Abstract
Purpose
To assess the influence of different hormonal profiles on the cardiorespiratory response to exercise in endurance-trained females.
Methods
Forty-seven eumenorrheic females, 38 low-dose monophasic oral contraceptive (OC) users and 13 postmenopausal women, all of them endurance-trained, participated in this study. A DXA scan, blood sample tests and a maximal aerobic test were performed under similar low-sex hormone levels: early follicular phase for the eumenorrheic females; withdrawal phase for the OC group and at any time for postmenopausal women. Cardiorespiratory variables were measured at resting and throughout the maximal aerobic test (ventilatory threshold 1, 2 and peak values). Heart rate (HR) was continuously monitored with a 12-lead ECG. Blood pressure (BP) was measured with an auscultatory method and a calibrated mercury sphygmomanometer. Expired gases were measured breath-by-breath with the gas analyser Jaeger Oxycon Pro.
Results
One-way ANCOVA reported a lower peak HR in postmenopausal women (172.4 ± 11.7 bpm) than in eumenorrheic females (180.9 ± 10.6 bpm) (p = 0.024). In addition, postmenopausal women exhibited lower VO2 (39.1 ± 4.9 ml/kg/min) compared to eumenorrheic females (45.1 ± 4.4 ml/kg/min) in ventilatory threshold 2 (p = 0.009). Nonetheless, respiratory variables did not show differences between groups at peak values. Finally, no differences between OC users and eumenorrheic females’ cardiorespiratory response were observed in endurance-trained females.
Conclusions
Cardiorespiratory system is impaired in postmenopausal women due to physiological changes caused by age and sex hormones’ decrement. Although these alterations appear not to be fully compensated by exercise, endurance training could effectively mitigate them. In addition, monophasic OC pills appear not to impact cardiorespiratory response to an incremental running test in endurance-trained females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Females experience cyclical changes in sex hormone levels throughout their menstrual cycle. These fluctuations, especially 17β-estradiol (E2) and progesterone, can affect female athletes in several ways. On the one hand, E2 is known to increase vasodilatation of blood vessels (dos Santos et al. 2014), pulmonary diffusion capacity as well as plasma volume, and this in turn increases blood supply to the heart and muscles (Mattu et al. 2019). In addition, this sex hormone is associated with increments in growth hormone secretion, epinephrine levels as well as glycogen sparing and fat oxidation (Ashley et al. 2000; Mattu et al. 2019; Packard et al. 2011). Furthermore, E2 regulates mechanical functioning and ventricular myocytes’ proteomic profiles (dos Santos et al. 2014) and appears to stimulate parasympathetic tone because of the presence of E2 receptors in the nucleus tractus solitarius of the medulla oblongata (Subhashri et al. 2019a; Weissman et al. 2009). On the other hand, high levels of progesterone have been linked to decrements in respiratory exchange ratio (RER) and lactate values (Burrows and Bird 2000). Furthermore, this sex hormone enhances water retention, fat utilisation, glycogen sparing (Burrows and Bird 2000; Packard et al. 2011), core temperature and heart rate (HR) (Janse de Jonge 2003; Lebrun 1993). Retrospective studies strongly suggested that progesterone increases chemosensitivity of hypothalamus chemoreceptors, lowering the threshold of the medullary respiratory centre, leading to an increase in ventilation (Ve) (Boukari et al. 2017; Constantini et al. 2005; Godbole et al. 2016; Goldsmith and Glaister 2020; Janse de Jonge 2003; Samsudeen and Rajagopalan 2016; Williams and Krahenbuhl 1997). In addition, these increments in Ve could be accompanied by a rise in oxygen consumption (VO2) (Goldsmith and Glaister 2020; Williams and Krahenbuhl 1997).
Natural endogenous sex hormone secretion is suppressed in women taking oral contraceptive (OC) pills because of the intake of exogenous ethinyl estradiol and progestin (Joyce et al. 2013). In relation to exogenous sex hormones, there is less knowledge about the effects of their administration on females’ physiology. Recent studies reported that ethinyl estradiol increases lipid and reduces glucose metabolism (Burrows and Peters 2007; Mattu et al. 2019; Packard et al. 2011; Rechichi et al. 2009), whereas progestin has been linked with increments in Ve and body temperature (Burrows and Peters 2007; Rechichi et al. 2009), which may result in an increase in cardiovascular strain (Janse de Jonge 2003). Similarly, another research study found significantly higher Ve and breath frequency during the exogenous administration phase (Barba-Moreno et al. 2019). In addition, ethinyl estradiol has mineralocorticoid actions, which activates the renin–angiotensin–aldosterone system encouraging Na+ and fluid retention, and this in turn would increase blood pressure (BP). On the contrary, progestin has anti-mineralocorticoid actions, which antagonises the effect of Na+ and fluid retention (Grandi et al. 2014; Meendering et al. 2009; Torgrimson et al. 2007).
Due to the different hormonal environment presented in OC users regarding eumenorrheic females, it is speculated that differences in cardiorespiratory response may exist between both groups. In this sense, some authors found a lower maximal HR (Gordon et al. 2018) with the use of OC pills, whereas some others agree in no effect of monophasic OC pills on cardiovascular system (Giribela et al. 2012; Grandi et al. 2014; Middlekauff et al. 2012; Nisenbaum et al. 2014; Teixeira et al. 2012). Even though a drop in VO2 max (Casazza et al. 2002; Lebrun 1993; Rechichi et al. 2009) and glucose metabolism (Burrows and Peters 2007) has been associated with the use of these pills, there is also some literature concluding that there is no effect on maximal VO2 and RER with the use of OC pills (Casazza et al. 2002; Gordon et al. 2018; Mattu et al. 2019; Packard et al. 2011; Vaiksaar et al. 2011). These conflicting findings can largely be explained by methodological shortcomings, such as not considering the OC’s formulation and different days of measurements over the menstrual and OC cycle.
Moving on to postmenopausal women, a drastic fall in sex hormone production takes place after menopause due to the loss of the ovarian function (Karsenty 2012). This in turn elicits some differences in postmenopausal women compared to their premenopausal counterparts, such as a reduction in bone mineral density, muscle mass, strength, aerobic capacity (Bondarev et al. 2018) and HR (Neufeld et al. 2015). Moreover, as E2 enhances vagal activity (Mattu et al. 2019) and vasodilatation of blood vessels (dos Santos et al. 2014; Mattu et al. 2019), its decrease with age is associated with arterial stiffness and vascular resistance, and this in turn increases BP in this population (Farinatti et al. 2018). Exercise is advocated to be one of the best tools to enhance cardiovascular function (Green et al. 2017) and improve respiratory parameters (Moazami and Farahati 2013). Consequently, sex hormones’ influence on the cardiorespiratory system could be covered in trained females due to the positive effect exercise has on these tissues. Furthermore, with regard to cardiorespiratory response to exercise, it has been speculated that sex hormones influence on these physiological variables may be masked when training at high intensities (Barba-Moreno et al. 2019; Janse de Jonge 2003; Mattu et al. 2019).
As previously mentioned, there are some controversial results when studying the impact of endogenous and exogenous sex hormones on premenopausal females as well as the effect of low concentrations of E2 and progesterone in postmenopausal women regarding the cardiorespiratory system. Therefore, the aim of this study was to assess the influence of different hormonal environments (eumenorrheic females, low-dose monophasic OC users and postmenopausal women) on endurance-trained females’ cardiorespiratory response to exercise.
Materials and methods
Participants
Forty-seven eumenorrheic females (cycles of 24–35 days in length), 38 low-dose monophasic OC users (at least 6 months intaking them) and 13 postmenopausal women (at least 1 year without menstruation) participated in this study. Brands and formulation of OC pills are presented in Table 1. Exogenous sex hormone concentration mean for the OC group was 0.03 ± 0.01 mg/day of ethinyl estradiol and 1.79 ± 1.28 mg/day of progestin. At the start of the data collection, all participants completed a questionnaire gathering information about training status, health conditions, dietary supplement consumption and type of OC pills when appropriate. All of them were endurance trained (Table 2). Females with metabolic pathologies, hormonal disorders, smoking habits, and intaking supplementation or with injuries/surgeries in the last 6 months were excluded from this study. To be included in the study participants were required to be healthy adult females, without iron deficiency anaemia (serum ferritin < 20 μg/l, haemoglobin < 115 μg/l and transferrin saturation < 16%), non-pregnant or oophorectomized, not consuming medication that alters vascular function (e.g. tricyclic antidepressants, α-blockers, and β-blockers) and they had to perform endurance training between 3 and 12 h per week. An informed consent was obtained from each participant with all the information about the procedures and risks involved. The experimental protocol was approved by the Ethical Committee of the Universidad Politécnica de Madrid and is in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) (Association 2002). Lastly, the study was registered on clinicaltrials.gov (ID: NCT04458662).
Experimental protocol
All measurements were carried out on the same day for each participant. To measure all participants under similar low-hormone conditions, eumenorrheic females were evaluated between the 2nd and 5th day of the menstrual cycle, the onset of the cycle being the first day of menstrual bleeding, while OC users were evaluated between the 3rd and the 7th day of the withdrawal phase (Sims and Heather 2018). Finally, any time was established for postmenopausal women, since their hormonal status does not vary. Volunteers refrain from physical activity and caffeine intake 24 h prior to the test.
Dual-energy X-ray absorptiometry scan
A dual-energy X-ray absorptiometry (DXA) scan (Version 6.10.029GE Encore 2002, GE Lunar Prodigy; GE Healthcare, Madison, WI, USA) was performed between 8 and 10 a.m. in fasting state to obtain body composition variables such as weight, fat mass (FM) and lean mass (LM), considering LM as body weight minus FM and minus bone mineral content. Calibration and evaluation procedures were realised by recommendations of the manufacturer and certified technicians.
Blood samples
To avoid ultradian rhythm variations (Janse de Jonge 2003), blood sample tests were done at the same time for all volunteers, between 8 and 10 a.m. They were obtained with venipuncture into a vacutainer containing clot activator. Following inversion and clotting, the whole blood was centrifuged (Biosan LMC-3000 version V.5AD) for 10 min at 3000 rpm. After that, serum was transferred into Eppendorf tubes and stored frozen at − 80 ºC until further analysis. Within 1–15 days after testing, the serum samples were delivered to the clinical laboratory of the Spanish National Centre of Sport Medicine (Madrid, Spain) to determine sex hormones to verify hormonal profiles. Total E2, progesterone, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were measured via ADVIA Centaur ® solid-phase competitive chemiluminescent enzymatic immunoassay (Siemens City, Germany). Inter- and intra-assay coefficients of variation (CV) reported by the laboratory for each variable were, respectively: 11.9% and 8.5% at 93.3 pg/ml and 6.8% and 4.7% at 166 pg/ml for E2; 23.1% and 11.8% at 0.7 ng/ml and 5.2% and 2.5% at 9.48 ng/ml for progesterone, 5.3% and 1.8% at 1.2 mIU/ml for FSH and 5.2% and 1.8% at 0.54 mIU/ml for LH.
Maximal aerobic test
At least 2 h after the last food intake, a maximal aerobic test was performed with a computerised treadmill (H/P/COSMOS 3PW 4.0, H/P/Cosmos Sports & Medical, Nussdorf—Traunstein, Germany) to determine their peak oxygen uptake (VO2peak). The gradient of the treadmill was set at 1% to simulate outdoor running (Goldsmith and Glaister 2020). Expired gases were measured breath-by-breath with the gas analyser Jaeger Oxycon Pro (Erich Jaeger, Viasys Healthcare, Germany), the validity and reliability of which has been previously demonstrated (Carter and Jeukendrup 2002; Foss and Hallen 2005). Heart response was continuously monitored with a 12-lead ECG. After a warm-up of 3 min at 6 km/h, the test started at 8 km/h. The speed increased 0.2 km/h every 12 s up to volunteer’s exhaustion. Maximal speed was considered reached at the last completed step of 12 s. The highest value from the last 30 s of the test was set as peak Ve and RER. Finally, peak HR was the highest value throughout the test. Standing BP was measured both at resting and at volunteers’ exhaustion in the maximal aerobic test, always by the same researcher, using the auscultatory method with a calibrated mercury sphygmomanometer. First and second ventilatory thresholds (VT1 and VT2, respectively) were set by the same researcher, following maximum agreement in the literature (Rabadan et al. 2011). Finally, 220-age equation was used to analyse the difference between the theoretical maximal HR and the peak HR reached during the maximal aerobic test (theoretical HRmax–HRpeak).
After a recovery phase of 5 min (3 min walking at 6 km/h and 2 min sitting on a chair), a confirmatory test was carried out to verify VO2peak was reached (Nolan et al. 2014; Poole and Jones 2017). This consisted of 3 min’ warm-up (2 min at 50% followed by 1 min at 70% of the maximal speed reached in the maximal aerobic test) (Nolan et al. 2014). Then, volunteers ran at the speed of 110% up to exhaustion (Astorino et al. 2018). If volunteers did not run at least 1 min at this speed, the confirmatory test was not considered for VO2peak determination and it was obtained only from the maximal aerobic test. Lastly, participants performed a 2 min’ recovery at 6 km/h.
VO2peak was determined as the mean of the three highest and continuous 15-s interval VO2 measurements in the maximal aerobic test (Cortes et al. 2014). This value was considered if its difference with the VO2peak obtained in the confirmatory test was lower than 3%. If the difference was higher, the value obtained in the confirmatory phase was considered.
Statistical analysis
All data are reported as mean ± standard deviation (SD). Data showed a normal distribution, thus analyses comparing groups (eumenorrheic, monophasic OC users and postmenopausal) were performed by one-way ANCOVA and age was used as a covariable. The Scheffé test was applied to examine the pairwise comparison. Effect size was calculated by partial eta-squared (ηp2) and small, moderate and large effect corresponded to values equal or greater than 0.001, 0.059, and 0.138, respectively (Cohen et al. 1988). All tests were conducted with a 5% significance level. Statistical analyses were performed using SPSS software for windows, version 20.1 (SPSS Inc, Chicago, IL, USA).
Results
One-way ANCOVA test showed a mean effect among all groups in age (F2,96 = 138.716) and theoretical maximal HR (F2,96 = 136.211) whereas no differences were reported for height (F2,96 = 1.364), weight (F2,96 = 2.337), body mass index (BMI) (F2,96 = 1.436), FM (F2,96 = 0.179), and LM (F2,96 = 0.420). With regard to training status, no significant differences were found for experience (F2,126 = 0.868), sessions per week (F2,126 = 0.906) or time per session (F2,126 = 0.178) among study groups. FSH (F2,84 = 102.147) and LH (F2,84 = 153.415) were, as expected, different among groups, postmenopausal women presenting higher values than both eumenorrheic females and OC users. Nevertheless, neither E2 (F2,84 = 2.337), progesterone (F2,84 = 2.542) nor E2/progesterone ratio (F2,84 = 0.957) reported differences among the study groups (Table 1).
Resting values
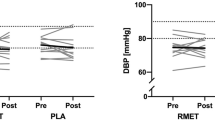
Cardiovascular resting values (Table 3 and Fig. 1) were no different between study groups either for systolic BP (SBP) (F2,71 = 1.110), diastolic BP (DBP) (F2,71 = 0.615) or HR (F2,69 = 0.338; p = 0.715; η2 = 0.010). Likewise, respiratory resting variables (Table 3 and Fig. 2) such as VO2 (F2,94 = 0.572; p = 0.566; η2 = 0.012), Ve (F2,94 = 0.473) and RER (F2,94 = 1.145) did not exhibit differences among eumenorrheic females, OC users and postmenopausal women.
HR response throughout a maximal aerobic test among trained females with different hormonal profile (eumenorrheic females, OC users and postmenopausal women). HR heart rate, OC oral contraceptive, VT1 ventilatory threshold 1, VT2 ventilatory threshold 2. £, Significant differences between postmenopausal females and eumenorrheic females
VO2 response throughout a maximal aerobic test among trained females with different hormonal profile (eumenorrheic females, OC users and postmenopausal women). VO2 oxygen consumption, oral contraceptive, VT1 ventilatory threshold 1, VT2 ventilatory threshold 2. £, Significant differences between postmenopausal females and eumenorrheic females
Ventilatory threshold 1
Heart response to exercise in VT1 reported differences among the study groups (F2,86 = 3.348; p = 0.040; η2 = 0.072) (Fig. 1). Specifically, postmenopausal women presented lower values than eumenorrheic females (p = 0.035; η2 = 0.042). The respiratory response to exercise for this threshold (Table 3 and Fig. 2) showed no significant differences either for VO2 (F2,94 = 1.886; p = 0.157; η2 = 0.039), Ve (F2,94 = 0.804), RER (F2,94 = 2.657) or %VO2 peak (F2,94 = 0.412). Nonetheless, speed (F2,94 = 6.067) was higher in the postmenopausal compared to the OC (p = 0.003; η2 = 0.145) and eumenorrheic (p = 0.026; η2 = 0.077) groups, and eumenorrheic females had higher values than OC users (p = 0.016; η2 = 0.083).
Ventilatory threshold 2
This threshold did not show differences in HR (Fig. 1) among study groups (F2,85 = 2.754; p = 0.069; η2 = 0.061). Nonetheless, VT2 showed differences in VO2 (F2,94 = 6.121; p = 0.003; η2 = 0.115), the postmenopausal group presenting lower values than the eumenorrheic one (p = 0.009; η2 = 0.111) (Fig. 2). Likewise, postmenopausal women reported lower values of %VO2 peak (F2,94 = 4.680) compared to eumenorrheic females (p = 0.019; η2 = 0.102). On the contrary, no differences were reported among study groups either for Ve (F2,94 = 1.229) or RER (F2,94 = 2.250). Finally, although speed reported differences among groups (F2,94 = 3.972), pairwise comparisons were not statistically different.
Peak values
Cardiovascular response at peak values (Table 3) reported no significant differences for SBP (F2,61 = 0.229) and DBP (F2,61 = 0.881), whereas HR (Fig. 1) was different among groups (F2,88 = 4.038; p = 0.021; η2 = 0.084), showing postmenopausal women having lower values than eumenorrheic females (p = 0.024; η2 = 0.077). In addition, the difference between theoretical maximal HR minus peak HR (F2,88 = 3.968) was lower in postmenopausal women compared to eumenorrheic females (p = 0.026; η2 = 0.077). Specifically, postmenopausal women reached a peak HR higher than their theoretical values. Finally, neither VO2 (F2,95 = 1.742; p = 0.181; η2 = 0.035), Ve (F2,93 = 0.124) RER (F2,93 = 2.917) nor speed (F2,95 = 2.325) showed differences among eumenorrheic females, OC users and postmenopausal women..
Discussion
The aim of this study was to assess the impact of different hormonal profiles on cardiorespiratory response to exercise in endurance-trained females. The main finding was the similar cardiorespiratory response obtained by postmenopausal and premenopausal endurance-trained females during a maximal aerobic test. In line with this, cardiorespiratory variables were not different in low-dose monophasic OC users compared to eumenorrheic females either at rest or during exercise.
In accordance with previous studies carried out in healthy but sedentary postmenopausal women, this population exhibited increments in resting HR, DBP (Subhashri et al. 2019a, b) and SBP (Subhashri et al. 2019a, b; Von Holzen et al. 2016), leading to higher cardiovascular risk and mortality in this population (Subhashri et al. 2019a). The deficiency in ovarian sex hormones after menopause (Karsenty 2012), along with other hormonal and physiological changes (e.g. bone mineral density, muscle mass, aerobic capacity and HR reduction) caused by age (Bondarev et al. 2018; Neufeld et al. 2015), could explain these increments in resting cardiovascular parameters. The positive and protector effect that ovarian sex hormones, specially E2, have over the cardiovascular system is lost after menopause. Thereby, postmenopausal females appear to suffer HR increments as well as arterial stiffness and vascular resistance, and this in turn increases BP (Farinatti et al. 2018). However, the present study did not show differences in resting cardiovascular parameters when comparing postmenopausal with premenopausal endurance-trained females. As far as we are aware, there is only one study in which active postmenopausal women reported higher HR and SBP than premenopausal women (Tapadar and Tapadar 2019). In this study, women practised yoga or walking for at least 3 months, which may have not been stimulus enough to compensate the cardiovascular system’s changes experienced due to the menopause. In fact, a recent review concluded that, in comparison to moderate-intensity exercise, high-intensity interval training elicits superior responses, such as an increase in maximal oxygen uptake and an enhanced capacity for oxidative metabolism owing to an increase in mitochondria (Gibala 2020).
Discrepancies between literature data and our outcomes could be explained by the key role exercise plays in cardiovascular system protection (Green et al. 2017; Moazami and Farahati 2013; Roldán et al. 2019), which may compensate physiological changes caused by age and sex hormones’ decrement. There is a strong basis for proposing that exercise induces structural changes, such as the growth and stretching of endothelial cells in the walls of the vascular system, leading to a reduction in artery stiffness (Green et al. 2017). Athletes also exhibit a remodelling of conduit arteries, such as an increase in artery diameters to increase blood flow and couple with metabolic demands when exercising (Green et al. 2017). Thus, these physiological effects after menopause appear to be compensated by exercise. Finally, it is worth mentioning the similar low-sex hormone concentrations in all groups when testing. Thereby, it could be hypostasized that there may not be a chronic effect of sex hormones on cardiorespiratory response to exercise in endurance-trained females, but there might be an acute effect. Thus, measurements in another phase of the menstrual cycle (e.g. the late follicular phase, when E2 reaches its peak, or the mid-luteal phase, with high levels of E2 and progesterone) or OC cycle (e.g. the active pill phase) could have influenced the results. Retrospective studies strongly suggest that both ovarian sex hormones, specially E2, upregulate very important mediators of vascular relaxation, such as nitric oxide, prostacyclin and endothelium-derived hyperpolarizing factors (dos Santos et al. 2014). In addition, E2 enhances vagal activity (Subhashri et al. 2019a) and regulates mechanical functioning and ventricular myocytes’ proteomic profiles (dos Santos et al. 2014). Consequently, these sex hormones influence on non-reproductive tissues may result in an acute effect on both the cardiovascular and respiratory systems of females.
Cardiovascular response to exercise in postmenopausal endurance-trained women did not report consistent differences compared to premenopausal females, but did so for VT1 and peak HR, where postmenopausal women reported lower values. As previously mentioned, the drastic fall in sex hormones after menopause has been related to increments in myocardial stiffness and drops in myocardial distensibility. With regard to intropic effects, lower β-adrenergic stimulation occurs with ageing (Christou and Seals 2008; Farinatti et al. 2018). Consequently, during vigorous exercise, when great cardiovascular work is required, this could be jeopardised (Christou and Seals 2008; Farinatti et al. 2018), preventing postmenopausal women from reaching HR values as high as premenopausal females. Nonetheless, a previous study (Farinatti et al. 2018), carried out with light-to-moderate physically active women (65 years), reported maximal HR values of 140 bpm (90.3% of their theoretical maximal HR (220-age)), whereas our endurance-trained females (51 years) achieved 172 bpm (101.8% of their theoretical maximal HR). Furthermore, the present study did not report differences in maximal BP among groups, whereas previous research found higher maximal SBP in healthy sedentary postmenopausal women (Farinatti et al. 2018; Teixeira et al. 2015b). Thereby, some of the changes in maximal cardiovascular parameters after menopause seem to be partially compensated by exercise. Moving on to respiratory response to exercise, the previously lower VO2 peak reported in postmenopausal women compared to premenopausal females (Bondarev et al. 2018; Farinatti et al. 2018; Fleg et al. 2005) has not been observed in the present study. Differences in physical activity status should be considered, as most of the previous studies were carried out with sedentary (Fleg et al. 2005) or light-to-moderate physically active women (Farinatti et al. 2018), whereas our postmenopausal participants were endurance-trained women, evidenced by their high-oxygen consumption (eumenorrheic females 49.7 ± 4.2; OC users 48.8 ± 5.7; postmenopausal women 46.1 ± 9.9 ml/kg/min) compared to the previously cited studies (Farinatti et al. 2018; Fleg et al. 2005). The cardiovascular adaptations to exercise (e.g. growth and strengthening of endothelial cells in the vessel walls as well as the increase in the arteries’ diameter) (Green et al. 2017; Moazami and Farahati 2013; Roldán et al. 2019) could explain this lack of decay in the respiratory system. It has been suggested that reductions in artery stiffness (Ferreira et al. 2003) and increments in artery diameter (Miyachi et al. 2001) are strongly related to an increase in maximal oxygen consumption. Therefore, the lack of difference in VO2 peak reported in the present study could be related to the cardiovascular adaptations caused by the regular practice of physical activity. Nonetheless, the lower VO2 observed in the present study in the postmenopausal group in VT2 should be highlighted. This could be explained by a different buffer system that postmenopausal woman may have, since they also presented at this threshold a higher, but not significant, RER.
Resting cardiovascular response was not different when comparing OC users and eumenorrheic females in the present study. Our results are supported by previous studies, which reported no impact of low-dose OC pills in healthy sedentary females on resting HR (Giribela et al. 2012; Middlekauff et al. 2012; Nisenbaum et al. 2014; Teixeira et al. 2012) and BP (Giribela et al. 2012; Grandi et al. 2014; Nisenbaum et al. 2014). The same results were obtained when studying physically active OC users (Rebelo et al. 2010). Nonetheless, it is worth highlighting a study carried out with physically active women, where OC pills (no dosages specified) had no impact on HR and DBP, whereas SBP was higher with the use of these pills (Teixeira et al. 2015a). Discrepancies in results regarding SBP could be explained by sex hormone dosages in OC pills and volunteers’ training status. Although it is well known that physical activity reduces BP (Green et al. 2017), training status was not analysed in Teixeira’s study when comparing OC users and non-OC users. This confounding variable was taken into consideration in the present study, reporting no differences in training status between OC users and eumenorrheic females.
Cardiovascular response to exercise did not report any difference comparing low-dose monophasic OC pill users and eumenorrheic females either. In line with our results, recent studies concluded no impact of these pills on maximal HR and BP (Joyce et al. 2013; Mattu et al. 2019; Rebelo et al. 2010). The lack of OC pills’ effect on BP could be explained by the counteraction between ethinyl estradiol (with mineralocorticoid actions) and progestin (with anti-mineralocorticoid actions) contained in these pills (Grandi et al. 2014; Meendering et al. 2009; Torgrimson et al. 2007). Respiratory response to exercise when comparing OC users and eumenorrheic females in the present study is supported by previous studies, which concluded no effect of low-dose monophasic OC pills on maximal VO2 (Mattu et al. 2019), Ve and RER (Casazza et al. 2002; Gordon et al. 2018; Joyce et al. 2013; Rebelo et al. 2010; Redman et al. 2005; Santos et al. 2008; Vaiksaar et al. 2011) in active females. The absence of influence of OC pills in the female respiratory system could be explained by the current low dosages of exogenous sex hormones in OC pills (White et al. 2011), which might not be enough to alter all the adjustments that take place in this complex system when exercising (Rebelo et al. 2010). Nonetheless, Lebrun and colleagues observed a decrease in VO2 max in highly active females with the use of triphasic OC pills (Lebrun et al. 2003). This type of OC pill seems to cause a higher impact on females’ physiology than monophasic pills (Burrows and Peters 2007). Finally, regardless of the OC formulation and training status, it should be pointed out that all groups were measured under the same hormonal environment, low-sex hormone levels. Thus, our outcomes suggest that there is no chronic effect with the use of low-dose monophasic OC pills on cardiorespiratory response but there might be an acute effect. In fact, it has been reported that, after oral administration, ethinyl estradiol has an initial peak in plasma after 2–4 h, followed by a secondary peak at about 12 h and no long detectable in plasma after 24 h (Nilsson and Nygren 1978; Westhoff et al. 2015). Therefore, if ethinyl estradiol has an acute effect on females’ physiology, it was not detectable in the present study, as participants were measured between the 3rd and the 7th day of the withdrawal phase.
The current study attempts to address a gap in the research through the investigation of important cardiorespiratory variables in endurance-trained females. The strengths of our study included different hormonal profiles and the recruitment of a homogeneous group for all of them: eumenorrheic females, OC users and postmenopausal women (active and healthy women with no differences either in training status or in body composition) and the control of OC dosages. Nevertheless, it should be pointed out that there was no difference in sex hormone levels among study groups at the time of testing and, therefore, measuring them in another phase of their OC and menstrual cycle may have reported other results. A limitation of the study might be the use of 220-age to predict HRmax in an athletic population since it may not be the most accurate method to estimate it. In addition, longitudinal studies with an intra-subject design should be carried out to explore the influence of the hormonal changes throughout the lifespan. It should be noted that it might also be interesting to analyse different hormonal stages throughout the menstrual cycle and OC cycle in trained females.
Conclusion
According to our results, endurance-trained postmenopausal women have a similar cardiorespiratory response to exercise compared to premenopausal females. Due to the age-related physiological changes, along with the sex hormone decrease, postmenopausal maximal HR cannot increase as much as premenopausal values. Therefore, although exercise cannot fully compensate biological changes in postmenopausal women, it could effectively attenuate them. In addition, cardiorespiratory response in low-dose monophasic OC users do not differ from the eumenorrheic response either at rest or during exercise in endurance-trained females. Further research is recommended to provide a better understanding of the potential effects of different hormonal profiles in cardiorespiratory system when studying physically active women, especially with a focus on the different types of OC.
Abbreviations
- ANCOVA:
-
Analysis of covariance
- BP:
-
Blood pressure
- DBP:
-
Diastolic blood pressure
- DXA:
-
Dual X-ray absorptiometry
- E2:
-
17β-Estradiol
- FM:
-
Fat mass
- LM:
-
Lean mass
- HR:
-
Heart rate
- OC:
-
Oral contraceptive
- RER:
-
Respiratory exchange ratio
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- Theorical HRmax–HRpeak :
-
Difference between theorical maximal heart rate (220-age) and peak heart rate
- VCO2 :
-
Carbon dioxide production
- Ve:
-
Ventilation
- VO2 :
-
Oxygen consumption
- VT:
-
Ventilatory threshold
References
Ashley CD, Bishop P, Smith JF, Reneau P, Perkins C (2000) Menstrual phase effects on fat and carbohydrate oxidation during prolonged exercise in active females. J Exerc Physiol 3:67–73
Association WM (2002) World medical association declaration of Helsinki ethical principles for medical research involving human subjects. Nurs Ethics 9:105. https://doi.org/10.1191/0969733002ne486xx
Astorino TA et al (2018) Increased cardiac output and maximal oxygen uptake in response to ten sessions of high intensity interval training. J Sports Med Phys Fitn 58:164–171. https://doi.org/10.23736/s0022-4707.16.06606-8
Barba-Moreno L, Cupeiro R, Romero-Parra N, Janse de Jonge XAK, Peinado AB (2019) cardiorespiratory responses to endurance exercise over the menstrual cycle and with oral contraceptive use. J Strength Cond Res. https://doi.org/10.1519/jsc.0000000000003447
Bondarev D et al (2018) Physical performance in relation to menopause status and physical activity. Menopause 25:1432–1441. https://doi.org/10.1097/gme.0000000000001137
Boukari R, Laouafa S, Ribon-Demars A, Bairam A, Joseph V (2017) Ovarian steroids act as respiratory stimulant and antioxidant against the causes and consequences of sleep-apnea in women. Resp Physiol Neurobi 239:46–54. https://doi.org/10.1016/j.resp.2017.01.013
Burrows M, Bird S (2000) The physiology of the highly trained female endurance runner. Sports Med 30:281–300. https://doi.org/10.2165/00007256-200030040-00004
Burrows M, Peters CE (2007) The influence of oral contraceptives on athletic performance in female athletes. Sports Med 37:557–574. https://doi.org/10.2165/00007256-200737070-00001
Carter J, Jeukendrup AE (2002) Validity and reliability of three commercially available breath-by-breath respiratory systems. Eur J Appl Physiol 86:435–441
Casazza GA, Suh S-H, Miller BF, Navazio FM, Brooks GA (2002) Effects of oral contraceptives on peak exercise capacity. J Appl Physiol 93:1698–1702. https://doi.org/10.1152/japplphysiol.00622.2002
Christou DD, Seals DR (2008) Decreased maximal heart rate with aging is related to reduced β-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J Appl Physiol 105:24–29. https://doi.org/10.1152/japplphysiol.90401.2008
Cohen J, Faul F, Erdfelder E, Lang A, Buchner A (1988) Statistical power analysis for the behavioral sciences. Routledge, New York
Constantini NW, Dubnov G, Lebrun CM (2005) The menstrual cycle and sport performance. Clin Sports Med 24:e51–e82. https://doi.org/10.1016/j.csm.2005.01.003
Cortes N, Onate J, Morrison S (2014) Differential effects of fatigue on movement variability. Gait Posture 39:888–893. https://doi.org/10.1016/j.gaitpost.2013.11.020
dos Santos RL, da Silva FB, Ribeiro RF, Stefanon I (2014) Sex hormones in the cardiovascular system. Horm Mol Biol Clin Invest 18:89–103. https://doi.org/10.1515/hmbci-2013-0048
Farinatti P, Monteiro W, Oliveira R, Crisafulli A (2018) Cardiorespiratory responses and myocardial function within incremental exercise in healthy unmedicated older vs. young men and women. Aging Clin Exp Res 30:341–349. https://doi.org/10.1007/s40520-017-0776-x
Ferreira I, Twisk JWR, Stehouwer CDA, Van Mechelen W, Kemper HCG (2003) Longitudinal changes in V̇O2max: associations with carotid IMT and arterial stiffness. Med Sci Sports Exerc 35:1670–1678. https://doi.org/10.1249/01.mss.0000089247.37563.4b
Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG (2005) Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112:674–682. https://doi.org/10.1161/CIRCULATIONAHA.105.545459
Foss Ø, Hallen J (2005) Validity and stability of a computerized metabolic system with mixing chamber. Int J Sports Med 26:569–575
Gibala MJ (2020) Physiological basis of interval training for performance enhancement. Exp Physiol. https://doi.org/10.1113/EP088190
Giribela CR, Melo NR, Silva RC, Hong VM, Guerra GM, Baracat EC, Consolim-Colombo FM (2012) A combined oral contraceptive containing drospirenone changes neither endothelial function nor hemodynamic parameters in healthy young women: a prospective clinical trial. Contraception 86:35–41. https://doi.org/10.1016/j.contraception.2011.08.017
Godbole G, Joshi A, Vaidya SM (2016) Effect of female sex hormones on cardiorespiratory parameters. J Fam Med Prim Care 5:822. https://doi.org/10.4103/2249-4863.201148
Goldsmith E, Glaister M (2020) The effect of the menstrual cycle on running economy. J Sports Med Phys Fitness. https://doi.org/10.23736/S0022-4707.20.10229-9
Gordon D, Scruton A, Barnes R, Baker J, Prado L, Merzbach V (2018) The effects of menstrual cycle phase on the incidence of plateau at and associated cardiorespiratory dynamics. Clin Physiol Funct Imaging 38:689–698. https://doi.org/10.1111/cpf.12469
Grandi G, Xholli A, Napolitano A, Piacenti I, Bellafronte M, Cagnacci A (2014) Prospective measurement of blood pressure and heart rate over 24 h in women using combined oral contraceptives with estradiol. Contraception 90:529–534. https://doi.org/10.1016/j.contraception.2014.05.011
Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH (2017) Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97:495–528. https://doi.org/10.1152/physrev.00014.2016
Janse de Jonge XA (2003) Effects of the menstrual cycle on exercise performance. Sports Med 33:833–851. https://doi.org/10.2165/00007256-200333110-00004
Joyce S, Sabapathy S, Bulmer A, Minahan C (2013) Effect of long-term oral contraceptive use on determinants of endurance performance. J Strength Cond Res 27:1891–1896. https://doi.org/10.1519/JSC.0b013e3182736935
Karsenty G (2012) The mutual dependence between bone and gonads. J Endocrinol 213:107–114
Lebrun CM (1993) Effect of the different phases of the menstrual cycle and oral contraceptives on athletic performance. Sports Med 16:400–430. https://doi.org/10.2165/00007256-199316060-00005
Lebrun CM, Petit MA, McKenzie DC, Taunton JE, Prior JC (2003) Decreased maximal aerobic capacity with use of a triphasic oral contraceptive in highly active women: a randomised controlled trial. Br J Sports Med 37:315–320. https://doi.org/10.1136/bjsm.37.4.315
Mattu AT, Iannetta D, MacInnis MJ, Doyle-Baker PK, Murias JM (2019) Menstrual and oral contraceptive cycle phases do not affect submaximal and maximal exercise responses. Scand J Med Sci Sports 00:1–13. https://doi.org/10.1111/sms.13590
Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT (2009) Ethinyl estradiol-to-desogestrel ratio impacts endothelial function in young women. Contraception 79:41–49. https://doi.org/10.1016/j.contraception.2008.07.025
Middlekauff HR, Park J, Gornbein JA (2012) Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol-Heart Circul Physiol 302:H2560–H2566
Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S (2001) Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol 90:2439–2444. https://doi.org/10.1152/jappl.2001.90.6.2439
Moazami M, Farahati S (2013) The effects of aerobic training on pulmonary function in postmenopausal women. Int J Sport Std 3:169–174
Neufeld IW, Kiselev AR, Karavaev AS, Prokhorov MD, Gridnev VI, Ponomarenko VI, Bezruchko BP (2015) Autonomic control of cardiovascular system in pre-and postmenopausal women: a cross-sectional study. J Turk Ger Gynecol Assoc 16:11. https://doi.org/10.5152/jtgga.2015.15201
Nilsson S, Nygren K-G (1978) Ethinyl estradiol in peripheral plasma after oral administration of 30 μg and 50 μg to women. Contraception 18:469–475. https://doi.org/10.1016/0010-7824(78)90031-8
Nisenbaum MG et al (2014) Effects of a contraceptive containing drospirenone and ethinyl estradiol on blood pressure and autonomic tone: a prospective controlled clinical trial. Eur J Obstet Gynecol Reprod Biol 175:62–66. https://doi.org/10.1016/j.ejogrb.2014.01.006
Nolan P, Beaven M, Dalleck L (2014) Comparison of intensities and rest periods for VO2max verification testing procedures. Int J Sports Med 35:1024–1029. https://doi.org/10.1055/s-0034-1367065
Packard KA, Lenz T, Elder B, Godfrey C, Holcomb R, Windle E (2011) Oral contraceptive use may attenuate menstrual cycle-induced ventilatory changes in endurance trained runners. J Sports Med 5:19–25. https://doi.org/10.2174/1874387001105010019
Poole DC, Jones AM (2017) Measurement of the maximum oxygen uptake VO2max: VO2peak is no longer acceptable. J Appl Physiol 122:997–1002. https://doi.org/10.1152/japplphysiol.01063.2016
Rabadan M, Diaz V, Calderon FJ, Benito PJ, Peinado AB, Maffulli N (2011) Physiological determinants of speciality of elite middle- and long-distance runners. J Sports Sci 29:975–982. https://doi.org/10.1080/02640414.2011.571271
Rebelo ACS, Zuttin RS, Verlengia R, Cesar MDC, de Sá MFS, da Silva E (2010) Effect of low-dose combined oral contraceptive on aerobic capacity and anaerobic threshold level in active and sedentary young women. Contraception 81:309–315. https://doi.org/10.1016/j.contraception.2009.11.005
Rechichi C, Dawson B, Goodman C (2009) Athletic performance and the oral contraceptive. Int J Sport Physiol 4:151–162. https://doi.org/10.1123/ijspp.4.2.151
Redman LM, Scroop GC, Westlander G, Norman RJ (2005) Effect of a synthetic progestin on the exercise status of sedentary young women. J Clin Endocrinol Metab 90:3830–3837. https://doi.org/10.1210/jc.2004-2401
Roldán A, Cordellat A, Monteagudo P, García-Lucerga C, Blasco-Lafarga NM, Gomez-Cabrera MC, Blasco-Lafarga C (2019) Beneficial effects of inspiratory muscle training combined with multicomponent training in elderly active women. Res Q Exerc Sport 90:547–554. https://doi.org/10.1080/02701367.2019.1633009
Samsudeen N, Rajagopalan A (2016) Effect of different phases of menstrual cycle on cardio-respiratory efficiency in normal, overweight and obese female undergraduate students. J Clin Diagn Res. https://doi.org/10.7860/JCDR/2016/23080.8954
Santos M, Rebelo A, Zuttin R, César M, Catai A, Silva E (2008) Influence of oral contraceptive use on lipid levels and cardiorespiratory responses among healthy sedentary women. Braz J Phys Ther 12:188–194
Sims ST, Heather AK (2018) Myths and Methodologies: reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol 103:1309–1317. https://doi.org/10.1113/EP086797
Subhashri S, Pal P, Pal GK (2019a) Sympathovagal imbalance and cognitive deficit in postmenopausal women: a mini review. Int J Clin Exp Physiol 6:38–41. https://doi.org/10.5530/ijcep.2018.6.2.11
Subhashri S, Pal P, Papa D, Nanda N, Pal GK, Packirisamy RM (2019b) Assessment of heart rate variability in early post-menopausal women. Int J Clin Exp Physiol 6:11–14. https://doi.org/10.5530/ijcep.2019.6.1.4
Tapadar S, Tapadar S (2019) A study on the effect of exercise on menopausal women. IOSR-JDMS 18:37–42. https://doi.org/10.9790/0853-1806133742
Teixeira ALS, Júnior WF, Moraes EM, Alves HB, Damasceno VDO, Dias MRC (2012) Effects of menstrual cycle phase on resting heart rate in healthy women. J Exerc Physiol. https://doi.org/10.7860/JCDR/2015/13795.6592
Teixeira AL, Ramos PS, Vianna LC, Ricardo DR (2015a) Effects of ovarian hormones and oral contraceptive pills on cardiac vagal withdrawal at the onset of dynamic exercise. PLoS ONE. https://doi.org/10.1371/journal.pone.0119626
Teixeira AL, Ramos PS, Vianna LC, Ricardo DR (2015b) Heart rate variability across the menstrual cycle in young women taking oral contraceptives. Psychophysiol 52:1451–1455. https://doi.org/10.1111/psyp.12510
Torgrimson BN, Meendering JR, Kaplan PF, Minson CT (2007) Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol 288:H103-110. https://doi.org/10.1152/ajpheart.00762.2006
Vaiksaar S, Jürimäe J, Mäestu J, Purge P, Kalytka S, Shakhlina L, Jürimäe T (2011) No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J Strength Cond Res 25:1571–1578. https://doi.org/10.1519/JSC.0b013e3181df7fd2
Von Holzen J, Capaldo G, Wilhelm M, Stute P (2016) Impact of endo-and exogenous estrogens on heart rate variability in women: a review. Climacteric 19:222–228. https://doi.org/10.3109/13697137.2016.1145206
Weissman A, Lowenstein L, Tal J, Ohel G, Calderon I, Lightman A (2009) Modulation of heart rate variability by estrogen in young women undergoing induction of ovulation. Eur J Appl Physiol 105:381–386. https://doi.org/10.1007/s00421-008-0914-4
Westhoff CL, Pike MC, Tang R, DiNapoli MN, Sull M, Cremers S (2015) Estimating systemic exposure to ethinyl estradiol from an oral contraceptive. Am J Obstet Gynecol 212(614):e611-614. https://doi.org/10.1016/j.ajog.2014.12.007
White CP, Hitchcock CL, Vigna YM, Prior JC (2011) Fluid retention over the menstrual cycle: 1-year data from the prospective ovulation cohort. Obs Gynecol Int. https://doi.org/10.1155/2011/138451
Williams TJ, Krahenbuhl GS (1997) Menstrual cycle phase and running economy. Med Sci Sports Exerc 29:1609–1618. https://doi.org/10.1097/00005768-199712000-00010
Funding
NRP and VMAM are funded by Universidad Politécnica de Madrid. EAC is funded by Universidad Católica de la Santísima Concepción. The IronFEMME Study takes place with the financial support of the Ministerio de Economía y Competitividad, Convocatoria de ayudas I + D 2016, Plan Estatal de Investigación Científica y Técnica y de Innovación 2013–2016 (Contract DEP2016-75387-P).
Author information
Authors and Affiliations
Consortia
Contributions
BRD contributed to data collecting, data analysis, manuscript drafting and submitting the manuscript. VMAM, NRP, LBM and EAC contributed to data collecting and critically revised the manuscript. AB and RC contributed to the conception and design of the study, data acquisition and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Kirsty Elliott-Sale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rael, B., Barba-Moreno, L., Romero-Parra, N. et al. Cardiorespiratory response to exercise in endurance-trained premenopausal and postmenopausal females. Eur J Appl Physiol 121, 903–913 (2021). https://doi.org/10.1007/s00421-020-04574-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04574-4