Abstract
Purpose
Previous work suggests that endurance-trained athletes have superior pulmonary vasculature function as compared to untrained individuals, which may contribute to their greater maximal oxygen uptake (\(\dot{\text{V}}\)O2max). Inhaled nitric oxide (iNO) reduces pulmonary vascular resistance in healthy individuals, which could translate into greater cardiac output and improved \(\dot{\text{V}}\)O2max, particularly in untrained individuals. The purpose of the study was to examine whether iNO improved \(\dot{\text{V}}\)O2max in endurance trained and untrained individuals.
Methods
Sixteen endurance-trained and sixteen untrained individuals with normal lung function completed this randomized double-blind cross-over study over four sessions. Experimental cardiopulmonary exercise tests were completed while breathing either normoxia (placebo) or 40 ppm of iNO, on separate days (order randomized). On an additional day, echocardiography was used to determine pulmonary artery systolic pressure at rest and during sub-maximal exercise (60 Watts) while participants breathed normoxia or iNO.
Results
Right ventricular systolic pressure was significantly reduced by iNO during exercise (Placebo: 34 ± 7 vs. iNO: 32 ± 7; p = 0.04). \(\dot{\text{V}}\)O2max was greater in the endurance trained group (Untrained: 3.1 ± 0.7 vs. Endurance: 4.3 ± 0.9 L min−1; p < 0.01), however, there was no effect of condition (p = 0.79) and no group by condition interaction (p = 0.68). Peak cardiac output was also unchanged by iNO in either group.
Conclusion
Despite a reduction in right ventricular systolic pressure, the lack of change in \(\dot{\text{V}}\)O2max with iNO suggests that the pulmonary vasculature does not limit \(\dot{\text{V}}\)O2max in young healthy individuals, regardless of fitness level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is traditionally assumed that maximal oxygen consumption (\(\dot{\text{V}}\)O2max) in healthy individuals is determined by the left ventricle (LV) and its ability to increase cardiac output sufficiently to meet metabolic demand (Wagner 1996). However, there is a growing body of work that suggests the right ventricle (RV) may limit cardiac output during heavy exercise in healthy adults (La Gerche et al. 2011, 2017). Stroke volume is, in part, dependent upon ventricular wall stress (i.e. afterload). At rest, pulmonary artery pressure and RV wall-stress are low; however, with incremental exercise, there is a disproportional increase in pulmonary artery pressure and, therefore, RV wall-stress as compared to systemic arterial pressure and LV wall-stress (La Gerche et al. 2011). As compared to the left ventricle, the RV is a thinner, less muscular ventricle, and therefore the greater relative increase in afterload may limit RV output and ultimately exercise performance (La Gerche et al. 2017).
Although it is assumed that lung function does not adapt to exercise training, there is evidence of training-induced changes to the pulmonary vasculature. In animal models, exercise training results in increased pulmonary vasoreactivity (Chen and Li 1993), and lower resting right ventricular systolic pressures (RVSP) (Weissmann et al. 2014). Though previous studies have challenged the role exercise training has on diffusing capacity (Flaherty et al. 2014; Reuschlein et al. 1968), a high resting diffusing capacity for carbon monoxide (DLCO) is correlated with greater \(\dot{\text{V}}\)O2max suggesting that either capillary blood volume (\(\dot{\text{V}}\)C) and/or membrane diffusing capacity (DM) may be influenced by fitness or exercise training (Coffman et al. 2017; Zavorsky and Smoliga 2017; Lalande et al. 2012; Tedjasaputra et al. 2016). Resting \(\dot{\text{V}}\)C has been shown to be predictive of \(\dot{\text{V}}\)O2max (Lalande et al. 2012), and endurance trained athletes have been found to have greater resting \(\dot{\text{V}}\)C as well as greater DLCO response to exercise, secondary to enhanced DM (Tedjasaputra et al. 2016). Since DM cannot be measured in un-perfused alveoli, the increased DM in endurance trained athletes likely represents increased pulmonary capillary recruitment (Johnson and Hsia 1994; Lalande et al. 2012). Importantly, endurance trained athletes demonstrate similar or even lower pulmonary vascular pressures for a given cardiac output at rest and during exercise (Stickland et al. 2006; Levine et al. 1991). The higher resting \(\dot{\text{V}}\)c as well as the greater exercise DLCO and DM would suggest that endurance trained athletes have greater pulmonary vasoreactivity and/or lower resting pulmonary vascular tone as compared to untrained individuals. The greater pulmonary vascular function may reduce RV afterload/wall-stress during exercise and ultimately facilitate greater RV output (i.e. stroke volume) and \(\dot{\text{V}}\)O2max.
Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator that has been shown to reduce pulmonary vascular resistance and pulmonary artery pressure at rest and during exercise (Koizumi et al. 1994; Pepke-Zaba et al. 1991; Frostell et al. 1993). In pathological conditions of elevated pulmonary vascular resistance (i.e. pulmonary hypertension or chronic heart failure), iNO has been shown to increase \(\dot{\text{V}}\)̇O2peak (Matsumoto et al. 1997). Previous work using sildenafil to induce pulmonary vasodilation under hypoxic conditions has been shown to increase \(\dot{\text{V}}\)O2max in healthy (mostly male) subjects (Ghofrani et al. 2004; Hsu et al. 2006; Faoro et al. 2007). While the RV and pulmonary circulation appear to limit exercise capacity in some clinical populations, no study to date has examined the impact of acutely reducing pulmonary artery pressure on \(\dot{\text{V}}\)O2max in healthy individuals. Further, studies have only examined systemic vasodilators (i.e. Sildenafil) in mostly active males and therefore it is unclear the impact of reducing pulmonary artery pressure alone on exercise capacity. Therefore, the purpose of the current study was to determine the effect of iNO on \(\dot{\text{V}}\)O2max in untrained and endurance trained individuals. As data suggest that exercise training may improve pulmonary vascular function (i.e. greater DLCO, \(\dot{\text{V}}\)C and DM), it was hypothesized that the improvement in \(\dot{\text{V}}\)O2max with iNO would be greater in untrained participants as compared to trained participants. Should \(\dot{\text{V}}\)O2max increase with iNO in either group, this would demonstrate that the RV and pulmonary circulation could, in part, be a limiting factor of exercise capacity in young healthy individuals. Further, should the endurance-trained group respond to iNO to a lesser extent than the untrained group, this would support a difference in pulmonary vascular reactivity with chronic exercise training.
Methods
Participants
Sixteen untrained individuals (8 females) and sixteen endurance trained individuals (8 females) were recruited. All participants were under 40 years of age, were non-smokers, and had no known history of pulmonary, metabolic, or cardiovascular disease. Untrained individuals reported participating in structured physical activity ≤ 2 times a week and had a \(\dot{\text{V}}\)O2max between 30 and 45 ml kg−1 min−1, which is considered average for healthy inactive males and females (Kaminsky et al. 2015). Endurance trained individuals reported training ≥ 5 times a week for at least 4 years and had a V̇O2max greater than 55 ml kg−1 min−1 for females and 60 ml kg−1 min−1 for males.
Study design
This study was part of a large series of projects examining the pulmonary circulation in health and disease, and work examining iNO in chronic obstructive pulmonary disease patients has already been published (Phillips et al. 2021). The study was a randomized, double-blind, placebo-controlled, crossover design (ClinicalTrials.gov Identifier: NCT03679312) and received ethical approval from Health Canada and the University of Alberta Health Research Ethics Board (Biomedical Panel: Pro00078715). After providing written informed consent, all participants completed four visits. Day 1 included medical history screening, pulmonary function testing, and an incremental cardiopulmonary cycle exercise test (CPET) to volitional exhaustion to determine \(\dot{\text{V}}\)O2max. On days 2 and 3, participants completed experimental CPETs while breathing either normoxia (placebo, day 2) or 40 parts per million (ppm) iNO (day 3). The order of days 2 and 3 were randomized. On Day 4, echocardiograms were conducted at rest and during sub-maximal exercise to evaluate cardiac function and right ventricular systolic pressure (RVSP) while breathing placebo and iNO. Both days 2 and 3 were separated by ≥ 24 h and ≤ 72 h. Participants were asked to abstain from caffeine for at least 6 h prior to testing and abstain from alcohol and any exercise 12 h prior to testing. It was requested that females performed both experimental days within the same phase of their respective menstrual cycle; however, a specific menstrual cycle phase was not targeted between female participants.
Intervention
A 40-ppm dose of iNO was given using a customized NO delivery system (SoKINOX, Vitalaire, Ontario, Canada). Briefly, the device consisted of a non-rebreather circuit connected to a flow sensor and NO was delivered with medical grade normoxic air (~ 21% O2 and balance N2), with O2 titrated in to maintain 21% inspired oxygen throughout the test. The placebo condition consisted of participants breathing medical grade normoxic gas which was delivered by the same non-rebreathing system (SoKINOX) as the iNO condition. All identifying information was covered and both cylinder tanks appeared identical. The lead researcher and participant were blinded to the condition of the trial. Only the research assistant and supervising physician were aware of the condition (placebo or iNO). A 5-min wash-in was completed prior to all experimental exercise trials, regardless of condition. Concentrations of inspired O2, NO, and nitrogen dioxide (NO2) were continuously monitored from a sample line on the mouthpiece. NO2 was monitored using the same SoKINOX system and did not rise above 2-ppm during any trial.
Cardiopulmonary testing
Consistent with previous work (Tedjasaputra et al. 2016; Bouwsema et al. 2017; Michaelchuk et al. 2019), the screening CPET consisted of a 2-min steady state resting period for baseline measurements. Initial power output was set to 50 W and power output was increased by 25 W every 2 min until ventilatory threshold, at which point power output increased 25 W every minute until test termination. Peak work rate was defined as the highest work rate that the participant was able to maintain for greater than 30 s. The highest 30-s average for oxygen consumption was accepted as \(\dot{\text{V}}\)O2max (Lewthwaite et al. 2020). Attainment of \(\dot{\text{V}}\)O2max was based on meeting three of the four following criteria: (1) volitional exhaustion, (2) respiratory exchange ratio greater than 1.1, (3) attainment of age predicted maximum heart rate (208 – (0.7 × age)) (Tanaka et al. 2001), and (4) increase in oxygen consumption < 100 ml min−1 with an increase in power output (Stickland et al. 2012).
Experimental CPET’s consisted of 5-min of steady-state baseline, followed by 2-min of exercise at 60 W. Exercise intensity was then increased to 60% of peak work rate obtained during the screening CPET and increased 10–25 W every minute until volitional exhaustion. The modified experimental CPET protocol was designed such that CPET duration would be similar between endurance and untrained participants (i.e. target 10 min) (Guenette et al. 2007). The identical protocol for each participant was used for both the iNO and placebo trials (Day 2 and 3). Participants rated their perceived breathing and leg discomfort followed by an inspiratory capacity maneuver every 2-min and at the end of the exercise, using the modified Borg categorical ratio scale (Borg 1982). All standard ventilatory and cardiovascular measurements were continuously recorded and averaged in 30-s intervals. Measurements were expressed as absolute values and percent predicted normal values when applicable (Neder et al. 1999).
All exercise tests were performed on an electronically braked cycle ergometer (Ergoselect II 1200 Ergoline, Blitz, Germany) using a cardiorespiratory metabolic measurement system (Encore229 Vmax, SensorMedics, Yorba Linda, CA, USA). Arterial oxygen saturation (SpO2) and methemoglobin (MET-Hb) were estimated using finger pulse oximetry (N-595; Nellcor Oximax, Boulder, CO, USA). Heart rate was measured using single-lead electrocardiography (CardioSoft, GE Medical Systems, Milwaukee, WI, USA), and blood pressure was taken by manual auscultation of the brachial artery. Cardiac output was continuously monitored beat-by-beat and recorded in 30-s averages with impedance cardiography (PhysioFlow, Manatec, Paris, France). Impedance cardiography has been validated against the gold standard Fick method at rest (Charloux et al. 2000) and during exercise (Richard et al. 2001).
Pulmonary function
Spirometry, plethysmography, and DLCO measurements were completed as per current guidelines (Graham et al. 2017) (Encore229 Vmax, SensorMedics, Yorda Linga, CA, USA). Measurements were expressed as absolute values and percentage of predicted normal values (Quanjer et al. 2012; Stanojevic et al. 2017).
Echocardiography
Transthoracic echocardiographic images were collected to assess cardiac structure and function in accordance with current guidelines (Lang et al. 2015). Images were collected using a commercially available ultrasound (Vivid Q, GE Healthcare, Fairfield, CT, USA). Participants were fitted to a supine cycle ergometer (Ergoselect 1200 Stress Echo Supine Ergometer, Blitz, Germany) for the entirety of imaging. During testing, the participant started in an optimal tilted position at rest and then proceeded to perform exercise at 60 W. A single-lead electrocardiograph was used to determine heart rate. Before baseline measurements, participants rested quietly for a minimum of 10 min. Echocardiography data were first collected while participants breathed normoxia. Echocardiographic measurements were then repeated while breathing iNO at rest, and while the participant exercised at a standardized 60 W work rate breathing normoxia and iNO. All echocardiograms were performed by a single experienced sonographer and were acquired within a range of 70–90 frames per second. Five consecutive cardiac cycles were recorded, and measurements were made in triplicate for each condition. Data analysis was completed by the same sonographer, blinded to the experimental condition (EchoPAC, GE Healthcare, Fairfield, CT, USA).
The apical 4-chamber view (Simpson’s monoplane) was used to determine LV end-diastolic volume (EDV) and end-systolic volume (ESV), as well as right-ventricular end-diastolic area (EDA) an end-systolic area (ESA) (Lang et al. 2015; Rudski et al. 2010). LV ejection fraction was calculated using the following formula:
Right ventricle fractional area change (RV FAC) was calculated using the following formula:
RVSP was calculated using tricuspid regurgitant peak velocity measured using continuous-wave Doppler, and estimated right atrial pressure (RAP), in the following formula (Lang et al. 2015; Rudski et al. 2010):
RAP was estimated through imaging of the inferior vena cava (IVC) from a subcostal view at rest and during an inspiratory sniff. A value of 3 mmHg was given for RAP if the diameter of the IVC was < 2.1 cm and collapsed > 50% with a sniff test. If IVC diameter was > 2.1 cm and collapsed < 50% with a sniff test, RAP was estimated as 15 mmHg. If either IVC diameter was > 2.1 or the collapsibility was less than 50%, RAP was estimated to be 8 mmHg (Lang et al. 2015). Tricuspid annular plane systolic excursion (TAPSE) was acquired by placing an M-mode cursor through the tricuspid annulus and measure the amount of longitudinal motion of the annulus at peak systole (Rudski et al. 2010).
Statistical analysis
The sample size was calculated from previous work in patients with chronic heart failure which demonstrated a 2.5 ml kg−1 min−1 (pooled standard deviation of 1.7) improvement in \(\dot{\text{V}}\)O2max with iNO, (Matsumoto et al. 1997). We assumed an effect size of approximately half of that observed in heart failure and an a priori sample size calculation determined 16 untrained individuals would be sufficient in detecting an effect of iNO on \(\dot{\text{V}}\)O2max (α = 0.05, β = 0.80) while accounting for a potential 20% drop-out. Sixteen endurance trained individuals were used for comparison (total sample = 32).
Data are presented as mean ± standard deviation (SD) unless otherwise stated. Statistical significance was set a priori at p < 0.05. Unpaired student T-tests were used to evaluate participant characteristics, pulmonary function, and baseline peak CPET data between groups. A two-way repeated-measures analysis of the variance (ANOVA) was used to analyze the effect of placebo versus iNO on \(\dot{\text{V}}\)O2max (primary outcome) and secondary outcomes in endurance trained and untrained individuals. Additionally, submaximal (2, 4, 6 min) parameters were compared using a multifactorial repeated-measures ANOVA. An exploratory analysis examining the effect of placebo versus iNO on \(\dot{\text{V}}\)O2max between males and females was conducted using a two-way repeated-measures ANOVA. Shapiro–Wilk tests were used to test normality in all outcomes prior to ANOVA. If a main effect or interaction was found, multiple comparison Bonferroni T-tests were completed to locate the differences. All statistical analysis was performed using IBM SPSS Statistics 24 (IBM Corporation, Armonk, NY).
Results
Participants
Descriptive characteristics for the untrained and endurance trained groups are displayed in Table 1. There were no between-group differences in age, height, and pulmonary function. The endurance trained group had a significantly lower BMI than the untrained. Baseline \(\dot{\text{V}}\)O2max (Untrained: 40 ± 4 vs. Endurance: 63 ± 7 ml kg−1 min−1; p < 0.01) and maximal power output (Untrained: 231 ± 49 vs. Endurance: 328 ± 64 W; p < 0.01) were greater in endurance-trained as compared to the untrained group.
Primary outcome
\(\dot{\text{V}}\)O2max in placebo and iNO conditions are reported in Fig. 1. \(\dot{\text{V}}\)O2max was greater in the high endurance trained group (Untrained: 3.1 ± 0.7 vs. Endurance: 4.3 ± 0.9 L min−1; p < 0.01), however, there was no effect of condition (p = 0.79) and no group by condition interaction (p = 0.68).
Hemodynamic responses
All peak hemodynamic data are displayed in Table 2, while incremental \(\dot{\text{V}}\)O2, cardiac output, and SpO2 data are reported in Fig. 2. Peak cardiac output was significantly higher in endurance trained group (Untrained: 17 ± 4 vs. Endurance: 20 ± 6 L min−1; p = 0.02), however, no difference across treatment condition (p = 0.11) or group by condition interaction (p = 0.67) was observed. No significant difference in peak heart rate was observed between groups (p = 0.08), condition (p = 0.95) or group by condition interaction (p = 0.62).
\(\dot{\text{V}}\)O2 response to placebo and iNO at baseline and during exercise at 60 watts, 4-min iso-time, 6-min iso-time, and \(\dot{\text{V}}\)O2max in untrained (A) and endurance trained individuals (B). Cardiac output response to placebo and iNO at baseline and during exercise at 60 watts, 4-min iso-time, 6-min iso-time, and \(\dot{\text{V}}\)O2max in untrained (C) and endurance trained individuals (D). Arterial oxygen saturation response to placebo and iNO at baseline and during exercise at 60 watts, 4-min iso-time, 6-min iso-time, and \(\dot{\text{V}}\)O2max in untrained (E) and endurance trained individuals (F). Values are expressed as mean ± SE
Cardiac output at submaximal intensities was significantly different between groups (Untrained: 12 ± 4 vs. Endurance: 15 ± 5 L min−1; p = 0.03) and condition (Placebo: 13 ± 4 vs. iNO: 14 ± 5 L min−1; p = 0.03), but no group by condition interaction was observed (p = 0.48). Of note, submaximal intensities between endurance trained and untrained individuals were not matched for workload, which would explain the higher cardiac output in endurance trained individuals as compared to untrained individuals. No significant difference was determined during submaximal exercise in heart rate between groups (p = 0.49), condition (p = 0.35), or group by condition interaction (p = 0.41). Importantly, no differences were observed during submaximal exercise in mean arterial pressure between group (Untrained: 96 ± 9 vs. Endurance: 94 ± 9 mmHg; p = 0.66), condition (Placebo: 95 ± 9 vs. iNO: 95 ± 10 mmHg; p = 0.97) or group by condition (p = 0.63), indicating iNO had no systemic effects on participants during the exercise trials.
Respiratory responses
Respiratory variables are reported in Table 2. Peak ventilation was significantly higher in the endurance trained group (Untrained: 107 ± 24 vs. Endurance: 137 ± 32 L min−1; p < 0.01), however, no effect for condition (Placebo: 123 ± 33 vs. iNO: 121 ± 32 L min−1; p = 0.78) or group by condition interaction (p = 0.87) was observed. Breathing frequency was greater in the endurance trained group at peak exercise as compared to the untrained group (Untrained: 44 ± 7 vs. Endurance: 52 ± 7 breaths min−1; p = < 0.01) while no significant effect for condition (Placebo: 48 ± 8 vs. iNO: 48 ± 8 breaths min−1; p = 0.23) or group by condition interaction (p = 0.86) was observed. In contrast, tidal volume was not significantly different at peak exercise between groups (p = 0.14), condition (p = 0.84), or group by condition interaction (p = 0.69). Ventilatory equivalent for carbon dioxide production (\(\dot{\text{V}}\)̇E/\(\dot{\text{V}}\)CO2) was significantly different between the two groups at peak exercise (Untrained: 29 ± 4 vs. Endurance: 27 ± 2; p = 0.03), but no condition (Placebo: 28 ± 3 vs. iNO: 28 ± 3; p = 0.54) or group by condition (p = 0.84) effect was observed. Peak end-tidal pressure of carbon dioxide was not different between groups (p = 0.06), condition (p = 0.49), or group by condition interaction (p = 0.93). Lastly, peak SpO2 was not different between groups (Untrained: 95 ± 3 vs. Endurance: 94 ± 3%; p = 0.49), condition (Placebo: 95 ± 3 vs. iNO: 94 ± 4%; p = 0.14) or group by condition interaction (p = 0.97).
Examining potential sex differences
When comparing all males to all females, an exploratory sex difference analysis demonstrated a significantly higher \(\dot{\text{V}}\)O2max in males as compared to females (Male: 4.3 ± 0.9 vs. Female: 3.0 ± 0.7 L min−1; p < 0.01). However, there was no sex by condition interaction (p = 0.55), indicating that the iNO response was not different between males and females.
Echocardiography
Echocardiography data were successfully acquired on a subset of participants (n = 13 and n = 10 at rest and exercise, respectively) in which a clear tricuspid regurgitation signal could be obtained, and data are presented in Table 3. Due to the small sample size, no comparisons between endurance trained vs. untrained participants were conducted. Pooled data of all participants demonstrated that iNO reduced RVSP at rest (Placebo: 25 ± 2 vs. iNO: 22 ± 3 mmHg; p < 0.01) and during exercise (Placebo: 34 ± 7 vs. iNO: 32 ± 7 mmHg; p = 0.04). There was no rest by exercise interaction (p = 0.41), indicating that iNO reduced RVSP similarly at rest and submaximal exercise.
Discussion
The current study aimed to evaluate the effects of iNO on \(\dot{\text{V}}\)O2max in healthy endurance trained and untrained individuals. It was hypothesized that iNO would increase \(\dot{\text{V}}\)O2max in both the endurance trained and untrained groups, with the untrained group demonstrating a greater increase in \(\dot{\text{V}}\)O2max with iNO. Despite a reduction in RVSP, iNO had no effect on \(\dot{\text{V}}\)O2max in either the untrained or endurance trained groups. These results suggest that, regardless of fitness level, the pulmonary vasculature is not a limiting factor to maximal exercise in healthy individuals.
Effect of iNO on hemodynamic function during exercise- endurance trained response
The cardiovascular adaptations in endurance athletes from chronic exercise training have been well documented (Gledhill et al. 1994; Levine et al. 1991; Stickland et al. 2006), however, potential pulmonary vascular adaptations are relatively under studied. In response to maximal exercise, the RV wall stress (i.e. afterload) is significantly higher in endurance trained athletes as compared to untrained individuals (D'Andrea et al. 2015; La Gerche et al. 2011, 2017). D’Andrea et al. found that pulmonary artery systolic pressure in endurance trained athletes may reach upwards of 40 mmHg at maximal exercise (D'Andrea et al. 2011), while La Gerche et al. have previously reported that RV wall stress/afterload is ~ 25% greater in endurance athletes as compared to non-athletes at peak exercise (La Gerche et al. 2011). It is speculated that this greater afterload is due to the greater overall workload that endurance athletes can achieve (La Gerche et al. 2011; Burger et al. 2001). Though evidence that prolonged elevations in wall stress, such as that experienced during competitive endurance competitions, can lead to short-term impairment in RV function (La Gerche et al. 2012), an acute increase in RV wall stress would increase RV end-systolic volume, resulting in lower RV stroke volume (Burger et al. 2001). A fundamental principle of cardiac function is that cardiac output is dependent on the stroke volume of both ventricles (La Gerche et al. 2017), thus the high RV wall stress observed in endurance athletes could limit global cardiac output and importantly, \(\dot{\text{V}}\)O2max. Further, recent work examining RV/LV responses to exercise has demonstrated that the mechanism of SV augmentation is different between the two ventricles (Ruijsink et al. 2020). In the RV, the increase in SV is accomplished by contracting to a lower ESV, whereas the LV increased EDV in order to augment SV. In discussing RV/LV interaction, Ruijsink et al. suggested that the greater RV systolic ejection (i.e. drop in RV-ESV) may be the dominant factor improving LV filling, and therefore SV, during exercise (Ruijsink et al. 2020).
As shown in Fig. 3B, iNO resulted in a modest reduction in RVSP of approximately 3-mmHg during 60 watts of exercise. Assuming the reduction in RVSP during submaximal exercise with iNO translated to a similar reduction in RVSP up to peak exercise, this should have resulted in a lower RV end systolic volume and correspondingly greater RV stroke volume, cardiac output and \(\dot{\text{V}}\)O2max. While cardiac output was increased in trained participants during submaximal exercise with iNO, this did not translate to an increase in peak cardiac output or \(\dot{\text{V}}\)O2max. With respect to the elevation in cardiac output with no change in \(\dot{\text{V}}\)O2 during submaximal exercise, previous work has suggested that a shunting of blood within the musculature could result in the uncoupling of the \(\dot{\text{V}}\)O2max-cardiac output relationship which would explain the elevated submaximal cardiac output, without a change in \(\dot{\text{V}}\)O2max in the current study (Lewis et al. 2011). It has been suggested that vasodilators such as sildenafil may disrupt peripheral autoregulation which could lead to a reduction in oxygen extraction (Claessen et al. 2015). However, iNO should act only on the pulmonary vasculature, and did not affect systemic blood pressure in the current study, suggesting that it is unlikely that iNO affected peripheral autoregulation. The lack of change in \(\dot{\text{V}}\)O2max or peak cardiac output would be consistent with previous research which demonstrated a reduction in pulmonary vascular resistance with sildenafil in endurance trained males, but no changes observed in normoxic exercise capacity (Hsu et al. 2006; Faoro et al. 2007). The lack of effect of iNO on peak cardiac output and \(\dot{\text{V}}\)O2max, despite the reduction in RVSP, suggests that the typical RV afterload experienced during maximal exercise does not limit cardiac output/\(\dot{\text{V}}\)O2max in highly endurance trained individuals.
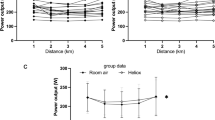
Effect of iNO and placebo on right ventricular systolic pressure at rest (A) and during exercise at 60 watts (B). *p < 0.05 versus placebo condition. Due to the lack of right ventricular systolic pressure data, the two groups were combined and evaluated as one group. Values are expressed as mean ± SE
Effect of iNO on hemodynamic function during exercise-untrained response
Previous work suggests that untrained individuals have reduced pulmonary capillary recruitment, demonstrated by a lower DM (Tedjasaputra et al. 2016), at a given perfusion pressure as compared to endurance trained athletes (Stickland et al. 2006; Levine et al. 1991). Pulmonary vascular recruitment and distention are critical in minimizing RV afterload/wall stress (La Gerche et al. 2010), and necessary for the attainment of maximum cardiac output during exercise. La Gerche et al. observed that individuals with greater pulmonary vascular reserve (i.e. pulmonary capillary recruitment/distention) demonstrated lower RVSP and achieved greater \(\dot{\text{V}}\)O2max (La Gerche et al. 2010). Untrained individuals therefore may be unable to achieve maximal pulmonary artery dilation/capillary recruitment during exercise. Exogenous iNO has shown to reduce pulmonary vascular resistance and pulmonary artery systolic pressure in healthy individuals at rest (Frostell et al. 1993), and increased pulmonary vascular reserve during maximal exercise would potentially facilitate greater peak cardiac output, and ultimately increased \(\dot{\text{V}}\)O2max. Interestingly, unlike the endurance trained athletes, iNO did not appear to have any effect on submaximal cardiac output, and while speculative, suggests that untrained individuals are less sensitive to reductions in RV afterload; however, this requires further investigation. Despite a reduction in exercise RVSP, there was no increase in cardiac output or \(\dot{\text{V}}\)O2max in untrained individuals, suggesting that the pulmonary vasculature does not limit \(\dot{\text{V}}\)O2max, regardless of fitness level.
Effect of iNO on pulmonary gas exchange during exercise
Previous work using iNO in endurance-trained populations have focused on pulmonary gas exchange responses during exercise (Durand et al. 1999; Sheel et al. 2001). Though not the primary outcome of the current study, ventilatory outcomes were not impacted by iNO in either the untrained or endurance trained groups. In the current study, SpO2 was unaffected by iNO; however, pulse oximetry is insensitive to small changes in the arterial partial pressure of oxygen (Mardirossian and Schneider 1992). The lack of change in SpO2 with iNO would support the previous invasive work by Durand et al. and Sheel et al. that iNO does not affect gas exchange during normoxic exercise in healthy participants.
Limitations
Within the untrained group, the difference in \(\dot{\text{V}}\)O2max between placebo and iNO was only 0.00 ± 0.05 L min−1 (p = 0.990) which would have no physiological significance. A post-hoc sample size calculation determined that more than 200 participants would have to be recruited to find a significant difference (power = 0.80) between iNO and placebo in the untrained group. Thus, we can conclude that the lack of change in \(\dot{\text{V}}\)O2max demonstrated with iNO in untrained individuals is due to the small observed effect size and not an inadequate sample size.
Impedance cardiography was used as a non-invasive technique to estimate cardiac output during the CPETs. Using a non-invasive method for estimating cardiac output avoided unnecessary discomfort from invasive cardiac output measurement and did not compromise the validity of the primary outcome (i.e. \(\dot{\text{V}}\)O2max). Though not as robust as the direct Fick method, which is considered the gold standard, cardiac output derived from impedance cardiography has been shown to be highly reliable (Richard et al. 2001) and strongly correlated with the direct Fick method at rest (r = 0.89) (Charloux et al. 2000) and during exercise in health (r = 0.94) (Richard et al. 2001). Further, Physioflow has also been shown to be a reliable method of estimating cardiac output with interventions that examine pulmonary vasodilators (Hsu et al. 2006). When prediction equations are used to estimate cardiac output based on measured \(\dot{\text{V}}\)O2max (Agostoni et al. 2017), PhysioFlow underestimated cardiac output in the current study by 20% and 12% in the endurance trained and untrained groups, respectively, which falls within the 95% confidence interval reported by Richard et al. (−27 to 21%). Importantly, impedance cardiography was used to describe cardiac output (i.e. a secondary study outcome), and any inaccuracy with the technique would not impact the primary outcome (i.e. \(\dot{\text{V}}\)O2max) which was determined by directly measured metabolic data.
Nitric oxide binds to hemoglobin, and therefore iNO interferes with carbon monoxide techniques to evaluated diffusing capacity. As a result, it was not possible to conduct Roughton and Forster’s (Roughton and Forster 1957) multiple-FiO2 technique to examine changes in \(\dot{\text{V}}\)C while breathing iNO. As such, we were unable to determine whether iNO modulated \(\dot{\text{V}}\)C or DM at rest or during exercise.
During exercise left atrial pressure increases (Stickland et al. 2006) which would also increase RV afterload independent of the pulmonary vasculature, and this increase in RV afterload would be unaffected by iNO. Consistent with previous iNO work (Durand et al. 1999; Matsumoto et al. 1997; Pepke-Zaba et al. 1991; Sheel et al. 2001; Tolle et al. 2008; Phillips et al. 2021), 40 ppm of iNO was used, which was successful at reducing RVSP by ~ 3.0 mmHg during exercise. It is possible that a higher dose of iNO may have resulted in greater reductions in RVSP and translated into measurable effects on \(\dot{\text{V}}\)O2max. However, with increased iNO concentration there is a greater risk of MET-Hb accumulation which could lead to reduced arterial content and exercise intolerance. Importantly, mean arterial pressure did not differ between placebo and iNO trials (see Table 2), demonstrating iNO had no systemic effects during exercise.
Due to the low prevalence and challenges in obtaining echocardiographic images of tricuspid regurgitation in young healthy participants, data were successfully obtained in only thirteen individuals. As a result, no between-group comparisons (i.e. trained vs. untrained) of the cardiac/RVSP response to iNO were conducted. While echocardiography has been shown to underestimate cardiac RV area (Kovacs et al. 2010), it is very reproducible (Rowland 2008), and therefore represents the best technique available to evaluate cardiac function/RVSP during whole-body exercise.
Summary
This study examined the effects of iNO on \(\dot{\text{V}}\)O2max in untrained and endurance trained individuals. Though previous literature has shown that healthy individuals, particularly endurance trained athletes, have disproportionately elevated RV afterload at maximal exercise as compared to the left ventricle (La Gerche et al. 2011), a moderate reduction in RVSP with iNO did not translate into increased \(\dot{\text{V}}\)O2max. Further, despite evidence to suggest that untrained individuals may have reduced pulmonary vascular function compared to endurance trained individuals (Tedjasaputra et al. 2016; Lalande et al. 2012), a modest reduction in RVSP with iNO did not improve \(\dot{\text{V}}\)O2max. In conclusion, despite a reduction in RVSP, no changes in maximal cardiac output or \(\dot{\text{V}}\)O2max were observed suggesting that the pulmonary vasculature does not limit \(\dot{\text{V}}\)O2max in healthy individuals regardless of aerobic fitness level.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CPET:
-
Cardiopulmonary exercise test
- DLCO :
-
Diffusing capacity for carbon monoxide
- D M :
-
Membrane diffusing capacity
- EDA:
-
End-diastolic area
- EDV:
-
End-diastolic volume
- EF:
-
Ejection fraction
- ESA:
-
End-systolic area
- ESV:
-
End-systolic volume
- FAC:
-
Fractional area change
- iNO:
-
Inhaled nitric oxide
- IVC:
-
Inferior vena cava
- LV:
-
Left ventricle
- MET-Hb:
-
Methemoglobin
- RAP:
-
Right atrial pressure
- RV:
-
Right ventricle
- RVSP:
-
Right ventricular systolic pressure
- SpO2 :
-
Arterial oxygen saturation
- TAPSE:
-
Tricuspid annular plane systolic excursion
- \(\dot{\text{V}}\)̇E/\(\dot{\text{V}}\)CO2 :
-
Ventilatory equivalent for carbon dioxide production
- \(\dot{\text{V}}\) C :
-
Capillary blood lung volume
- \(\dot{\text{V}}\)O2max :
-
Maximal oxygen consumption
References
Agostoni P, Vignati C, Gentile P, Boiti C, Farina S, Salvioni E, Mapelli M, Magri D, Paolillo S, Corrieri N, Sinagra G, Cattadori G (2017) Reference values for peak exercise cardiac output in healthy individuals. Chest 151(6):1329–1337. https://doi.org/10.1016/j.chest.2017.01.009
Borg GAV (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Bouwsema MM, Tedjasaputra V, Stickland MK (2017) Are there sex differences in the capillary blood volume and diffusing capacity response to exercise? J Appl Physiol 122(3):460–469. https://doi.org/10.1152/japplphysiol.00389.2016
Burger W, Jockwig B, Rucker G, Kober G (2001) Influence of right ventricular pre- and afterload on right ventricular ejection fraction and preload recruitable stroke work relation. Clin Physiol 21(1):85–92. https://doi.org/10.1046/j.1365-2281.2001.00300.x
Charloux A, Lonsdorfer-Wolf E, Richard R, Lampert E, Oswald-Mammosser M, Mettauer B, Geny B, Lonsdorfer J (2000) A new impedance cardiography device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol 82(4):313–320. https://doi.org/10.1007/s004210000226
Chen HI, Li HT (1993) Physical conditioning can modulate endothelium-dependent vasorelaxation in rabbits. Arterioscler Thromb 13(6):852–856
Claessen G, La Gerche A, Dymarkowski S, Claus P, Delcroix M, Heidbuchel H (2015) Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc 4(3):e001602. https://doi.org/10.1161/JAHA.114.001602
Coffman KE, Carlson AR, Miller AD, Johnson BD, Taylor BJ (2017) The effect of aging and cardiorespiratory fitness on the lung diffusing capacity response to exercise in healthy humans. J Appl Physiol 122(6):1425–1434. https://doi.org/10.1152/japplphysiol.00694.2016
D’Andrea A, Naeije R, D’Alto M, Argiento P, Golia E, Cocchia R, Riegler L, Scarafile R, Limongelli G, Di Salvo G, Citro R, Caso P, Russo MG, Calabro R, Bossone E (2011) Range in pulmonary artery systolic pressure among highly trained athletes. Chest 139(4):788–794. https://doi.org/10.1378/chest.10-1260
D’Andrea A, La Gerche A, Golia E, Teske AJ, Bossone E, Russo MG, Calabro R, Baggish AL (2015) Right heart structural and functional remodeling in athletes. Echocardiography 32(Suppl 1):S11-22. https://doi.org/10.1111/echo.12226
Durand F, Mucci P, Safont L, Prefaut C (1999) Effects of nitric oxide inhalation on pulmonary gas exchange during exercise in highly trained athletes. Acta Physiol Scand 165(2):169–176. https://doi.org/10.1046/j.1365-201x.1999.00480.x
Faoro V, Lamotte M, Deboeck G, Pavelescu A, Huez S, Guenard H, Martinot JB, Naeije R (2007) Effects of sildenafil on exercise capacity in hypoxic normal subjects. High Alt Med Biol 8(2):155–163. https://doi.org/10.1089/ham.2007.1058
Flaherty JM, Smoliga JM, Zavorsky GS (2014) The effect of increased physical activity on pulmonary diffusing capacity in unfit women. Exp Physiol 99(3):562–570. https://doi.org/10.1113/expphysiol.2013.076406
Frostell CG, Blomqvist H, Hedenstierna G, Lundberg JO, Zapol WM (1993) Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology 78:8
Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, Seeger W, Grimminger F (2004) Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med 141(3):169–177
Gledhill N, Cox D, Jamnik R (1994) Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc 26(9):1116–1121
Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, MacIntyre NR, Thompson BR, Wanger J (2017) 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 49(1):1600016. https://doi.org/10.1183/13993003.00016-2016
Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW (2007) Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581(Pt 3):1309–1322. https://doi.org/10.1113/jphysiol.2006.126466
Hsu AR, Barnholt KE, Grundmann NK, Lin JH, McCallum SW, Friedlander AL (2006) Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol 100(6):2031–2040. https://doi.org/10.1152/japplphysiol.00806.2005
Johnson RL Jr, Hsia CC (1994) Functional recruitment of pulmonary capillaries. J Appl Physiol 76(4):1405–1407
Kaminsky LA, Arena R, Myers J (2015) Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin Proc 90(11):1515–1523. https://doi.org/10.1016/j.mayocp.2015.07.026
Koizumi T, Gupta R, Banerjee M, Newman JH (1994) Changes in pulmonary vascular tone during exercise. Effects of nitric oxide (NO) synthase inhibition, L-arginine infusion, and NO inhalation. J Clin Invest 94(6):2275–2282. https://doi.org/10.1172/JCI117590
Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Hesse C, Troester N, Salmhofer W, Stauber R, Fuerst FC, Thonhofer R, Ofner-Kopeinig P, Gruenig E, Olschewski H (2010) Assessment of pulmonary arterial pressure during exercise in collagen vascular disease: echocardiography vs right-sided heart catheterization. Chest 138(2):270–278. https://doi.org/10.1378/chest.09-2099
La Gerche A, MacIsaac AI, Burns AT, Mooney DJ, Inder WJ, Voigt JU, Heidbuchel H (1985) Prior DL (2010) Pulmonary transit of agitated contrast is associated with enhanced pulmonary vascular reserve and right ventricular function during exercise. J Appl Physiol 109(5):1307–1317. https://doi.org/10.1152/japplphysiol.00457.2010
La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL (2011) Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc 43(6):974–981. https://doi.org/10.1249/MSS.0b013e31820607a3
La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H, Prior DL (2012) Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33(8):998–1006. https://doi.org/10.1093/eurheartj/ehr397
La Gerche A, Rakhit DJ, Claessen G (2017) Exercise and the right ventricle: a potential Achilles’ heel. Cardiovasc Res 113(12):1499–1508. https://doi.org/10.1093/cvr/cvx156
Lalande S, Yerly P, Faoro V, Naeije R (2012) Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 590(17):4279–4288. https://doi.org/10.1113/jphysiol.2012.234310
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39. https://doi.org/10.1016/j.echo.2014.10.003
Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG (1991) Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84(3):1016–1023. https://doi.org/10.1161/01.cir.84.3.1016
Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, Systrom DM, Semigran MJ (2011) Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail 4(3):276–285. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959437
Lewthwaite H, Benedetti A, Stickland MK, Bourbeau J, Guenette JA, Maltais F, Marciniuk DD, O’Donnell DE, Smith BM, Tan WC, Jensen D, Can CCRG, the Canadian Respiratory Research N (2020) Normative peak cardiopulmonary exercise test responses in Canadian adults aged >/=40 years. Chest 158(6):2532–2545. https://doi.org/10.1016/j.chest.2020.06.074
Mardirossian G, Schneider RE (1992) Limitations of pulse oximetry. Anesth Prog 39(6):194–196
Matsumoto A, Momomura S, Hirata Y, Aoyagi T, Sugiura S, Omata M (1997) Inhaled nitric oxide and exercise capacity in congestive heart failure. Lancet 349(9057):999–1000. https://doi.org/10.1016/s0140-6736(05)62897-8
Michaelchuk WW, Tedjasaputra V, Bryan TL, van Diepen S, Stickland MK (2019) The effect of dopamine on pulmonary diffusing capacity and capillary blood volume responses to exercise in young healthy humans. Exp Physiol 104(12):1952–1962. https://doi.org/10.1113/EP088056
Neder JA, Nery LE, Castelo A, Andreoni S, Lerario MC, Sachs A, Silva AC, Whipp BJ (1999) Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: a randomised study. Eur Respir J 14(6):1304–1313. https://doi.org/10.1183/09031936.99.14613049
Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J (1991) Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 338(8776):1173–1174. https://doi.org/10.1016/0140-6736(91)92033-x
Phillips DB, Brotto AR, Ross BA, Bryan TL, Wong EYL, Meah VL, Fuhr DP, van Diepen S, Stickland MK, Canadian Respiratory Research N (2021) Inhaled nitric oxide improves ventilatory efficiency and exercise capacity in patients with mild COPD: a randomized-control cross-over trial. J Physiol. https://doi.org/10.1113/JP280913
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, Stocks J (2012) Multi-ethnic reference values for spirometry for the 3–95-year age range: the global lung function 2012 equations. Eur Respir J 40(6):1324. https://doi.org/10.1183/09031936.00080312
Reuschlein PS, Reddan WG, Burpee J, Gee JB, Rankin J (1968) Effect of physical training on the pulmonary diffusing capacity during submaximal work. J Appl Physiol 24(2):152–158
Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B, Lonsdorfer J (2001) Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 85(3–4):202–207. https://doi.org/10.1007/s004210100458
Roughton FJ, Forster RE (1957) Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 11(2):290–302
Rowland T (2008) Echocardiography and circulatory response to progressive endurance exercise. Sports Med 38(7):541–551. https://doi.org/10.2165/00007256-200838070-00002
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010 (quiz 688–786)
Ruijsink B, Velasco Forte MN, Duong P, Asner L, Pushparajah K, Frigiola A, Nordsletten D, Razavi R (2020) Synergy in the heart: RV systolic function plays a key role in optimizing LV performance during exercise. Am J Physiol Heart Circ Physiol 319(3):H642–H650. https://doi.org/10.1152/ajpheart.00256.2020
Sheel AW, Edwards MR, Hunte GS, McKenzie DC (2001) Influence of inhaled nitric oxide on gas exchange during normoxic and hypoxic exercise in highly trained cyclists. J Appl Physiol 90(3):926–932
Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, Hall GL (2017) Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. https://doi.org/10.1183/13993003.00010-2017
Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M, Haykowsky MJ (2006) Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol 100(6):1895–1901. https://doi.org/10.1152/japplphysiol.01485.2005
Stickland MK, Butcher SJ, Marciniuk DD, Bhutani M (2012) Assessing exercise limitation using cardiopulmonary exercise testing. Pulm Med 2012:824091. https://doi.org/10.1155/2012/824091
Tanaka H, Monahan KD, Seals DR (2001) Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37(1):153–156
Tedjasaputra V, Bouwsema MM, Stickland MK (2016) Effect of aerobic fitness on capillary blood volume and diffusing membrane capacity responses to exercise. J Physiol 594(15):4359–4370. https://doi.org/10.1113/JP272037
Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM (2008) Exercise-induced pulmonary arterial hypertension. Circulation 118(21):2183–2189. https://doi.org/10.1161/CIRCULATIONAHA.108.787101
Wagner PD (1996) Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58:21–50. https://doi.org/10.1146/annurev.ph.58.030196.000321
Weissmann N, Peters DM, Klopping C, Kruger K, Pilat C, Katta S, Seimetz M, Ghofrani HA, Schermuly RT, Witzenrath M, Seeger W, Grimminger F, Mooren FC (2014) Structural and functional prevention of hypoxia-induced pulmonary hypertension by individualized exercise training in mice. Am J Physiol Lung Cell Mol Physiol 306(11):L986-995. https://doi.org/10.1152/ajplung.00275.2013
Zavorsky GS, Smoliga JM (2017) The association between cardiorespiratory fitness and pulmonary diffusing capacity. Respir Physiol Neurobiol 241:28–35. https://doi.org/10.1016/j.resp.2017.03.007
Acknowledgements
The authors would like to acknowledge all participants for taking part in the study. Without their involvement and willingness to participate, the study would not have been possible.
Funding
This study was partly funded by the Canadian Institutes for Health Research.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: AB, DP, SD, RB, MS; formal analysis and investigation: AB, DP, RB, VM, BR, DF; writing—original draft preparation: AB, DP, MS; writing—review and editing: AB, DP, VM, BR, RB, DF, SD, MS; Funding acquisition: MS and supervision: SD, MS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Alberta Health Research Ethics Board (Biomedical Panel: Pro00078715).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of all data and figures incorporated within the current manuscript.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brotto, A.R., Phillips, D.B., Meah, V.L. et al. Inhaled nitric oxide does not improve maximal oxygen consumption in endurance trained and untrained healthy individuals. Eur J Appl Physiol 122, 703–715 (2022). https://doi.org/10.1007/s00421-021-04866-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04866-3