conducting clinical trials on xenotransplantation of encapsulated porcine islets in different countries. However, preliminary results were discussed and indicate overall safety of this xenotransplantation approach while efficacy data failed to achieve the desired success [45].
Encapsulation material alternatives
While the vast majority of published islet encapsulation studies used alginate-encapsulated islets, this material might not be the most suitable. Alternatives, such as agarose gel as done by Iwata’s group, have shown some promise [46]. Due to slower crosslinking when compared to alginate, they employ an emulsion approach that results in centered islets with smaller volumes. Another approach of multi-component coatings has been developed by Taylor Wang and has shown great success in rodents [47]. Large animal studies are ongoing and seem promising [48].
Another interesting alternative to natural alginate and agarose polymers is synthetic polymer approaches, which provide a higher degree of control in purity, functionalization, and permeability. A possible synthetic material for biomaterial applications is poly(ethylene glycol) (PEG). PEG forms a thin coating on the surface of each islet by radical-induced polymerization [49–51]. Small animal therapeutic studies were very successful [52], unfortunately the translation to large animal models failed due to bioincompatibility issues. Following modifications on the PEG formulation, subcutaneous allogeneic grafts of PEG-coated baboon islets were highly successful in diabetic recipients achieving insulin independency for up to two years without any immunosuppression [53]. Based upon that, innovative approaches using PEG and microfluidic platforms have recently been developed by Hubbell et al. with very promising albeit preliminary results [54].
Nano-encapsulation or layer-by-layer coating
Further alternative to microencapsulation or conformal coating is the formation of nanoscale polymeric layers directly onto the islet surface via layer-by-layer assembly. Several groups have developed unique approaches, such as PEG-lipid coatings, streptavidin/biotin layers, and covalently linked hyperbranched polymers [55–57]. These approaches are mostly in the very early stage albeit auspicious [58, 59].
Immunprotection
Although islet transplantation has demonstrated its potential use in treating type 1 diabetes, this remains limited to a small subset of patients with brittle diabetes suffering from severe hypoglycemia [60]. This restriction is at least in part due to the chronic need for immunosuppression to establish graft acceptance, which is potentially associated with severe side effects. Macro- and microencapsulation of islets may enable the transplantation of pancreatic islets in the absence of immunosuppression by protecting the graft through a mechanical barrier. This protection may even allow for the transplantation of animal tissue or novel insulin-producing cells and opens up the perspective of using animal donors or stem cells as a means to solve the problem of organ shortage.
Transplantation of allogeneic or xenogeneic islet grafts induces complex interactions between the foreign islet graft and the recipient that involve non-immunological factors as well as innate and adaptive immunity. These reactions can cause gradual or rapid functional graft loss. In patients with type 1 diabetes, the situation becomes even more complex since this is an autoimmune disease, where destruction of the transplanted beta cells may occur due to recurrence of autoimmunity. Islet encapsulation approaches may have the potential to attenuate or even abrogate these mechanisms. Yet, encapsulation systems themselves may induce at least some of these reactions due to biocompatibility issues.
Inflammatory reactions
Islet transplantation is usually performed by intraportal delivery to the liver [61]. However, the exposure of isolated allogeneic or xenogeneic donor islets to the recipient’s circulating blood triggers a rapid thrombo-inflammatory reaction termed instant blood-mediated inflammatory reaction (IBMIR). The activation of coagulation and the complement systems, accompanied by the activation and infiltration of platelets and innate immune cells to the transplantation site, constitutes major components of IBMIR that dramatically affect the survival and function of the transplanted islets [8]. The extent of the inflammatory events can be even greater in xenograft models [62] due to molecular incompatibilities between the coagulation systems of different species [63]. Encapsulation may have the potential to protect the islets against IBMIR. In addition, islets may directly be shielded from IBMIR by PEGylation, as an increasing number of studies over the past years could show [64–70]. As discussed above, PEGylation involves the addition of linear PEG molecules to the surface of islets. This method allows attaching multiple molecules to the surface of an islet. Teramura and Iwata presented several studies, e.g., coupling with bioactive molecules such as urokinase, thrombomodulin, complement receptor 1, and heparin, and could demonstrate very promising results in mammals with significant inhibition of IBMIR effects on islet grafts [36, 57, 66, 70]. Another interesting approach is to use PEG-based shielding systems and low-dose immunosuppression to protect islet grafts from inflammation and allograft rejection [71].
Overall, early inflammatory reactions after islet transplantation are a well-known phenomenon that is possibly deleterious for graft survival and function. Approaches to prevent components of IBMIR might be of high interest for encapsulated islets as well as traditional islet transplantation. However, more work is needed to evaluate the described shielding procedures for their benefit on islet graft survival in relevant preclinical model systems.
Immunprotection against allografts
Upon islet transplantation, both the innate and the adaptive immune systems are activated to destroy the foreign graft. It has been shown that both allo- and autoimmunity influence beta cell survival after islet allo-transplantation in type 1 diabetes [72, 73]. Macro- as well as microencapsulation techniques have been shown to create an immune barrier that protects islet allografts in various experimental and preclinical models and first clinical trials [30, 35, 74, 75]. Currently, there are five clinical trials retrievable form ClinicalTrials.gov (Table 1) that recruit patients with type 1 diabetes mellitus for encapsulated islet transplantation without or with minor immunosuppression [https://clinicaltrials.gov/]. These studies will hopefully help to understand the current role and future potential for encapsulated islet transplantation for diabetes therapy.
Immunprotection against xenografts
The response of the immune system against xenografts is generally more aggressive than in allo-transplantation due to the presence of preformed antibodies [76], e.g., pig islets that are transplanted into non-immunosuppressed nonhuman primates are rejected by both humoral and cellular immune reactions [28, 77, 78]. Thus, encapsulation technologies that allow for sufficient immune shielding of the islet graft are of extreme interest in the xenogeneic setting since a clinically acceptable regimen against xeno-responses has not been attained yet.
Macro- and microencapsulation systems have been demonstrated to prolong xenogeneic islet graft survival in several experimental and preclinical studies [10, 27–29, 39, 79]. As described above, the first clinical trials with microencapsulated neonatal insulin-producing porcine islet cells have been conducted by Living Cell Technology® but adequate information on these trials is urgently awaited.
A specific appeal of xenotransplantation is that it is possible to genetically modify donor tissue to promote islet isolation performance and reproducibility and islet engraftment and to protect or hide the xenograft from immune attacks. The combination of encapsulation and local immunosuppression may open up another novel and potent strategy. Given that the use of systemic immunosuppression should be avoided when possible, localized immunosuppression, which seeks to deliver immunoprotective agents adjacent to or from within the encapsulating device, may be sufficient to protect the islets. Polymers could be used to provide a controlled release for local immunosuppression or immunomodulatory cells, such as mesenchymal stem cells (MSC), and could be co-encapsulated for local modulation of the graft microenvironment. Alternatively, the use of a genetically modified islet source, such as transgenic pigs expressing LEA294, a high affinity variant of T cell co-stimulating inhibitor CTLA-4 Ig, may provide local immunosuppression for encapsulated islet transplants [80].
Kinetics of insulin release and transplantation sites
The primary goal of islet transplantation is the reconstitution of physiological glucose homeostasis that is maintained by insulin production and secretion from pancreatic beta cells. Although a variety of hormones and neurotransmitters can induce insulin release, glucose is the main physiological insulin secretagogue [81]. It has been shown that in healthy beta cells the release of insulin is oscillatory with relatively stable pulses of variable amplitude [82, 83]. Maintaining this subtle regulation within an encapsulated islet is one of the major challenges in this field. The production, secretion, and diffusion of insulin through a capsule/chamber system depend on a variety of biological and physico-chemical factors [75, 84]. Extensive research has been done to identify the most optimal conditions and select appropriate capsule components [75, 79] with reasonable success in insulin release upon glucose in vitro and in vivo. The composition of the Beta O2® device is an example for almost unrestricted hormonal response without relevant diffusion delay [26, 28]. Nevertheless, the relatively large surface-to-volume ratio of macrocapsules may interfere with optimal interexchange of nutrients and hormones, thus compromise a narrow metabolic homeostasis. In this regard, microencapsulation might be superior to any kind of macro device. Indeed, microencapsulated islets from various sources have generally shown appropriate competence for insulin release, but the kinetics are often compromised with delayed insulin response in vitro and in vivo [75, 85–88]. This might be related to the size of the capsules (layer-by-layer encoated nanocapsules show improvement in insulin kinetics [59]), the composition of encapsulation material, and possibly pericapsular fibrotic overgrowth. The latter is understood to be attributed to an immune response against microcapsules themselves or to antigen shedding through microcapsule pores from encapsulated islet tissue [89]. It might also be possible that the dynamics of insulin release from microencapsulated islets are affected by the intraperitoneal transplantation site. It has been shown that insulin infusion into the peritoneal cavity leads to a markedly delayed and reduced increase in peripheral blood insulin levels when compared to intraportal insulin infusion [90].
The transplantation site for encapsulated islets is actually a controversial and highly critical issue to be considered. In clinical islet transplantation, free islets are commonly infused into the liver via the portal vein [61]. The insulin release from grafted islets into the portal circulation matches the physiologic route. In the case of microencapsulation, the intraperitoneal space is the most popular site for implantation. The main reason therefore is the ease of access via laparascopy and the less restriction on the volume of material that can be transplanted. However, as mentioned above, the location per se might be suboptimal in regards to insulin kinetics. The lack of close contact to a vascular network and the difficulty in retrieval of implanted islets are additional negative features. Moreover, in contrast to the situation in rodent and pig models, microencapsulated islets transplanted into the peritoneal cavity show a tendency to gravity-dependent clumping in upright nonhuman primates and man [91]. The most promising alternative site for transplantation of microcapsules appears a surgically created omental pouch [92]. Although it is a more complex procedure, the advantages are obvious due to the physiological route of insulin delivery, the close proximity to extensive vasculature and the relatively easy retrieval of grafted capsules.
In the case of macroencapsulation, most trials were performed in the subcutaneous space. It is easily accessible for implantation, retrieval, and even biopsy. However, the subcutaneous space is a bradytroph area, and while various rodent models of subcutaneous macro-device implantation were successful, they failed after upscaling to large animal models. The rare vascularization is causing poor oxygen supply to the encapsulated islets and prohibits a relevant route for insulin diffusion. Beta O2® has developed a system that allows a controlled oxygen supply to the islet graft by means of an integrated oxygen reservoir that can be refilled regularly and can maintain oxygen pressure. Thereby, a sufficient supply of oxygen for maintaining optimal islet function can simultaneously ensure functional potency and immunprotection [26–30]. Another approach is the prevascularization of the transplantation site either by local administration of vascular growth or trophic factors or by the implantation of a foreign material to induce strong neovascularization prior to implanting the islet-containing devices several weeks later [93, 94]. Prevascularization has been evaluated to mimic the native microarchitecture of the islets, where each beta cell is in intimate contact with the surrounding microvasculature [95, 96].
Future perspectives
Islet transplantation represents a potential cure for diabetes with limitations caused by insufficient long-term success, the risks associated with chronic immunosuppression, and donor organ shortage. Islet encapsulation is an expanding field that attempts to overcome these hurdles by providing the means to transplant islets without immunosuppression, creating a beneficial microenvironment for long-term function, and may enable the safe usage of animal tissue or stem cells. We have presented an overview of current research lines in the field of islet encapsulation and several small and large animal models that have demonstrated the promise of encapsulated islet transplantation. The key issues that need to be addressed are the definition of the optimal capsule configuration to effectively deliver oxygen and nutrients to the islet graft, the use of anti-inflammatory agents to prohibit graft loss and promote sustained islet function, and optimized biomaterials and encapsulation methods to eliminate the need for immunosuppression. A major challenge is to further reach consensus about appropriate animal models. It is a matter of common knowledge that rodent models although indispensable cannot predict success in large animal models or man. Therefore, clinically relevant large animal models are of great relevance in order to adequately evaluate the clinical potency of encapsulation systems and eventually satisfy regulatory agencies in order to proceed to clinical studies. However, also the putative gold standard model of human/pig islet transplantation into nonhuman primates has been shown to be highly critical both regarding efficacy as well as safety [97, 98].
In our view, the possibly highest potential of islet encapsulation is due to the exploitation of alternative cell sources. Numerous studies have demonstrated the potential of encapsulated islet xenotransplantation to treat diabetes. Porcine islets are the species of choice as pigs are readily available and have a glucose physiology similar to humans and pig insulin has a long history of use in humans. Furthermore, in recent years, effective methods have been established for genetic modification of pigs. Somatic cell nuclear transfer using genetically modified donor cells facilitates the generation of tailored pig models for xenotransplantation. However, despite all advances in the field of xenotransplantation, there is a serious even though possibly irrational reservation against xenotransplantation which is mainly associated with safety concerns. Here, macro- and microencapsulation may offer a safe strategy that will lead to a clinically viable therapy that is both effective and safe.
Stem cells are another attractive alternative, virtually unlimited source for transplantation. Viable insulin-producing beta cells can be derived from various kinds of stem cells (human embryonic [hESC], induced pluripotent [iPSC], etc.), and over the last years, various encouraging studies in the field of beta cell differentiation have been published. However, the lack of sustained insulin independence using beta cells generated from stem cells in appropriate preclinical models is a matter of concern. Moreover, one has to keep two principle issues in mind that have not been resolved yet. Beta cells generated from hESC/iPSCs show a high persistence of undifferentiated cells with the competence to proliferate rapidly in an unpredictable manner by undergoing malignant transformation. Further, transplanted stem cells may be recognized by the host immune system and subsequently attacked and destroyed. Moreover, if presenting key features of beta cells, autoimmunity might reoccur in type 1 diabetes patients and further push destruction of the transplant. However, encapsulation techniques may allow safe enclosing of SC-derived insulin-producing cells and tumor formation, and immune attack can be prevented.
Conclusions
With a world diabetes prevalence of 330 million and an estimated increase to 550 million in 20 years, there is an urgent need for therapy. It is reasonable to envision that within the next decade, the cure of diabetes will rely on the conjoined application of pharmacological- and cell-based treatments. The field of islet encapsulation may provide important contributions and fulfill the own pretension of establishing a simple and safe therapy as a functional cure for diabetes.
References
Gruessner RW, Gruessner AC (2014) What defines success in pancreas and islet transplantation-insulin independence or prevention of hypoglycemia? A review. Transplant Proc 46(6):1898–1899
Maffi P, Secchi A (2015).Clinical results of islet transplantation. Pharmacol Res
Balamurugan AN, Naziruddin B, Lockridge A, Tiwari M, Loganathan G, Takita M, Matsumoto S, Papas K, Trieger M, Rainis H, Kin T, Kay TW, Wease S, Messinger S, Ricordi C, Alejandro R, Markmann J, Kerr-Conti J, Rickels MR, Liu C, Zhang X, Witkowski P, Posselt A, Maffi P, Secchi A, Berney T, O’Connell PJ, Hering BJ, Barton FB (2014) Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999–2010. Am J Transplant 14(11):2595–2606
Robertson RP (2015) Islet transplantation for type 1 diabetes, 2015: what have we learned from alloislet and autoislet successes? Diabetes Care 38(6):1030–1035
Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle J, Onaca N, Naziruddin B, Levy MF (2011) Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant 20(10):1641–1647
Fridell JA, Rogers J, Stratta RJ (2010) The pancreas allograft donor: current status, controversies, and challenges for the future. Clin Transpl 24(4):433–449
Stegall MD, Dean PG, Sung R, Guidinger MK, McBride MA, Sommers C, Basadonna G, Stock PG, Leichtman AB (2007) The rationale for the new deceased donor pancreas allocation schema. Transplantation 83(9):1156–1161
Nilsson B, Ekdahl KN, Korsgren O (2011) Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant 16(6):620–626
Krishnan R, Alexander M, Robles L, Foster CE 3rd, Lakey JR (2014) Islet and stem cell encapsulation for clinical transplantation. Rev Diabet Stud 11(1):84–101
Scharp DW, Mason NS, Sparks RE (1984) Islet immuno-isolation: the use of hybrid artificial organs to prevent islet tissue rejection. World J Surg 8(2):221–229
Algire GH, Weaver JM, Prehn RT (1954) Growth of cells in vivo in diffusion chambers. I. Survival of homografts in immunized mice. J Natl Cancer Inst 15(3):493–507
Weaver JM, Algire GH, Prehn RT (1955) The growth of cells in vivo in diffusion chambers. II. The role of cells in the destruction of homografts in mice. J Natl Cancer Inst 15(6):1737–1767
Algire GH, Weaver JM, Prehn RT (1957) Studies on tissue homotransplantation in mice, using diffusion-chamber methods. Ann N Y Acad Sci 64(5):1009–1013
Algire GH, Borders ML, Evans VJ (1958) Studies of heterografts in diffusion chambers in mice. J Natl Cancer Inst 20(6):1187–1201
Algire GH (1957) Summary of studies of transplantation of homologous tissues. Fed Proc 16(2):601–602
Algire GH (1957) Diffusion-chamber techniques for studies of cellular immunity. Ann N Y Acad Sci 69(4):663–667
Monaco AP, Maki T, Ozato H, Carretta M, Sullivan SJ, Borland KM, Mahoney MD, Chick WL, Muller TE, Wolfrum J et al (1991) Transplantation of islet allografts and xenografts in totally pancreatectomized diabetic dogs using the hybrid artificial pancreas. Ann Surg 214(3):339–360, discussion 361-332
Hymer WC, Wilbur DL, Page R, Hibbard E, Kelsey RC, Hatfield JM (1981) Pituitary hollow fiber units in vivo and in vitro. Neuroendocrinology 32(6):339–349
Zekorn T, Siebers U, Filip L, Mauer K, Schmitt U, Bretzel RG, Federlin K (1989) Bioartificial pancreas: the use of different hollow fibers as a diffusion chamber. Transplant Proc 21(1 Pt 3):2748–2750
Maki T, Ubhi CS, Sanchez-Farpon H, Sullivan SJ, Borland K, Muller TE, Solomon BA, Chick WL, Monaco AP (1991) Successful treatment of diabetes with the biohybrid artificial pancreas in dogs. Transplantation 51(1):43–51
Maki T, Mullon CJ, Solomon BA, Monaco AP (1995) Novel delivery of pancreatic islet cells to treat insulin-dependent diabetes mellitus. Clin Pharmacokinet 28(6):471–482
Rafael E, Wernerson A, Arner P, Wu GS, Tibell A (1999) In vivo evaluation of glucose permeability of an immunoisolation device intended for islet transplantation: a novel application of the microdialysis technique. Cell Transplant 8(3):317–326
Rafael E, Wernerson A, Arner P, Tibell A (1999) In vivo studies on insulin permeability of an immunoisolation device intended for islet transplantation using the microdialysis technique. Eur Surg Res 31(3):249–258
Scharp DW, Marchetti P (2014) Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 67–68:35–73
de Groot M, Schuurs TA, van Schilfgaarde R (2004) Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res 121(1):141–150
Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y, Yavriyants K, Azarov D, Zimermann B, Maimon S, Shabtay N, Balyura M, Rozenshtein T, Vardi P, Bloch K, de Vos P, Rotem A (2013) Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant 22(8):1463–1476
Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, Vardi P, German T, Shabtay N, Rotem A, Evron Y, Neufeld T, Mimon S, Ludwig S, Brendel MD, Bornstein SR, Barkai U (2010) A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res 42(13):918–922
Neufeld T, Ludwig B, Barkai U, Weir GC, Colton CK, Evron Y, Balyura M, Yavriyants K, Zimermann B, Azarov D, Maimon S, Shabtay N, Rozenshtein T, Lorber D, Steffen A, Willenz U, Bloch K, Vardi P, Taube R, de Vos P, Lewis EC, Bornstein SR, Rotem A (2013) The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One 8(8):e70150
Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen A, Ludwig S, Chavakis T, Reichel A, Azarov D, Zimermann B, Maimon S, Balyura M, Rozenshtein T, Shabtay N, Vardi P, Bloch K, de Vos P, Schally AV, Bornstein SR, Barkai U (2012) Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci U S A 109(13):5022–5027
Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E, Solimena M, Gendler Z, Rotem A, Barkai U, Bornstein SR (2013) Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A 110(47):19054–19058
Lanza RP, Beyer AM, Staruk JE, Chick WL (1993) Biohybrid artificial pancreas. Long-term function of discordant islet xenografts in streptozotocin diabetic rats. Transplantation 56(5):1067–1072
Lanza RP, Borland KM, Lodge P, Carretta M, Sullivan SJ, Muller TE, Solomon BA, Maki T, Monaco AP, Chick WL (1992) Treatment of severely diabetic pancreatectomized dogs using a diffusion-based hybrid pancreas. Diabetes 41(7):886–889
Lanza RP, Borland KM, Staruk JE, Appel MC, Solomon BA, Chick WL (1992) Transplantation of encapsulated canine islets into spontaneously diabetic BB/Wor rats without immunosuppression. Endocrinology 131(2):637–642
Merani S, Toso C, Emamaullee J, Shapiro AM (2008) Optimal implantation site for pancreatic islet transplantation. Br J Surg 95(12):1449–1461
de Vos P, Spasojevic M, Faas MM (2010) Treatment of diabetes with encapsulated islets. Adv Exp Med Biol 670:38–53
Teramura Y, Iwata H (2010) Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv Drug Deliv Rev 62(7–8):827–840
Soon-Shiong P, Feldman E, Nelson R, Komtebedde J, Smidsrod O, Skjak-Braek G, Espevik T, Heintz R, Lee M (1992) Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation 54(5):769–774
Lanza RP, Ecker DM, Kuhtreiber WM, Marsh JP, Ringeling J, Chick WL (1999) Transplantation of islets using microencapsulation: studies in diabetic rodents and dogs. J Mol Med (Berl) 77(1):206–210
Dufrane D, Gianello P (2012) Macro- or microencapsulation of pig islets to cure type 1 diabetes. World J Gastroenterol 18(47):6885–6893
Calafiore R (1992) Transplantation of microencapsulated pancreatic human islets for therapy of diabetes mellitus. A preliminary report. Asaio J 38(1):34–37
Paredes Juarez GA, Spasojevic M, Faas MM, de Vos P (2014) Immunological and technical considerations in application of alginate-based microencapsulation systems. Front Bioeng Biotechnol 2:26
Opara EC, McQuilling JP, Farney AC (2013) Microencapsulation of pancreatic islets for use in a bioartificial pancreas. Methods Mol Biol 1001:261–266
Calafiore R, Basta G (2014) Clinical application of microencapsulated islets: actual prospectives on progress and challenges. Adv Drug Deliv Rev 67–68:84–92
De Vos P, Van Straaten JF, Nieuwenhuizen AG, de Groot M, Ploeg RJ, De Haan BJ, Van Schilfgaarde R (1999) Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes 48(7):1381–1388
Matsumoto S, Tan P, Baker J, Durbin K, Tomiya M, Azuma K, Doi M, Elliott RB (2014) Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc 46(6):1992–1995
Matsuda T, Kitamura T, Iwata H, Takano H, Akutsu T (1988) A hybrid artificial vascular graft based upon an organ reconstruction model. Significance and design criteria of an artificial basement membrane. ASAIO Trans 34(3):640–643
Wang T, Lacik I, Brissova M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC (1997) An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol 15(4):358–362
Wang T, Adcock J, Kuhtreiber W, Qiang D, Salleng KJ, Trenary I, Williams P (2008) Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 85(3):331–337
Drumheller PD, Elbert DL, Hubbell JA (1994) Multifunctional poly(ethylene glycol) semi-interpenetrating polymer networks as highly selective adhesive substrates for bioadhesive peptide grafting. Biotechnol Bioeng 43(8):772–780
Sawhney AS, Pathak CP, Hubbell JA (1993) Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials 14(13):1008–1016
Sawhney AS, Pathak CP, Hubbell JA (1994) Modification of islet of langerhans surfaces with immunoprotective poly(ethylene glycol) coatings via interfacial photopolymerization. Biotechnol Bioeng 44(3):383–386
Hill RS, Cruise GM, Hager SR, Lamberti FV, Yu X, Garufis CL, Yu Y, Mundwiler KE, Cole JF, Hubbell JA, Hegre OD, Scharp DW (1997) Immunoisolation of adult porcine islets for the treatment of diabetes mellitus. The use of photopolymerizable polyethylene glycol in the conformal coating of mass-isolated porcine islets. Ann N Y Acad Sci 831:332–343
Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA (1999) In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant 8(3):293–306
Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A (2014) Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A 111(29):10514–10519
Wilson JT, Cui W, Chaikof EL (2008) Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett 8(7):1940–1948
Gattas-Asfura KM, Stabler CL (2013) Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers. ACS Appl Mater Interfaces 5(20):9964–9974
Teramura Y, Kaneda Y, Iwata H (2007) Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials 28(32):4818–4825
Rengifo HR, Giraldo JA, Labrada I, Stabler CL (2014) Long-term survival of allograft murine islets coated via covalently stabilized polymers. Adv Healthcare Mater 3(7):1061–1070
Zhi ZL, Kerby A, King AJ, Jones PM, Pickup JC (2012) Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia 55(4):1081–1090
Ludwig B, Ludwig S, Steffen A, Saeger HD, Bornstein SR (2010) Islet versus pancreas transplantation in type 1 diabetes: competitive or complementary? Curr Diab Rep 10(6):506–511
van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK (2008) The choice of anatomical site for islet transplantation. Cell Transplant 17(9):1005–1014
van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK (2007) Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation 14(4):288–297
Robson SC, Cooper DK, d’Apice AJ (2000) Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 7(3):166–176
Kim JH, Oh BJ, Lee HN, Park HS, Park SG, Park KS (2011) Endothelial colony-forming cell coating of pig islets prevents xenogeneic instant blood-mediated inflammatory reaction. Cell Transplant 20(11-12):1805–1815
Hwang JW, Jung HS, Lee DY (2011) Inhibition of platelet adhesion onto intrahepatically transplanted islets using PEGylation for attenuating instant blood-mediated inflammatory reaction (IBMIR). J Control Release 152(Suppl 1):e213–e214
Teramura Y, Iwata H (2011) Improvement of graft survival by surface modification with poly(ethylene glycol)-lipid and urokinase in intraportal islet transplantation. Transplantation 91(3):271–278
Luan NM, Teramura Y, Iwata H (2011) Immobilization of soluble complement receptor 1 on islets. Biomaterials 32(20):4539–4545
Luan NM, Teramura Y, Iwata H (2011) Layer-by-layer co-immobilization of soluble complement receptor 1 and heparin on islets. Biomaterials 32(27):6487–6492
Chen H, Teramura Y, Iwata H (2011) Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)-conjugated phospholipid. J Control Release 150(2):229–234
Teramura Y, Iwata H (2009) Surface modification of islets with PEG-lipid for improvement of graft survival in intraportal transplantation. Transplantation 88(5):624–630
Im BH, Jeong JH, Haque MR, Lee DY, Ahn CH, Kim JE, Byun Y (2013) The effects of 8-arm-PEG-catechol/heparin shielding system and immunosuppressive drug, FK506 on the survival of intraportally allotransplanted islets. Biomaterials 34(8):2098–2106
Hilbrands R, Huurman VA, Gillard P, Velthuis JH, De Waele M, Mathieu C, Kaufman L, Pipeleers-Marichal M, Ling Z, Movahedi B, Jacobs-Tulleneers-Thevissen D, Monbaliu D, Ysebaert D, Gorus FK, Roep BO, Pipeleers DG, Keymeulen B (2009) Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes 58(10):2267–2276
Harlan DM, Kenyon NS, Korsgren O, Roep BO (2009) Current advances and travails in islet transplantation. Diabetes 58(10):2175–2184
Calafiore R, Basta G, Luca G, Lemmi A, Racanicchi L, Mancuso F, Montanucci MP, Brunetti P (2006) Standard technical procedures for microencapsulation of human islets for graft into nonimmunosuppressed patients with type 1 diabetes mellitus. Transplant Proc 38(4):1156–1157
O’Sullivan ES, Vegas A, Anderson DG, Weir GC (2011) Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev 32(6):827–844
Pierson RN 3rd (2009) Antibody-mediated xenograft injury: mechanisms and protective strategies. Transpl Immunol 21(2):65–69
Kirchhof N, Shibata S, Wijkstrom M, Kulick DM, Salerno CT, Clemmings SM, Heremans Y, Galili U, Sutherland DE, Dalmasso AP, Hering BJ (2004) Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation 11(5):396–407
Soderlund J, Wennberg L, Castanos-Velez E, Biberfeld P, Zhu S, Tibell A, Groth CG, Korsgren O (1999) Fetal porcine islet-like cell clusters transplanted to cynomolgus monkeys: an immunohistochemical study. Transplantation 67(6):784–791
de Vos P, Marchetti P (2002) Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med 8(8):363–366
Klymiuk N, van Buerck L, Bahr A, Offers M, Kessler B, Wuensch A, Kurome M, Thormann M, Lochner K, Nagashima H, Herbach N, Wanke R, Seissler J, Wolf E (2012) Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes 61(6):1527–1532
Henquin JC (2000) Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49(11):1751–1760
Hellman B, Gylfe E, Bergsten P, Grapengiesser E, Lund PE, Berts A, Tengholm A, Pipeleers DG, Ling Z (1994) Glucose induces oscillatory Ca2+ signalling and insulin release in human pancreatic beta cells. Diabetologia 37(Suppl 2):S11–S20
Marchetti P, Scharp DW, McLear M, Gingerich R, Finke E, Olack B, Swanson C, Giannarelli R, Navalesi R, Lacy PE (1994) Pulsatile insulin secretion from isolated human pancreatic islets. Diabetes 43(6):827–830
Buchwald P, Cechin SR, Weaver JD, Stabler CL (2015) Experimental evaluation and computational modeling of the effects of encapsulation on the time-profile of glucose-stimulated insulin release of pancreatic islets. Biomed Eng Online 14(1):28
Blasi P, Luca G, Mancuso F, Schoubben A, Calvitti M, Giovagnoli S, Basta G, Becchetti E, Ricci M, Calafiore R (2013) Conformal polymer coatings for pancreatic islets transplantation. Int J Pharm 440(2):141–147
Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacik I, Shapiro AM, Pedraz JL (2003) Cell encapsulation: promise and progress. Nat Med 9(1):104–107
Orive G, Gascon AR, Hernandez RM, Igartua M, Luis Pedraz J (2003) Cell microencapsulation technology for biomedical purposes: novel insights and challenges. Trends Pharmacol Sci 24(5):207–210
de Vos P, Hamel AF, Tatarkiewicz K (2002) Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia 45(2):159–173
Vaithilingam V, Kollarikova G, Qi M, Lacik I, Oberholzer J, Guillemin GJ, Tuch BE (2011) Effect of prolonged gelling time on the intrinsic properties of barium alginate microcapsules and its biocompatibility. J Microencapsul 28(6):499–507
De Vos P, Vegter D, De Haan BJ, Strubbe JH, Bruggink JE, Van Schilfgaarde R (1996) Kinetics of intraperitoneally infused insulin in rats. Functional implications for the bioartificial pancreas. Diabetes 45(8):1102–1107
Yang HK,Yoon KH (2015) Current status of encapsulated islet transplantation. J Diabet Complications
Pareta R, McQuilling JP, Sittadjody S, Jenkins R, Bowden S, Orlando G, Farney AC, Brey EM, Opara EC (2014) Long-term function of islets encapsulated in a redesigned alginate microcapsule construct in omentum pouches of immune-competent diabetic rats. Pancreas 43(4):605–613
Juang JH, Bonner-Weir S, Ogawa Y, Vacanti JP, Weir GC (1996) Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 61(11):1557–1561
De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R (1997) Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation 63(6):824–830
Eberhard D, Kragl M, Lammert E (2010) ‘Giving and taking’: endothelial and beta-cells in the islets of Langerhans. Trends Endocrinol Metab 21(8):457–463
Lammert E, Cleaver O, Melton D (2001) Induction of pancreatic differentiation by signals from blood vessels. Science 294(5542):564–567
Denner J,Graham M (2015) Xenotransplantation of islet cells: what can the non-human primate model bring for the evaluation of efficacy and safety? Xenotransplantation
Graham ML, Bellin MD, Papas KK, Hering BJ, Schuurman HJ (2011) Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation 18(6):328–342
Acknowledgments
This work was supported by the University Hospital Carl Gustav Carus, Department of Medicine III, and Paul Langerhans Institute Dresden of Helmholtz Centre Munich at University Clinic Carl Gustav Carus of TU Dresden Faculty of Medicine, Technische Universität Dresden, DZD- German Centre for Diabetes Research and by grants from the Deutsche Forschungsgemeinschaft SFB/TR127 (to B.L.).
Conflicts of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ludwig, B., Ludwig, S. Transplantable bioartificial pancreas devices: current status and future prospects. Langenbecks Arch Surg 400, 531–540 (2015). https://doi.org/10.1007/s00423-015-1314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1314-y