Abstract
Purpose
We sought to determine the effects of heat acclimation on endurance exercise-induced hepcidin elevation under hot conditions.
Methods
Fifteen healthy men were divided into two groups: endurance training under hot conditions (HOT, 35 °C, n = 8) and endurance training under cool conditions (CON, 18 °C, n = 7). All subjects completed 10 days of endurance training (8 sessions in total), consisting of 60 min of continuous exercise at 50% of maximal oxygen uptake (\(\dot{V}{\text{O}}_{2\max }\)) under their assigned environment condition. Subjects completed a heat stress exercise test (HST, 60 min exercise at 60% \(\dot{V}{\text{O}}_{2\max }\)) to evaluate the exercise-induced thermoregulatory and hepcidin responses under hot conditions (35 °C) before (pre-HST) and after (post-HST) the training period.
Results
Core temperature during exercise in the post-HST decreased significantly in the HOT group compared to pre-HST (P = 0.004), but not in the CON group. The HOT and CON groups showed augmented exercise-induced plasma interleukin-6 (IL-6) elevation in the pre-HST (P = 0.002). Both groups had significantly attenuated increases in exercise-induced IL-6 in the post-HST; however, the reduction of exercise-induced IL-6 elevation was not different significantly between both groups. Serum hepcidin concentrations increased significantly in the pre-HST and post-HST in both groups (P = 0.001), no significant difference was observed between both groups during each test or over the study period.
Conclusion
10 days of endurance training period under hot conditions improved thermoregulation, whereas exercise-induced hepcidin elevation under hot conditions was not attenuated following the training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron deficiency (ferritin < 20 ng/mL) is frequently observed among endurance athletes, and reduces endurance capacity (Beard and Tobin 2000; Eliakim et al. 2002; Sim et al. 2019). Although several factors have been suggested to explain iron deficiency, iron loss through exercise training (Mclnnis et al. 1998; Babic et al. 2001; DeRuisseau et al. 2002) and insufficient dietary iron intake (Hinton 2014) strongly affect higher risk of iron deficiency. In addition to the above factors, previous studies proposed that post-exercise increases in the iron regulatory hormone, hepcidin, may alter iron metabolism post-exercise (Peeling et al. 2009a, b; Sim et al. 2019). Hepcidin is a peptide hormone that has a negative effect on iron metabolism (Nemeth et al. 2004b). Elevated hepcidin concentrations suppress the absorption of dietary iron in duodenal enterocytes and iron recycling by macrophages (Nemeth et al. 2004b. Hepcidin secretion has been suggested to be upregulated by exercise-induced elevation of interleukin-6 (IL-6) (Peeling et al. 2009a; Nemeth et al. 2004a), hemolysis (Peeling et al. 2009b), and normal to higher iron status (Peeling et al. 2014). Furthermore, as hepcidin is synthesized primarily by hepatocytes, it was recently suggested that a reduction of the liver glycogen level may be related to hepcidin production (Vecchi et al. 2014; Badenhorst et al. 2015, 2019).
Approximately 5–14 days of repeated heat exposure during endurance exercise induces heat acclimation (HA), which is associated with several specific adaptations, including plasma volume (PV) expansion, increased sweating rate and skin blood flow, and lower body temperature and cardiovascular strain during exercise under hot conditions (Périard et al. 2015, 2016). Moreover, carbohydrate (CHO) metabolism during endurance exercise under hot conditions appears to be altered after HA (Febbraio et al. 1994, 2001). Febbraio et al. (1994) reported that HA reduced muscle glycogen utilization and attenuated plasma catecholamine elevation during endurance exercise under hot conditions compared with before HA. As greater muscle glycogen utilization during exercise enhances IL-6 elevation (Keller et al. 2001; Steensberg et al. 2001), exercise-induced IL-6 elevation under hot conditions may also be attenuated after HA. Furthermore, HA augments the expression of heat shock protein 72 (HSP72), which exerts anti-inflammatory effects by blocking nuclear factor-κB activation (Ghosh et al. 1998; Lee and Thake 2017). Lee and Thake (2017) investigated the influence of HA on the baseline HSP72 level and exercise-induced IL-6 elevation under hot conditions; 10 days of HA augmented the baseline plasma HSP72 level and attenuated exercise-induced plasma IL-6 elevation under hot conditions compared to the same exercise before HA. Therefore, exercise-induced hepcidin elevation under hot conditions may be attenuated after HA due to the reduction of post-exercise IL-6 concentrations. Moreover, a previous study has investigated the influence of a single session of endurance exercise under a hot condition (35 °C) on IL-6 and hepcidin elevations. The results showed that endurance exercise under the hot condition augmented IL-6 elevation, whereas hepcidin elevation was not different compared with same exercise under the neutral condition (Mckay et al. 2021). However, the effects of HA on exercise-induced hepcidin elevations remains unclear. Endurance training under hot conditions (i.e., HA training) is a well-established method to improve thermoregulatory capacity and endurance performance (Lorenzo et al. 2010; Tyler et al. 2016; Saunders et al. 2019; Waldron et al. 2021), and is widely accepted as a useful form of training among various types of endurance athletes. A better understanding of hepcidin activity in association with iron metabolism is important, as endurance athletes commonly incorporate several days of endurance training under hot conditions into their training schedule (i.e., training camps).
The present study was performed to examine the effects of HA on endurance exercise-induced hepcidin elevation under hot conditions compared to the same training under cool conditions. We hypothesized that HA would attenuate endurance exercise-induced hepcidin elevation under hot conditions.
Methods
Subjects
For determination of sample size, we referred to previous studies that demonstrated large effects of thermoregulatory adaptations (e.g., reduction of core temperature during endurance exercise under hot conditions) after several days of HA (Travers et al. 2020; Gerrett et al. 2021). Moreover, the present study was conducted over a relatively short period (i.e., December to January), to avoid potential effects of seasonal HA and completion of all training sessions. Furthermore, as a result of the strict limitation on the number of subjects in the environmental chamber due to the COVID-19 pandemic, we recruited 15 unacclimated subjects as the minimal sample size. The mean ± standard deviation (SD) for age, height, and body mass were 23 ± 2 years, 170.8 ± 7.2 cm, and 66.6 ± 9.4 kg, respectively. All subjects were physically active and had been involved in recreational resistance exercise or endurance exercise. They were informed of the experimental procedures and possible risks involved in this study, and provided informed consent. The present study was approved by the Ethics Committee for Human Experiments at Ritsumeikan University (BKC-IRB-2021-014), and was conducted in accordance with the Declaration of Helsinki.
Experimental design
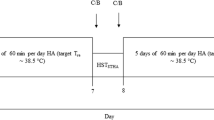
After the maximal oxygen uptake (\(\dot{V}{\text{O}}_{2\max }\)) test, subjects were divided into two groups: endurance training under hot conditions [HOT, 35 °C, 50% relative humidity (RH), n = 8] and endurance training under cool conditions (CON, 18 °C, 50% RH, n = 7). All subjects completed 10 days of endurance training (8 sessions in total), consisting of 60 min of continuous exercise at 50% of \(\dot{V}{\text{O}}_{2\max }\) under their assigned environment condition. As shown in Fig. 1, subjects performed the heat stress exercise test (HST) before and before and the day following the completion of the endurance training to evaluate the exercise-induced thermoregulatory response under hot conditions (35 °C, 50% RH). Blood samples were collected before and after HST to determine changes in the exercise-induced serum hepcidin level, plasma IL-6 concentration, and other blood variables.
Heat stress test
Before and the day following the completion of the endurance training, subjects arrived at the laboratory at 09:00 after an overnight fast, and rested for 20 min before baseline blood drawing and rectal temperature (Trec) measurement. After completing baseline measurements, subjects started the HST to evaluate changes in exercise-induced thermoregulatory and hepcidin responses. HST consisted of 60 min of endurance exercise under hot conditions (35 °C, 50% RH) using a cycle ergometer (Aerobike 75XLIII; Konami Corp., Tokyo, Japan). The exercise intensity was set at 60% of \(\dot{V}{\text{O}}_{2\max }\), which was sufficient to evoke exercise-induced plasma IL-6 and serum hepcidin elevations under hot conditions (Hayashi et al. 2020). During HST, Trec, mean skin temperature (Tsk) and heart rate (HR) were recorded every 1 min. Thermal sensation (TS) and rate of perceived exertion (RPE) were measured at the end of HST. Before and immediately after HST, body weight was evaluated to calculate the total sweat volume. After completing HST, the subjects rested on a chair for 3 h under thermoneutral conditions (23 °C, 50% RH). Blood samples were collected before, immediately after, and 1 and 3 h after completing the exercise.
Training protocols
During endurance training period (days 2–11), all training was conducted using a cycle ergometer (Aerobike 75XLIII; Konami Corp.) in an environmentally controlled chamber. To prevent the development of training-induced fatigue, there were 2 designated rest days (days 6 and 7) during the training period (Fig. 1). In each training session, the subjects performed 60 min of endurance exercise at 50% of \(\dot{V}{\text{O}}_{2\max }\) in the HOT (35 °C, 50% RH) and CON (18 °C, 50% RH) groups. During the daily 60 min of endurance training, all subjects consumed 500 mL of water to avoid severe dehydration. The Trec, HR, TS, RPE, and total sweat volume data at the end of exercise were collected on days 2, 5, and 11 during endurance training.
Measurements
Maximal oxygen uptake (preliminary measurement)
The \(\dot{V}{\text{O}}_{2\max }\) test was conducted under cool conditions (18 °C, 50% RH). The test began at 1.0 kp, and the load was increased progressively in 0.5 kp increments every 2 min until exhaustion (60 rpm). During the test, expired gases were collected and analyzed using an automatic gas analyzer (AE300S; Minato Medical Science Co., Ltd, Tokyo, Japan). The respiratory data were averaged every 30 s. HR was measured continuously during the test using a wireless HR monitor (Accurex Plus; Polar Electro Oy, Kempele, Finland).
Measurements on HST
Thermoregulatory variables, rate of perceived exertion, and thermal sensation
Trec was measured using a wired rectal thermistor (ITP010-11; Nikkiso Therm Co., Ltd., Tokyo, Japan) inserted to a depth of 10 cm beyond the anal sphincter. Tsk was measured at four sites (chest, arm, thigh, and calf) using probe-type thermometers (NK543; Nikkiso Therm Co., Ltd.) and the Tsk was calculated using the following equation (Ramanathan 1964):
Tsk was measured halfway between the nipple and clavicle for the chest, at 60% of the distance between the acromion and elbow for the arm, halfway between the greater trochanter and knee for the thigh, and at 30% of the distance between the knee and lateral malleolus for the calf. Trec and Tsk were monitored at 0.5 Hz throughout the experiment using a data logger (N543; Nikkiso Therm Co., Ltd.) and recorded every 1 min before HST (rest Trec) and during HST. The increase in Trec (ΔTrec) during HST was calculated by subtracting Trec at rest from the peak Trec value during 60 min exercise as an index of heat storage in the body. HR was recorded every 1 min during HST, and the average value during the last 1 min (59–60 min) of HST was determined. The subjects indicated their RPE on a scale of 0 (none at all) to 10 (maximal exertion) (Wilson and Jones 1991), and TS on a scale of 1 (very cold) to 10 (very hot) (Zhang et al. 2004) at the end of HST. Total sweat volume during HST was calculated from the change in body weight after exercise compared to before exercise.
Blood variables
The subjects came to the laboratory at 09:00 following an overnight fast, and after a 20 min rest, a polyethylene catheter was inserted into an antecubital vein to obtain a baseline blood sample. Blood samples were collected before, and immediately, 1 h, and 3 h, after completing the 60 min exercise. All blood samples for determination of blood hemoglobin (Hb) concentration and hematocrit (Hct) level were collected using a 2.5-mL syringe containing heparin to calculate changes in plasma volume (PV). Blood was collected using a 10-mL syringe, and serum and plasma samples were obtained by centrifugation for 10 min at 3.000 rpm (4 °C) followed by storage at − 80 °C until analysis.
Blood glucose, lactate, serum iron, ferritin, hepcidin, plasma IL-6 concentration, and blood gas variables were measured in the obtained blood samples. Blood glucose and lactate concentrations were measured using a glucose analyzer (FreeStyle; Nipro Co., Osaka, Japan) and a lactate analyzer (Lactate Pro; ARKRAY Co., Kyoto, Japan) immediately after blood sample collection. The serum iron and ferritin concentrations were measured at a clinical laboratory (SRL Inc., Tokyo, Japan). The intra-assay coefficients of variation were 2.2% and 1.3% for serum iron and ferritin concentrations, respectively. Serum hepcidin concentrations were analyzed with an enzyme-linked immuno-sorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), and the intra-assay coefficient of variation was 4.5%. The plasma IL-6 concentration was measured with an ELISA kit (R&D Systems), and the intra-assay coefficient of variation was 2.1%. All samples for hepcidin and IL-6 measurement were analyzed in duplicate, and the average values were determined. Blood Hb concentration and Hct level were analyzed with an automatic blood gas analyzer (OPTI CCA TS; Sysmex Co., Kobe, Japan). These analyses were completed within 15 min after collecting blood, and samples were put on ice until analysis. The changes in PV from baseline were calculated using the Dill and Costill equation (Dill and Costill 1974), as follows:
where Hct is hematocrit (in %) and Hb is hemoglobin (in g/dL).
Measurements during the training period (days 2, 5, and 11)
Physiological responses and rates of perceived exertion and thermal sensation during endurance training
Trec and HR were recorded every 1 min during exercise, and the average values during the last 1 min (59–60) of the 60 min exercise period were determined. RPE and TS were collected at the end (last 1 min) of 60 min of endurance training. Total sweat volume during exercise was calculated from changes in body weight before and after exercise.
Statistical analysis
Data are expressed as means ± SD. Two-way repeated-measures [one between- (group) and two within- (training period) subjects factors] analysis of variance (ANOVA) was used to test for significant effects of exercise training on the variables in each group. Similarly, three-way [one between- (group) and two within- (training period and time) subjects factors] ANOVA was used to test for any significant effects of exercise training on blood variables in each group, at any time point during HST. When ANOVA revealed a significant interaction or main effect, the Tukey–Kramer test was performed as a post hoc analysis. In all analyses, P < 0.05 was taken to indicate statistical significance.
Results
\(\dot{V}{\text{O}}_{2\max }\) and pedaling workload
There was no difference in \(\dot{V}{\text{O}}_{2\max }\) before the training period between the two groups (CON: 47.7 ± 4.0 mL/kg/min, HOT: 49.3 ± 8.0 mL/kg/min). Consequently, the pedaling workloads during training (CON: 1.7 ± 0.2 kp, HOT: 1.8 ± 0.4 kp) and HST (CON: 2.2 ± 0.2 kp, HOT: 2.4 ± 0.5 kp) were not different between the two groups.
Thermoregulatory variables, RPE, and TS during the training period
Table 1 shows the peak Trec, HR, TS, RPE, and total sweat volume during endurance training in HOT and CON groups (on days 2, 5, and 11). The peak Trec (P = 0.046), HR (P = 0.003), TS (P < 0.001), RPE (P = 0.007), and total sweat volume (P = 0.008) were significantly higher in the HOT group than the CON group throughout the training period. Moreover, the HOT group showed reductions in peak Trec (P = 0.031), HR (P = 0.001), TS (P = 0.013), and RPE (P = 0.005) on day 11 compared to day 2, with no significant interaction of training period × group for any variable.
Thermoregulatory variables, RPE and TS during HST
Table 2 shows changes in Trec (rest and peak), ΔTrec, Peak Tsk, HR, RPE and TS during HST. The Trec at rest did not change significantly before and after training in either group (P = 0.073), and there was no significant main effect of group (P = 0.707) or a training period × group interaction (P = 0.434). After the training period, peak Trec (P = 0.004) and ΔTrec (P = 0.040) decreased significantly in the HOT group in comparison to before the training period, but the CON group showed no such effect. However, we found no significant main effect for group (peak Trec: P = 0.703, ΔTrec: P = 0.628) and no significant training period × group interaction (peak Trec: P = 0.114, ΔTrec: P = 0.236). The peak Tsk during exercise did not change before and after the training period in either group (P = 0.412). Moreover, we found no significant main effect for training period (P = 0.625) and no training period × group interaction (P = 0.352). HR at the end of HST decreased significantly in both groups after the training period (P < 0.001), and there was no significant main effect for group (P = 0.996) and no significant training period × group interaction (P = 0.660). The HOT group showed reductions of TS (P = 0.049) and RPE (P = 0.027) after compared to before the training period. However, no significant main effect for group (TS: P = 0.852, RPE: P = 0.934) or training period × group interaction (TS: P = 0.566, RPE: P = 0.432) was observed.
After the endurance training, blood Hb concentrations (P = 0.015) at baseline decreased significantly in the HOT group (pre-HST: 14.9 ± 0.7 g/dL, post-HST: 14.2 ± 0.6 g/dL) compared with before the endurance training, but the CON group showed no such effect (pre-HST: 14.6 ± 1.2 g/dL, post-HST: 14.2 ± 0.9 g/dL). The HOT group had significant reductions (P = 0.015) in hematocrit value at baseline (pre-HST: 44.5 ± 2.0%, post-HST: 42.8 ± 1.7%), but this was not seen in the CON group (pre-HST: 43.8 ± 3.5%, post-HST: 42.7 ± 2.5%). There was no significant main effect for training period × group interaction (Hb: P = 0.416, Hct: P = 0.554). After the training period, the HOT group had increased PV at baseline (7.7% ± 7.2%) of the post-HST (P = 0.016) compared to baseline at pre-HST, but this was not seen in the CON group (4.6% ± 9.9%). However, no significant main effect of group (P = 0.501) or training period × group interaction (P = 0.501) was observed. Furthermore, exercise-induced PV change (data not shown) with HST did not differ before and after the training period (P = 0.457) in both groups, with no significant main effect for group (P = 0.395) or training period × group interaction (P = 0.831). Total sweat volume (data not shown) during exercise did not change before and after the training period in either group (P = 0.618), and there was no significant main effect of group (P = 0.599) or training period × group interaction (P = 0.130).
Blood variables
Table 3 shows the changes in blood lactate, glucose, plasma IL-6, serum iron, and ferritin concentrations during HST. Blood lactate concentrations increased significantly after exercise in both groups (P < 0.001). However, there was no significant difference in exercise-induced blood lactate elevation before and after training (P = 0.176) in either groups, and there was no significant main effect for group (P = 0.671) or training period × group interaction (P = 0.831). Blood glucose concentrations decreased significantly after exercise in both groups (P = 0.002). However, there was no significant main effect of training period (P = 0.534) or training period × group interaction (P = 0.343). Before the training period, plasma IL-6 concentrations were significantly elevated immediately after HST in both the HOT and CON groups (P = 0.002). However, the exercise-induced plasma IL-6 elevations were attenuated after the training period in both groups (P = 0.012), although we found no significant main effect of group (P = 0.564) or group × training period interaction (P = 0.250). The HOT and CON groups showed increased serum iron concentrations during the post-exercise period (P = 0.003). However, we found no significant main effect of training period (P = 0.159) or training period × group interaction (P = 0.509). The serum ferritin concentrations at baseline were significantly reduced after the training period in both groups (P = 0.015), and there was no significant main effect of group (P = 0.798) or training period × group interaction (P = 0.918).
As shown in Fig. 2, the serum hepcidin concentrations increased significantly 3 h after HST in both groups before and after the training period (P < 0.0001). However, we found no significant main effect for training period (P = 0.152) and no training period × group interaction (P = 0.899). When the exercise-induced elevation of serum hepcidin concentrations was plotted individually, no outlier was observed among 15 subjects.
Discussion
The present study was performed to examine the effects of HA on endurance exercise-induced hepcidin elevation under hot conditions. The results showed that 10 days of endurance training under hot conditions improved thermoregulation, whereas exercise-induced hepcidin elevation under hot conditions was not attenuated following the training period.
After two weeks of HA training, Trec was lower during HST in the HOT group but not in the CON group. Furthermore, the HOT group showed increased baseline PV after the training period. These responses indicated that two weeks of endurance training under hot conditions improved thermoregulatory function. The majority of previous studies have established that several days (5–14 days) of endurance training under hot conditions causes thermoregulatory adaptations, including lower core temperature and PV expansion (Tyler et al. 2016; Périard et al. 2015, 2016), which were consistent with the present findings. However, thermoregulatory adaptations after the training period in the HOT group were modest compared with previous studies that have used a greater heat stress (i.e., higher environmental temperature) during training sessions (Lorenzo et al. 2010; Neal et al. 2016; Travers et al. 2020). Indeed, the peak Trec during training sessions in the HOT group was 38.30 °C ± 0.32 °C (CON group: 37.96 °C ± 0.12 °C) in the present study, which was lower than in previous studies (e.g., Trec > 38.5 °C). Moreover, although previous studies demonstrated that HA training induced a greater reduction of HR and increased the sweating rate during exercise under hot conditions compared to the same training under neutral conditions (Périard et al. 2015, 2016), we found no such changes after the training period in the HOT group in the present study.
Before the training period, plasma IL-6 concentrations increased significantly immediately after HST in both groups. However, exercise-induced IL-6 elevation was attenuated after the training period in both groups in comparison with the pre-training period. The results of the present study may reflect modifications of energy metabolism and inflammatory response during HST, probably mediated by improved aerobic capacity and anti-inflammatory function independent of environmental temperature during training sessions. Unfortunately, we did not evaluate \(\dot{V}{\text{O}}_{2\max }\) after the training period. However, HR during exercise decreased significantly after the training period in both groups, suggesting that relative exercise intensity during HST after the training period was lower than before the training period potentially due to increased aerobic capacity in both groups. In this regard, reduction of exercise-induced IL-6 elevation following the training period did not differ significantly between the two groups. Elevation in plasma IL-6 post-exercise mainly reflects augmented muscle-derived IL-6 production (muscle glycogen utilization), which plays a role in activating signaling in adipose tissue and the liver, thereby facilitating lipolysis and gluconeogenesis (Pedersen et al. 2001). Although muscle glycogen utilization was not evaluated in the present study, considering that post-exercise blood lactate elevation was comparable between the HOT and CON groups after the training period, muscle glycogen utilization during HST would not be likely to differ markedly between the two groups. Therefore, the effects of exercise training on muscle metabolism would be comparable between the HOT and CON groups, which may explain the lack of difference in exercise-induced IL-6 elevation between the two groups.
As shown above, the exercise-induced plasma IL-6 elevation was attenuated after the training period in both groups. Therefore, we expected a reduction in exercise-induced serum hepcidin elevations following the training period, because exercise-induced IL-6 elevation increases the blood hepcidin concentration (Nemeth et al. 2004a; Peeling et al. 2009a). In contrast, the present findings do not show a change in hepcidin activity in line with changes in IL-6, a result that is similar to some previously reported studies (McKay et al. 2019, 2021). McKay et al. (2019) investigated the impact of acute low CHO ingestion on post-exercise IL-6 and hepcidin responses. They showed that low CHO ingestion before and during endurance exercise augmented exercise-induced plasma IL-6 elevation compared to high CHO ingestion. However, augmented IL-6 elevation by low CHO ingestion did not exacerbate post-exercise serum hepcidin concentrations. Similarly, endurance exercise under hot conditions (35 °C) caused greater plasma IL-6 concentrations immediately after exercise compared to same exercise under cool conditions (18 °C), with no difference in hepcidin elevation during the post-exercise period between the two trials (McKay et al. 2021). In the present study, HA (i.e., endurance training under hot conditions) did not attenuate exercise-induced hepcidin elevations compared to the same training under cool conditions. This is not surprising based on the previous findings that hepcidin response after a single session of exercise did not differ between hot condition and neutral condition (McKay et al. 2021; Zheng et al. 2021). Exercise-induced upregulation of hepcidin may be due to several factors, including hemolysis (Peeling et al. 2009b), higher blood iron and ferritin levels (Peeling et al. 2014), negative energy balance (Badenhorst et al. 2019; Hennigar et al. 2021), and lower muscle and liver glycogen contents (Vecchi et al. 2014; Hennigar et al. 2021). In the present study, the HOT and CON groups both showed high serum iron concentrations after the HST both before and after the training period, which could be explained by increased hemolysis rather than increased iron stores in the body (Buchman et al. 1998). Since blood iron concentration plays an important role in regulating hepcidin activity, the increases in serum iron concentrations associated with HST observed in both groups after the training period may have contributed to the similar elevations in hepcidin concentrations (Peeling et al. 2009b). On the other hand, baseline blood ferritin concentrations also affect exercise-induced blood hepcidin elevation. After the training period, baseline serum ferritin concentrations decreased in both groups, while post-exercise serum hepcidin concentrations did not decrease. In the present study, all subjects were iron-sufficient (i.e., baseline ferritin concentrations > 100 ng/mL), presenting with high serum ferritin concentrations after the training period (i.e., > 80 ng/mL), which may have prevented any reductions in hepcidin after the training period. Thus, exercise-induced hepcidin elevation might not be blunted after the training period.
The present study had some limitations. First, the sample size was relatively small (HOT: n = 8; CON: n = 7). The sample size was comparable to previous studies, which determined thermoregulatory (Travers et al. 2020) or hepcidin responses to endurance training (Ishibashi et al. 2020; Zügel et al. 2020). Moreover, inter-individual variations in the respective parameters (i.e., serum hepcidin concentrations, Fig. 2) were small. However, further determination with increased number of endurance athletes may be valuable. Second, heat stress during all training sessions in the HOT groups appeared to be modest compared with previous studies (Lorenzo et al. 2010; Neal et al. 2016; Travers et al. 2020), because ambient temperature was set at 35 °C in the HOT group. In contrast, many previous studies utilized 38–40 °C to produce heat stress during the HA protocol (Lorenzo et al. 2010; Neal et al. 2016; Travers et al. 2020). This may be a reason why the HA was modest in the HOT group. Thus, further research is needed to investigate the exercise-induced hepcidin elevation after HA when utilizing greater heat stress (e.g., 38–40 °C) during HA protocols. Finally, the findings of the present study may be specific to iron-sufficient subjects (i.e., ferritin > 60 ng/mL). As hepcidin responses following exercise have been suggested to be influenced by iron status (Peeling et al. 2014), the impact of hepcidin responses to endurance training under hot conditions should be clarified in such populations (e.g., ferritin < 20–30 ng/mL). Moreover, we did not evaluate blood ferritin concentrations after HST, which affect post-exercise blood hepcidin concentrations.
Conclusion
10 days of endurance training under hot conditions improved thermoregulation, whereas endurance exercise-induced hepcidin elevation under hot conditions was not attenuated compared to before the training period. The present findings suggest that short-term HA training under hot conditions does not affect iron metabolism, at least in terms of hepcidin activity. Therefore, endurance training under hot conditions can improve thermoregulatory capacity and endurance capacity (Lorenzo et al. 2010; Tyler et al. 2016; Saunders et al. 2019; Waldron et al. 2021) without affecting iron metabolism.
Abbreviations
- ANOVA:
-
Analysis of variance
- CHO:
-
Carbohydrate
- CON:
-
Endurance training under cool conditions
- ELISA:
-
Enzyme-linked immune sorbent assay
- HA:
-
Heat acclimation
- Hb:
-
Hemoglobin
- Hct:
-
Hematocrit
- HOT:
-
Endurance training under hot conditions
- HR:
-
Heart rate
- HSP72:
-
Heat shock protein 72
- HST:
-
Heat stress test
- IL-6:
-
Interleukin-6
- PV:
-
Plasma volume
- RH:
-
Relative humidity
- RPE:
-
Rate of perceived exertion
- SD:
-
Standard deviations
- T rec :
-
Rectal temperature
- TS:
-
Thermal sensation
- T sk :
-
Skin temperature
- \(\dot{V}{\text{O}}_{2\max }\) :
-
Maximal oxygen uptake
References
Babic Z, Papa B, Sikirika-Bosnjakovi M, Prkacin I, Misigoj-Durakovic M, Katicic M (2001) Occult gastrointestinal bleeding in rugby player. J Sports Med Phys Fitness 41:399–402
Badenhorst CE, Dawson B, Cox GR, Laarakkers CM, Swinkels DW, Peeling P (2015) Acute dietary carbohydrate manipulation and the subsequent inflammatory and hepcidin responses to exercise. Eur J Appl Physiol 115(12):2521–2530
Badenhorst CE, Black KE, O’Brien WJ (2019) Hepcidin as a prospective individualized biomarker for individuals at risk of low energy availability. Int J Sport Nutr Exerc Metab 29(6):671–681
Beard J, Tobin B (2000) Iron status and exercise. Am J Clin Nutr 72:594S-S597
Buchman AL, Keen C, Commisso J et al (1998) The effect of a marathon run on plasma and urine mineral and metal concentrations. J Am Coll Nutr 17(2):124–127
DeRuisseau KC, Cheuvront SN, Haymes EM, Sharp RG (2002) Sweat iron and zinc losses during prolonged exercise. Int J Sport Nutr Exerc Metab 12(4):428–437
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
Eliakim A, Nemet D, Constantini N (2002) Screening blood tests in members of the Israeli National Olympic team. J Sports Med Phys Fit 42:250–255
Febbraio MA (2001) Alterations in energy metabolism during exercise and heat stress. Sports Med 31(1):47–59
Febbraio MA, Snow RJ, Hargreaves M, Stathis CG, Martin IK, Carey MF (1994) Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol (1985) 76(2):589–97
Gerrett N, Alkemade P, Daanen H (2021) Heat Reacclimation Using Exercise or Hot Water Immersion. Med Sci Sports Exerc 53(7):1517–1528
Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260
Hayashi N, Yatsutani H, Mori H, Ito H, Badenhorst CE, Goto K (2020) No effect of supplemented heat stress during an acute endurance exercise session in hypoxia on hepcidin regulation. Eur J Appl Physiol 120(6):1331–1340
Hennigar SR, McClung JP, Hatch-McChesney A et al (2021) Energy deficit increases hepcidin and exacerbates declines in dietary iron absorption following strenuous physical activity: a randomized-controlled cross-over trial. Am J Clin Nutr 113(2):359–369
Hinton PS (2014) Iron and the endurance athlete. Appl Physiol Nutr Metab 39(9):1012–1018
Ishibashi A, Kojima C, Tanabe Y et al (2020) Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol Rep 8(12):e14494
Keller C, Steensberg A, Pilegaard H et al (2001) Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15:2748–2750
Lee BJ, Thake CD (2017) Heat and hypoxic acclimation increase monocyte heat shock protein 72 but do not attenuate inflammation following hypoxic exercise. Front Physiol 8:811
Lorenzo S, Halliwill JR, Sawka MN, Minson CT (2010) Heat acclimation improves exercise performance. J Appl Physiol (1985) 109(4):1140–7
McInnis MD, Newhouse IJ, von Duvillard SP, Thayer R (1998) The effect of exercise intensity on hematuria in healthy male runners. Eur J Appl Physiol Occup Physiol 79(1):99–105
McKay AKA, Peeling P, Pyne DB et al (2019) Acute carbohydrate ingestion does not influence the post-exercise iron-regulatory response in elite keto-adapted race walkers. J Sci Med Sport 22(6):635–640
McKay AKA, McCormick R, Tee N, Peeling P (2021) Exercise and heat stress: inflammation and the iron regulatory response. Int J Sport Nutr Exerc Metab 29:1–6
Neal RA, Massey HC, Tipton MJ, Young JS, Corbett J (2016) Effect of permissive dehydration on induction and decay of heat acclimation, and temperate exercise performance. Front Physiol 7:564
Nemeth E, Rivera S, Gabayan V et al (2004a) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig 113:1271–1276
Nemeth E, Tuttle MS, Powelson J et al (2004b) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Pedersen BK, Steensberg A, Schjerling P (2001) Muscle-derived interleukin-6: possible biological effects. J Physiol 536(Pt 2):329–337
Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck ET, Swinkels DW (2009a) Effects of exercise on hepcidin response and iron metabolism during recovery. Int J Sport Nutr Exerc Metab 19(6):583–597
Peeling P, Dawson B, Goodman C et al (2009b) Training surface and intensity: inflammation, hemolysis, and hepcidin expression. Med Sci Sports Exerc 41(5):1138–1145
Peeling P, Sim M, Badenhorst CE, Dawson B, Govus AD, Abbiss CR (2014) Iron status and the acute post-exercise hepcidin response in athletes. PLoS ONE 9:e93002
Périard JD, Racinais S, Sawka MN (2015) Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand J Med Sci Sports 1:20–38
Périard JD, Travers GJS, Racinais S, Sawka MN (2016) Cardiovascular adaptations supporting human exercise-heat acclimation. Auton Neurosci 196:52–62
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Saunders PU, Garvican-Lewis LA, Chapman RF, Périard JD (2019) Special environments: altitude and heat. Int J Sport Nutr Exerc Metab 29(2):210–219
Sim M, Garvican-Lewis LA, Cox GR et al (2019) Iron considerations for the athlete: a narrative review. Eur J Appl Physiol 119(7):1463–1478
Steensberg A, Febbraio MA, Osada T et al (2001) Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537:633–639
Travers G, Nichols D, Riding N, González-Alonso J, Périard JD (2020) Heat acclimation with controlled heart rate: influence of hydration status. Med Sci Sports Exerc 52(8):1815–1824
Tyler CJ, Reeve T, Hodges GJ, Cheung SS (2016) The Effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med 46(11):1699–1724
Vecchi C, Montosi G, Garuti C et al (2014) Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 146(4):1060–1069
Waldron M, Fowler R, Heffernan S, Tallent J, Kilduff L, Jeffries O (2021) Effects of heat acclimation and acclimatisation on maximal aerobic capacity compared to exercise alone in both thermoneutral and hot environments: a meta-analysis and meta-regression. Sports Med 51(7):1509–1525
Wilson RC, Jones PW (1991) Long-term reproducibility of Borg scale estimates of breathlessness during exercise. Clin Sci (Lond) 80:309–312
Zhang H, Huizenga C, Arens E, Wang D (2004) Thermal sensation and comfort in transient non-uniform thermal environments. Eur J Appl Physiol 92(6):728–733
Zheng H, Badenhorst CE, Lei TH, Liao YH, Che Muhamed AM, Fujii N, Kondo N, Mündel T (2021) Menstrual phase and ambient temperature do not influence iron regulation in the acute exercise period. Am J Physiol Regul Integr Comp Physiol 320(6):R780–R790
Zügel M, Treff G, Steinacker JM, Mayer B, Winkert K, Schumann U (2020) Increased hepcidin levels during a period of high training load do not alter iron status in male elite junior rowers. Front Physiol 10:1577
Acknowledgements
We would like to appreciate all participants who completed experimental trials. We also thank the laboratory members for the grateful technical support. The present study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science
Funding
This study was supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (17H02147).
Author information
Authors and Affiliations
Contributions
DS contributed to the study design, data collection, analysis, and manuscript writing. HN contributed to the data collection, analysis. KM and KO contributed to the study design, data interpretation and manuscript preparation. KG contributed to the study design, data collection, analysis, and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by George Havenith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sumi, D., Nagatsuka, H., Matsuo, K. et al. Heat acclimation does not attenuate hepcidin elevation after a single session of endurance exercise under hot condition. Eur J Appl Physiol 122, 1965–1974 (2022). https://doi.org/10.1007/s00421-022-04974-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-04974-8