Abstract
Purpose
Athlete’s heart encompasses multiple physiological cardiac adaptations, although less is known at atrial level. How sex may influence the type and extent of atrial adaptations to exercise stimuli is also unknown. Our objective was to compare gender differences of echocardiographic atrial function indices in response to exercise in endurance athletes (EAs).
Methods
Highly trained (> 10 h/week) endurance athletes performed a maximal cardiopulmonary exercise test (CPET). Echocardiographic evaluation was performed at rest and immediately after exercise. Atria analysis consisted of standard and speckle-tracking echocardiographic assessment of atrial dimensions and contractile, reservoir, and conduit functions with myocardial deformation.
Results
80 EAs (55% women) were enrolled and performed excellent CPET (129.6% of predicted VO2 maximal consumption). At rest, left atrial (LA) volumes and strain were similar between men and women. Women had lower right atrial (RA) volumes (26.7 vs 32.9 ml/m2, p < 0.001) and higher reservoir and conduit strain absolute values. After exercise, women exhibited a larger improvement in reservoir and conduit LA strain, and the same trend was observed for the RA. In EAs with LA dilatation on baseline (~ 50%), women persistently showed higher increase in reservoir and conduit strain profile with exercise compared to men.
Conclusion
In highly trained EAs, women have similar or even lower atrial dimensions remodelling compared to men, but better function based on reservoir and conduit strain values both at rest and in response to exercise. This phenomenon should be confirmed in larger studies and its potential role in the development of supraventricular arrhythmias, addressed in a specifically designed protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prolonged participation in high-intensity physical activities is associated with multiple electrical and structural cardiac adaptations (Kovacs and Baggish 2016). The type and extent of cardiac remodelling in athletes is multifactorial, but one of its main determinants is related to the type of sport involvement (Baggish et al. 2008; Pluim Babette et al. 2000). In athletes engaged in endurance sports, biventricular dilatation has been well described as a physiological adaptations (Kovacs & Baggish, 2016). A similar adaptation at the atrial level, namely bi-atrial dilatation, has been described (Cuspidi et al. 2019), although less is known about its underlying determinants. Sex is a major determinant of classical cardiac remodelling (left-ventricular hypertrophy and dilatation, and right-ventricular dilatation) (Pelliccia and Adami 2017), but its precise influence and impact on exercise-induced atrial remodelling has not been eluded yet.
Multiple studies have addressed the relationship between exercise and increased incidence of atrial fibrillation (AF). Observational data have shown that athletes involved in long-term endurance training appear at higher risk of AF (Nielsen et al. 2013). The precise physiopathology remains to be defined, but left atrial remodelling could be contributory (Estes & Madias, 2017). A dose–response relationship between volume endurance exercise training and the incidence of AF has been also demonstrated (Andersen et al. 2013; Wilhelm et al. 2011). This dose–response relationship seems to be also true for the atrial adaptation to an acute exercise. After a cross country race, a significant impairment in right and left atrial performance was shown in athletes completing a long-distance race, while no changes were observed in athletes running the short distance race. However, all the above-mentioned data come from studies in which female athletes were systematically underrepresented (Sanz-de la Garza et al. 2016).
On the other hand, the relationship between high-endurance training loads and AF in women athletes appears different than in men athletes. In a recent meta-analysis integrating data of more than 650 000 athletes from 22 studies (Mohanty et al. 2016), prolonged high-intensity endurance exercise involvement was a clear risk factor of AF in male athletes, while it appeared to be protective for women athletes. This further increases the interest on the precise influence of sex on exercise-induced atrial remodelling.
Therefore, the aim of our study was to comprehensively evaluate atrial structural and functional parameters at rest and after an exercise stimulus in a cohort of highly trained endurance athletes of both sexes. We hypothesized that female athletes would show a better atrial performance profile in response to exercise and less pronounced atrial remodelling than male athletes—in line with the difference in AF incidence.
Methods
Endurance athletes were enrolled between October 2015 and October 2016 as part of a study aimed at evaluating sex-related differences in cardiac adaptations both at rest and stress (Sanz-de la Garza et al. 2016). Study participants were volunteers answering to an advertisement in local triathlon clubs. The exclusion criteria were significant history of cardiovascular or lung diseases and family history of sudden cardiac death or early cardiomyopathy.
All participants were highly trained—defined as more than 10 h of training a week for at least 5 years at time of recruitment. Training load was verified based on answers provided on their International Physical Activity Questionnaire (IPAQ) (Ainsworth et al. 2011). All participants gave verbal and written informed consent. This study was performed in accordance with local ethic committee approval and in respect with Helsinki declaration.
All participants underwent a medical survey, a resting conventional 12-lead ECG, and a standard physical examination including anthropometric measurements performed by a trained physician.
Exercise protocol
Subjects underwent an incremental cardiopulmonary exercise testing to exhaustion on an up-right cycle ergometer (Ergoselected 100, Ergoline, Bitz, Germany). Ramp protocol was calculated for each subject based on sex and age (Hansen et al. 1984) with a pedalling frequency kept constant between 60 and 80 rotations/minute. Gas composition and ventilation volume measurements were done using breath-by-breath mode (Ergocard Professional, Medisoft, Sorinnes, Belgium) ("ATS/ACCP Statement on cardiopulmonary exercise testing," 2003). Maximal oxygen consumption was determined by an identifiable plateau of the VO2–workload relationship at maximum exercise. Before testing, the spirometer and gas analyser were calibrated following international standardisation. During exercise, subjects were also monitored using a 12-lead electrocardiogram, pulse oximetry and a non-invasive blood pressure monitor.
Baseline echocardiography
After at least 20 min of rest in pre-prandial condition, a standard basal echocardiography on a commercially available system (Vivid Q; GE Medical; Milwaukee, USA) was performed according to guidelines (Lang et al. 2015) and included focused apical views of the atria. For each acquisition, a three consecutive cardiac cycles’ cine loop was recorded for offline analysis with commercially available software EchoPac, version 202.41.0, Milwaukee, WI, USA).
Left ventricle (LV) volumes and ejection fraction were calculated using the biplane Simpson method. Right ventricle (RV) size and function was evaluated from an apical modified four-chamber view, tracing RV endocardium borders in end-diastole and end-systole and calculating RV fractional area change (Lang et al. 2015). RV and LV diastolic function was assessed using respective atrioventricular valves’ Doppler inflow and tissue-Doppler values as per guidelines (Nagueh et al. 2016). Cardiac chamber dimensions were indexed for body surface area according to the Dubois formula.
Exercise echocardiography
Image acquisition was repeated immediately after the peak of exercise in a left-lateral decubitus position. Focus was put on acquisition of apical views for longitudinal strain assessment. Images acquired within 2 min of exercise termination were kept for analysis.
Echocardiographic atrial evaluation
Atrial parameters’ measurements were performed offline using the same software as for ventricular measurements (EchoPac, version 202.41.0, Milwaukee, WI, USA). All measurements were acquired at rest and after maximal exercise when feasible in the same conditions as described above for the standard echocardiographic measurements. Atrial volumes (left and right) were measured using the four-chamber view, tracing the endocardial borders and using the modified Simpson method at end-systole, before the P wave and end-diastole, of which were derived ejection fraction calculations. Left (LA) and right atrial (RA) dilatation were defined as per guidelines (LA dilatation: > 34 ml/m2 for both sexes, RA dilatation: ≥ 27 ml/m2 for women, ≥ 32 ml/m2 for men) (Lang et al. 2015).
Atrial function was also assessed using atrial longitudinal strain. Atrial strain was performed using the EchoPAC left-ventricular software algorithm. Endocardial borders were traced for both atria taking extra-caution to make sure the atrial walls were well tracked through the whole cardiac cycle, as assessed by the software algorithm and visually. Cardiac cycle points of reference used were the onset of the P wave to the next one (as opposed to QRS onset to next QRS). Atrial strain (in %) and strain rate (in s−1) values were then transformed to the QRS-to-QRS points of reference as recommended in guidelines (Badano et al. 2018).
Statistical analysis
Data are reported as mean with standard deviations or median with interquartile range as appropriate using the Shapiro–Wilk test for data normality distribution assessment. Baseline and stress values for all athletes were compared using the paired T test for normally distributed and Mann–Whitney U test for non-normally distributed variables. Comparisons between men and women were done using the unpaired T test or Wilcoxon rank sum test, as appropriate. Pearson’s or Spearman’s correlations were also calculated for continuous variables.
Impact of height on different atrial size and strain parameters was first assessed by linear regression. Height was then transformed from a continuous variable to terciles. Subsequently, a two-way analysis of variance (ANOVA) was performed to evaluate height and sex as potential covariables of previously identified significant atrial parameters on linear regression.
Intra and inter-observer reliability measurements were measured using intra-class correlation on ten studies that were re-measured by two different operators (FS, MSG). Statistical significance was set at a p value < 0.05. All analysis were done on IBM SPSS Statistics version 25.0 (Chicago, IL, USA, 2017).
Results
Baseline population characteristics and exercise parameters
Eighty athletes (44 women, 55%) were enrolled in this study. Both groups (women vs men) were of similar age and were engaged in an equivalent amount of endurance training. Male athletes had higher anthropometric measurements, slower baseline heart rate, and higher systolic and diastolic blood pressures compared to female athletes (Table 1).
Both groups exhibited, as expected, excellent cardiovascular fitness achieving 129.6% of their predicted VO2 maximal consumption and an adjusted to weight mean consumption of 44.7L/min*kg−1. There was also substantial and equivalent heart rate and blood pressure increase in both groups (Table 1). Heart rate at time of echocardiographic images acquisition after exercise was similar in both groups (90.2 vs 96.9 beats per minute, p > 0.05).
Baseline echocardiographic ventricular measurements
On baseline studies, female athletes exhibited lower left ventricle (LV) wall thickness, diameters, and volumes compared to men (Table 1). Women also showed mildly higher LV ejection fraction (56.0 vs 53.4%, p = 0.008) and right-ventricular fractional area change (47.3 vs 44.6%, p = 0.005). LV E and e’ septal waves' values were slightly increased in women, but overall E/e’ ratio and other diastolic parameters at rest were similar between both sexes (Table 1).
Baseline atrial echocardiographic characteristics
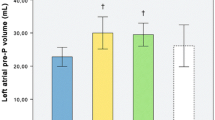
At rest, indexed left atrial (LA) volume and left atrial strain parameters were similar between female and male athletes, while LA ejection fraction was slightly higher in women (68.0 vs 64.0%, p = 0.010) (Tables 2, 3 and Fig. 1A) as compared to men. Right atrial (RA) indexed volumes were significantly lower in women vs men (26.7 vs 32.9 ml/m2, p < 0.001) (Table 2). Female athletes also exhibited higher RA function values as compared to male athletes at baseline (Table 4 and Fig. 2A).
Atrial performance after exercise
As expected, exercise promoted a significant increase in most LA functional parameters in both sexes. However, the extent of this improvement was greater in female athletes as compared to male athletes for both LA reservoir and conduit strain. No significant changes in LA volume were documented after exercise in neither of the two groups. (Tables 2, 3).
As for the LA, the exercise stimulus induced a significant improvement of all RA functional parameters. In female athletes, the exercise-induced increase in RA reservoir and conduit strains showed a trend to be greater than in male athletes (although not statistically significant), while no differences between sexes were documented for the change in other RA functional parameters or RA volume. (Table 4 and Fig. 2).
Nineteen (53%) male athletes and 22 (50%) female athletes had dilated LA (Tables 2, 3 and Fig. 1B). Both female and male athletes with a dilated LA tended to have worst LA strain reservoir and conduit function compared to athletes with normal LA size. However, when comparing female and male athletes with dilated left atrium, women exhibited significantly increased LA reservoir and conduit strain values (Fig. 1B). Fewer athletes had RA dilatation: 11 athletes, 6 men (17%), and 5 women (11%). Male athletes from this subgroup seemed to have a lower conduit function change compared to women, albeit not reaching statistical difference (Fig. 2B).
Impact of height to atrial response with exercise was explored because of its known relation with atrial dimension (Levin et al. 2020). When adjusting atrial volumes and strain values to height instead of body surface area, baseline atrial volumes were different between men and women (Table 2). Height was weakly correlated to the same strain parameters that are different between men and women (percent change in LA reservoir and conduit strain and baseline RA reservoir and conduit strain). Using a two-way ANOVA and dividing athletes in terciles based on their height, the relationship between height and these atrial strain parameters was not statistically significant anymore, while it remained significant for sex (Supplementary Table 1).
Atrial evaluation and more particularly strain assessment were possible in 99% of cases at rest and more than 87% of cases after exercise (93% left atrium, 83% right atrium). Intra-class correlation revealed excellent intra-observer reproducibility of bi-atrial strain both at rest and exercise (both of 0.99). Good inter-observer agreement was also observed (intra-class correlation of bi-atrial strain at rest and with exercise of 0.87 and 0.83, respectively) (Koo and Li 2016).
Discussion
This study aimed to give a more detailed description of the different cardiac adaptations to high-intensity endurance exercise training between sexes at the atrial level. We believe that our cohort is of great interest because of our restricted inclusion criteria regarding exercise status (more than 10 h a week in the past 5 years) and the equal sex representation. Two key messages are arising from our observations. First, sex influences atrial remodelling in athletes involved in long-term endurance training. Women athletes have similar LA volumes and strain parameters compared to men at rest but show significantly less RA dilatation and better RA reservoir and conduit function. Second, the magnitude of left atrial strain improvement with exercise stimulus is significantly higher in women athletes. This is true even in athletes with dilated LA and the same trend is observed for the RA.
Sex influence on long-term exercise-induced atrial remodelling
Data addressing the specific influence of sex on chronic exercise-induced atrial remodelling remain limited. In 1996, Pelliccia et al. reported echocardiographic characteristics of a cohort of 600 female high-level athletes of 27 different disciplines. They found significant LA dilatation compared to their sex-matched sedentary control group. However, the degree of dilatation was significantly less than what was observed in their cohort of male athletes, suggesting that women engaged in long-term exercise have cardiac structural adaptations, although to a lesser extent than their male counterpart (Pelliccia et al. 1996).
Atrial structural adaptations to exercise in women was specifically studied in a prospective cohort of 24 high-level handball players. After 16 weeks of intensive exercise (16 h a week), D’Ascenzi et al. reported that female athletes can develop significant LA enlargement (Flavio D'Ascenzi et al. 2018). The same relation was observed for the RA. While still within normal range, after intensive training, LA strain values also tended to slightly drop compared to baseline values, a phenomenon though not observed for the RA. Hence, this study strongly suggests that exercise can induce significant atrial structural, and possibly functional, changes in women. Of note, this study was not designed to evaluate sex difference, if any, in the extent of atrial adaptations to exercise.
More recently, our group (Sanchis et al. 2017) also studied bi-atrial dimensions and mechanics in 40 controls and 40 highly selected athletes (training > 10 h per week for at least 5 years). Both athlete and control groups had sex parity. Interestingly, as opposed to Pelliccia’s findings (Pelliccia et al. 1996), women athletes did have increased LA volumes compared to the control group, but there was no difference between men and women athletes in the degree of LA dilatation. LA function, evaluated using strain and strain rates profiles, was also similar between male and women athletes. Regarding the RA, women athletes had lower RA chamber dilatation compared to men athletes and better RA myocardial deformation indices.
Put together, our data support that female athletes do present significant atrial remodelling when engaged in long-term intensive exercise. This remodelling appears to be both structural and functional and similar to male athletes, although women show significantly less RA dilatation and better atrial function profiles based on strain evaluation.
Sex influence on atrial performance in response to exercise
A major difference was observed between sexes regarding immediate LA deformation parameters after exercise. In fact, women showed increased LA myocardial deformation and, theoretically, better performance compared to men. Moreover, the same sex discrepancy was observed in athletes with dilated LA. The same trend was observed, although not reaching statistical significance, in the group of athletes with dilated RA. Immediate atrial response to exercise is known to be highly variable between individuals (Gabrielli et al. 2016; Sanz-de la Garza et al. 2016) and, together with the relatively small subgroup size, possibly explains why statistical significance was not reached.
Prolonged periods of endurance exercise mandate a significant increase in cardiac output and the “athlete’s heart” capacity to achieve high stroke volume is not solely related to ventricular dilatation. Diastolic function is also a significant component of heart adaptations in athletes enrolled in prolonged isotonic exercise (Kovacs and Baggish 2016) allowing a fast and optimal ventricular filling at low pressure. Diastolic function is classically studied using Doppler mitral inflow in tissue-Doppler velocities, although limitations of these parameters are well known (Park and Marwick 2011). Some studies suggest that atrial volumes and functions are probably more closely linked to diastolic function and, arguably, better marker of early diastolic dysfunction or increase in ventricular filling pressure (Singh et al. 2017; Thomas et al. 2019).
As depicted in Fig. 1 and Table 3, both sexes have similar LA strain parameters at rest, but women athletes significantly improve their LA reservoir and conduit function with exercise, while men exhibit milder or no improvement of these same atrial function components. For the right atrium (Fig. 2 and Table 4), women athletes exhibit better RA strain reservoir and conduit function at rest and they tend to show a better profile with exercise stimulus compared to men. In our cohort, men athletes could be described as showing a potentially negative atrial remodelling given that during exercise, an important goal of the atria is to help increase cardiac output with minimal increase in ventricular filling pressures.
This different atrial response to exercise between sexes remains of an unknown underlying physiology. Nevertheless, the evidence accumulating on the sex’s disparities on cardiac exercise-induced remodelling (not only at atrial level (D’Ascenzi et al. 2020; Antonio Pelliccia and Adami 2017)) merit revisiting known pathophysiologic concepts of volume and pressure overload and their different impacts based on sex. Oestrogens could be playing a central role in modulating these differential adaptations, since they are known to influence proinflammatory cytokines, fibroblast growth, and myocytes’ apoptosis, and have an antihypertrophic effect (Ainsworth et al. 2011; Piro et al. 2010) which possibly induce differences in myocardial contractility and diastolic responses to acute and chronic exercise stimuli. Other factors, such as the environment, should also be taken into account (Guasch and Mont 2015).
Influence of height on atrial characteristics in athletes
Because of geometric considerations, strain is closely related to the dimension of the cardiac chamber under study—it tends to be lower (more negative) with a smaller cavity size (Voigt and Cvijic 2019). Considering that men are on average taller than woman for multiple genetic, hormonal, and environmental reasons (Ellis et al. 2001) and that height also influences left atrial size (Levin et al. 2020), it could be argued that the differences we observed in different atrial strain parameters between sexes are in fact related to the height discrepancy of both groups. However, in our cohort, while height was associated with atrial dimensions and a few strain parameters, its influence was limited compared to the role of sex in our observations, since the latter was the only parameter that remained statistically significant in a two-way ANOVA model (Supplementary Table 1).
Clinical implications
Our study confirms that female athletes develop atrial dilatation (D’Ascenzi et al. 2014; Sanchis et al. 2017) but mainly adds, to our opinion, a better description of the underlying differential phenotypic atrial remodelling triggered by high-intensity endurance exercise between sexes. To our knowledge, it is the first time that the impact of sex on atrial performance in response to the exercise stimulus has been evaluated. These findings add to the already known differences between sexes regarding differential ventricular adaptations to chronic aerobic exercise.
It is certainly precocious to make any link between these atrial parameters’ differences and the known difference in incidence of supraventricular arrhythmias between sexes in endurance athletes. Dedicated studies comparing the occurrence of supraventricular arrhythmias and atrial function profiles in athletes are needed before making any assumption. Nevertheless, we believe that our results merit further consideration and could eventually help to better understand the underlying determinants of this dichotomous incidence of atrial fibrillation between sexes in endurance athletes, especially given the number of reports addressing left atrial mechanical alterations and their association with atrial fibrillation incidence in other contexts (Olsen et al. 2019; Thomas et al. 2020). It should be emphasized that to this date, no relation between transitory atrial dysfunction induced by exercise and incidence of atrial arrhythmias has been demonstrated (Cavigli et al. 2022).
Study limitations
Our study has limitations that need to be considered. Despite being one of the biggest cohort of athletes specifically addressing the differences in atrial characteristics between men and women athletes, our findings should be confirmed by other groups to better document the precise relationship between sex and exercise-induced atrial remodelling. Also, by its cross-sectional design, our study cannot provide a proper understanding and explanation of the differences we observed in both groups. Nevertheless, our findings are at least hypothesis-generating regarding hormonal influence on atrial function.
Atrial volumetric quantification is an alternative method to assess atrial function and has been previously used to investigate atrial remodelling in athletes (F. D'Ascenzi et al. 2015). More recently, myocardial deformation has been recognized as a robust tool to analyse atrial function (Hoit Brian, 2022). Despite the limitations of exercise images and with the use of high frame acquisition modalities, we nevertheless managed to acquire proper data in a substantial proportion of individuals with a good-to-excellent agreement in intra- and inter-observer measurements. Interestingly and logically, similarities in terms of atrial function component affected by exercise remodelling can be found between both methods (the absence of significant change in contractile function for example (D'Ascenzi et al. 2015)).
Finally, we acquired stress echocardiographic images only in the immediate recovery after maximal exercise timeframe. While it does not affect the pertinence and the significance of our results, this causes an intrinsic limitation of not being able to identify and describe a more precise stage and load of exercise that leads to the observations that we described. This could have been of value considering echocardiographic diastolic study at various exercise stages during a stress test may offer incremental value (Pellikka et al. 2020).
Conclusion
In a cohort of highly trained endurance exercise athletes, we found significantly less exercise-induced atrial remodelling in women compared to men. More specifically, significantly lower right atrial volumes and higher myocardial deformation performance parameters were observed in female athletes. Female athletes also demonstrated a significantly better left atrial performance profile after exercise compared to male athletes and the same trend was observed for the right atrium. These observations add to our understanding of the athlete’s heart features and the differences known between sexes, although additional data to confirm our findings are needed. Moreover, a specifically designed study addressing the hypothetic relationship between exercise-induced atrial remodelling and incidence of atrial arrhythmias in endurance athletes, with particular attention to possible sex disparities, is needed.
Abbreviations
- AF:
-
Atrial fibrillation
- ANOVA:
-
Analysis of variance
- LA:
-
Left atria
- LV:
-
Left ventricle
- RA:
-
Right atria
- RV:
-
Right ventricle
- VO2 :
-
Oxygen consumption
References
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Leon AS (2011) 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43(8):1575–1581. https://doi.org/10.1249/MSS.0b013e31821ece12
Andersen K, Farahmand B, Ahlbom A, Held C, Ljunghall S, Michaëlsson K, Sundström J (2013) Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 34(47):3624–3631. https://doi.org/10.1093/eurheartj/eht188
ATS, ACCP (2003) Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167(2):211–277. https://doi.org/10.1164/rccm.167.2.211
Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T (2018) Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 19(6):591–600. https://doi.org/10.1093/ehjci/jey042
Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Wood MJ (2008) Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol 104(4):1121–1128. https://doi.org/10.1152/japplphysiol.01170.2007
Cavigli L, Zorzi A, Spadotto V, Mandoli GE, Melani A, Fusi C, D’Ascenzi F (2022) The acute effects of an ultramarathon on atrial function and supraventricular arrhythmias in master athletes. J Clin Med. https://doi.org/10.3390/jcm11030528
Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G (2019) Left atrial function in elite athletes: a meta-analysis of two-dimensional speckle tracking echocardiographic studies. Clin Cardiol 42(5):579–587. https://doi.org/10.1002/clc.23180
D’Ascenzi F, Pelliccia A, Natali Benedetta M, Zacà V, Cameli M, Alvino F, Mondillo S (2014) Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circulation 7(2):222–229. https://doi.org/10.1161/CIRCIMAGING.113.001345
D’Ascenzi F, Biella F, Lemme E, Maestrini V, Giacinto BD, Pelliccia A (2020) Female athlete’s heart. Circulation 13(12):e011587. https://doi.org/10.1161/CIRCIMAGING.120.011587
D’Ascenzi F, Pelliccia A, Natali BM, Cameli M, Lisi M, Focardi M, Henein M (2015) Training-induced dynamic changes in left atrial reservoir, conduit, and active volumes in professional soccer players. Eur J Appl Physiol 115(8):1715–1723. https://doi.org/10.1007/s00421-015-3151-7
D’Ascenzi F, Anselmi F, Focardi M, Mondillo S (2018) Atrial enlargement in the athlete’s heart: assessment of atrial function may help distinguish adaptive from pathologic remodeling. J Am Soc Echocardiogr 31(2):148–157. https://doi.org/10.1016/j.echo.2017.11.009
Ellis JA, Stebbing M, Harrap SB (2001) Significant population variation in adult male height associated with the y chromosome and the aromatase gene. J Clin Endocrinol Metab 86(9):4147–4150. https://doi.org/10.1210/jcem.86.9.7875
Estes NAM, Madias C (2017) Atrial fibrillation in athletes: a lesson in the virtue of moderation. JACC 3(9):921–928. https://doi.org/10.1016/j.jacep.2017.03.019
Gabrielli L, Bijnens BH, Brambila C, Duchateau N, Marin J, Sitges-Serra I, Sitges M (2016) Differential atrial performance at rest and exercise in athletes: potential trigger for developing atrial dysfunction? Scand J Med Sci Sports 26(12):1444–1454. https://doi.org/10.1111/sms.12610
Guasch E, Mont L (2015) Exercise, sex and atrial fibrillation: arrhythmogenesis beyond Y-chromosome? Heart 101(20):1607. https://doi.org/10.1136/heartjnl-2015-308036
Hansen JE, Sue DY, Wasserman K (1984) Predicted values for clinical exercise testing. Am Rev Respir Dis 129(2 Pt 2):S49-55. https://doi.org/10.1164/arrd.1984.129.2P2.S49
Hoit Brian D (2022) Left atrial reservoir strain. JACC 15(3):392–394. https://doi.org/10.1016/j.jcmg.2021.10.003
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Kovacs R, Baggish AL (2016) Cardiovascular adaptation in athletes. Trends Cardiovasc Med 26(1):46–52. https://doi.org/10.1016/j.tcm.2015.04.003
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Voigt J-U (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 28(1):1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Levin MG, Judy R, Gill D, Vujkovic M, Verma SS, Bradford Y, Damrauer SM (2020) Genetics of height and risk of atrial fibrillation: a Mendelian randomization study. PLoS Med 17(10):e1003288. https://doi.org/10.1371/journal.pmed.1003288
Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, Natale A (2016) Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol 27(9):1021–1029. https://doi.org/10.1111/jce.13023
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Nielsen JR, Wachtell K, Abdulla J (2013) The relationship between physical activity and risk of atrial fibrillation-a systematic review and meta-analysis. J Atr Fibrillation 5(5):789. https://doi.org/10.4022/jafib.789
Olsen FJ, Møgelvang R, Jensen GB, Jensen JS, Biering-Sørensen T (2019) Relationship between left atrial functional measures and incident atrial fibrillation in the general population: the copenhagen city heart study. JACC Cardiovasc Imaging 12(6):981–989. https://doi.org/10.1016/j.jcmg.2017.12.016
Park J-H, Marwick TH (2011) Use and limitations of e/e’ to assess left ventricular filling pressure by echocardiography. Journal of Cardiovascular Ultrasound 19(4):169–173. https://doi.org/10.4250/jcu.2011.19.4.169
Pelliccia A, Adami PE (2017) The female side of the heart: sex differences in athlete’s heart. JACC 10(9):973–975. https://doi.org/10.1016/j.jcmg.2016.08.010
Pelliccia A, Maron BJ, Culasso F, Spataro A, Caselli G (1996) Athlete’s heart in women. Echocardiographic characterization of highly trained elite female athletes. JAMA 276(3):211–215. https://doi.org/10.1001/jama.276.3.211
Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG (2020) Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the american society of echocardiography. J Am Soc Echocardiogr 33(1):1-41.e48. https://doi.org/10.1016/j.echo.2019.07.001
Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F (2010) Sex-related differences in myocardial remodeling. J Am Coll Cardiol 55(11):1057–1065. https://doi.org/10.1016/j.jacc.2009.09.065
Pluim Babette M, Zwinderman Aeilko H, van der Laarse A, van der Wall Ernst E (2000) The athlete’s heart. Circulation 101(3):336–344. https://doi.org/10.1161/01.CIR.101.3.336
Sanchis L, Sanz-de La Garza M, Bijnens B, Giraldeau G, Grazioli G, Marin J, Sitges M (2017) Gender influence on the adaptation of atrial performance to training. Eur J Sport Sci 17(6):720–726. https://doi.org/10.1080/17461391.2017.1294620
Sanz-de la Garza M, Grazioli G, Bijnens BH, Sarvari SI, Guasch E, Pajuelo C, Sitges M (2016) Acute, exercise dose-dependent impairment in atrial performance during an endurance race: 2D ultrasound speckle-tracking strain analysis. JACC Cardiovasc Imaging 9(12):1380–1388. https://doi.org/10.1016/j.jcmg.2016.03.016
Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM (2017) LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 10(7):735–743. https://doi.org/10.1016/j.jcmg.2016.08.014
Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP (2019) Left atrial structure and function, and left ventricular diastolic dysfunction: jacc state-of-the-art review. J Am Coll Cardiol 73(15):1961–1977. https://doi.org/10.1016/j.jacc.2019.01.059
Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, Badano LP (2020) Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr 33(8):934–952. https://doi.org/10.1016/j.echo.2020.03.021
Voigt J-U, Cvijic M (2019) 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC 12(9):1849–1863. https://doi.org/10.1016/j.jcmg.2019.01.044
Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid JP, Saner H (2011) Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol 108(4):580–585. https://doi.org/10.1016/j.amjcard.2011.03.086
Acknowledgements
None.
Funding
This work was partially funded by grants from the Generalitat de Catalunya (FI-AGAUR 2014–2017) (RH 040991, M. Sanz-de la Garza), from the Spanish Government (Plan Nacional IþD, Ministerio de Economía y Competitividad DEP2013-44923-P) and Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (PI17/ 1515), Fondo Europeo de Desarrollo Regional (FEDER) Unión Europea. ‘‘Una manera de hacer Europa’’. This work has no relationship with industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Communicated by Ellen Adele Dawson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francois Simard and Maria Sanz-de la Garza are co-authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Simard, F., Sanz-de la Garza, M., Vaquer-Seguí, A. et al. Sex as a main determinant of bi-atrial acute and chronic adaptation to exercise. Eur J Appl Physiol 122, 2585–2596 (2022). https://doi.org/10.1007/s00421-022-05018-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-05018-x