Abstract
Purpose
To critically examine the research on novel supplements and strategies designed to enhance carbohydrate delivery and/or availability.
Methods
Narrative review.
Results
Available data would suggest that there are varying levels of effectiveness based on the supplement/supplementation strategy in question and mechanism of action. Novel carbohydrate supplements including multiple transportable carbohydrate (MTC), modified carbohydrate (MC), and hydrogels (HGEL) have been generally effective at modifying gastric emptying and/or intestinal absorption. Moreover, these effects often correlate with altered fuel utilization patterns and/or glycogen storage. Nevertheless, performance effects differ widely based on supplement and study design. MTC consistently enhances performance, but the magnitude of the effect is yet to be fully elucidated. MC and HGEL seem unlikely to be beneficial when compared to supplementation strategies that align with current sport nutrition recommendations. Combining carbohydrate with other ergogenic substances may, in some cases, result in additive or synergistic effects on metabolism and/or performance; however, data are often lacking and results vary based on the quantity, timing, and inter-individual responses to different treatments. Altering dietary carbohydrate intake likely influences absorption, oxidation, and and/or storage of acutely ingested carbohydrate, but how this affects the ergogenicity of carbohydrate is still mostly unknown.
Conclusions
In conclusion, novel carbohydrate supplements and strategies alter carbohydrate delivery through various mechanisms. However, more research is needed to determine if/when interventions are ergogenic based on different contexts, populations, and applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few decades, we have seen exponential growth in the field of endurance sport nutrition, with hundreds of studies published examining nutritional supplements and strategies for optimizing performance and training adaptations. These studies have generally focused on strategies for augmenting exogenous carbohydrate (CHO) oxidation and/or glycogen storage, altering fuel utilization patterns, and/or enhancing gastrointestinal tolerance to nutrient-dense interventions (Aulin et al. 2000; Leiper et al. 2000; Jeukendrup 2010; Roberts et al. 2011; Ormsbee et al. 2014; Rowlands et al. 2015; Baur et al. 2016, 2018, 2019). This work has resulted in dramatic advances in our understanding of the nutritional impacts of supplemental nutrients on human metabolism and performance. Based on this, nuanced recommendations have been published detailing precise nutrient intake strategies and hydration requirements for optimal performance and/or training outcomes (Sawka et al. 2007; Thomas et al. 2016).
Importantly, access to sport nutrition research has never been greater with open-access publishing, popular media reporting, and direct cooperation between athletes and researchers. For many athletes, this has aided in the attainment of record-breaking performances. Additionally, it has resulted in the development of dozens of new products and dietary strategies designed to better provide nutrients in line with recommendations. However, access to this valuable information and the ubiquity of sport nutrition supplements has also resulted in a “levelling of the nutritional playing field” in some ways. Athletes of all ability levels can no longer assume a competitive advantage from simply following sport nutrition guidelines. As such, there is a great demand for novel, innovative, and potentially paradigm-shifting nutritional strategies and/or products that may provide a competitive advantage for early adopters.
In response to this demand, a number of new products have been developed that purport to enhance performance relative to traditional nutritional supplements. Some of these products have a high degree of notoriety likely as a result of effective marketing and the early adoption by champion athletes. Another reason for their popularity may be that many of these products are highly innovative, utilizing new or reimagined technologies/processes and/or integrative scientific approaches that encompass multiple disciplines (e.g., physiology, food science, chemistry, biopharmaceuticals, etc.) for product development. A recent example is carbohydrate hydrogels (HGEL), which transform from liquid to gel when exposed to the pH levels present in the stomach, which purportedly results in more rapid carbohydrate delivery to the small intestine (Sutehall et al. 2018). Such advancements exemplify scientific progress in the field, which certainly warrants excitement, debate, and further examination.
In addition to newly developed CHO supplements, recent research has also emphasized dietary approaches aimed at improving performance via optimization of fuel metabolism to maximize CHO availability. For instance, dozens of studies have recently examined the impact of manipulating dietary CHO either chronically or periodically (Impey et al. 2018). Additionally, a number of investigations have assessed the impact of combining CHO with other presumably ergogenic substances to determine whether additive or synergistic benefits result (Jeukendrup et al. 1998; Saunders et al. 2004; Acker-Hewitt et al. 2012; Cox et al. 2016). As with novel supplements, these dietary and supplementation strategies have drawn substantial notoriety and have become hotly debated topics within popular and sport cultures (Burke et al. 2018).
Importantly, while enthusiasm is valuable for innovation and discovery, it can also potentiate myopia and dogmatism when it comes to critically evaluating new data. There are varying degrees of evidence supporting the ergogenic potential of these newly developed supplements and strategies. In some cases, a wealth of data exists, but it requires reexamination to accurately characterize the likely effects based on newly available research. In other instances, data are often limited, equivocal, or misinterpreted based on the findings of individual studies. Finally, little focus has been paid to how different strategies interact. For example, the effects of manipulating dietary CHO on the ergogenicity of acutely ingested CHO are still mostly undetermined. As such, there is a need for a review of studies in this area that holistically examines the data and reevaluates it from different perspectives. Therefore, the aims of this review are to: (1) determine the mechanistic underpinnings of recent findings and develop hypotheses for future studies and applications, (2) qualitatively evaluate the relative strength of evidence for given supplements/strategies and draw conclusions as to value/potential, (3) contextualize new data within the literature, and (4) consider the potential effects and interactions of different supplements/strategies used concurrently.
Multiple transportable carbohydrates
CHO intake during exercise is perhaps the most widely practiced nutritional strategy to influence endurance performance. Though not a unanimous finding, there is a substantial body of evidence, indicating that CHO ingestion can extend time to exhaustion (TTE) and enhance time-trial (TT) performance/power output in endurance events > 45 min in duration (Saunders and Luden 2012). Mechanistically, the effects of CHO on performance can be partially attributed to influences on the central nervous system, and related effects on perceived effort, affect, and reduced inhibition of central motor drive (Saunders and Luden 2012). This is best illustrated by numerous studies reporting that mouth rinsing with CHO (without swallowing) can significantly improve TT performance (Carter et al. 2004; Chambers et al. 2009). These central influences are likely the primary mechanism responsible for ergogenic effects of CHO during shorter endurance events (~ 45 to 75 min). However, with progressively longer exercise durations, the metabolic effects of CHO ingestion become more critical to performance. It has been known for well over a century that dietary intake can influence the magnitude of relative CHO/fat utilization (Zuntz and Loeb 1894). In fasted conditions, endogenous CHO reserves, primarily from skeletal muscle glycogen and blood glucose (derived from liver glycogen stores and gluconeogenesis), are the predominant energy substrate utilized by the working muscles during endurance exercise at moderate-vigorous intensities (Romijn et al. 1993). However, endogenous CHO stores can be significantly depleted during endurance exercise, limiting CHO availability and oxidation during the later stages of prolonged exercise (Coyle et al. 1986; Gonzalez et al. 2015). Depletion of endogenous CHO stores has been shown to contribute to fatigue and impaired exercise capacity (Bergstrom et al. 1967; Coyle et al. 1986). Furthermore, reduced CHO oxidation may negatively impact endurance performance even in the absence of critically low endogenous reserves, as the oxygen cost of constant-load exercise is increased with low CHO oxidation (and higher fat oxidation) (Burke et al. 2017). CHO ingestion during exercise maintains higher rates of total CHO oxidation throughout prolonged exercise, which is associated with improved performance in prolonged endurance events (Coyle et al. 1986; Smith et al. 2010). Improved CHO availability in late-exercise is due to increased oxidation of the exogenous CHO itself (Jeukendrup 2008; Smith et al. 2010), combined with greater availability of endogenous reserves due to sparing of hepatic glycogen (Jeukendrup et al. 1999; Gonzalez et al. 2015), and possibly muscle glycogen (Stellingwerff et al. 2007; De Bock et al. 2007), though the later finding is not consistently observed in the literature (Coyle et al. 1986; Gonzalez et al. 2015).
Oxidation of exogenous CHO is modulated by various factors related to the exercise bout (i.e., intensity and duration) and nutritional approach (i.e., amount, type, and timing of CHO ingestion). As indicated above, the metabolic influences of CHO ingestion are most important for performance during prolonged exercise (> 2 h, generally at intensities < 85% VO2max), because CHO availability can be limiting to energy demands during such events. During exercise of this type, the oxidation of ingested CHO is typically initiated within 5 min of the initial feeding and, presuming continued feedings at regular intervals, increases progressively over the first 75–90 min of exercise; thereafter, exogenous CHO oxidation rates typically plateau at high rates for the remainder of exercise (Jeukendrup and Jentjens 2000). Exogenous CHO oxidation rates are also dose-dependent. When glucose (or glucose polymers such as maltodextrin) alone is consumed during exercise, oxidation rates increase curvilinearly, reaching maximal rates at ingestion rates of ~ 66 g·h−1 (1.1 g·min−1) (Jeukendrup and Jentjens 2000). The factor(s) limiting this rate are not definitively known, but could potentially be influenced by rates of gastric emptying, digestion, and absorption, hepatic factors influencing passage into systemic blood supply, and glucose uptake/oxidation by the working muscles (Fuchs et al. 2019). However, intravenous glucose infusion studies have achieved CHO oxidation rates much higher than 66 g·h−1 (Hawley et al. 1994), suggesting that muscle glucose uptake/oxidation is not limiting. Similarly, gastric emptying rates of glucose have been shown to exceed maximal CHO oxidation rates (Rehrer et al. 1992). Thus, the major factor limiting maximal exogenous CHO oxidation is presumed to be intestinal absorption and hepatic limitations influencing release to systemic circulation (Rosset et al. 2017; Fuchs et al. 2019). Glucose is primarily absorbed across the intestinal mucosa by the transport protein sodium-dependent glucose transporter 1 (SGLT1) and transported from the splanchnic region via portal circulation to the liver, where it largely passes into systemic circulation and can be taken up by the working muscle for oxidation (Rosset et al. 2017; Fuchs et al. 2019). However, intestinal absorption of glucose becomes limited with saturation of the SGLT1 transporter, which occurs at glucose ingestion rates of ~ 66 g·h−1. Consuming higher rates of glucose (or other single CHO forms) does not elevate exogenous oxidation of CHO by the working muscle (Jeukendrup 2008). Furthermore, residual intestinal CHO is likely to contribute to gastrointestinal distress during exercise, which can impair performance (Rehrer et al. 1992; Pfeiffer et al. 2012).
Traditional nutritional guidelines from sport science groups such as the American College of Sports Medicine have generally recommended consuming 30–60 g·h−1 (0.5–1.0 g·min−1) of CHO during endurance exercise (Rodriguez et al. 2009). These guidelines are consistent with the aforementioned description of glucose metabolism, as these rates are sufficiently high to elicit meaningful rates of exogenous CHO oxidation, without exceeding the intestinal absorption threshold of glucose. It is generally presumed that higher intake rates of glucose within this range are associated with better effects on performance in prolonged events, though studies directly supporting this concept are minimal. The most convincing evidence comes from Smith et al. (2010), who examined the effects of four different rates of glucose ingestion (0, 15, 30, and 60 g·h−1) during exercise consisting of 2 h of constant-load cycling (~ 77% VO2peak), followed immediately by a simulated 20-km TT. Higher CHO intake was associated with stepwise increases in exogenous CHO oxidation rates and hepatic glycogen-sparing, and these metabolic effects were associated with improved TT performance with increasing dose (210 ± 36; 225 ± 40; 227 ± 40; and 232 ± 34 W, respectively) (Smith et al. 2010).
It has been consistently reported that higher than recommended rates of glucose ingestion do not elevate exogenous CHO oxidation beyond levels achieved at ~ 66 g·h−1 (Jeukendrup 2008). However, intestinal absorption of fructose occurs primarily via the GLUT5 transporter, which is unique for glucose. Fructose alone is not considered ideal for optimizing CHO availability, as exogenous oxidation rates during exercise are less than or equal to glucose (Massicotte et al. 1990; Adopo et al. 1994), and large amounts of fructose ingestion are associated with gastrointestinal distress (Truswell et al. 1988). However, because these two monosaccharides are absorbed via non-competitive pathways, the consumption of high levels of glucose (or glucose polymers) combined with fructose during exercise has repeatedly been shown to increase maximal exogenous CHO oxidation (Jeukendrup 2008). Co-ingestion of glucose–fructose may elevate maximal exogenous oxidation > 40%, depending on the doses provided, up to maximal levels of ~ 105 g·h−1 (Jentjens and Jeukendrup 2005; Wallis et al. 2005). These high rates of exogenous CHO oxidation also occur when sucrose (a disaccharide that includes glucose and fructose molecules) is co-ingested with glucose (Trommelen et al. 2017). Unlike glucose, fructose is not believed to be directly oxidized by skeletal muscle at meaningfully high levels during exercise (Fuchs et al. 2019). As mentioned previously, the majority of glucose ingested during exercise is released into systemic circulation following intestinal absorption. By contrast, most fructose is rapidly converted into glucose and lactate in splanchnic organs (intestines and liver), and these substrates are then released into systemic circulation (Rosset et al. 2017). As a result, fructose ingestion elicits only minimal increases in plasma fructose concentrations, while the majority is converted to secondary substrates that are delivered to peripheral tissues and/or to contribute to liver glycogen synthesis (Rosset et al. 2017). The co-ingestion of high rates of fructose and glucose (48 and 72 g·h−1, respectively) during exercise has been shown to significantly elevate the rate of appearance of systemic glucose/lactate versus isocaloric amounts of glucose alone (Lecoultre et al. 2010), and the oxidation of these substrates may completely account for the elevated exogenous CHO oxidation rates reported with glucose and fructose co-ingestion (Gonzalez et al. 2017). Therefore, the utilization of multiple transportable CHO (MTC) appears to substantially elevate maximal rates of exogenous CHO oxidation during prolonged exercise versus single CHO sources. There is also evidence that glucose and fructose co-ingestion reduces gastrointestinal malabsorption and symptoms of gastrointestinal discomfort in comparison to fructose (Latulippe and Skoog 2011) or glucose alone (Jentjens et al. 2006; Triplett et al. 2010; Rowlands et al. 2012). In addition, the more rapid intestinal absorption of MTC may reduce feedback inhibition to the stomach, resulting in faster gastric emptying and fluid delivery compared to glucose alone (Jeukendrup and Moseley 2010). Collectively, these factors have the potential to positively influence endurance performance, which is discussed further below (Fig. 1). In addition, the increased CHO availability induced by ingesting large doses of MTC have been hypothesized to augment glycogen repletion rates during post-exercise recovery. However, this topic is beyond the scope of the present paper, as it has reviewed recently elsewhere (Fuchs et al. 2019).
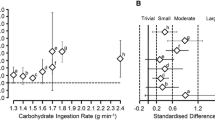
There is a variety of evidence supporting the concept that MTC augments performance in prolonged endurance activities. Of particular interest are studies comparing the effects of MTC to isocaloric amounts of glucose (see Table 1), so that performance differences can be attributed to the combination of MTC, rather than additional CHO calories alone. Currell and Jeukendrup (2008), were the first to report performance gains with consumption of MTC versus isocaloric amounts of glucose, during a protocol consisting of 2 h of constant-load cycling followed by a simulated 1-h TT. Specifically, high doses of glucose and fructose co-ingestion (72 and 36 g·h−1, respectively) resulted in 8% faster TT performance versus when glucose alone was ingested (108 g·h−1). Numerous studies have subsequently examined performance outcomes when MTC are consumed during prolonged exercise at intake rates > 60 g·h−1, in comparison to isocaloric amounts of glucose. Most studies have reported better mean performance outcomes with MTC, though not all effects have been statistically significant (Tarpey et al. 2013; Lee et al. 2014; Hill and Bosch 2017; Baur et al. 2019). Ergogenic effects have been reported with MTC in laboratory trials consisting of pre-loaded cycling TT with total durations of 2.75 to > 4 h (Currell and Jeukendrup 2008; Tarpey et al. 2013; Baur et al. 2014; Roberts et al. 2014), a pre-loaded running trial (~ 2.5 h; Wilson and Ingraham 2015), a simulated 100-km (> 3 h) cycling TT (Triplett et al. 2010), and a pre-loaded cycling sprint test (total duration: ~ 3 h; Rowlands et al. 2012). In addition, performance gains with MTC versus isocaloric glucose have also been reported in field-based performance tests, including a simulated mountain bike race (~ 2.5 h; Rowlands et al. 2012) and long-distance triathlon (~ 5 h; Rowlands and Houltham 2017).
The magnitude of ergogenic effects reported in studies comparing MTC versus isocaloric amounts of glucose may be at least partly influenced by the total CHO ingestion rates utilized in individual studies (Rowlands et al. 2015). For example, in the six trials which utilized CHO ingestion rates of 78–90 g·h−1, the mean performance improvement with MTC ranged from 0.5 to 3.0% (Rowlands et al. 2012 [2 sub-studies]; Baur et al. 2014, 2019; Wilson and Ingraham 2015; Rowlands and Houltham 2017). However, the four trials that utilized ingestion rates from 102 to 144 g·h−1 reported mean performance improvements of 5.0–8.0% with MTC versus glucose (Currell and Jeukendrup 2008; Triplett et al. 2010; Tarpey et al. 2013; Roberts et al. 2014). On one hand, this information fits appropriately with the mechanisms proposed for MTC efficacy, whereby higher doses of glucose–fructose ingestion are associated with greater increases in exogenous CHO oxidation (and fluid absorption) compared to glucose alone, which could potentially explain the greater performance effects in these studies. However, it is important to recognize that these higher ingestion rates for MTC also require higher ingestion rates in the glucose-only control beverages of these studies, to maintain an isocaloric comparison between beverages. The control beverages used in the latter group of studies necessitated glucose ingestion rates which exceeded the presumed maximal intestinal uptake of glucose (~ 66 g·h−1) by at least 50%. Thus, it is likely that significant CHO malabsorption was present in the glucose-only trials of these studies, leading to potentially negative effects on gastrointestinal comfort, or other inhibitory factors that could impair performance (Triplett et al. 2010; Rowlands et al. 2012). As a result, the “real-world” benefits of MTC on performance could be substantially overstated, in comparison to what may be expected when compared to ecologically relevant glucose beverages (i.e., those consumed at rates ≤ 60 g·h−1). Only one study to date has directly compared the effects of a glucose–fructose beverage (60 and 30 g·h−1, respectively) versus both an isocaloric glucose-only beverage (90 g·h−1) and glucose-matched beverage (60 g·h−1 with no fructose) (Baur et al. 2014). Performance was assessed in a simulated 30-km TT which followed 120 min of constant-load cycling at 55% Wmax. Performance in the glucose-fructose trial (50.4 ± 2.2 min; 244 ± 27 W) was likely improved (3.0%) versus the 90 g·h−1 glucose trial (52.0 ± 3.7 min; 229 ± 38 W), but no statistically clear benefit (1.2%) was observed versus the 60 g·h−1 glucose trial (51.1 ± 2.4 min; 237 ± 30 W). These findings suggest that a large portion of the reported performance benefits with MTC could be attributable to excess glucose in control beverages, rather than a true beneficial effect versus recommended glucose beverages. A recent study from King et al. (2018) provides additional insight on this issue, as they compared performance differences between a glucose–fructose beverage (60 and 30 g·h−1) and a glucose-matched beverage (60 g·h−1), as well as additional beverages containing higher glucose (75 g·h−1) and higher glucose–fructose (75 and 37.5 g·h−1), using a similar pre-loaded cycling trial (30 min TT following 120 min constant-load cycling). Compared to Baur et al. (2014), these investigators observed a more robust effect of glucose–fructose (225 ± 45 W) versus the 60 g·h−1 glucose trial (206 ± 41 W), which was an 8.9% (likely) improvement (King et al. 2018). However, mean power output decreased by 5% in the higher glucose trial (75 g·h−1; 196 ± 46 W), magnifying the observed performance effect between glucose–fructose and the higher glucose beverage (15.2%; very likely improvement). In addition, a performance decrement was also observed in the higher glucose–fructose trial (75 and 37.5 g·h−1; 213 ± 43 W) compared to when glucose and fructose were provided at presumably appropriate levels for gastrointestinal uptake (60 g·h−1and 30 g·h−1; 225 ± 45 W).
Collectively, the data above suggest that the use of control beverages with glucose content that exceeds gastrointestinal absorption rates (> 60 g·min−1) may distort the magnitude of the reported ergogenic effects of MTC beverages, especially when levels exceed 90 g·h−1. In addition, the findings of King et al. (2018) indicate that excessive CHO ingestion from either glucose or fructose (alone or in combination) may negatively impact performance. This concept is supported by data from Smith et al. (2013) who conducted a multi-laboratory study investigating the effects of 12 different MTC beverages (3 per laboratory; doses ranged from 10 to 120 g·h−1 in 10 g increments, provided at 2:1 ratios of glucose + maltodextrin:fructose) on pre-loaded TT performance versus a placebo beverage. Based on regression analyses, they reported that CHO influenced performance (total duration ~ 2.5 h) in a curvilinear dose-responsive manner, with the best performance occurring with MTC provided at ~ 78 g·h−1. In a recent similar study assessing MTC (glucose–fructose 2:1) dose–response effects, King et al. (2019) reported a similar curvilinear trend albeit with optimal performance occurring at a higher CHO ingestion rate (90 g·h−1 rather than 78 g·h−1). Specifically, 30-min power output (following 180 min constant-load cycling) improved as the MTC dose increased from 78 g·h−1 (219 ± 48 W) to 90 g·h−1 (228 ± 37 W), but then decreased by 7% when the MTC dose was further elevated to 102 g·h−1 (212 ± 48 W). Interestingly, despite exogenous CHO oxidation being highest in the 102 g·h−1 trial, the authors also reported greater reliance on muscle glycogen utilization (with no differences in liver glycogen use) with this dose—which could provide another potential mechanism (beyond gastrointestinal limitations) for impaired performance with high CHO doses.
In total, there is clear evidence that ingesting MTC at high rates can elevate peak exogenous CHO oxidation rates beyond those achievable with single CHO sources. In addition, there is also evidence that fluid uptake and gastrointestinal comfort may be improved with MTC use. These effects have been associated with improvements in performance during prolonged endurance exercise lasting > 2.5 h, versus isocaloric glucose beverages. However, further investigation is required to quantify the beneficial effects of MTC beverages versus glucose doses ingested at rates that do not exceed gastrointestinal uptake limits, as isocaloric comparisons magnify the presumed benefits of MTC versus lower glucose doses. Furthermore, some recent studies have provided evidence refuting the presence of augmented performance with CHO doses above moderate (39.6 g·h−1) intake rates (Newell et al. 2018). Thus, there remains a need for additional well-powered studies investigating the dose–response effects of MTC ingestion (and CHO ingestion in general) on endurance performance. Dose–response studies of MTC should also consider how varying ratios of glucose:fructose may influence performance. As reviewed elsewhere, there is some evidence that MTC beverages containing higher fructose ratios than commonly used in the literature (i.e., increasing the proportion of fructose from 0.5 to 0.8–1.0 versus glucose) may elicit improved intestinal absorption and exogenous oxidation (Rowlands et al. 2015). Finally, future studies should assess how prior dietary status and exposure to high-dose CHO consumption (i.e., “gut training”) may influence responses to different MTC doses. As recently reviewed (Jeukendrup 2017), repeated exposure to glucose or fructose can increase gastric emptying rates of these monosaccharides, and elevated CHO intake levels in the diet can increase SGLT1 transporter activity and intestinal absorption of CHO. Further exploration in these areas can provide athletes and coaches with greater insights to personalize MTC dosage recommendations under specific conditions.
Modified carbohydrates
Based on findings from studies of MTC, it is clear that metabolic and performance outcomes can be mediated by CHO type. In this case, consuming specific combinations of CHO monomers or rapidly digested polymers (i.e., glucose, maltodextrin, and fructose) can increase CHO absorption and oxidation, thereby enhancing CHO delivery and performance. Besides the likely ergogenic effects already noted, simple forms of CHO such as are utilized in MTC are logical ingredients for sport supplements due to their typically high degree of solubility and palatability. More complex forms of CHO (e.g., polysaccharides like starches) have historically lacked practical application for athletes as most are derived from whole foods like oats, corn, potato, and barley, which are not commonly consumed by athletes before/during competition, possibly because they are more likely to cause gastrointestinal distress (Pfeiffer et al. 2010, 2012; Guillochon and Rowlands 2017). However, the variety of sources and diversity of physicochemical structures across different types of complex CHO provide a wide range of digestion/absorption profiles that can alter metabolism in multiple potentially advantageous ways (e.g., increasing/decreasing CHO/fat oxidation, glycogen synthesis, blood glucose concentrations, etc.). Importantly, a number of newly developed supplements employ complex CHO in traditional and easily consumable forms (e.g., beverages, gels, and bars). Moreover, advanced processing and modification techniques have been utilized to alter their digestibility to enhance metabolic and performance outcomes. Several studies have investigated the effects of consuming modified CHO supplements (MC) on physiology and performance.
Complex carbohydrates and their modification
Most recently developed MC are starch-based. Starches are the primary storage form of CHO in plants and can be found in pollen, leaves, fruits, tubers, bulbs, stems, roots, etc. (Lehmann and Robin 2007). Based on the botanical origin, starches can have a wide variety of structures and physicochemical properties that mediate digestibility and metabolism. The primary structural components of starches are the glucose polymer chains amylose and amylopectin (Buléon et al. 1998). Amylose chains are essentially linear and helical, composed almost entirely (> 99%) of α-(1–4) bonds. Conversely, a relatively large amount of branching in amylopectin results in a greater proportion (5%) of linkages being derived from α-(1–6) bonds (Buléon et al. 1998). These structural differences seem to play an important role in mediating digestibility. Specifically, the lack of branching in amylose reduces the surface area for hydrolysis, which slows and/or prevents digestion compared to the more densely branched and typically faster digesting amylopectin (O’Dea et al. 1980; Goddard et al. 1984).
Non-starch complex CHO like trehalose, maltose, and isomaltulose have also been examined for potential ergogenic effects (Jentjens and Jeukendrup 2003; Venables et al. 2008; Oosthuyse et al. 2015; König et al. 2016). These CHO are composed of two or more monomers and bound by linkages similar to those found in starches (e.g., α-[1–4] and α-[1–6]) as well as others (e.g., α-[1–1], α-[1–2], etc.) (Higashiyama 2002; Maresch et al. 2017). As with starches, the non-starch CHO glycosidic bonds can impact digestibility by altering the surface area for hydrolysis. Digestion is also impacted by hydrolytic enzyme availability/activity. Whereas starch digestion is primarily dependent on α-amylase (i.e., hydrolysis of polysaccharides to the disaccharide maltose) and maltases (i.e., hydrolysis of maltose to glucose), non-starch CHO are hydrolyzed by a number of enzymes that can differ widely in their availability and activity. Certain enzymes, like trehalase for example, are substantially less active relative to other hydrolytic enzymes (e.g., maltase) (Dahlqvist and Thomson 1963). As a result, digestion of trehalose, the substrate for trehalase, is slower relative to maltose (Venables et al. 2008). As with starches, the distinctive characteristics of non-starch complex CHO result in a range of digestion/absorption rates depending on the source.
Due to the diversity of sources and hydrolytic rates of different complex CHO, various methods have been developed to categorize CHO based on their digestibility and absorption profiles. The glycemic index is utilized as the measure of CHO digestion/absorption in vivo. With this method, CHO digestibility is based on the measurement of the incremental area under the curve of blood glucose concentrations following ingestion (Jenkins et al. 1981). The greater the elevation in blood glucose, the higher is the glycemic index of the CHO. Most simple CHO (e.g., glucose or dextrose) such as can be found in traditional sport supplements have high glycemic indices due to their rapid absorption profiles (Wolever and Jenkins 1986). While some starches or non-starch CHO can also have high glycemic indices particularly following processing, there is a wide range of complex CHO that are slow or resistant to digestion resulting in low glycemic indices (Wolever and Jenkins 1986; Atkinson et al. 2008). Importantly, CHO that differ in their glycemic indices can have substantially different metabolic effects. For example, high glycemic index CHO enhance glycogen storage and CHO oxidation rates, whereas low glycemic index CHO are associated with enhanced fat utilization (Burke et al. 1993; Leijssen et al. 1995; Stevenson et al. 2009). Based on this, there has been a wealth of research examining how the glycemic index of CHO may influence health outcomes. For example, low glycemic index CHO have been extensively evaluated as a means of maintaining/improving metabolic health (i.e., glucose stability and insulin sensitivity) in diabetic patients due to the attenuated glycemic and insulinemic responses post-ingestion (Brand-Miller et al. 2009). The glycemic index is also potentially of interest to athletes as will be discussed below (see “Pre-exercise modified carbohydrates”).
Of interest, complex CHO can be selectively modified to alter their digestibility. This is typically accomplished by exposing CHO to heat, moisture, and/or different chemicals/enzymes for a given amount of time, which changes their fine properties (e.g., linkage type and branching density) without altering the overall structure (Lehmann and Robin 2007). Modification is common practice in the food industry to alter shelf-life, cooking outcomes, etc. However, it has only recently been employed in the development of endurance supplements. This application is logical and exciting, because it permits supplement designers to target certain metabolic outcomes without necessarily changing nutrient structure and function. For example, hydrothermal modification of corn starch results in a low glycemic index CHO supplement (GI = 30) despite its very high amylopectin content (> 99%), a characteristic associated with rapid digestion and a high glycemic index (GI = 70) when in a unmodified state (Correia et al. 2008). Modification can also be utilized to alter molecular weight and stability. For example, enzymatic treatment of potato starch results in a starch with high molecular weight (500,000–700,000) that gels in solution (Brynolf et al. 1995; Leiper et al. 2000). Both examples have been employed as CHO supplements in efforts to alter fuel utilization patterns and gastric emptying, respectively.
In terms of evaluating the research on MC, it is most useful to categorize the studies based on the timing of intake employed, as this relates to the hypothesized metabolic effects. Modified CHO supplements designed to absorb slower (i.e., low glycemic index; SMC) are more typically employed before exercise as this characteristic would conceivably lead to fewer glycemic and insulinemic perturbations and prolong glucose absorption and availability throughout exercise. Conversely, faster digesting/absorbing modified CHO supplements (FMC) can provide rapid access to CHO, which may enhance exogenous CHO oxidation and/or glycogen resynthesis during- and/or post-exercise. The following review will first examine supplements more suitable to pre-exercise applications (i.e., SMC) followed by an analysis focusing on post-exercise supplements (i.e., FMC). During exercise, ingestion of MC is relatively rare in the research, but will be addressed where relevant.
Pre-exercise modified carbohydrates
The perceived benefits of consuming SMC align with the theoretical benefits of consuming unmodified low glycemic index CHO. As described above, the structure and composition of a CHO influences the speed of digestion and absorption. The slower the digestion/absorption, the lower is typically the glycemic index. Many unmodified complex CHO such as can be found in foods like lentils and oats have low glycemic indices, meaning that blood glucose and insulin responses following ingestion are attenuated relative to high glycemic index simple CHO like glucose (Atkinson et al. 2008). This is potentially of interest prior to exercise as large elevations in blood glucose and insulin can lead to rebound hypoglycemia when exercise commences (Costill et al. 1977). Moreover, insulin is antilipolytic leading to attenuated fat availability and oxidation during exercise, a finding consistently reported with high vs. low glycemic index pre-exercise CHO (DeMarco et al. 1999; Stevenson et al. 2005; Choi et al. 2010). For these reasons, low glycemic index CHO would seem to confer an advantage in terms of endurance by enhancing overall fuel availability during exercise (i.e.. blood glucose and fat) relative to high glycemic index CHO. Importantly, research assessing the impact of the glycemic index of CHO on endurance capacity (i.e., TTE) does seem to confirm this notion (Thomas et al. 1991; DeMarco et al. 1999; Kirwan et al. 2001; Karamanolis et al. 2011; Moore et al. 2013), albeit not unanimously (Hargreaves et al. 1987; Wee et al. 1999; Stannard et al. 2000). While intriguing, the practical value for endurance athletes is limited, because unmodified low glycemic index CHO are typically found in whole foods that may lack palatability in proximity to exercise. Moreover, whole foods are less likely to be consumed by endurance athletes during exercise (Pfeiffer et al. 2012). SMC potentially address both concerns, since they can be made in traditional supplement forms such as beverages that can be consumed before and/or during exercise.
While individual modified CHO have distinct compositions (e.g., amylose:amylopectin ratios) and physicochemical properties that may uniquely influence digestion, most research investigating the effects of SMC aligns with findings from studies investigating low glycemic index unmodified starches and complex CHO (Thomas et al. 1991; Febbraio et al. 2000; Kirwan et al. 2001) (see Fig. 1 and Table 2). In the first study to examine potential exercise applications for SMC, ingestion of an acid/alcohol-modified corn starch (70/30% amylose:amylopectin) prior to cycling (2 h at ~ 65% VO2max) resulted in attenuated pre-exercise blood glucose and insulin concentrations relative to dextrose (Johannsen and Sharp 2007). Similarly, pre-exercise ingestion of a hydrothermally modified corn starch (95% amylopectin, 5% amylose) or isomaltulose (i.e., enzymatically modified sucrose) reduced resting blood glucose and insulin response relative to high glycemic index CHO (i.e.. maltodextrin and sucrose) (Roberts et al. 2011; König et al. 2016; Baur et al. 2018). Of interest, some evidence suggests that these attenuated blood glucose and insulin responses may lead to improved glucose stability (Johannsen and Sharp 2007), or even higher glucose concentrations (König et al. 2016) during exercise relative to high glycemic index CHO control presumably as a result of an attenuated rebound glycemic response. These findings would seem to confirm the hypothesis that pre-exercise SMC ingestion results in an extended and prolonged absorption of glucose, which enhances glucose availability throughout exercise.
Importantly, SMC glycemia and insulinemia outcomes are associated with potentially advantageous changes in fuel utilization patterns. Both hydrothermally modified corn starch and isomaltulose ingestion have been reported to increase pre- and/or during-exercise free fatty acid (FFA)/glycerol concentrations and fat oxidation relative to high glycemic index CHO (Roberts et al. 2011; König et al. 2016; Baur et al. 2018). These findings suggest that pre-exercise SMC enhances access to adipose tissue fuel stores, which greatly exceed CHO stores (Acheson et al. 1988). This is of obvious interest to endurance athletes as enhanced adipose tissue lipolysis, FFA delivery, and oxidation reduces muscle glycogen utilization, which has been associated with enhanced performance (Vukovich et al. 1993; Pitsiladis et al. 1999). However, the impact of SMC on adipose tissue metabolism is yet to be fully elucidated. In a study utilizing the microdialysis technique to assess subcutaneous abdominal adipose tissue lipolysis, hydrothermally modified corn starch ingestion (75 g; 30 min prior to exercise) did not alter resting or exercise lipolytic rates despite reduced pre-exercise insulin concentrations and elevated fat oxidation (Baur et al. 2018). It is possible that the increased fat oxidation noted in this study and others with low glycemic index treatments is due to an increased reliance on intramuscular triglycerides, or that changes in fat metabolism may occur in other depots besides subcutaneous abdominal adipose tissue. However, Trenell et al. (2008) reported decreased reliance on intramuscular triglycerides when a low glycemic index diet was consumed following exercise (90-min cycling at 70% VO2peak) and for 12 h preceding a second exercise bout relative to a high glycemic index diet. Moreover, adipose tissue is more lipolytically active and contributes more FFA for oxidation during exercise than other depots (e.g., visceral and femoral), suggesting that any impact on whole-body lipolysis would presumably be revealed via measurement of this specific depot (Arner et al. 1990; Nielsen et al. 2004). It is also possible that low glycemic index CHO reduces FFA reesterification without impacting lipolysis relative to high glycemic index CHO (Enevoldsen et al. 2004). This hypothesis is supported by the significant interaction for plasma FFA despite unchanged plasma glycerol in Baur et al. (2018). More research is clearly warranted to determine the precise mechanism underlying changes in fuel utilization with SMC or other unmodified low glycemic index CHO.
Whether SMC-mediated alterations in fuel use enhance performance is equivocal. Most studies report no improvements in running or cycling performance following SMC ingestion despite seemingly beneficial metabolic responses (Roberts et al. 2011; Parks et al. 2018; Baur et al. 2018; Dudar et al. 2020). König et al. (2016) found a likely 2.7% enhancement of pre-loaded (90-min cycling at 60% VO2max) TT performance with pre-exercise isomaltulose ingestion (75 g; 45 min prior). While intriguing, the lack of external validity in this study makes assessment of the practical value of isomaltulose challenging. For any event > 1 h, it is recommended that athletes consume CHO both before and during exercise to optimize performance (Thomas et al. 2016). As such, consuming a supplement like isomaltulose prior to 2 h or more of exercise without consuming additional CHO during exercise is inadvisable and likely to run counter to common and best practices (Pfeiffer et al. 2012). Thus, confirmation of ergogenic effects within a more externally valid design is necessary. Importantly, studies employing more realistic designs for a pre-exercise-only intervention (30–90 min in duration) have reported null performance effects with SMC that have similar metabolic effects to isomaltulose (Parks et al. 2018; Baur et al. 2018; Dudar et al. 2020).
It is worth considering from a practical standpoint whether SMC produce ergogenic effects when consumed before and during exercise. However, combining pre- and during-exercise ingestion of SMC does not seem to benefit performance. Only one study to date has examined combined pre- and during-exercise SMC ingestion (Baur et al. 2016). In this study, pre-exercise (30 min prior) CHO intake was held constant (60 g; hydrothermally modified SMC or sucrose/dextrose). During exercise (~ 3-h intermittent high-intensity cycling), subjects ingested isocaloric CHO doses (60-g·h−1 SMC or sucrose/dextrose). In a third condition, subjects ingested a low dose of SMC (30 g·h−1) during exercise to determine whether the purported slow absorption/prolonged glucose release of SMC permitted the intake of less total CHO while maintaining performance. Of interest, the combination of pre-exercise SMC with both during-exercise doses enhanced fat oxidation and attenuated blood glucose responses relative to pre- and during-exercise sucrose/dextrose. However, repeated sprint performance was unchanged or impaired with the isocaloric and low-dose condition versus sucrose/dextrose, respectively. Additionally, SMC ingestion was associated with increased gastrointestinal distress, which likely had a negative impact on performance. These findings are supported by Oosthuyse et al. (2015) who reported increased gastrointestinal distress and impaired performance with during-exercise (2 h cycling at 60% VO2max + 16 km time-trial) ingestion of isomaltulose versus glucose/fructose (63 g·h−1). Collectively, these findings are not surprising considering the slow absorption rates and low glycemic index of the SMC utilized in these studies. When CHO is consumed at rates in excess of their maximal absorption capacity, malabsorption occurs which is associated with gastrointestinal distress (Triplett et al. 2010; Rowlands et al. 2012). Furthermore, osmolality is also thought to be a primary contributor to gastrointestinal distress owing to osmotic fluid secretion and gastric distension (Rehrer et al. 1992; Rowlands et al. 2015). With this in mind, it is noteworthy that gastrointestinal distress occurred in both studies despite very low (37–53 vs. 278–363 m·Osm·kg−1) (Baur et al. 2016) or approximately matched (245 vs. 212 m·Osm·kg−1) (Oosthuyse et al. 2015) solution osmolality with SMC versus control beverages. These findings indicate that osmolality may play a secondary role in CHO absorption in manifesting gastrointestinal distress. Finally, with these results in mind, it may seem logical that combining pre-exercise SMC or low glycemic index CHO with during-exercise high glycemic index CHO may represent a compromise that maximizes gastrointestinal comfort and performance. However, this nutritional strategy negates the metabolic effects of low glycemic index CHO and does not further enhance performance relative to a high glycemic index CHO-only nutritional strategy (Burke et al. 1998).
The prolonged glucose release associated with SMC also potentiates elevated blood glucose availability in the morning following pre-sleep SMC ingestion. If true, this application may be beneficial for morning events in which many athletes may be reluctant to consume nutrients prior to competing due to low gastrointestinal tolerability. Dudar et al. (2020) recently investigated this question in trained runners completing an incremental exercise test followed by a 5-km TT 7–9 h (including sleep) after consuming 75 g of SMC, a sucrose-based supplement, or non-nutritive placebo. Of interest, the authors noted a trend for increased CHO oxidation with SMC relative to the other treatments, but there were no differences in blood glucose or running performance. Thus, this study lends some support to the concept that SMC can prolong metabolic effects, even overnight, but not in a way that meaningfully impacts performance. As with similar and previously described studies, this finding is not surprising considering the duration and consequent intensity of exercise prescribed (Parks et al. 2018; Baur et al. 2018), which is likely glycogen-dependent, but not glycogen-limited making blood glucose release profiles to be of relatively less import (Romijn et al. 1993).
When considered together, these limited data suggest that the above-described SMC have limited utility for endurance athletes. Despite presumably advantageous metabolic effects, performance is unchanged or impaired in practically relevant scenarios. These include short-moderate duration (30–90 min) exercise in which one might conceivably consume CHO-only before exercise, or with prolonged (> 90 min) exercise requiring combined pre- and during-exercise ingestion. Nevertheless, a number of studies have indicated enhanced fat utilization with pre-exercise SMC (Roberts et al. 2011; König et al. 2016; Baur et al. 2018). While an increased reliance on fat may not necessarily translate into enhanced performance, it has been associated with increased endurance capacity (Thomas et al. 1991; Pitsiladis et al. 1999; DeMarco et al. 1999), which may be of interest to certain populations that are frequently engaged in a prolonged low-intensity exercise with limited access to during-exercise nutrients (i.e., wildland firefighters, soldiers, etc.). Thus, determination of the effects of pre-exercise SMC on endurance capacity and potential ergogenic effects in different populations/contexts is warranted.
Post-exercise modified carbohydrates
For endurance athletes, post-competition or training nutrition typically focuses on maximizing glycogen restoration for subsequent competition/training (Thomas et al. 2016). Glycogen can be synthesized rapidly following exercise with CHO intake due to enhanced insulin sensitivity and insulin-independent GLUT4 translocation to the sarcolemma (Richter et al. 1982; Lund et al. 1995; Thorell et al. 1999). Rates of glycogen synthesis seem to be heavily mediated by the timing and amount of CHO/nutrients consumed. Glycogen restoration rates are highest when CHO is consumed within the first 2 h of recovery at rates of 1.0–1.2 g·kg−1·h−1 but decline thereafter likely due to attenuated GLUT4 translocation (Ivy et al. 1988; Goodyear et al. 1990). The type of nutrients consumed is also a critical consideration for glycogen restoration. Faster absorbing high glycemic index CHO enhance glycogen restoration relative to low glycemic CHO likely because of higher insulin production (Blom et al. 1987; Burke et al. 1993; Jozsi et al. 1996). This theory is supported by the fact that the addition of 0.3–0.5 g·protein−1·kg−1·h−1 to a post-exercise CHO beverage enhances the insulin response and glycogen synthetic rate relative to energy-matched CHO alone (van Loon et al. 2000; Ivy and Goforth 2002; Williams et al. 2003; Berardi et al. 2006). Taken together, an effective supplement for post-exercise glycogen synthesis would ideally be well-tolerated/easy to consume in large quantities, fast to absorb, and maximize insulin production. All of these characteristics can be potentially targeted by CHO modification.
Studies suggest that CHO modification may provide a means of enhancing post-exercise glycogen restoration and subsequent exercise performance (see Table 3). All of the available research in this area employ high amylopectin (78–98%) starches derived through fractionation [acid modification at high temperatures (110–140 °C)] of various native starches (e.g., potato, wheat, corn, and barley) (Brynolf et al. 1995). The resultant FMC has very high molecular weight ~ 500,000 to 700,000 and thus lower osmolality (~ 20–80 m·Osm·L−1) when compared to simple CHO that are typically found in sport supplements (e.g., glucose, maltodextrin, and fructose; ~ 150–1400 m·Osm·L−1 depending on dose/formulation). This is noteworthy, because osmolality can impact gastric emptying. Specifically, low osmolality solutions empty faster from the stomach when matched for energy content, particularly at high CHO concentrations and/or when compared to hypertonic solutions with osmolalities exceeding ~ 350 mOsm·kg−1 (Rehrer et al. 1992; Shi et al. 2017). As such, low osmolality CHO solutions could conceivably enhance glycogen restoration due to faster delivery of CHO to the small intestine, and there is evidence that apparently supports this notion. Aulin et al. (2000) reported a 70% higher glycogen synthesis rate within 2 h post-exercise with ingestion of the aforementioned FMC compared to maltodextrin (2.1 g·kg−1·h−1 for the first 2 h of a 4 h measurement period). Findings from a follow-up study suggest higher rates of glycogen synthesis may be related to the low osmolality of the FMC and its influence on gastric emptying. Leiper et al. (2000) reported 80% faster gastric emptying rates with FMC versus a maltodextrin control within the first 10 min following ingestion (75 g CHO). Cumulatively, these studies point to faster delivery of CHO to the small intestine, enhancing CHO availability for glycogen restoration (Fig. 1).
While these findings are intriguing, further examination of the data from these studies and others reveals that potential ergogenic effects derived from this FMC may be transient and dependent on the dosing strategy. Specifically, the primary effects in both above-described studies seem confined to the early post-prandial period. For example, the study by Aulin et al. (2000) found that, despite enhanced glycogen synthesis from 0 to 2 h with FMC, glycogen concentrations were equal at 4 h post-suggesting that glycogen synthesis rates “caught up” with the control solution over the final 2 h. Similarly, Leiper et al. (2000) reported that the gastric emptying rate of FMC lowered to control levels after the first 10-min measurement period and remained at this level for the remainder of the experiment (10–60 min). While speculative, this suggests that the FMC is delivered rapidly to the intestine following an initial dose, but then slows over time or with repeated doses. Importantly, this potential slowing seems to impact CHO absorption in a way that may nullify the benefits of fast initial gastric emptying. Both Aulin et al. (2000) and Leiper et al. (2000) reported similar blood glucose and insulin concentrations post-ingestion between FMC and the control solution signifying similar (or even slower, considering the faster initial gastric emptying rate) rates of CHO absorption. In support, three studies that assessed exogenous CHO oxidation rates with during-exercise ingestion of the same FMC reported that oxidation rates were either the same (Rowlands et al. 2005) or lower versus a maltodextrin control solution (Rowlands and Clarke 2011) or MTC solutions (Pettersson et al. 2020). Again, this points to similar or even impaired absorption of glucose derived from this FMC.
As several authors have speculated (Leiper et al. 2000; Rowlands and Clarke 2011), there are two mechanisms that may explain slower glucose absorption with this FMC relative to maltodextrin. In the first, digestion and absorption may be delayed with FMC due to the time needed for hydrolysis from amylopectin to maltose and from maltose to glucose. Second, the high molecular weight of the FMC may contribute to gel formation in the small intestine slowing the movement of glucose into the intestinal villi for absorption (Johnson and Gee 1981). If true, this slowed absorption would likely also contribute to accumulation of CHO in the small intestine, stimulation of gut receptors sensitive to nutrient density, and feedback inhibition of gastric emptying thereby slowing the delivery of CHO from any future doses (Brener et al. 1983). This mechanism is supported by reports of increased gastrointestinal distress with during- or post-exercise ingestion of this FMC (McGlory and Morton 2010; Rowlands and Clarke 2011). Whatever the precise mechanism, collectively, these data suggest that the primary benefits to be gained from ingestion of FMC (i.e., enhanced glycogen synthesis) may be almost entirely explained by the initial CHO dose and that further ingestion may actually lead to slower CHO delivery compared to a traditional CHO supplement. This mechanistic analysis is instructive in terms of contextualizing later findings.
Recent findings show that post-exercise FMC may be beneficial in specific scenarios. Two studies have reported ergogenic effects with post-exercise ingestion of FMC. In the first, recreationally active males cycled to exhaustion at 75% VO2max and then immediately consumed 100 g of FMC, maltodextrin, or a non-caloric placebo (Stephens et al. 2008). Participants then rested for 2 h, and then completed a 15-min TT. Performance was enhanced ~ 10% with modified starch relative to all other treatments. In addition to this substantial ergogenic effect, post-exercise FMC ingestion increased blood glucose and insulin concentrations relative to maltodextrin. This is noteworthy as it contrasts with prior findings (Aulin et al. 2000; Leiper et al. 2000; Rowlands et al. 2005), but also because it suggests enhanced CHO absorption with FMC. Based on the above discussion, an explanation for this glycemia/insulinemia finding is challenging, but may be related to sampling technique as Stephens et al. assessed arterialized rather than venous blood and sampled more frequently than prior studies (Stephens et al. 2008). This hypothesis is plausible as no other studies measured arterialized blood (Aulin et al. 2000; Leiper et al. 2000; Rowlands et al. 2005; McGlory and Morton 2010; Rowlands and Clarke 2011; Oliver et al. 2016). Moreover, arterialization of blood results in meaningful differences in glucose/insulin relative to venous blood, and differences can be magnified by exercise and/or feeding (Edinburgh et al. 2017; Chen et al. 2018). If true, the increased glucose and insulin concentrations reported in this study would provide a potential mechanism for the enhanced glycogen restoration reported by Aulin et al. (2000). Nevertheless, firm conclusions in terms of a mechanism are elusive due to methodological differences between studies that may have impacted gastric emptying and/or CHO absorption (i.e., CHO dose/concentration, single versus repeated doses, etc.). More research is clearly needed.
The other study reporting ergogenic effects with modified starch utilized a similar dosing strategy. In this study, resistance-trained males completed a glycogen-depleting intermittent ride on an cycle ergometer (60 min at 70% VO2max + high-intensity intervals) and then immediately consumed 1.2 g·kg−1 of FMC, maltodextrin, or a non-caloric placebo (Oliver et al. 2016). After 2-h recovery, subjects performed repeated back squats (5 × 10; 75% 1RM) in which power output was measured with a force plate. Post-exercise FMC enhanced average back squat power by 3.1% and 4.9% relative to maltodextrin and placebo, respectively. This performance effect occurred despite similar glucose and insulin concentrations post-ingestion for both CHO treatments, which contrasts with the above study and further complicates the determination of a mechanism. Nevertheless, these studies seem to support the post-exercise use of FMC for endurance or resistance training performance in subsequent exercise. However, the designs of both studies (and subsequent research) seemingly limit extrapolation to unique, and potentially unrealistic, situations. Both Stephens et al. (2008) and Oliver et al. (2016) employed designs in which subjects ingested a single bolus of CHO (or placebo) (Stephens et al. 2008; Oliver et al. 2016). Moreover, the timing between exercise bouts was relatively short (2 h). These design choices contrast with current recommendations for optimal post-exercise refueling, which state that athletes should consume 1.0–1.2 g·kg−1·h−1 over a 4-h period following exercise (4.0–4.8 g·kg−1 total) preferably as multiple smaller doses (Burke et al. 2004; Thomas et al. 2016). Thus, it would seem that utilizing FMC under the conditions in which it was shown to be effective would result in sub-optimal post-exercise refueling relative to current best practices. Importantly, the existing study designs would seem to magnify the potential benefits of FMC, as ergogenic effects seem to primarily derive from the initial bolus ingested as described above.
When modified starch is employed in a study design that more closely adheres to best practices, effects may be less apparent. McGlory and Morton (2010) investigated the impact of post-exercise ingestion of FMC on subsequent running endurance utilizing a refueling strategy that meets current recommendations. Following prolonged running (1 h 70% VO2max), subjects consumed three treatment doses over a 3-h period (1.2 g·kg−1 each; 3.6 g·kg−1 total) of FMC, maltodextrin/dextrose, or a non-caloric placebo. Following this, intermittent running endurance was assessed. While both CHO treatments improved endurance and increased blood glucose concentrations relative to placebo, there were no differences between CHO conditions. Additionally, FMC ingestion was associated with increased gastrointestinal distress. Taken together, these results suggest that ingesting FMC in a manner consistent with recommendations results in no additional benefits relative to traditional CHO supplements.
Additionally, recent research suggests that post-exercise FMC does not benefit female endurance athletes, even when employed in the most likely beneficial manner (i.e., single dose; 2-h recovery) (Mock et al. 2018). In this study, female cyclists consumed a placebo or 1.2 g·kg−1 CHO (i.e., FMC or maltodextrin/dextrose/fructose) immediately following a ride to exhaustion at 75% VO2max. After 2-h rest, subjects completed a 15-min TT. Both CHO treatments resulted in increased CHO oxidation as determined via RER, but no other variables were different including performance. It is possible that the lack of ergogenic effects in this study stems from sex-specific differences in fuel utilization that favor fat oxidation over CHO utilization (Tarnopolsky 2000). Additionally, some research has suggested that rates of glycogen storage may be different across sexes (Tarnopolsky et al. 1995, 2001; Walker et al. 2000). However, differences in substrate utilization and glycogen storage in females have been found to be the result of cyclical changes in 17β-estradiol, a hormone measured and statistically controlled for in this study (Kendrick et al. 1987; Ruby et al. 2002; Maher et al. 2010). Moreover, recent research found that females restore glycogen at similar rates compared to males with post-exercise ingestion of various forms of CHO suggesting that any enhancement of glycogen restoration present in males should theoretically also occur in females (Flynn et al. 2020). Thus, more research is required to determine if there are sex differences in response to FMC or other post-exercise supplements designed to restore glycogen.
Collectively, research examining post-exercise FMC points to a number of conclusions. First, it seems that FMC enhances gastric emptying possibly due to its molecular weight and osmolality. This faster rate of CHO delivery to the small intestine also seems to contribute to faster glycogen synthesis. However, these benefits appear limited to the early post-prandial period (2 h), and seem to diminish over time and with additional CHO doses. As such, performance benefits may occur when a single dose is ingested post-exercise and subsequent exercise commences relatively soon thereafter (within 2 h). This may be a relevant scenario for athletes such as soccer players seeking to quickly refuel at halftime prior to a subsequent 45-min period of exercise that may be glycogen-limited. However, in scenarios commonly experienced by endurance athletes (i.e., refueling between training/competition) who are following best practices for refueling (consuming large amounts of CHO across multiple doses and hours), FMC does not appear to be beneficial and may actually be detrimental due to its potential to induce gastrointestinal distress.
Carbohydrate hydrogel
As discussed above, early research on CHO supplementation revealed the importance of maintaining CHO availability during exercise to maximize endurance performance. The “next generation” of CHO supplements (MTC, MC, HGEL, etc.) has seemingly been designed to further enhance CHO availability by targeting the presumed primary limiters of CHO delivery—gastric emptying and CHO absorption. The recently developed “CHO hydrogel” supplement would seem to represent a convergence of these mechanisms for enhancing CHO delivery and is therefore of great interest as a potential ergogenic aid. Along these same lines, the excitement surrounding this supplement has grown substantially of late based on reports that it has been utilized by champion athletes in record-breaking performances (e.g., see www.maurten.com/achievements).
The distinctive characteristics of hydrogels are derived from the ingredients alginate and pectin. These soluble dietary fibers are typically derived from brown seaweed and citrus fruits, respectively (Sriamornsak 2011; Lee and Mooney 2012). Due to their distinctive physicochemical properties, alginate and pectin can form three-dimensional cross-linked aqueous gels when exposed to certain stimuli (e.g., temperature, pH, ionic strength, etc.) (Lee and Mooney 2012). As such, these fibers have been utilized for a number of applications in the food and cosmetics industries often as thickening or stabilizing agents. However, the application most relevant to this review is drug delivery. Alginate and pectin are frequently utilized as a means of protecting or encapsulating drugs that are ingested to ensure delivery and release at a specific site (Sriamornsak 2011; Lee and Mooney 2012). This is possible via preparation (e.g., addition of HCl, Ca2+, NaCl, etc.) that causes gel formation in certain regions of the body that have specific characteristics (e.g., low/high pH, temperature, etc.). A common example is a hydrogel that protectively forms around a drug at the low pH levels present in the stomach and then dissolves in the neutral pH environment of the small intestine allowing for drug release and absorption (Colinet et al. 2009).
Through use of this mechanism, hydrogels could potentially be employed to enhance CHO delivery while simultaneously maintaining fluid delivery and gastrointestinal comfort. As mentioned, current recommendations advise consuming large amounts (30–90 g·h−1) of CHO during prolonged (> 60 min) exercise, which typically necessitates consuming highly concentrated beverages even if fluid intake is near optimal (0.4–0.8 L·h−1) (Thomas et al. 2016). While this has been shown to optimize performance, it also reduces gastric emptying, fluid delivery, and gastrointestinal comfort (Vist and Maughan 1995; Pfeiffer et al. 2012). Gastric emptying is the result of a balance between feed-forward stimulation (stomach volume) and feedback inhibition (nutrient density) (Thomson et al. 2001). Feedback inhibition of gastric emptying is thought to occur via osmo- and/or chemoreceptors in the duodenum (Brener et al. 1983; Thomson et al. 2001). Theoretically, pectin and alginate hydrogels could be utilized to enhance gastric emptying of concentrated solutions through encapsulation of CHO in the stomach to prevent detection of nutrient density by duodenal receptors. If true, faster gastric emptying may enable earlier and/or faster absorption of fluid and CHO. This is particularly true if employed in a solution containing MTC, which have already been shown to enhance both gastric emptying and absorption relative isocaloric amounts of glucose/maltodextrin (Jeukendrup and Moseley 2010). Such a supplement would hypothetically enhance performance relative to other CHO supplements due to enhanced CHO/fluid delivery and attenuated gastrointestinal distress owing to enhanced CHO absorption efficiency (i.e., attenuated malabsorption (Fig. 1)) (Rowlands et al. 2015).
Early reports (see Table 4) on a newly developed hydrogel supplement that contains MTC (HGEL; maltodextrin + fructose; 1:0.5–0.7 ratio) lend support to this hypothesis based on findings relating to gastrointestinal tolerance. Sutehall et al. (2018) observed no incidences of gastrointestinal distress among Kenyan distance runners consuming a highly concentrated HGEL (~ 90 g·h−1; 18–30%) during a ~ 95-min training run. In agreement, a recent laboratory study reported no differences in gastrointestinal comfort with ingestion of HGEL consumed at an even higher rate of 132 g·h−1 (18% solution) during cross-country skiing (2 h at ~ 70% VO2max) compared to a non-caloric flavor-matched placebo (Pettersson et al. 2019). This is noteworthy as several prior studies have reported severe gastrointestinal distress among cyclists and runners with similar, or even lower, doses and concentrations of CHO (Triplett et al. 2010; Wilson and Ingraham 2015).
While intriguing, the mechanism for enhanced gastrointestinal comfort with HGEL is yet to be fully elucidated. Flood et al. (2020) recently investigated whether HGEL attenuated gastrointestinal damage during exercise (90-min cycling 45% VO2max followed by a 15-min TT) in hot conditions (32 ○C; 70% humidity). Exercise in such conditions has been reported to increase intestinal permeability and potentiate endotoxemia (Rowell et al. 1968; Lambert et al. 2008; ter Steege and Kolkman 2012; Zuhl et al. 2014; de Oliveira et al. 2014). HGEL (90 g·h−1; 16%; 1:0.73 ratio) and the CHO-matched control attenuated markers of gut damage (i.e., intestinal fatty acid-binding protein and the percent ratio of lactulose to rhamnose) relative to water. However, there were no differences between CHO treatments. Thus, any benefits of HGEL in terms of gastrointestinal tolerance relative to traditional CHO supplements do not seem related to enhanced maintenance of intestinal wall integrity and/or prevention of endotoxemia.
Another possible explanation for the reported enhanced gastrointestinal comfort with HGEL is related to enhanced gastric emptying and/or CHO absorption. In a recent study by Sutehall et al. (2020), gastric emptying rates were assessed via doubling-sampling technique following ingestion of an HGEL (90 g; 18%; 732 mOsmol·kg−1) versus isocaloric and ratio-matched (1:0.7) control solutions: osmolality-matched maltodextrin + fructose (727 mOsmol·kg−1) and high osmolality glucose + fructose (1392 mOsmol·kg−1). Gastric emptying (i.e., half-emptying time) was faster with HGEL (21 ± 9 min) compared to the non-hydrogel, osmotically matched control solution (37 ± 8 min) and the high osmolality control (51 ± 15 min). Clearly, this suggests that the addition of alginate and pectin to an MTC solution is effective in terms of enhancing CHO delivery to the small intestine. Moreover, this effect is presumably the result of hydrogel formation, which has been confirmed in vitro and in vivo at pH levels found in stomach (Marciani et al. 2019; McCubbin et al. 2020). While this suggests enhanced CHO delivery with HGEL, gastric emptying is only one of the limiters of CHO and fluid uptake. For HGEL to truly confer ergogenic effects, faster gastric emptying would likely need to be coupled with enhanced CHO and fluid absorption.
While data are limited, evidence at present suggests that HGEL increases intestinal absorption relative to CHO-matched controls is equivocal. Pettersson et al. (2020) recently assessed exogenous CHO oxidation rates during the first 100 min of cycling (3 h at 55% Wmax) with HGEL (95 g·h−1; 14%; 1:0.72 ratio) relative to iso-carbohydrate FMC and a maltodextrin + sucrose control. Interestingly, the authors noted enhanced exogenous CHO oxidation with HGEL relative to both control beverages. While enhanced exogenous CHO oxidation with HGEL relative to FMC was expected as this has been previously reported with MTC versus the same FMC (Rowlands and Clarke 2011), the increased oxidation relative to maltodextrin + sucrose is notable as this would seemingly indicate enhanced CHO delivery versus a traditional MTC solution. However, this interpretation is challenged by the fact that these solutions were not matched for CHO type. Specifically, the comparison beverage contained less fructose than HGEL (10.2 g·h−1 vs. 40.8 g·h−1, respectively). As the authors acknowledged, this resulted in substantially different maltodextrin:fructose ratios (1:0.25 vs. 1:0.75). As MTC ratios closer to 1:0.8 have been reported to increase fructose oxidation efficiency and total exogenous CHO oxidation relative to lower ratios (O’Brien et al. 2013), it is perhaps no surprise that HGEL enhanced exogenous CHO oxidation relative to the maltodextrin + sucrose solution. This notion is supported by the lower blood glucose concentrations reported in the HGEL trial at the end of exercise relative to the control solutions. Since CHO supplementation ceased at 100 min, the higher levels of blood glucose at the end of exercise with the control solutions can be interpreted as evidence of slower glucose absorption prolonging glucose appearance in the blood, but also likely contributing to attenuated exogenous CHO oxidation. Taken together, it is impossible to conclude, based on this study, that alginate and pectin enhance CHO absorption and oxidation without comparing HGEL to a solution matched for CHO type and quantity. Notably, another recent study assessed exogenous CHO oxidation rates during running (2 h at 60% VO2peak) between HGEL (90 g·h−1; 16%; 1:0.72 ratio) and a non-hydrogel, but otherwise identical, MTC control solution. Importantly, exogenous CHO oxidation rates were not different between HGEL and the isocaloric MTC control. These data indicate that when CHO type and quantity is matched, alginate and pectin provide no additional enhancement of exogenous CHO oxidation. More evidence for a lack of benefit to CHO absorption comes from studies assessing the impact of HGEL on blood glucose and insulin concentrations. None have found differences in these variables relative to CHO-matched controls (Baur et al. 2019; McCubbin et al. 2020; Barber et al. 2020; Mears et al. 2020; Sutehall et al. 2020; Flood et al. 2020). When combined with gastric emptying and exogenous CHO oxidation data, these findings indicate that HGEL may arrive earlier to the small intestine, but absorption is delayed or slowed resulting in similar exogenous CHO oxidation rates and blood glucose appearance relative CHO-matched solutions.
Despite limited data, there are possible mechanisms explaining the lack of difference in CHO absorption between HGEL and MTC. As noted by Sutehall et al. (2020), gastric emptying kinetics following HGEL ingestion closely mirrored those reported by Leiper et al. (2000) with ingestion of a high amylopectin FMC. Both studies reported faster gastric emptying compared to solutions containing maltodextrin, fructose, and glucose. However, this difference seemed to stem almost entirely from a faster initial emptying (10–40 min following ingestion) that slowed to control levels thereafter. In addition, neither study reported differences in blood glucose or insulin concentrations, suggesting that faster initial gastric emptying did not lead to appreciable increases in CHO absorption versus control solutions, a notion mostly confirmed by studies of exogenous CHO oxidation rates for these treatments (Rowlands et al. 2005; Rowlands and Clarke 2011; Barber et al. 2020). Collectively, these data suggest that the gel-forming properties inherent to HGEL and presumed present with this FMC enhance gastric emptying in the immediate post-ingestion period due to either CHO encapsulation-induced attenuation of duodenal feedback inhibition or feed-forward stimulation of mechanoreceptors in the stomach wall resulting from the volume expansion that occurs with gel formation (Powley and Phillips 2004). Following initial emptying, absorption of both treatments appears slowed possibly due to the increased viscosity of the gels inhibiting infiltration by hydrolytic enzymes or slowing movement of contained glucose and/or fructose to the intestinal epithelia (Johnson and Gee 1981). Due to this delay, stimulation of intestinal osmoreceptors may be increased, leading to subsequent slowing of further gastric emptying (Brener et al. 1983; Vist and Maughan 1995). This hypothesis is supported by reports of increased fullness with HGEL ingestion (Georg Jensen et al. 2012; Wanders et al. 2013; Baur et al. 2019; Mears et al. 2020). Higher ratings of fullness could be interpreted as representative of synergistic effects on both feed-forward and feedback mechanisms initiated by volume expansion in the stomach and reinforced by nutrient sensing in the intestinal track that induces release of satiety hormones like glucagon‐like peptide 1 and peptide YY (Maljaars et al. 2007). However, this is purely speculative. Further study is needed to confirm the precise mechanism, but the available evidence indicates that HGEL does not alter CHO availability relative to non-hydrogel, but CHO-matched controls. Moreover, confirmation that hydrogels increase satiety is warranted as such a finding would presumably discourage use of this supplement for endurance athletes aiming to consume as many calories as feasibly possible during exercise.
Additional indirect evidence for the hypothesis that HGEL does not meaningfully enhance CHO availability relative to non-hydrogel CHO solutions comes from studies assessing metabolic and performance responses with HGEL ingestion during exercise. Across the studies assessing these outcomes, it has been consistently reported that CHO hydrogel solutions do not impact fuel utilization or performance (Baur et al. 2019; McCubbin et al. 2020; Mears et al. 2020; Flood et al. 2020). This appears to be the case in runners (McCubbin et al. 2020) and cyclists (Baur et al. 2019; Mears et al. 2020; Flood et al. 2020) with a range of CHO ingestion rates (68–90 g·h−1), high and low CHO concentrations (7.8–16%), and varied fluid ingestion rates (~ 500 mL·h−1 vs. 1 L·h−1). Moreover, similar results were reported regardless of the performance protocol employed (TT or repeated sprinting). While these findings are certainly discouraging and suggest that HGEL is not ergogenic despite likely faster gastric emptying, it is worth noting that data are limited. Moreover, the variety of study designs and beverage formulations employed make drawing firm conclusions challenging.
It is also worth considering in terms of performance responses that potential HGEL-mediated ergogenic effects are non-metabolic in nature and obscured by the study designs employed thus far. Specifically, it is possible that any beneficial effects of HGEL will stem from enhanced gastrointestinal tolerance to very high CHO doses. Indeed, it has already been noted that ingesting highly concentrated HGEL at very high CHO delivery rates (132 g·h−1) resulted in no differences in gastrointestinal distress versus a flavor-matched placebo (Pettersson et al. 2019). With this in mind, it is important to note that all studies that have assessed performance responses thus far have utilized CHO ingestion rates in line with current recommendations (60–90 g·h−1) (Baur et al. 2019; McCubbin et al. 2020; Mears et al. 2020; Flood et al. 2020). In these studies, there were no reported differences in gastrointestinal comfort between HGEL and CHO-matched solutions (Baur et al. 2019; McCubbin et al. 2020; Mears et al. 2020). A likely explanation for similar findings is that non-hydrogel MTC solutions are already well tolerated at these ingestion rates and cannot be further improved by the addition of pectin and alginate (Rowlands et al. 2015). Perhaps, detection of possible benefits with HGEL requires substantially higher CHO intake rates that exceed recommendations, but appear to be well tolerated (Pettersson et al. 2019).
It may seem counterintuitive to consume CHO at rates far exceeding recommendations and known maximal absorption capacities, as it would simply lead to reduced oxidation efficiency and accumulation of CHO in the gut. However, there may be practical value to athletes who either do not have access to or are unwilling/unable to consume CHO consistently during exercise. For example, distance runners rarely consume fluid at recommended levels during training or competition, which compromises both fluid and CHO delivery (Pfeiffer et al. 2012). In the field study described above, Kenyan distance runners consumed fluid at a rate of only ~ 185 mL·h−1 (Sutehall et al. 2018). With such low fluid intake rates, any fluid ingested would need to be highly concentrated to deliver CHO at recommended levels (30–90 g·h−1). However, attempting to meet CHO needs with infrequent, but highly concentrated, CHO doses is not well tolerated. Stocks et al. (2016) assessed the impact of consuming different concentrations (high = 24%; moderate = 12%) of MTC (maltodextrin + fructose; 1:1 ratio) and dosing frequencies (high = 6 doses per 140 min; low = 2 doses per 140 min) on metabolism and performance in cross-country skiers, another population prone to under-consumption of fluid/CHO due to logistical challenges. The authors reported that consuming the highest concentration (24%) at the lowest frequently (2 doses within 140 min) resulted in higher average gastrointestinal distress relative to all other conditions (i.e., high concentration—high frequency, moderate concentration—low frequency, and moderate concentration—high frequency) (Stocks et al. 2016). While 30-km TT performance was unaffected, the authors estimated a probable 60-s advantage with the low-frequency dosing strategy due to less time spent slowing to collect/consume beverages. Viewed in this light, the similar performance with the high concentration and low-frequency condition could be viewed as a performance impairment. Moreover, there was an order effect reported that may have obscured gastrointestinal-distress-induced reductions in performance. Regardless, it is obvious that gastrointestinal distress during endurance exercise is undesirable and potentially harmful (Rowlands et al. 2012; Baur et al. 2016). From this study, one could conclude that there may be potential advantages inherent to a supplement that is well tolerated even when consumed in highly concentrated and infrequent doses. Importantly, the limited evidence suggests that HGEL may fit this characterization. Indeed, in the same field study of Kenyan runners described above, the three that consumed the highly concentrated hydrogel supplement (18–30%) were still able to ingest CHO at a rate of ~ 30 to 55 g·h−1 despite the very low (~ 185 mL·h−1) fluid intake rates (Sutehall et al. 2018). Moreover, they reported no gastrointestinal symptoms. Nevertheless, it is worth noting that with during-exercise HGEL (90 g·h−1; 1:0.7 ratio) in the heat (32 ○C; 70% humidity) (i.e., another scenario in which gastrointestinal distress is likely to be increased), some have reported hydrogels to actually increase gastrointestinal distress relative to a CHO-matched control and water (Flood et al. 2020). Collectively, these findings indicate that HGEL tolerability may be condition-specific (i.e., temperate vs. hot) even when intake rates are within recommended ranges. Clearly, more research is needed in this area to establish under which, if any, scenario HGEL-mediated CHO tolerance is beneficial.
In total, the limited studies conducted investigating the effects of adding pectin and alginate to CHO solutions do not indicate ergogenic effects. No studies to date have reported benefits to performance or metabolic alterations indicative of enhanced CHO availability. Nevertheless, the noted high gastrointestinal tolerance to very high CHO concentrations/doses requires further research to evaluate potential scenarios in which hydrogels may be advantageous.
Supplementation strategies
The basis for the above-described research seems to be that CHO supplementation is effective, and that harnessing any additional CHO-based ergogenic effects requires manipulation of CHO type, physicochemical properties, and/or form to enhance CHO availability. This is a sound assumption and hypothesis. Decades of research (Jeukendrup 2004; Temesi et al. 2011; Vandenbogaerde and Hopkins 2011; Cermak and Van Loon 2013; Stellingwerff and Cox 2014) support CHO supplementation for endurance performance enhancement making it logical to assume further benefits by refinement of delivery methods. As described, there is some evidence in support of this theory. However, it is conceivable, and perhaps likely based on the above review, that the potential ergogenic effects of acute CHO supplementation have reached a point of diminishing returns as a result of extensive experimentation. With this in mind, it is prudent to consider alternative strategies for nutritionally augmenting endurance performance beyond that which is afforded by acute CHO supplementation alone. Specifically, combining CHO with other presumably ergogenic nutrients/substances may additively or synergistically enhance performance. Additionally, dietary alterations may influence CHO metabolism in ways that influence performance (Table 5).
It is worth noting that many of the below topics have been reviewed elsewhere (and readers will be directed to relevant reviews where appropriate). As a result, the below discussion of these topics will be concise and the focus will remain on how these approaches may interact with CHO supplementation to potentially alter metabolism and performance. Moreover, research in these areas, and particularly with said focus, is somewhat limited. Therefore, some of the discussion that follows will be necessarily speculative and hypothetical.
Caffeine
The use of caffeine as an ergogenic aid is well supported in the literature (Tarnopolsky 1994; Burke 2008). Recent systematic reviews and meta-analyses report 2–4% improvements in endurance performance with ingestion of moderate doses of caffeine (2–5 mg·kg−1) (Ganio et al. 2009; Shearer and Graham 2014; Souza et al. 2017; Southward et al. 2018), and there appears to be no additional benefit from higher doses (Graham and Spriet 1995; Desbrow et al. 2012). A number of potential mechanisms for this performance effect have been suggested. These include muscle glycogen-sparing via enhanced fat oxidation (Costill et al. 1978; Ivy et al. 1978; Graham and Spriet 1991; Cox et al. 2002), adenosine receptor antagonism resulting in attenuation of pain receptor stimulation and perceived effort (Davis et al. 2003; Aguiar et al. 2020), increased motor output via simulation of oral caffeine and/or bitter taste receptors (Beaven et al. 2013), and enhanced skeletal muscle force production via increased mobilization of myofibrillar calcium and subsequent increased nitric oxide production (Cappelletti et al. 2015). Based on these well-documented effects, the ease of supplementing foods/beverages with caffeine, and the fact that many of these purported mechanisms are separate from those impacted by CHO, it is logical to hypothesize that combining caffeine with CHO supplementation may result in superior endurance performance compared to either substance alone.
Support for a combined ergogenic effect comes from studies examining the impact of caffeine supplementation on CHO metabolism. However, this support seems confined to exercise studies. Early research on resting subjects suggested that caffeine may actually counteract the ergogenic effects of CHO. Indeed, studies employing an oral glucose tolerance test or hyperinsulinemic–euglycemic clamp indicated that caffeine supplementation resulted in attenuated muscle glucose uptake and glycogen synthase activity via insulin desensitization (Graham et al. 2001; Greer et al. 2001; Thong et al. 2002). The reduced insulin sensitivity noted in these studies would seemingly reduce the availability of ingested CHO negatively impacting exogenous CHO oxidation during exercise or glycogen restoration following exercise. While seemingly undesirable, it is important to note that exercise both enhances insulin sensitivity and increases muscle glucose uptake via insulin-independent GLUT4 translocation (Richter et al. 1982; Lund et al. 1995; Thorell et al. 1999; Thong et al. 2002). The combined contribution of these mechanisms may explain why data from exercise studies seemingly contradict the above-described findings from resting studies.
A number of exercise studies indicate that caffeine may enhance exogenous CHO delivery to skeletal muscle. For instance, studies have reported faster intestinal absorption (23%) and increased exogenous CHO oxidation rates (26%) with during-exercise ingestion of caffeine and CHO versus CHO alone (Van Nieuwenhoven et al. 2000; Yeo et al. 2005). These effects may also be present post-exercise. Pedersen et al. (2008) reported the highest glycogen synthesis rates ever recorded (60 mmol·kg−1·dry weight−1·h−1); 66% higher compared to CHO alone) with post-exercise CHO and caffeine ingestion (1.0 g·kg−1·h−1 and 2 mg·kg−1·h−1, respectively). While a mechanism to explain these findings is still lacking, it has been suggested that caffeine-induced increases in cAMP concentrations may enhance SGLT1 and/or GLUT2 transporter activity in the intestinal enterocytes, thereby augmenting intestinal glucose absorption (Van Nieuwenhoven et al. 2000; Yeo et al. 2005). If true, this mechanism has potentially important implications for MTC-based research focusing on maximal exogenous CHO oxidation rates as the fructose transporter GLUT5 is similarly regulated by cAMP levels (Douard and Ferraris 2008). While these studies are intriguing, it is important to note that follow-up studies have failed to replicate these findings. Specifically, studies have reported similar exogenous CHO oxidation rates when either pre- or during-exercise caffeine is combined with during-exercise CHO (Hulston and Jeukendrup 2008; Desbrow et al. 2009). Moreover, others have observed similar glycogen synthesis rates (31 mmol·kg dry weight−1·h−1) following exercise when CHO was consumed with or without added caffeine (Beelen et al. 2012). While it is possible that the conflicting findings across studies are the result of methodological differences (e.g., timing and amount of CHO/caffeine, the magnitude of prior glycogen depletion, etc.), these data indicate that metabolic responses with combined caffeine and CHO are variable with a range of possible effects. At worst, caffeine appears to have no effect on CHO metabolism, which potentially implies additive ergogenic effects as the two substances seem to augment performance via distinct mechanisms. At best, caffeine may augment CHO delivery possibly leading to synergistic performance enhancements.
Studies assessing performance responses to combined caffeine and CHO mostly support this notion of additive or synergistic effects. However, firm conclusions are challenging to make based on study designs. Most studies in this area compare combined caffeine and CHO with CHO alone (either before and/or during exercise or post-exercise followed by subsequent exercise). These studies consistently report enhanced performance or attenuated fatigue with combined caffeine and CHO versus CHO alone (Kovacs et al. 1998; Bell and McLellan 2002, 2003; Cureton et al. 2007; Hogervorst et al. 2008; Hulston and Jeukendrup 2008; Taylor et al. 2011; Cooper et al. 2014), a conclusion supported by a meta-analysis of 21 such studies (Conger et al. 2011). This same meta-analysis also suggested that the nature of the ergogenic effect was likely additive albeit not perfectly so [i.e., the additional performance benefit from adding caffeine to CHO (ES = 0.26) was smaller than the effect of caffeine compared to placebo (ES = 0.51) (Conger et al. 2011)]. Nevertheless, the true nature of the ergogenic effect (i.e., whether it is additive or synergistic) is difficult to fully elucidate without crossover studies comparing combined caffeine and CHO to both CHO alone and caffeine alone. In one of the few studies to make both comparisons, Acker-Hewitt et al. (2012) reported that combined pre-exercise caffeine and CHO ingestion enhanced pre-loaded (20 min 60% Wmax) 20-km cycling TT performance relative to a placebo, while caffeine alone and CHO alone did not. As neither substance enhanced performance independently, these data suggest that caffeine and CHO can synergistically enhance endurance performance. While it is worth noting that a similarly designed study (Slivka et al. 2008) did not report synergistic effects (i.e., null effects of caffeine/CHO vs. other treatments), subjects in this study also exercised while in negative energy balance (1.1 kg body mass loss over 2 days), which may have influenced metabolic responses in a way that negated potential performance improvements. In general, it seems clear that adding caffeine to CHO solutions is beneficial for performance. However, the magnitude and nature of the benefit is yet to be fully elucidated. This is particularly true in light of recent research examining genetic polymorphisms that influence caffeine metabolism, sex-based differences, the effects of time of day, and the degree to which caffeine habituation affects responses (see review: Pickering and Grgic 2019). More research is clearly warranted to determine the metabolic effects, performance outcomes, and individual characteristics that may predict responsiveness to combined caffeine and CHO.
Protein
Within the past 20 years, researchers have investigated the potential ergogenic effects of co-ingesting CHO and protein (CHO + PRO) during endurance exercise (> 90 min). The findings in this area have been mixed. Three early studies reported sizable improvements (13–37%) in cycling TTE with CHO + PRO versus CHO beverages (Ivy et al. 2003; Saunders et al. 2004, 2007), while others have observed no augmented performance with CHO + PRO (van Essen and Gibala 2006; Osterberg et al. 2008; Breen et al. 2010). The specific reasons for these varied findings are not clear, but are likely related to different performance protocols and nutrient ingestion rates between studies. Studies reporting the largest performance gains with CHO + PRO (Ivy et al. 2003; Saunders et al. 2004, 2007) all utilized TTE protocols, which tend to magnify performance variability between trials, in comparison to protocols using timed-trials over a fixed distance (or amount of work) (Hinckson and Hopkins 2005). In addition, the aforementioned studies compared treatment beverages which were matched for CHO content, and consumed at ingestion rates of 37–47 g·h−1 (i.e., below maximal exogenous oxidation rates for CHO, as discussed previously). Thus, the CHO + PRO beverages contained additional calories from protein.
Other studies have compared beverages in which some CHO calories were replaced with protein in the CHO + PRO beverage, to create an isocaloric comparison (Romano-Ely et al. 2006; Gui et al. 2017), or utilized CHO + PRO treatments that included fewer total calories than the CHO treatment (Ferguson-Stegall et al. 2010; Martinez-Lagunas et al. 2010). In these studies, no overall treatment differences were reported for TTE (Romano-Ely et al. 2006; Ferguson-Stegall et al. 2010; Martinez-Lagunas et al. 2010) or time-trial performance (Gui et al. 2017). Because this group of studies compared beverages consumed at sub-maximal CHO ingestion rates, it could be argued that the protein provided in the CHO + PRO beverages provided an ergogenic effect that effectively replaced the potential benefits of the substituted CHO calories. However, this hypothesis is speculative, and it is not possible to ascertain from these studies if protein provided any calorically-independent ergogenic effects. To address this issue, a number of studies have now examined the effects of protein added to CHO beverages consumed at rates ≥ 60 g·h−1. At these ingestion rates, most studies have reported no beneficial effects of CHO + PRO versus CHO on time-trial performance (van Essen and Gibala 2006; Osterberg et al. 2008; Breen et al. 2010; Oosthuyse and Millen 2016) or cycling TTE (Valentine et al. 2008); though one study reported a 3% improvement in late-exercise performance with CHO + PRO (Saunders et al. 2009).
The findings above may lead some to speculate that the enhanced TTE observed in some studies may be simply related to additional calories provided from protein. Amino acids derived from dietary protein are utilized in a variety of biochemical processes during exercise, including oxidation for ATP resynthesis, conversion to Kreb’s Cycle intermediates, and substrate for gluconeogenesis (Dohm 1986). However, there is no compelling evidence that protein supplementation (without CHO co-ingestion) is ergogenic, particularly when considered within the context of prior CHO + PRO studies. A few studies have reported that branched-chain amino acid (BCAA) supplementation may extend endurance by attenuating central fatigue (Blomstrand et al. 1991; Mittleman et al. 1998). However, most studies have reported no influence of BCAA supplementation on endurance performance (Van Hall et al. 1996; Cheuvront et al. 2004; Watson et al. 2004), and it is unlikely that the aforementioned CHO + PRO studies contained sufficient BCAA to influence central fatigue, since those reporting the largest ergogenic effects provided ≤ 12 g·h−1 whey protein (containing ~ 3 g·h−1 BCAA) (Ivy et al. 2003; Saunders et al. 2004, 2007). Similarly, the amino acid alanine has been investigated for ergogenic properties, due to its potential to be oxidized as a skeletal muscle fuel or utilized as a gluconeogenic precursor. Despite observations that significant amounts of exogenous alanine are decarboxylated during prolonged exercise (Korach-André et al. 2002), alanine supplementation has been reported to have no effects on performance (Klein et al. 2009; Schroer et al. 2014). Furthermore, in perhaps the only study directly investigating the ergogenic effects of protein consumed at intake rates similar to those of CHO, Schroer et al. (2014) reported that ingestion of 45 g·h−1 of whey protein hydrolysate had no effect on endurance cycling performance compared to a placebo. Based on these studies, it seems unlikely that the purported ergogenic effects of CHO + PRO are due to protein ingestion per se. This suggests that these effects may be due to synergistic effects between protein and CHO. For example, post-exercise consumption of CHO + PRO has been reported to elicit higher insulin levels than CHO alone, which has been associated with augmented muscle glycogen resynthesis in some feeding protocols (Alghannam et al. 2018), and could hypothetically influence bioenergetic pathways during exercise. However, insulin levels do not appear to be significantly altered by CHO + PRO ingestion during exercise (versus CHO alone), at least at doses pertinent to existing studies (Ivy et al. 2003). Similarly, Betts et al. (2008) observed that post-exercise CHO + PRO ingestion resulted in increased CHO oxidation during subsequent exercise, compared to a CHO-matched beverage without protein. However, Rowlands and Wadsworth (2012) reported that CHO + PRO coingestion during exercise did not elevate exogenous (or total) CHO oxidation versus CHO alone. Thus, the potential synergistic effects of CHO + PRO ingestion, and their effects on endurance performance, remain unclear and warrant further investigation.
Based on the evidence above, the prevailing view is that CHO + PRO ingestion does not augment endurance time-trial performance beyond levels elicited when optimal levels of CHO are consumed (Van Loon 2014). However, evidence that protein added to sub-optimal CHO doses may extend endurance (and that performance is preserved when some CHO calories are replaced with protein) has some potentially useful applications for athletes, when considered alongside evidence that protein ingestion may improve post-exercise recovery. The effects of CHO + PRO on recovery in endurance athletes have been reviewed elsewhere (Saunders 2011; Alghannam et al. 2018). Briefly, prior studies have reported that post-exercise CHO + PRO ingestion improved markers of post-exercise recovery, including muscle glycogen replenishment, protein turnover, plasma markers of muscle disruption (i.e., CK, myoglobin), and muscle soreness (Saunders 2011). Importantly, CHO + PRO ingestion following heavy endurance exercise has also been reported to enhance subsequent exercise performance by some studies (Berardi et al. 2008; Ferguson-Stegall et al. 2011; Alghannam 2011; Lunn et al. 2012), though this finding is not unanimous (Betts et al. 2005; Berardi et al. 2006; Romano-Ely et al. 2006). Furthermore, a few studies have reported that CHO + PRO ingestion during exercise positively influenced markers of post-exercise recovery (Valentine et al. 2008; Hall et al. 2013; Saunders et al. 2018). Therefore, it is tempting to speculate that CHO + Pro ingestion during exercise may provide some positive recovery outcomes for endurance athletes during periods of heavy training/competition. However, at least two studies have investigated the effects of protein supplementation during exercise in endurance cyclists performing multiple days of intensified training (Hansen et al. 2016; D’Lugos et al. 2016), and neither reported significant differences in performance versus CHO treatments. Future study in this area is warranted to determine the mechanisms by which protein affects recovery/performance in endurance athletes, and how the influences of varied exercise demands and protein doses may alter the potential efficacy of CHO + PRO.
Medium-chain triglycerides and ketone salts/esters
Similar to combining CHO supplementation with protein, a number of studies have examined the effects of co-ingesting CHO with lipids/lipid species. There is some compelling evidence that exogenous fat could serve as an additional substrate during exercise. Specifically, early studies examining the effects of pre-/during-exercise fat ingestion/infusion combined with heparin infusion reported elevations in blood FFA concentrations and fat oxidation, attenuated muscle glycogen utilization, and enhanced endurance (Costill et al. 1977; Ravussin et al. 1986; Hargreaves et al. 1991; Dyck et al. 1993, 1996; Vukovich et al. 1993; Romijn et al. 1995; Odland et al. 1998, 2000; Pitsiladis et al. 1999; Hawley et al. 2000; Jacobson et al. 2001). However, attempts to replicate these findings via ingestion of fat alone mostly failed to meaningfully alter metabolism (i.e., in a way that impacted endogenous CHO utilization and/or performance) (Okano et al. 1996, 1998; Whitley et al. 1998; Rowlands and Hopkins 2002a; Paul et al. 2003), with one exception (Murakami et al. 2012). This is likely because fat, particularly long-chain FFA (> 12 carbon atoms), slows gastric emptying and FFA absorption likely to a degree that limits availability for exercising muscle (Satabin et al. 1987; Cunningham and Read 1989; Houghton et al. 1990). Importantly, co-ingesting fat with CHO is not likely to improve the availability and/or utilization of either substrate. Most research indicates that fat slows glucose absorption, which would presumably attenuate exogenous CHO oxidation and/or glycogen synthesis (Welch et al. 1987; Cunningham and Read 1989; Houghton et al. 1990). Moreover, CHO ingestion-induced insulin elevations reduce fat oxidation likely due to increased acetylcarnitine formation stemming from enhanced glycolytic flux, which reduces the availability of free carnitine for carnitine transferase 1 transport of FFA into the mitochondria (Jeppesen and Kiens 2012). Of interest, certain lipid moieties like medium-chain triglycerides and/or ketone salts/esters are more rapidly digested and absorbed than long-chain FFA. This both potentiates more meaningful metabolic effects and increases the possibility of additive or synergistic effects when co-ingested with CHO. Nevertheless, most research in this area does not suggest ergogenic effects.
Medium-chain triglycerides (6–12 carbon atoms) have intriguing characteristics that suggest possible benefits when combined with CHO. In contrast to long-chain triglycerides (which are transported in chylomicrons via the lymphatic system), medium-chain triglycerides are rapidly emptied from the stomach and are absorbed directly into the blood leading to comparable oxidation rates to glucose during exercise (Massicotte et al. 1992; Jeukendrup and Aldred 2004). Moreover, medium-chain triglycerides can be transported into the mitochondria independently of carnitine (Jong-Yeon et al. 2002). Taken together with the fact that combining medium-chain triglycerides with CHO both enhances gastric emptying over CHO alone and increases medium-chain triglyceride oxidation over medium-chain triglyceride alone (Beckers et al. 1992; Jeukendrup et al. 1995), it is no surprise that many researchers hypothesized synergistic or additive ergogenic effects when these substrates were consumed together. However, almost all subsequent research [except for one study (Van Zyl et al. 1996)] revealed that combining medium-chain triglycerides with CHO has little effect on metabolism (e.g., muscle glycogen utilization) or endurance performance relative to CHO alone regardless of the amount consumed, timing, or endogenous CHO availability (see reviews: Hawley 2002; Jeukendrup and Aldred 2004). Moreover, many studies have reported substantially increased rates of gastrointestinal distress with medium-chain triglyceride ingestion often in combination with impaired performance (Jeukendrup et al. 1998; Goedecke et al. 1999, 2005; Thorburn et al. 2006, 2007). Importantly, chronic intake of medium-chain triglycerides has been reported to acutely enhance fat oxidation, reduce endogenous CHO, and improve gastrointestinal tolerance (Thorburn et al. 2006). However, these adaptations are also associated with impaired performance, which were likely related to gastrointestinal symptoms, despite their being slightly attenuated versus acute supplementation (Thorburn et al. 2006). Collectively, there is very little evidence to support the use of combined medium-chain triglycerides and CHO before or during exercise.
Ketone salts/esters also have characteristics that would seemingly potentiate synergistic or additive effects when combined with CHO. As recently reviewed by Margolis and O’Fallon (2020), exogenous ketones can induce ketosis [> 0.5 mM β hydroxybutyrate (βHB)] and thereby potentially provide an alternative fuel source during exercise. Moreover, ketones may be a more efficient substrate than CHO based on higher in vitro energy production per mole and free energy release from ketone-derived ATP (Sato et al. 1995; Veech 2004). Nevertheless, it is well understood that ketones are not oxidized at high rates by skeletal muscle with naturally occurring ketosis (i.e., with high-fat diets or starvation) (Hagenfeldt and Wahren 1971; Fery and Balasse 1983; Phinney et al. 1983). This calls into question the metabolic utility of exogenous ketones. However, the combination of supplemental ketones and exogenous/endogenous-sufficient CHO (i.e., non-starved and/or glycogen-depleted) represents a previously impossible physiological state in which maintained glycolytic and tri-carboxylic acid cycle flux likely sustains the production of intermediates that are required for ketone body oxidation (Russell and Taegtmeyer 1991). If true, consuming ketones in combination with CHO may synergistically enhance ketone utilization, thereby sparing endogenous CHO. Importantly, there is some evidence to support this hypothesis.
Cox et al. (2016) observed high rates of ketone utilization (16–18% of oxygen consumed) during exercise in glycogen replete (non-starved) subjects. Additionally, the authors observed glycogen-sparing and enhanced cycling TT performance with during-exercise ingestion of a mixed ketone monoester and CHO solution relative to CHO alone (Cox et al. 2016). While intriguing, no subsequent studies have replicated these findings, and some have reported impaired performance and severe gastrointestinal distress (O’Malley et al. 2017; Rodger et al. 2017; Leckey et al. 2017; Waldman et al. 2018; Evans and Egan 2018; Shaw et al. 2019; Scott et al. 2019a; Evans et al. 2019; Prins et al. 2020). As speculated by others (Margolis and O’Fallon 2020), the conflicting data may be the result of the degree of ketosis achieved (i.e., peak levels of βHB), which may have been impacted by the ketone supplement type and/or dietary controls (i.e., fasted vs. post-prandial). The majority of studies reporting null/impaired performance reported βHB levels below 2.0 mM (O’Malley et al. 2017; Rodger et al. 2017; Leckey et al. 2017; Waldman et al. 2018; Shaw et al. 2019; Scott et al. 2019a; Evans et al. 2019; Prins et al. 2020). Additionally, an explanation for unchanged/impaired performance in most studies may be explained by reductions in pH induced by ketone ingestion (Dearlove et al. 2019). Poffé et al. (2020) recently reported enhanced cycling TT performance with ingestion of ketone monoester combined with sodium bicarbonate and CHO versus a control solution. Importantly, neither sodium bicarbonate/CHO nor ketone monoester/CHO enhanced performance suggesting a synergistic effect with ketone monoester/bicarbonate/CHO possibly owing to better maintenance of blood pH (Poffé et al. 2020). However, it should be noted that this study did not include a CHO-only trial. Thus, it is impossible to determine whether ketone monoester/bicarbonate/CHO would have enhanced performance relative to CHO alone. Finally, it is possible that ketone/salts and CHO are actually metabolically antagonistic, such that CHO oxidation inhibits ketone oxidation, thereby minimizing any potential effect. Petrick et al. (2020) recently investigated the mitochondrial respiration of ketones (βHB and lithium acetoacetate) and reported that ketone oxidation was minimized in the presence of pyruvate likely owing to product inhibition. Moreover, the authors noted that ketone oxidation, even at supra-physiological intramuscular concentrations (~ 10 mM), represented a minor proportion of mitochondrial respiration (2–10%) compared to maximal pyruvate respiration. The investigators were unable to detect ketone respiration at biologic ketone concentrations. It is worth noting that this was an in vitro study, which does not account for the numerous interactive factors that influence metabolism in vivo. However, when considered together with the studies conducted on trained subjects, the evidence strongly suggests that combining ketone salts/esters with CHO is not ergogenic. Nevertheless, the findings of Cox et al. (2016), Poffé et al. (2020), and the noted variability between studies warrant further investigation to determine under what, if any, conditions exogenous ketones can impact metabolism and performance.
Dietary nitrate
Dietary nitrate also represents an interesting potential candidate to combine with supplemental CHO. The ergogenic effects of dietary nitrate have been reported in numerous studies (see reviews and meta-analyses: Hoon et al. 2013; Van De Walle and Vukovich 2018; Senefeld et al. 2020). Moreover, dietary nitrate appears to exert effects via mechanisms distinct from carbohydrate. Specifically, most studies suggest that it reduces the oxygen cost of exercise by enhancing the efficiency of skeletal muscle contraction (i.e., reduced phosphocreatine utilization) and/or mitochondrial oxidative phosphorylation (i.e., more ATP resynthesized per oxygen consumed; see reviews: Jones 2014; Jones et al. 2018). As a result, the intensity of exercise at a given absolute work output is reduced, thereby increasing exercise tolerance or the highest sustainable pace in a TT (Senefeld et al. 2020). While it is worth noting that there is significant variability across studies, and that the efficacy of dietary nitrate seems dependent on a number of factors including supplementation duration, dose, training status, etc. (Jones et al. 2018), the available evidence is strongly suggestive of potential additive ergogenic effects when combined with CHO. However, to our knowledge, no studies have investigated this question. It is interesting to note that a number of studies have combined dietary nitrate with caffeine. Two studies have reported no effects of dietary nitrate (i.e., beetroot juice) on the ergogenic effects of caffeine (Lane et al. 2014; Glaister et al. 2015). One study reported non-significant additive effects with pre-exercise ingestion of caffeine and beetroot juice (Handzlik and Gleeson 2013). Collectively, this, at least, provides some proof of concept that the effects of dietary nitrate may not interfere with or supersede those of other ergogenic substances. Thus, future studies should investigate this hypothesis in the context of combined CHO and dietary nitrate.
Some evidence also indicates the potential for synergistic effects from combining dietary nitrate with CHO. Studies in animals (Li et al. 2016; Hezel et al. 2016; Gheibi et al. 2017) and obese men (Beals et al. 2017) have reported enhanced glucose disposal and insulin sensitivity with dietary nitrate supplementation combined with a glucose load. Moreover, some have reported exercise responses that indicate potentially enhanced delivery of CHO during- or post-exercise. Specifically, several studies in animals and humans have reported enhanced blood flow to exercising skeletal muscle (Richards et al. 2018), Type II muscle fibers (Ferguson et al. 2013), and the gastrointestinal tract (Petersson et al. 2007). Moreover, some evidence suggests enhanced blood glucose uptake and relative carbohydrate oxidation during exercise (Holloszy and Narahara 1967; Larsen et al. 2011; Roux‐Mallouf et al. 2019). While these findings are not universally supported (Trexler et al. 2019; Hughes et al. 2020), they at least warrant further investigation as the effects could conceivably enhance exogenous CHO oxidation during exercise or glycogen storage post-exercise. Surprisingly, only one study has examined the interaction between dietary nitrate and glucose/glycogen metabolism during exercise (Betteridge et al. 2016). In this study, trained cyclists consumed beetroot juice (8 mM nitrate) prior to prolonged exercise (60 min; 65% VO2max) during which glucose was infused to measure its metabolic fate. Importantly, beetroot juice ingestion had no impact on metabolism including blood metabolites (i.e., blood glucose, lactate, FFA, or insulin) or fuel utilization (i.e., blood glucose uptake/oxidation, total carbohydrate oxidation, or glycogen utilization). Nevertheless, the authors also reported no impact of beetroot juice on the oxygen cost of exercise, which contrasts with dozens of other studies in this area (Pawlak-Chaouch et al. 2016). As such, further study is warranted to determine if the enhanced exercise efficiency often observed with dietary nitrate is associated with potentially advantageous effects that may support synergistic enhancement of exogenous CHO metabolism or glycogen storage. In conclusion, no research to date has examined the effects of combining dietary nitrate with supplemental CHO despite the logic and ease of the pairing (e.g., both substances support exercise efficiency in seemingly complementary ways; logistical ease of combined supplementation [does not need to occur simultaneously within the same solution]) and intriguing mechanistic evidence for potential additive or synergistic effects.
Dietary manipulation of carbohydrate availability
Aside from CHO supplementation, no other research area within sport nutrition has received more focus and notoriety in recent decades than the dietary manipulation of CHO. In this area, a number of strategies have been evaluated to determine whether altering CHO intake can influence substrate utilization in ways that preserve endogenous CHO (e.g., by increasing fat utilization) and/or enhance metabolic flexibility (i.e., the capacity to switch between fuel sources during exercise depending on intensity). Recent examples include ketogenic/low-CHO diets, dietary periodization, and fat adaptation with CHO restoration. The central tenet of all these approaches is the selective withdrawal of CHO availability to stimulate metabolic adaptations that optimize fuel utilization patterns. The primary differentiating factor between the approaches is the degree and duration of CHO restriction. Ketogenic/low-CHO diets require long-term adherence (i.e., weeks to years) and chronic CHO restriction (< 20–50 g·day−1 CHO) (Burke 2015). Dietary periodization involves the selective exclusion of dietary and/or peri-exercise CHO throughout a training cycle based on the fuel needs of a given training session (Impey et al. 2018). Finally, fat adaptation is a short-term approach requiring 5–10 days adherence to a high-fat diet followed by 1–3 days of a high CHO diet with the aim of restoring muscle glycogen stores (Yeo et al. 2011).
In terms of ergogenic effects, dietary manipulation of CHO availability has clear metabolic effects that do not consistently translate into performance benefits. Research spanning the various dietary approaches consistently reports a shift towards increased fat utilization. Specifically, studies assessing ketogenic/low-CHO diets (see review: Burke 2015), CHO periodization (see review: Impey et al. 2018), and fat adaptation with CHO restoration (see review: Yeo et al. 2011) report enhanced fat oxidation, upregulation of enzyme activity (e.g., carnitine palmitoyl transferase 1, β-hydroxyacyl-CoA dehydrogenase, etc.), increased transcription of proteins involved in FFA uptake and oxidation (e.g., fatty acid translocase, fatty acid-binding protein, etc.), and reduced muscle glycogen and total CHO oxidation. Nevertheless, there is little evidence that these dietary approaches enhance endurance performance. Two studies have reported benefits with ketogenic diets (Phinney et al. 1983; McSwiney et al. 2018). However, enhanced performance in Phinney et al. (1983) seemed to be primarily the result of dramatic improvements in one subject while other subjects’ performance was unchanged/impaired; and the analytical approach and data interpretation from McSwiney et al. (2018) has been questioned (Pickering 2018). More recent work has reported impaired performance in competitive race-walkers on a ketogenic diet likely owing to reduced exercise efficiency (Burke et al. 2017, 2020). Minimal evidence also exists in support of ergogenic effects following CHO periodization or fat adaptation with CHO restoration. One study reported enhanced performance with CHO periodization (Marquet et al. 2016), but many recent well-controlled studies in highly trained athletes have failed to replicate this finding when compared to high CHO diets (Burke et al. 2017, 2020; Gejl et al. 2017; Riis et al. 2019). Additionally, one study has reported enhanced TT performance with fat adaptation and CHO restoration (Lambert et al. 2001), but the majority report no benefits (Carey et al. 2001; Burke et al. 2002; Rowlands and Hopkins 2002b), or even impaired performance (Havemann et al. 2006).
There are a number of potential explanations for the lack of an obvious performance benefit with these different approaches that have been reviewed or discussed elsewhere (Yeo et al. 2011; Burke 2015; Burke et al. 2020) and some of which will be discussed below. However, it seems most likely that a major part of the explanation lies with the potentially incorrect assumption that an increase in fat utilization is ergogenic. Despite popular conceptions, most endurance events (and thus exercise intensities in performance assessments employed in studies) are performed either wholly or partly at high intensities (> 75% VO2max), and the ability to reach and/or sustain these intensities typically determines race outcomes (Peronnet and Thibault 1989; Fernández-García et al. 2000). Exercise at these intensities is CHO-dependent (Romijn et al. 1993; Van Loon et al. 2001) and unlikely to be influenced by subtle changes in fat metabolism. As evidence, Leckey et al. (2016) reported no difference in TTE at half-marathon personal best pace (~ 78% VO2max) with/without CHO intake pre-/during-exercise and with/without nicotinic acid ingestion, which blunts lipolysis and fat oxidation. The authors reported exercise in all trials to be CHO-dependent (83–91% of energy expended) with no differences in substrate utilization between conditions regardless of nicotinic acid ingestion. A primary explanation for CHO dependence at high exercise intensities is that CHO requires less oxygen for ATP synthesis relative to fat making it the preferred fuel for exercise limited by oxygen availability (i.e., high-intensity exercise; Krogh and Lindhard 1920; Cole et al. 2014). With this in mind, diets that increase fat oxidation are likely to attenuate exercise efficiency thereby increasing the relative intensity for a given work output and reducing the highest sustainable pace in TT events/assessments, a finding observed and replicated recently by Burke et al. (2017,2020). Thus, there appears to be little support for the adoption of these diets based on equivocal performance responses, potentially detrimental metabolic adaptations, and the inherent challenges of adhering to diets that partially or completely restrict access to an entire macronutrient and can increase the perceived exertion of training (Helge et al. 1996; Stepto et al. 2002). This is particularly true when compared to traditional high CHO diets, which have robust support and popularity within the scientific and athletic communities, respectively (Burke 2001; Onywera et al. 2004; Vogt et al. 2005; Burke et al. 2011; Thomas et al. 2016; Helge 2017). Nevertheless, these dietary approaches remain highly popular, and it is still possible that metabolic adaptations derived from these diets can result in enhanced performance albeit in ways that are undetectable with typical study designs and sample sizes. Moreover, with any/all of these dietary approaches, many endurance athletes are still likely to utilize supplemental CHO during competition and/or high-intensity training sessions. Therefore, a consideration of how these diets and CHO supplementation may interact is warranted.
Interactions between dietary approaches and supplemental CHO are likely to be mediated by the degree of CHO restriction. Given a sufficient magnitude/duration of CHO restriction, exogenous CHO oxidation is likely to be impaired due to skeletal muscle adaptations that favor fat oxidation at the expense of glycolytic flux. Stellingwerff et al. (2006) reported attenuated pyruvate dehydrogenase activity in trained cyclists exercising at moderate (70% VO2peak) and sprint intensities (150% VO2peak) following 5 days of a high-fat/low-CHO diet (67% fat, 18% CHO) followed by 1 day of a high CHO diet (70% CHO) compared to a high CHO diet for all 6 days (70% CHO). Importantly, this reduced enzymatic flux occurred despite similar pre-exercise glycogen levels in both conditions, suggesting that CHO metabolism was impaired. While this adaptation is not surprising, considering that low glycogen levels stimulate pyruvate dehydrogenase kinase activity (Arkinstall et al. 2004), it is noteworthy that this effect occurred in such a short time frame, with a relatively moderate degree of CHO restriction (compared to ketogenic diets), and when glycogen levels were restored. With this in mind, it seems highly likely that similar or exacerbated reductions in glycolytic flux occur with ketogenic diets. In addition to reductions in glycolytic flux, exogenous CHO utilization may be altered by reported changes in the gut microbiome with adherence to CHO-restricted diets during heavy endurance training (Murtaza et al. 2019). While it is yet to be fully elucidated how these effects might influence CHO metabolism, it is possible that changes in gut bacteria may modulate intestinal absorption of CHO. This hypothesis is supported by a recent study reporting increased exogenous CHO oxidation in trained cyclists with 4 weeks of probiotic supplementation (Pugh et al. 2020). Along similar lines, 4 weeks of adherence to a high CHO diet (8.5 g·kg−1·day−1) increases exogenous CHO oxidation without influencing muscle GLUT4 concentrations suggesting non-muscular adaptations (e.g., the small intestine) augmenting CHO absorption. The influence of CHO-restricted diets on intestinal absorption has not been assessed, but it seems plausible that such diets would have the opposite effect. Collectively, it seems likely that the attenuated pyruvate dehydrogenase activity and possible reductions in CHO absorption induced by CHO-restricted diets are likely to reduce exogenous CHO oxidation and/or glycogen storage thereby attenuating the ergogenic effects of ingested CHO.
In theory, the selective supplementation of CHO characteristic of CHO periodization may counteract some of the possible maladaptations that likely occur with CHO-restricted diets, without compromising the presumed beneficial adaptations that upregulate fat utilization. For instance, two recent studies indicate that consuming CHO during exercise while in a low glycogen state does not attenuate the elevations in fat oxidation or signaling induced by low CHO availability (Margolis et al. 2019; Podlogar et al. 2020). Moreover, skeletal muscle signaling relating to upregulation of enzymes/proteins associated with fat metabolism seems to be maximized so long as pre-exercise glycogen levels are < 300 mmol·kg−1·dry weight−1, suggesting that any possible endogenous CHO sparing induced by during-exercise CHO supplementation is unlikely to compromise planned next day/workout "train low" sessions (Hearris et al. 2019; Margolis et al. 2019). It is even conceivable that supplementing with CHO during or after “training low” may actually enhance the uptake, storage, and/or oxidation of exogenous CHO. Zderic et al. (2004) observed a hierarchy of substrate utilization in which blood glucose uptake and oxidation was enhanced by the blunting of muscle glycogenolysis (i.e., via high-fat diet) and fat oxidation [i.e., via β blockade (propranolol)]. This intriguing study indicates that fuel metabolism is dependent on substrate availability and that sufficient scarcity of endogenous fuel may enhance the utilization of exogenous CHO, which may conceivably stimulate the expression of glucose transport proteins (e.g., GLUT4) when imposed chronically (Zorzano et al. 2005). However, this is speculative and seems unlikely. It is more likely that supplemental CHO employed within a CHO periodization dietary model would primarily serve to maintain exogenous CHO oxidative and intestinal absorption capacity (Cox et al. 2010). Indeed, Margolis et al. (2019) recently found that, in the absence of a β blockade (i.e., the physiologic state), fuel demands when exercising in a low glycogen state (~ 215 g·kg−1·dry weight−1) were met by increasing fat oxidation. Blood glucose and/or exogenous CHO oxidation rates were not different between low and adequate glycogen conditions (~ 400 g·kg−1·dry weight−1), which lends support to the notion that such a strategy would primarily serve to maintain CHO oxidative capacity, not enhance it. Finally, studies utilizing twice per day training models, in which the second training session is completed in a presumably low glycogen state, indicate potentially beneficial interactions with supplemental CHO. Specifically, some evidence suggests this approach results in enhanced resting muscle glycogen storage relative to once per day training (Hansen et al. 2005; Yeo et al. 2008). Moreover, enhanced glycogen storage with this approach has also been associated with enhanced fatigue resistance in previously untrained subjects (Hansen et al. 2005); however, no performance benefits were reported in trained cyclists/triathletes (Yeo et al. 2008). Thus, more research is needed to confirm ergogenic effects. Taken together, “training low” may benefit CHO metabolism in some ways (i.e., enhancing post-exercise glycogen storage), but it is most likely that supplemental CHO is primarily beneficial as a means to maintain exogenous CHO oxidative capacity, reduce perceived exertion, and enhance training quality without compromising targeted adaptations (i.e., upregulation of fat oxidation).
While selective CHO supplementation may enhance outcomes with CHO periodization, many questions still remain in terms of the most effective coupling of dietary CHO and supplementation. Ultimately, more research is warranted to determine whether any particular nutrient timing paradigm can sufficiently stimulate metabolic adaptations in ways that enhance performance. Until then, it seems prudent for any endurance athlete considering these dietary approaches to carefully weigh the risks and rewards of adopting any diet that restricts CHO intake as doing so is likely to add further stress to already highly demanding sports.
Considerations for special populations
It is worth noting that the vast majority of research on CHO supplementation utilizes male subjects without any specific special needs. This has been typical of the field throughout its history, and a number of justifications have been put forward (e.g., logistical challenges in controlling for menstrual cycle, limited subject pool, etc.) for the almost exclusive use of this population. While it is generally assumed, and some research supports (e.g., Davis et al. 1997), the notion that ergogenic effects reported among healthy, male subjects translate roughly equally to other populations, there is a paucity of research to confirm this hypothesis.
Most research indicates that CHO supplementation is ergogenic in females, but it is yet to be determined how physiological differences may impact the magnitude of performance benefits relative to males. As noted above (i.e., in the section titled “Post-exercise modified carbohydrate”), females exhibit distinct metabolic characteristics that may influence the ergogenicity of supplemental CHO (see review: Devries 2016). For instance, females oxidize more fat during exercise than males (Tarnopolsky 2000), which may alter the contribution of exogenous CHO during exercise. However, available data are equivocal with one study reporting increased exogenous CHO oxidation (Riddell et al. 2003), and two studies reporting no differences between sexes (M’Kaouar et al. 2004; Wallis et al. 2006). Moreover, the impact of supplemental CHO on endogenous CHO utilization in females is yet to be fully elucidated as studies have reported reduced (Riddell et al. 2003), unchanged (Wallis et al. 2006), or increased oxidation rates (Tremblay et al. 2010). Whatever the case may be, it is possible that differences in CHO utilization influence the ergogenic effects of CHO. There is some evidence to support this hypothesis. However, data are surprisingly limited. Glace et al. (2019) recently compared the effects of CHO (sucrose) ingestion during exercise (2 h; 65% VO2peak) on cycling TT performance between males and females and found ergogenic effects in males only. However, others have reported enhanced performance across both sexes and of a similar magnitude (Davis et al. 1997). While more research is clearly needed, it is possible that females have unique nutritional needs. For instance, relative fat-dependence in females may predispose them to ergogenic effects from low CHO and/or high-fat diets, low glycemic index CHO, or SMC. Additionally, it is also possible females may experience larger or smaller magnitude improvements in performance with supplements likely to maximize exogenous CHO like MTC and/or HGEL. Females may also be less able to synthesize muscle glycogen due to consuming an insufficient amount of dietary CHO to maximize glycogen stores (Tarnopolsky et al. 2001). As such, perhaps females would benefit to a greater degree from post-exercise CHO + PRO or FMC, as these strategies may enhance glycogen synthesis when CHO intake is sub-optimal. In general, more research is required to refine nutritional recommendations for female athletes based on sex differences in the response to ergogenic aids.
Little research also exists as to the effectiveness of supplemental CHO in athletes with type 1 diabetes (T1D). Similar to females, T1D athletes have unique metabolic characteristics that may influence the effects of ingested CHO (see review: Riddell et al. 2020). Specifically, individuals with T1D cannot produce insulin endogenously and have an altered counter-regulatory hormone response to exercise (e.g., attenuated glucagon release) that makes blood glucose regulation challenging. Moreover, the use of exogenous insulin, combined with an inability to rapidly alter circulating insulin concentrations following injection, further complicates glucose regulation (Riddell et al. 2020). As a result, it is common for individuals with T1D to experience hypoglycemia during moderate-intensity exercise owing to relative hyperinsulinemia combined with insulin-independent skeletal muscle glucose uptake. It is also common for T1D athletes to experience hyperglycemia during high-intensity exercise (i.e., due to elevated counter-regulatory hormone stimulation of liver glycogenolysis) and/or post-exercise (i.e., due to an inability to release insulin in response to increasing glucose concentrations stemming from a cessation of skeletal muscle glucose uptake) (Scott et al. 2019b). Because of these factors and due to large inter-individual differences in terms of gluco-regulatory responses to exercise/nutrition, it is challenging to develop and assess appropriate CHO supplementation strategies for athletes with T1D. A number of recent reviews have considered this challenge and made recommendations based mostly on mechanistic studies, case reports, and data from healthy subjects (Gallen et al. 2011; Scott et al. 2019b).
Nevertheless, a small number of studies have evaluated TID responses to CHO supplementation and the impact of CHO type on metabolic and performance responses. These studies indicate that T1D athletes may have a delayed increase in exogenous CHO oxidation, reduced uptake and oxidation of liver-derived glucose, and increased muscle glycogenolysis relative to healthy controls (Riddell et al. 2000; Robitaille et al. 2007). As such, it is possible that CHO supplementation may be less effective in individuals with T1D making fat availability/oxidation all the more important. This combined with the overall challenge of maintaining stable glucose levels during-/post-exercise raises the possibility that T1D athletes may benefit from low CHO diets, a strategy recently considered by Scott and colleagues (Scott et al. 2019c). Additionally, T1D might also benefit from low glycemic index CHO in proximity to exercise. Indeed, limited evidence suggests that isomaltulose may aid in the maintenance of euglycemia during-/post-exercise (West et al. 2011; Bracken et al. 2012). Alternatively, some evidence suggests that TID athletes could also benefit from high glycemic index CHO. Specifically, during-exercise ingestion of MTC was associated with increased fat oxidation and reduced muscle glycogen utilization relative to glucose alone in T1D subjects (Bally et al. 2017). Moreover, pre-exercise ingestion of FMC has been associated with enhanced late-exercise running performance versus dextrose (Gray et al. 2016). Overall, while T1D athletes are likely to always need to follow some degree of trial-and-error approach, it is clear that more research is necessary to improve recommendations and evaluate the effectiveness of specific supplements and strategies.
Conclusions and future directions
The value of CHO as an exercise fuel has been appreciated for over 100 years (Zuntz and Loeb 1894). Nevertheless, research over the last several decades has greatly expanded our understanding of the mechanisms by which CHO supplementation enhances performance. Studies examining the impact of novel CHO supplements have been crucial in this regard. These studies strongly indicate the importance of intestinal absorption of CHO as a primary limiter of ergogenic effects. This conclusion is based on myriad MTC-based investigations consistently reporting high rates of exogenous CHO oxidation coupled with enhanced performance. Further support comes from studies on supplements designed to influence other mechanisms of CHO delivery such as gastric emptying (e.g., FMC and HGEL) or the insulinemic response (e.g., SMC). These studies often report these supplements to be successful at influencing their target mechanisms albeit in ways that rarely benefit performance, at least in practical scenarios. Despite the weight of evidence, questions remain as to the precise value (e.g., magnitude of performance benefits, contexts that may reveal ergogenicity, etc.) of these supplements and whether these data indicate a point of diminishing returns for CHO-induced ergogenic effects.
With this in mind, forward-thinking sport nutrition research should consider how other substances and/or dietary approaches may influence or augment the effectiveness of supplemental CHO. For example, research examining the effects of combining CHO with caffeine and/or protein is promising and suggests potential additive or synergistic effects. However, few studies thus far have been designed to elucidate how the substances interact, which would facilitate the development of optimal supplementation protocols. Other combinations (i.e., medium-chain triglycerides and ketone salts/esters) appear less promising, but variability in study designs and metabolic responses complicate interpretation and warrant replication and/or studies in different populations/contexts. A number of other ergogenic substances (e.g., dietary nitrate) are mostly unexamined in this context and present intriguing research opportunities. Finally, despite a wealth of research examining dietary approaches aimed at enhancing endogenous CHO availability (i.e., which have resulted in mostly equivocal effects on performance), little attention has been paid to how these approaches may influence the metabolism of supplemental CHO, and how this interaction may affect training adaptations and/or performance. In conclusion, numerous questions remain to support the next generation of carbohydrate research.
Future areas of inquiry include the following:
-
1.
Optimal ingestion rates and ratios for multiple transportable carbohydrates.
-
2.
A comparison of the dose responsiveness of glucose/maltodextrin versus glucose/maltodextrin + fructose and their potential overlap.
-
3.
The impact of high, low, or periodized carbohydrate diets on multiple transportable carbohydrates oxidation efficiency, training adaptations, and performance.
-
4.
The mechanism underlying increased fat oxidation with pre-exercise modified carbohydrate and/or low glycemic index carbohydrates.
-
5.
Practical scenarios in which pre-exercise modified carbohydrates and/or low glycemic index carbohydrates may be beneficial.
-
6.
Conditions under which post-exercise modified carbohydrates are beneficial for glycogen restoration and subsequent performance.
-
7.
The impact of dose concentration and frequency on gastrointestinal distress and performance outcomes with carbohydrate hydrogels.
-
8.
Special population responsiveness to different CHO supplements and strategies including women and type 1 diabetics. Recent studies investigating the ergogenicity of caffeine provide an excellent model for future research in this area (Clarke et al. 2019; Skinner et al. 2019).
-
9.
Further research examining the effects of combining carbohydrate with other ergogenic supplements including dietary nitrate.
-
10.
The role of the microbiome, genetic factors, muscle fiber type, etc. in mediating carbohydrate-based ergogenic effects.
-
11.
The use of novel or exemplary experimental designs to elucidate subtle differences or confirm the magnitude of ergogenic effects in larger samples and/or “real-world” situations. For example, replicated crossovers, multi-site studies, and field studies have been previously utilized to great effect (Smith et al. 2013; Rowlands and Houltham 2017; Goltz et al. 2018).
Abbreviations
- βHB:
-
Beta hydroxybutyrate
- CHO:
-
Carbohydrate
- CHO + PRO:
-
Combined carbohydrate and protein solutions
- FFA:
-
Free fatty acid
- FMC:
-
Fast-absorbing modified carbohydrate or starch
- GLUT5:
-
Intestinal fructose transporter
- HGEL:
-
Carbohydrate hydrogel solution
- IFABP:
-
Intestinal fatty acid-binding protein
- L:R:
-
Lactulose to rhamnose ratio
- MC:
-
Modified carbohydrate or starch
- MCT:
-
Medium-chain triglycerid
- MTC:
-
Multiple transportable carbohydrate
- RER:
-
Respiratory exchange ratio
- RM:
-
Repetition maximum
- SGLT1:
-
Sodium-dependent glucose transporter 1
- SMC:
-
Slow-absorbing modified carbohydrate or starch
- T1D:
-
Type 1 diabetes
- TTE:
-
Time to exhaustion
- TT:
-
Time trial
- VO2max :
-
Maximal oxygen consumption
- VO2peak :
-
Peak oxygen consumption
- W max :
-
Peak power output
References
Acheson KJ, Schutz Y, Bessard T, Anantharaman K, Flatt JP, Jéquier E (1988) Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr 48:240–247
Acker-Hewitt TL, Shafer BM, Saunders MJ, Goh Q, Luden ND (2012) Independent and combined effects of carbohydrate and caffeine ingestion on aerobic cycling performance in the fed state. Appl Physiol Nutr Metab 37:276–283
Adopo E, Péronnet F, Massicotte D, Brisson GR, Hillaire-Marcel C (1994) Respective oxidation of exogenous glucose and fructose given in the same drink during exercise. J Appl Physiol 76:1014–1019
Aguiar AS, Speck AE, Canas PM, Cunha RA (2020) Neuronal adenosine A2A receptors signal ergogenic effects of caffeine. Sci Rep 10:13414
Alghannam AF (2011) Carbohydrate-protein ingestion improves subsequent running capacity towards the end of a football-specific intermittent exercise. Appl Physiol Nutr Metab 36:748–757
Alghannam AF, Gonzalez JT, Betts JA (2018) Restoration of muscle glycogen and functional capacity: role of post-exercise carbohydrate and protein co-ingestion. Nutrients 10:253
Arkinstall MJ, Tunstall RJ, Cameron-Smith D, Hawley JA (2004) Regulation of metabolic genes in human skeletal muscle by short-term exercise and diet manipulation. Am J Physiol Endocrinol Metab 287:E25-31
Arner P, Kriegholm E, Engfeldt P, Bolinder J (1990) Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 85:893–898
Atkinson FS, Foster-Powell K, Brand-Miller JC (2008) International tables of glycemic index and glycemic load values: 2008. Diabetes Care 31:2
Aulin KP, Söderlund K, Hultman E (2000) Muscle glycogen resynthesis rate in humans after supplementation of drinks containing carbohydrates with low and high molecular masses. Eur J Appl Physiol 81:346–351
Bally L, Kempf P, Zueger T, Speck C, Pasi N, Ciller C, Feller K, Loher H, Rosset R, Wilhelm M, Boesch C, Buehler T, Dokumaci AS, Tappy L, Stettler C (2017) Metabolic effects of glucose-fructose co-ingestion compared to glucose alone during exercise in type 1 diabetes. Nutrients 9:164
Barber JFP, Thomas J, Narang B, Hengist A, Betts JA, Wallis GA, Gonzalez JT (2020) Pectin-alginate does not further enhance exogenous carbohydrate oxidation in running. Med Sci Sport Exerc 52:1376–1384
Baur DA, Schroer A, Luden N, Womack C, Smyth S, Saunders M (2014) Glucose-fructose enhances performance versus isocaloric, but not moderate, glucose. Med Sci Sport Exerc 46:1778–1786
Baur DA, Vargas FCS, Bach CW, Garvey JA, Ormsbee MJ (2016) Slow-absorbing modified starch before and during prolonged cycling increases fat oxidation and gastrointestinal distress without changing performance. Nutrients 8:E392
Baur DA, Willingham BD, Smith KA, Kisiolek JN, Morrissey MC, Saracino PG, Ragland TJ, Ormsbee MJ (2018) Adipose lipolysis unchanged by preexercise carbohydrate regardless of glycemic index. Med Sci Sports Exerc 50:827–836
Baur DA, Toney HR, Saunders MJ, Baur KG, Luden ND, Womack CJ (2019) Carbohydrate hydrogel beverage provides no additional cycling performance benefit versus carbohydrate alone. Eur J Appl Physiol 119:2599–2608
Beals JW, Binns SE, Davis JL, Giordano GR, Klochak AL, Paris HL, Schweder MM, Peltonen GL, Scalzo RL, Bell C (2017) Concurrent beet juice and carbohydrate ingestion: influence on glucose tolerance in obese and nonobese adults. J Nutr Metab 2017:6436783
Beaven CM, Maulder P, Pooley A, Kilduff L, Cook C (2013) Effects of caffeine and carbohydrate mouth rinses on repeated sprint performance. Appl Physiol Nutr Metab 38:633–637
Beckers E, Jeukendrup A, Brouns F, Wagenmakers A, Saris W (1992) Gastric emptying of carbohydrate—medium chain triglyceride suspensions at rest. Int J Sport Med 13:581–584
Beelen M, Van Kranenburg J, Senden JM, Kuipers H, Van Loon LJC (2012) Impact of caffeine and protein on postexercise muscle glycogen synthesis. Med Sci Sports Exerc 44:692–700
Bell DG, McLellan TM (2002) Exercise endurance 1, 3, and 6 h after caffeine ingestion in caffeine users and nonusers. J Appl Physiol 93:1227–1234
Bell DG, McLellan TM (2003) Effect of repeated caffeine ingestion on repeated exhaustive exercise endurance. Med Sci Sport Exerc 35:1348–1354
Berardi JM, Price TB, Noreen EE, Lemon PWR (2006) Postexercise muscle glycogen recovery enhanced with a carbohydrate-protein supplement. Med Sci Sport Exerc 38:1106–1113
Berardi JM, Noreen EE, Lemon PW (2008) Recovery from a cycling time trial is enhanced with carbohydrate-protein supplementation vs. isoenergetic carbohydrate supplementation. J Int Soc Sport Nutr 5:24
Bergstrom J, Hermansen L, Hultman E, Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71:140–150
Betteridge S, Bescós R, Martorell M, Pons A, Garnham AP, Stathis CC, McConell GK (2016) No effect of acute beetroot juice ingestion on oxygen consumption, glucose kinetics, or skeletal muscle metabolism during submaximal exercise in males. J Appl Physiol 120:391–398
Betts JA, Stevenson E, Williams C, Sheppard C, Grey E, Griffin J (2005) Recovery of endurance running capacity: effect of carbohydrate-protein mixtures. Int J Sport Nutr Exerc Metab 15:590–609
Betts JA, Williams C, Boobis L, Tsintzas K (2008) Increased carbohydrate oxidation after ingesting carbohydrate with added protein. Med Sci Sport Exerc 40:903–912
Blom PCS, Høstmark AT, Vaage O, Kardel KR, Mæhlum S (1987) Effect of different post-exercise sugar diets on the rate of muscle glycogen synthesis. Med Sci Sport Exerc 19:491–496
Blomstrand E, Hassmen P, Ekblom B, Newsholme EA (1991) Administration of branched-chain amino acids during sustained exercise–effects on performance and on plasma concentration of some amino acids. Eur J Appl Physiol Occup Physiol 63:83–88
Bracken RM, Page R, Gray B, Kilduff LP, West DJ, Stephens JW, Bain SC (2012) Isomaltulose improves glycemia and maintains run performance in type 1 diabetes. Med Sci Sport Exerc 44:800–808
Brand-Miller J, McMillan-Price J, Steinbeck K, Caterson I (2009) Dietary glycemic index: health implications. J Am Coll Nutr 28(Suppl):446S-449S
Breen L, Tipton KD, Jeukendrup AE (2010) No effect of carbohydrate-protein on cycling performance and indices of recovery. Med Sci Sport Exerc 42:1140–1148
Brener W, Hendrix TR, McHugh PR (1983) Regulation of the gastric emptying of glucose. Gastroenterology 85:76–82
Brynolf M, Sandstrom R, Stahl A (1995) Energy formulation. Pat No. EP0745096A1
Buléon A, Colonna P, Planchot V, Ball S (1998) Starch granules: structure and biosynthesis. Int J Biol Macromol 23:85–112
Burke LM (2001) Nutritional practices of male and female endurance cyclists. Sport Med 31:521–532
Burke LM (2008) Caffeine and sports performance. Appl Physiol Nutr Metab 33:1319–1334
Burke LM (2015) Re-Examining high-fat diets for sports performance: did we call the ‘nail in the coffin’ too soon? Sport Med 45:33–49
Burke LM, Collier GR, Hargreaves M (1993) Muscle glycogen storage after prolonged exercise: effect of the glycemic index of carbohydrate feedings. J Appl Physiol 75:1019–1023
Burke LM, Claassen A, Hawley JA, Noakes TD (1998) Carbohydrate intake during prolonged cycling minimizes effect of glycemic index of preexercise meal. J Appl Physiol 85:2220–2226
Burke LM, Hawley JA, Angus DJ, Cox GR, Clark SA, Cummings NK, Desbrow B, Hargreaves M (2002) Adaptations to short-term high-fat diet persist during exercise despite high carbohydrate availability. Med Sci Sport Exerc 34:83–91
Burke LM, Kiens B, Ivy JL (2004) Carbohydrates and fat for training and recovery. J Sports Sci 22:15–30
Burke LM, Hawley JA, Wong SHS, Jeukendrup AE (2011) Carbohydrates for training and competition. J Sports Sci 29(Supp 1):S17-27
Burke LM, Ross ML, Garvican-Lewis LA, Welvaert M, Heikura IA, Forbes SG, Mirtschin JG, Cato LE, Strobel N, Sharma AP, Hawley JA (2017) Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol 595:2785–2807
Burke LM, Hawley JA, Jeukendrup A, Morton JP, Stellingwerff T, Maughan RJ (2018) Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab 28:451–463
Burke LM, Sharma AP, Heikura IA, Forbes SF, Holloway M, McKay AKA, Bone JL, Leckey JJ, Welvaert M, Ross ML (2020) Crisis of confidence averted: Impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS ONE 15:e0234027
Cappelletti S, Daria P, Sani G, Aromatario M (2015) Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol 13:71–88
Carey AL, Staudacher HM, Cummings NK, Stepto NK, Nikolopoulos V, Burke LM, Hawley JA (2001) Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. J Appl Physiol 91:115–122
Carter JMM, Jeukendrup AEE, Mann CH, Jones DAA (2004) The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Med Sci Sport Exerc 36:2107–2111
Cermak NM, Van Loon LJC (2013) The use of carbohydrates during exercise as an ergogenic aid. Sport Med 43:1139–1155
Chambers ES, Bridge MW, Jones DA (2009) Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol 587:1779–1794
Chen YC, Edinburgh RM, Hengist A, Smith HA, Walhin JP, Betts JA, Thompson D, Gonzalez JT (2018) Venous blood provides lower glucagon-like peptide-1 concentrations than arterialized blood in the postprandial but not the fasted state: consequences of sampling methods. Exp Physiol 103:1200–1205
Cheuvront SN, Iii RC, Kolka MA, Lieberman HR, Kellogg MD, Sawka MN (2004) Branched-chain amino acid supplementation and human performance when hypohydrated in the heat downloaded from. J Appl Physiol 97:1275–1282
Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ (2010) Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol 30:5009–5020
Clarke ND, Kirwan NA, Richardson DL (2019) Coffee ingestion improves 5 km cycling performance in men and women by a similar magnitude. Nutrients 11:2575
Cole M, Coleman D, Hopker J, Wiles J (2014) Improved gross efficiency during long duration submaximal cycling following a short-term high carbohydrate diet. Int J Sport Med 35:265–269
Colinet I, Dulong V, Mocanu G, Picton L, Le Cerf D (2009) New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur J Pharm Biopharm 73:345–350
Conger SA, Warren GL, Hardy MA, Millard-Stafford ML (2011) Does caffeine added to carbohydrate provide additional ergogenic benefit for endurance? Int J Sport Nutr Exerc Metab 21:71–84
Cooper R, Naclerio F, Allgrove J, Larumbe-Zabala E (2014) Effects of a carbohydrate and caffeine gel on intermittent sprint performance in recreationally trained males. Eur J Sport Sci 14:353–361
Correia CE, Bhattacharya K, Lee PJ, Shuster JJ, Theriaque DW, Shankar MN, Smit GPA, Weinstein DA (2008) Use of modified corn starch therapy to extend fasting in glycogen storage disease types Ia and Ib. Am J Clin Nutr 88:1272–1276
Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D (1977) Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol 43:695–699
Costill DL, Dalsky GP, Fink WJ (1978) Effects of caffeine ingestion on metabolism and exercise performance. Med Sci Sport Exerc 10:155–158
Cox GR, Desbrow B, Montgomery PG, Anderson ME, Bruce CR, Macrides TA, Martin DT, Moquin A, Roberts A, Hawley JA, Burke LM (2002) Effect of different protocols of caffeine intake on metabolism and endurance performance. J Appl Physiol 93:990–999
Cox GR, Clark SA, Cox AJ, Halson SL, Hargreaves M, Hawley JA, Jeacocke N, Snow RJ, Yeo WK, Burke LM (2010) Daily training with high carbohydrate availability increases exogenous carbohydrate oxidation during endurance cycling. J Appl Physiol 109:126–134
Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K (2016) Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 24:256–268
Coyle EF, Coggan AR, Hemmert MK, Ivy JL (1986) Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol 61:165–172
Cunningham KM, Read NW (1989) The effect of incorporating fat into different components of a meal on gastric emptying and postprandial blood glucose and insulin responses. Br J Nutr 61:285–290
Cureton KJ, Warren GL, Millard-Stafford ML, Wingo JE, Trilk J, Buyckx M (2007) Caffeinated sports drink: ergogenic effects and possible mechanisms. Int J Sport Nutr Exerc Metab 17:35–55
Currell K, Jeukendrup AE (2008) Superior endurance performance with ingestion of multiple transportable carbohydrates. Med Sci Sport Exerc 40:275–281
D’Lugos AC, Luden ND, Faller JM, Akers JD, McKenzie AI, Saunders MJ (2016) Supplemental protein during heavy cycling training and recovery impacts skeletal muscle and heart rate responses but not performance. Nutrients 8:550
Dahlqvist A, Thomson DL (1963) The digestion and absorption of maltose and trehalose by the intact rat. Acta Physiol Scand 59:111–125
Davis J, Jackson D, Broadwell M, Queary JL, Lambert C (1997) Carbohydrate drinks delay fatigue during intermittent, high-intensity cycling in active men and women. Int J Sport Nutr 7:261–273
Davis JM, Zhao Z, Stock HS, Mehl KA, Buggy J, Hand GA (2003) Central nervous system effects of caffeine and adenosine on fatigue. Am J Physiol Regul Integr Comp Physiol 284:R399-404
De Bock K, Derave W, Ramaekers M, Richter EA, Hespel P (2007) Fiber type-specific muscle glycogen sparing due to carbohydrate intake before and during exercise. J Appl Physiol 102:183–188
de Oliveira EP, Burini RC, Jeukendrup A (2014) Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sport Med 44:79–85
Dearlove DJ, Faull OK, Rolls E, Clarke K, Cox PJ (2019) Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol 10:290
DeMarco HM, Sucher KP, Cisar CJ, Butterfield GE (1999) Pre-exercise carbohydrate meals: application of glycemic index. Med Sci Sport Exerc 31:164–170
Desbrow B, Barrett CM, Minahan CL, Grant GD, Leveritt MD (2009) Caffeine, cycling performance, and exogenous CHO oxidation: a dose-response study. Med Sci Sports Exerc 41:1744–1751
Desbrow B, Biddulph C, Devlin B, Grant GD, Anoopkumar-Dukie S, Leveritt MD (2012) The effects of different doses of caffeine on endurance cycling time trial performance. J Sports Sci 30:115–120
Devries MC (2016) Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol 101:243–249
Dohm G (1986) Protein as a fuel for endurance exercise. Exerc Sport Sci Rev 14:143–173
Douard V, Ferraris RP (2008) Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295:E227
Dudar MD, Bode ED, Fishkin KR, Brown RA, Carre MM, Mills NR, Ormsbee MJ, Ives SJ (2020) Pre-sleep low glycemic index modified starch does not improve next-morning fuel selection or running performance in male and female endurance athletes. Nutrients 12:2888
Dyck DJ, Putman CT, Heigenhauser GJ, Hultman E, Spriet LL (1993) Regulation of fat-carbohydrate interaction in skeletal muscle during intense aerobic cycling. Am J Physiol 265:E852–E859
Dyck DJ, Peters SJ, Wendling PS, Chesley A, Hultman E, Spriet LL (1996) Regulation of muscle glycogen phosphorylase activity during intense aerobic cycling with elevated FFA. Am J Physiol 270:E116–E125
Edinburgh RM, Hengist A, Smith HA, Betts JA, Thompson D, Walhin JP, Gonzalez JT (2017) Prior exercise alters the difference between arterialised and venous glycaemia: implications for blood sampling procedures. Br J Nutr 117:1414–1421
Enevoldsen LH, Simonsen L, Macdonald IA, Bülow J (2004) The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J Physiol 561:871–882
Evans M, Egan B (2018) Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc 50:2330–2338
Evans M, McSwiney FT, Brady AJ, Egan B (2019) No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sport Exerc 51:2506–2515
Febbraio M, Keenan J, Angus D, Campbell S, Garnham A (2000) Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: effect of the glycemic index. J Appl Physiol 89:1845–1851
Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC (2013) Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591:547–557
Ferguson-Stegall L, McCleave EL, Ding Z, Kammer LM, Wang B, Doerner PG, Liu Y, Ivy JL (2010) The effect of a low carbohydrate beverage with added protein on cycling endurance performance in trained athletes. J Strength Cond Res 24:2577–2586
Ferguson-Stegall L, McCleave EL, Ding Z, Doerner PGI, Wang B, Liao Y-H, Kammer L, Liu Y, Hwang J, Dessard BM, Ivy JL (2011) Postexercise carbohydrate-protein supplementation improves subsequent exercise performance and intracellular signalling for protein synthesis. J Strength Cond Res 25:1210–1224
Fernández-García B, Pérez-Landaluce J, Rodríguez-Alonso M, Terrados N (2000) Intensity of exercise during road race pro-cycling competition. Med Sci Sport Exerc 32:1002–1006
Fery F, Balasse EO (1983) Ketone body turnover during and after exercise in overnight-fasted and starved humans. Am J Physiol Endocrinol Metab 8:E318–E325
Flood TR, Montanari S, Wicks M, Blanchard J, Sharpe H, Taylor L, Kuennen MR, Lee BJ (2020) Addition of pectin-alginate to a carbohydrate beverage does not maintain gastrointestinal barrier function during exercise in hot-humid conditions better than carbohydrate ingestion alone. Appl Physiol Nutr Metab 45:1145–1155
Flynn S, Rosales A, Hailes W, Ruby B (2020) Males and females exhibit similar muscle glycogen recovery with varied recovery food sources. Eur J Appl Physiol 120:1131–1142
Fuchs CJ, Gonzalez JT, van Loon LJC (2019) Fructose co-ingestion to increase carbohydrate availability in athletes. J Physiol 597:3549–3560
Gallen IW, Hume C, Lumb A (2011) Fuelling the athlete with type 1 diabetes. Diabetes Obes Metab 13:130–136
Ganio MS, Klau JF, Casa DJ, Armstrong LE, Maresh CM (2009) Effect of caffeine on sport-specific endurance performance: a systematic review. J Strength Cond Res 23:315–324
Gejl KD, Thams LB, Hansen M, Rokkedal-Lausch T, Plomgaard P, Nybo L, Larsen FJ, Cardinale DA, Jensen K, Holmberg HC, Vissing K, Ørtenblad N (2017) No superior adaptations to carbohydrate periodization in elite endurance athletes. Med Sci Sport Exerc 49:2486–2497
Georg Jensen M, Kristensen M, Belza A, Knudsen JC, Astrup A (2012) Acute effect of alginate-based preload on satiety feelings, energy intake, and gastric emptying rate in healthy subjects. Obesity 20:1851–1858
Gheibi S, Bakhtiarzadeh F, Jeddi S, Farrokhfall K, Zardooz H, Ghasemi A (2017) Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric Oxide 64:39–51
Glace BW, Kremenic IJ, McHugh MP (2019) Effect of carbohydrate beverage ingestion on central versus peripheral fatigue: a placebo-controlled, randomized trial in cyclists. Appl Physiol Nutr Metab 44:139–147
Glaister M, Pattison JR, Muniz-Pumares D, Patterson SD, Foley P (2015) Effects of dietary nitrate, caffeine, and their combination on 20-km cycling time trial performance. J Strength Cond Res 29:165–174
Goddard MS, Young G, Marcus R (1984) The effect of amylose content on insulin and glucose responses to ingested rice. Am J Clin Nutr 39:388–392
Goedecke JH, Elmer-English R, Dennis SC, Schloss I, Noakes TD, Lambert EV (1999) Effects of medium-chain triacylglycerol ingested with carbohydrate on metabolism and exercise performance. Int J Sport Nutr 9:35–47
Goedecke JH, Clark VR, Noakes TD, Lambert EV (2005) The effects of medium-chain triacylglycerol and carbohydrate ingestion on ultra-endurance exercise performance. Int J Sport Nutr Exerc Metab 15:15–27
Goltz FR, Thackray AE, King JA, Dorling JL, Atkinson G, Stensel DJ (2018) Interindividual responses of appetite to acute exercise: a replicated crossover study. Med Sci Sports Exerc 50:758–768
Gonzalez JT, Fuchs CJ, Smith FE, Thelwall PE, Taylor R, Stevenson EJ, Trenell MI, Cermak NM, van Loon LJC (2015) Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance-type exercise in trained cyclists. Am J Physiol Metab 309:E1032–E1039
Gonzalez J, Fuchs C, Betts J, van Loon L (2017) Glucose plus fructose ingestion for post-exercise recovery—greater than the sum of its parts? Nutrients 9:344
Goodyear LJ, Hirshman MF, King PA, Horton ED, Thompson CM, Horton ES (1990) Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J Appl Physiol 68:193–198
Graham TE, Spriet LL (1991) Performance and metabolic responses to a high caffeine dose during prolonged exercise. J Appl Physiol 71:2292–2298
Graham TE, Spriet LL (1995) Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J Appl Physiol 78:867–874
Graham TE, Sathasivam P, Rowland M, Marko N, Greer F, Battram D (2001) Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Can J Physiol Pharmacol 79:559–565
Gray BJ, Page R, Turner D, West DJ, Campbell MD, Kilduff LP, Stephens JW, Bain SC, Bracken RM (2016) Improved end-stage high-intensity performance but similar glycemic responses after waxy barley starch ingestion compared to dextrose in type 1 diabetes. J Sport Med Phys Fit 56:1392–1400
Greer F, Hudson R, Ross R, Graham T (2001) Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes 50:2349–2354
Gui Z, Sun F, Si G, Chen Y (2017) Effect of protein and carbohydrate solutions on running performance and cognitive function in female recreational runners. PLoS ONE 12:e0185982
Guillochon M, Rowlands DS (2017) Solid, gel, and liquid carbohydrate format effects on gut comfort and performance. Int J Sport Nutr Exerc Metab 27:247–254
Hagenfeldt L, Wahren J (1971) Human forearm muscle metabolism during exercise VI. Substrate utilization in prolonged fasting. Scand J Clin Lab Invest 27:299–306
Hall AH, Leveritt MD, Ahuja KDK, Shing CM (2013) Coingestion of carbohydrate and protein during training reduces training stress and enhances subsequent exercise performance. Appl Physiol Nutr Metab 38:597–604
Handzlik MK, Gleeson M (2013) Likely additive ergogenic effects of combined preexercise dietary nitrate and caffeine ingestion in trained cyclists. ISRN Nutr 2013:1–8
Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK (2005) Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol 98:93–99
Hansen M, Bangsbo J, Jensen J, Krause-Jensen M, Bibby BM, Sollie O, Hall UA, Madsen K (2016) Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. J Int Soc Sport Nutr 13:9
Hargreaves M, Costill DL, Fink WJ (1987) Effect of pre-exercise carbohydrate feedings on endurance cycling performance. Med Sci Sport Exerc 19:33–36
Hargreaves M, Kiens B, Richter EA (1991) Effect of increased plasma free fatty acid concentration on muscle metabolism in exercising men. J Appl Physiol 70:194–201
Havemann L, West SJ, Goedecke JH, Macdonald IA, St Clair Gibson A, Noakes TD, Lambert EV (2006) Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. J Appl Physiol 100:194–202
Hawley JA (2002) Effect of increased fat availability on metabolism and exercise capacity. Med Sci Sport Exerc 34:1485–1491
Hawley JA, Bosch AN, Weltan SM, Dennis SC, Noakes TD (1994) Glucose kinetics during prolonged exercise in euglycaemic and hyperglycaemic subjects. Pflügers Arch Eur J Physiol 426:378–386
Hawley JA, Burke LM, Angus DJ, Fallon KE, Martin DT, Febbraio MA (2000) Effect of altering substrate availability on metabolism and performance during intense exercise. Br J Nutr 84:829–838
Hearris MA, Hammond KM, Seaborne RA, Stocks B, Shepherd SO, Philp A, Sharples AP, Morton JP, Louis JB (2019) Graded reductions in preexercise muscle glycogen impair exercise capacity but do not augment skeletal muscle cell signaling: Implications for CHO periodization. J Appl Physiol 126:1587–1597
Helge JW (2017) A high carbohydrate diet remains the evidence based choice for elite athletes to optimise performance. J Physiol 595:2775
Helge JW, Richter EA, Kiens B (1996) Interaction of training and diet on metabolism and endurance during exercise in man. J Physiol 492(Pt 1):293–306
Hezel M, Peleli M, Liu M, Zollbrecht C, Jensen BL, Checa A, Giulietti A, Wheelock CE, Lundberg JO, Weitzberg E, Carlström M (2016) Dietary nitrate improves age-related hypertension and metabolic abnormalities in rats via modulation of angiotensin II receptor signaling and inhibition of superoxide generation. Free Radic Biol Med 99:87–98
Higashiyama T (2002) Novel functions and applications of trehalose. Pure Appl Chem 74:1263–1269
Hill L, Bosch AN (2017) Mixed drink increased carbohydrate oxidation but not performance during a 40 km time trial. S Afr J Sport Med 28:79–84
Hinckson E, Hopkins W (2005) Reliability of time to exhaustion analyzed with critical-power and log-log modeling. Med Sci Sport Exerc 37:696–701
Hogervorst E, Bandelow S, Schmitt J, Jentjens R, Oliveira M, Allgrove J, Carter T, Gleeson M (2008) Caffeine improves physical and cognitive performance during exhaustive exercise. Med Sci Sports Exerc 40:1841–1851
Holloszy JO, Narahara HT (1967) Nitrate ions: Potentiation of increased permeability to sugar associated with muscle contraction. Science (80-) 155:573–575
Hoon MW, Johnson NA, Chapman PG, Burke LM (2013) The effect of nitrate supplementation on exercise performance in healthy individuals: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab 23:522–532
Houghton LA, Mangnall YF, Read NW (1990) Effect of incorporating fat into a liquid test meal on the relation between intragastric distribution and gastric emptying in human volunteers. Gut 31:1226–1229
Hughes WE, Kruse NT, Ueda K, Feider AJ, Hanada S, Bock JM, Casey DP (2020) Dietary nitrate does not acutely enhance skeletal muscle blood flow and vasodilation in the lower limbs of older adults during single-limb exercise. Eur J Appl Physiol 120:1357–1369
Hulston CJ, Jeukendrup AE (2008) Substrate metabolism and exercise performance with caffeine and carbohydrate intake. Med Sci Sports Exerc 40:2096–2104
Impey SG, Hearris MA, Hammond KM, Bartlett JD, Louis J, Close GL, Morton JP (2018) Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sport Med 48:1031–1048
Ivy J, Goforth H (2002) Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. J Appl Physiol 93:1337–1344
Ivy JL, Costill DL, Fink WJ, Lower RW (1978) Influence of caffeine and carbohydrate feedings on endurance performance. Med Sci Sport Exerc 11:6–11
Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF (1988) Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol 64:1480–1485
Ivy JL, Res PT, Sprague RC, Widzer MO (2003) Effect of a carbohydrate-protein supplement on endurance performance during exercise of varying intensity. Int J Sport Nutr Exerc Metab 13:382–395
Jacobson TL, Febbraio MA, Arkinstall MJ, Hawley JA (2001) Effect of caffeine co-ingested with carbohydrate or fat on metabolism and performance in endurance-trained men. Exp Physiol 86:137–144
Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV (1981) Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 34:362–366
Jentjens RLPG, Jeukendrup AE (2003) Effects of pre-exercise ingestion of trehalose, galactose and glucose on subsequent metabolism and cycling performance. Eur J Appl Physiol 88:459–465
Jentjens RLPG, Jeukendrup AE (2005) High rates of exogenous carbohydrate oxidation from a mixture of glucose and fructose ingested during prolonged cycling exercise. Br J Nutr 93:485–492
Jentjens RLPG, Underwood K, Achten J, Currell K, Mann CH, Jeukendrup AE (2006) Exogenous carbohydrate oxidation rates are elevated after combined ingestion of glucose and fructose during exercise in the heat. J Appl Physiol 100:807–816
Jeppesen J, Kiens B (2012) Regulation and limitations to fatty acid oxidation during exercise. J Physiol 590:1059–1068
Jeukendrup AE (2004) Carbohydrate intake during exercise and performance. Nutrition 20:669–677
Jeukendrup AE (2008) Carbohydrate feeding during exercise. Eur J Sport Sci 8:77–86
Jeukendrup AE (2010) Carbohydrate and exercise performance: the role of multiple transportable carbohydrates. Curr Opin Clin Nutr Metab Care 13:452–457
Jeukendrup AE (2017) Training the gut for athletes. Sport Med 47:101–110
Jeukendrup AE, Aldred S (2004) Fat supplementation, health, and endurance performance. Nutrition 20:678–688
Jeukendrup AE, Jentjens R (2000) Oxidation of carbohydrate feedings during prolonged exercise: current thoughts, guidelines and directions for future research. Sport Med 29:407–424
Jeukendrup AE, Moseley L (2010) Multiple transportable carbohydrates enhance gastric emptying and fluid delivery. Scand J Med Sci Sport 20:112–121
Jeukendrup AE, Saris WH, Schrauwen P, Brouns F, Wagenmakers AJ (1995) Metabolic availability of medium-chain triglycerides coingested with carbohydrates during prolonged exercise. J Appl Physiol 79:756–762
Jeukendrup AE, Thielen JJ, Wagenmakers AJ, Brouns F, Saris WH (1998) Effect of medium-chain triacylglycerol and carbohydrate ingestion during exercise on substrate utilization and subsequent cycling performance. Am J Clin Nutr 67:397–404
Jeukendrup AE, Wagenmakers AJ, Stegen JH, Gijsen AP, Brouns F, Saris WH (1999) Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Am J Physiol 276:E672–E683
Johannsen NM, Sharp RL (2007) Effect of preexercise ingestion of modified corn starch on substrate oxidation during endurance exercise. Int J Sport Nutr Exerc Metab 17:232–243
Johnson IT, Gee JM (1981) Effect of gel-forming gums on the intestinal unstirred layer and sugar transport in vitro. Gut 22:398–403
Jones AM (2014) Dietary nitrate supplementation and exercise performance. Sport Med 44(Suppl 1):S35-45
Jones AM, Thompson C, Wylie LJ, Vanhatalo A (2018) Dietary nitrate and physical performance. Ann Rev Nutr 38:303–328
Jong-Yeon K, Hickner RC, Dohm GL, Houmard JA (2002) Long- and medium-chain fatty acid oxidation is increased in exercise-trained human skeletal muscle. Metabolism 51:460–464
Jozsi AC, Trappe TA, Starling RD, Goodpaster B, Trappe SW, Fink WJ, Costill DL (1996) The influence of starch structure on glycogen resynthesis and subsequent cycling performance. Int J Sport Med 17:373–378
Karamanolis IA, Laparidis KS, Volaklis KA, Douda HT, Tokmakidis SP (2011) The effects of pre-exercise glycemic index food on running capacity. Int J Sport Med 32:666–671
Kendrick ZV, Steffen CA, Rumsey WL, Goldberg DI (1987) Effect of estradiol on tissue glycogen metabolism in exercised oophorectomized rats. J Appl Physiol 63:492–496
King AJ, O’Hara JP, Morrison DJ, Preston T, King RFGJ (2018) Carbohydrate dose influences liver and muscle glycogen oxidation and performance during prolonged exercise. Physiol Rep 6:e13555
King AJ, O’Hara JP, Arjomandkhah NC, Rowe J, Morrison DJ, Preston T, King RFGJ (2019) Liver and muscle glycogen oxidation and performance with dose variation of glucose–fructose ingestion during prolonged (3 h) exercise. Eur J Appl Physiol 119:1157–1169
Kirwan JP, Cyr-Campbell D, Campbell WW, Scheiber J, Evans WJ (2001) Effects of moderate and high glycemic index meals on metabolism and exercise performance. Metabolism 50:849–855
Klein J, Nyhan WL, Kern M (2009) The effects of alanine ingestion on metabolic responses to exercise in cyclists. Amino Acids 37:673–680
König D, Zdzieblik D, Holz A, Theis S, Gollhofer A (2016) Substrate utilization and cycling performance following PalatinoseTMingestion: a randomized, double-blind controlled trial. Nutrients 8:390
Korach-André M, Burelle Y, Péronnet F, Massicotte D, Lavoie C, Hillaire-Marcel C, Korach-Andre M, Peronnet F (2002) Differential metabolic fate of the carbon skeleton and amino-N of [13C]alanine and [15N]alanine ingested during prolonged exercise. J Appl Physiol 93:499–504
Kovacs EMR, Stegen JHCH, Brouns F (1998) Effect of caffeinated drinks on substrate metabolism, caffeine excretion, and performance. J Appl Physiol 85:709–715
Krogh A, Lindhard J (1920) The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem J 14:290–363
Lambert E, Goedecke J, Zyl C (2001) High-fat diet versus habitual diet prior to carbohydrate loading: effects on exercise metabolism and cycling performance. Int J Sport Nutr Exerc Metab 11:209–225
Lambert GP, Lang J, Bull A, Pfeifer PC, Eckerson J, Moore C, Lanspa S, O’Brien J (2008) Fluid restriction during running increases GI permeability. Int J Sport Med 29:194–198
Lane SC, Hawley JA, Desbrow B, Jones AM, Blackwell JR, Ross ML, Zemski AJ, Burke LM (2014) Single and combined effects of beetroot juice and caffeine supplementation on cycling time trial performance. Appl Physiol Nutr Metab 39:1050–1057
Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E (2011) Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13:149–159
Latulippe ME, Skoog SM (2011) Fructose malabsorption and intolerance: effects of fructose with and without simultaneous glucose ingestion. Crit Rev Food Sci Nutr 51:583–592
Leckey JJ, Burke LM, Morton JP, Hawley JA (2016) Altering fatty acid availability does not impair prolonged, continuous running to fatigue: evidence for carbohydrate dependence. J Appl Physiol 120:107–113
Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM (2017) Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol 8:806
Lecoultre V, Benoit R, Carrel G, Schutz Y, Millet GP, Tappy L, Schneiter P (2010) Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am J Clin Nutr 92:1071–1079
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Lee MJC, Hammond KM, Vasdev A, Poole KL, Impey SG, Close GL, Morton JP (2014) Self-selecting fluid intake while maintaining high carbohydrate availability does not impair half-marathon performance. Int J Sports Med 35:1216–1222
Lehmann U, Robin F (2007) Slowly digestible starch—its structure and health implications: a review. Trends Food Sci Technol 18:346–355
Leijssen DP, Saris WH, Jeukendrup AE, Wagenmakers AJ (1995) Oxidation of exogenous [13C]galactose and [13C]glucose during exercise. J Appl Physiol 79:720–725
Leiper J, Piehl Aulin K, Soderlund K (2000) Improved gastric emptying rate in humans of a unique glucose polymer with gel-forming properties. Scand J Gastroenterol 35:1143–1149
Li T, Lu X, Sun Y, Yang X (2016) Effects of spinach nitrate on insulin resistance, endothelial dysfunction markers and inflammation in mice with high-fat and high-fructose consumption. Food Nutr Res 60:32010
Lund S, Holman GD, Schmitz O, Pedersen O (1995) Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci USA 92:5817–5821
Lunn WR, Pasiakos SM, Colletto MR, Karfonta KE, Carbone JW, Anderson JM, Rodriguez NR (2012) Chocolate milk and endurance exercise recovery: protein balance, glycogen, and performance. Med Sci Sport Exerc 44:682–691
M’Kaouar H, Péronnet F, Massicotte D, Lavoie C (2004) Gender difference in the metabolic response to prolonged exercise with [13C]glucose ingestion. Eur J Appl Physiol 92:462–469
Maher AC, Akhtar M, Tarnopolsky MA (2010) Men supplemented with 17β-estradiol have increased β-oxidation capacity in skeletal muscle. Physiol Genom 42:342–347
Maljaars J, Peters HPF, Masclee AM (2007) Review article: the gastrointestinal tract: neuroendocrine regulation of satiety and food intake. Aliment Pharm Ther 26(Suppl 2):241–250
Marciani L, Lopez-Sanchez P, Pettersson S, Hoad C, Abrehart N, Ahnoff M, Ström A (2019) Alginate and HM-pectin in sports-drink give rise to intra-gastric gelation in vivo. Food Funct 10:7892–7899
Maresch CC, Petry SF, Theis S, Bosy-Westphal A, Linn T (2017) Low glycemic index prototype isomaltulose—update of clinical trials. Nutrients 9:381
Margolis LM, O’Fallon KS (2020) Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr 11:412–419
Margolis LM, Wilson MA, Whitney CC, Carrigan CT, Murphy NE, Hatch AM, Montain SJ, Pasiakos SM (2019) Exercising with low muscle glycogen content increases fat oxidation and decreases endogenous, but not exogenous carbohydrate oxidation. Metabolism 97:1–8
Marquet LA, Brisswalter J, Louis J, Tiollier E, Burke LM, Hawley JA, Hausswirth C (2016) Enhanced endurance performance by periodization of carbohydrate intake: “Sleep Low” strategy. Med Sci Sport Exerc 48:663–672
Martinez-Lagunas V, Ding Z, Bernard JR, Wang B, Ivy JL (2010) Added protein maintains efficacy of a low-carbohydrate sports drink. J Strength Cond Res 24:48–59
Massicotte D, Péronnet F, Brisson G, Boivin L, Hillaire-Marcel C (1990) Oxidation of exogenous carbohydrate during prolonged exercise in fed and fasted conditions. Int J Sport Med 11:253–258
Massicotte D, Peronnet F, Brisson GR, Hillaire-Marcel C (1992) Oxidation of exogenous medium-chain free fatty acids during prolonged exercise: comparison with glucose. J Appl Physiol 73:1334–1339
Maughan RJ, Fenn CE, Leiper JB (1989) Effects of fluid, electrolyte and substrate ingestion on endurance capacity. Eur J Appl Physiol Occup Physiol 58:481–486
McCubbin AJ, Zhu A, Gaskell SK, Costa RJS (2020) Hydrogel carbohydrate-electrolyte beverage does not improve glucose availability, substrate oxidation, gastrointestinal symptoms or exercise performance, compared with a concentration and nutrient-matched placebo. Int J Sport Nutr Exerc Metab 30:25–33
McGlory C, Morton JP (2010) The effects of postexercise consumption of high-molecular-weight versus low-molecular-weight carbohydrate solutions on subsequent high-intensity interval-running capacity. Int J Sport Nutr Exerc Metab 20:361–369
McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L (2018) Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 81:25–34
Mears SA, Worley J, Mason GS, Hulston CJ, James LJ (2020) Addition of sodium alginate and pectin to a carbohydrate-electrolyte solution does not influence substrate oxidation, gastrointestinal comfort or cycling performance. Appl Physiol Nutr Metab 45:675–678
Mittleman KD, Ricci MR, Bailey SP (1998) Branched-chain amino acids prolong exercise during heat stress in men and women. Med Sci Sport Exerc 30:83–91
Mock MG, Hirsch KR, Blue MNM, Trexler ET, Roelofs EJ, Smith-Ryan AE (2018) Post-exercise ingestion of low or high molecular weight glucose polymer solution does not improve cycle performance in female athletes. J Strength Cond Res. https://doi.org/10.1519/JSC.0000000000002560
Moore L, Szpalek HM, McNaughton LR (2013) Preexercise high and low glycemic index meals and cycling performance in untrained females: randomized, cross-over trial of efficacy. Res Sport Med 21:24–36
Murakami I, Sakuragi T, Uemura H, Menda H, Shindo M, Tanaka H (2012) Significant effect of a pre-exercise high-fat meal after a 3-day high-carbohydrate diet on endurance performance. Nutrients 4:625–637
Murtaza N, Burke LM, Vlahovich N, Charlesson B, O’Neill H, Ross ML, Campbell KL, Krause L, Morrison M (2019) The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients 11:261
Newell M, Wallis G, Hunter A, Tipton K, Galloway S, Newell ML, Wallis GA, Hunter AM, Tipton KD, Galloway SDR (2018) Metabolic responses to carbohydrate ingestion during exercise: associations between carbohydrate dose and endurance performance. Nutrients 10:37
Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD (2004) Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588
O’Brien WJ, Stannard SR, Clarke JA, Rowlands DS (2013) Fructose-maltodextrin ratio governs exogenous and other cho oxidation and performance. Med Sci Sport Exerc 45:1814–1824
O’Dea K, Nestel PJ, Antonoff L (1980) Physical factors influencing postprandial glucose and insulin responses to starch. Am J Clin Nutr 33:760–765
O’Malley T, Myette-Cote E, Durrer C, Little JP (2017) Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab 42:1031–1035
Odland LM, Heigenhauser GJ, Wong D, Hollidge-Horvat MG, Spriet LL (1998) Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. Am J Physiol 274:R894–R902
Odland LM, Heigenhauser GJF, Spriet LL (2000) Effects of high fat provision on muscle PDH activation and malonyl-CoA content in moderate exercise. J Appl Physiol 89:2352–2358
Okano G, Sato Y, Takumi Y, Sugawara M (1996) Effect of 4 h preexercise high carbohydrate and high fat meal ingestion on endurance performance and metabolism. Int J Sport Med 17:530–534
Okano G, Sato Y, Murata Y (1998) Effect of elevated blood FFA levels on endurance performance after a single fat meal ingestion. Med Sci Sport Exerc 30:763–768
Oliver JM, Almada AL, Van Eck LE, Shah M, Mitchell JB, Jones MT, Jagim AR, Rowlands DS (2016) Ingestion of high molecular weight carbohydrate enhances subsequent repeated maximal power: a randomized controlled trial. PLoS ONE 11:e0163009
Onywera VO, Kiplamai FK, Tuitoek PJ, Boit MK, Pitsiladis YP (2004) Food and macronutrient intake of elite Kenyan distance runners. Int J Sport Nutr Exerc Metab 14:709–719
Oosthuyse T, Millen AME (2016) Comparison of energy supplements during prolonged exercise for maintenance of cardiac function: carbohydrate only versus carbohydrate plus whey or casein hydrolysate. Appl Physiol Nutr Metab 41:674–683
Oosthuyse T, Carstens M, Millen AM (2015) Ingesting isomaltulose versus fructose-maltodextrin during prolonged moderate-heavy exercise increases fat oxidation but impairs gastrointestinal comfort and cycling performance. Int J Sport Nutr Exerc Metab 25:427–438
Ormsbee MJ, Bach CW, Baur DA (2014) Pre-exercise nutrition: the role of macronutrients, modified starches and supplements on metabolism and endurance performance. Nutrients 6:1782–1808
Osterberg KL, Zachwieja JJ, Smith JW (2008) Carbohydrate and carbohydrate + protein for cycling time-trial performance. J Sport Sci 26:227–233
Parks RB, Angus HF, King DS, Sharp RL (2018) Effect of preexercise ingestion of modified amylomaize starch on glycemic response while cycling. Int J Sport Nutr Exerc Metab 28:82–89
Paul D, Jacobs KA, Geor RJ, Hinchcliff KW (2003) No effect of pre-exercise meal on substrate metabolism and time trial performance during intense endurance exercise. Int J Sport Nutr Exerc Metab 13:489–503
Pawlak-Chaouch M, Boissière J, Gamelin FX, Cuvelier G, Berthoin S, Aucouturier J (2016) Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: a systematic review and a meta-analysis. Nitric Oxide 53:65–76
Pedersen DJ, Lessard SJ, Coffey VG, Churchley EG, Wootton AM, Ng T, Watt MJ, Hawley JA (2008) High rates of muscle glycogen resynthesis after exhaustive exercise when carbohydrate is coingested with caffeine. J Appl Physiol 105:7–13
Peronnet F, Thibault G (1989) Mathematical analysis of running performance and world running records. J Appl Physiol 67:453–465
Petersson J, Phillipson M, Jansson EÅ, Patzak A, Lundberg JO, Holm L (2007) Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am J Physiol Gastrointest Liver Physiol 292:G718–G724
Petrick HL, Brunetta HS, Pignanelli C, Nunes EA, van Loon LJC, Burr JF, Holloway GP (2020) In vitro ketone-supported mitochondrial respiration is minimal when other substrates are readily available in cardiac and skeletal muscle. J Physiol. https://doi.org/10.1113/JP280032
Pettersson S, Edin F, Bakkman L, McGawley K (2019) Effects of supplementing with an 18% carbohydrate-hydrogel drink versus a placebo during whole-body exercise in −5 °C with elite cross-country ski athletes: a crossover study. J Int Soc Sports Nutr 16:46
Pettersson S, Ahnoff M, Edin F, Lingström P, Simark Mattsson C, Andersson-Hall U (2020) A hydrogel drink with high fructose content generates higher exogenous carbohydrate oxidation and lower dental biofilm pH compared to two other, commercially available, carbohydrate sports drinks. Front Nutr 7:88
Pfeiffer B, Cotterill A, Grathwohl D, Stellingwerff T, Jeukendrup AE (2009) The effect of carbohydrate gels on gastrointestinal tolerance during a 16–km run. Int J Sport Nutr Exerc Metab 19:485–503
Pfeiffer B, Stellingwerff T, Zaltas E, Jeukendrup AE (2010) Oxidation of solid versus liquid CHO sources during exercise. Med Sci Sport Exerc 42:2030–2037
Pfeiffer B, Stellingwerff T, Hodgson AB, Randell R, Pottgen K, Res P, Jeukendrup AE (2012) Nutritional intake and gastrointestinal problems during competitive endurance events. Med Sci Sport Exerc 44:344–351
Phinney SD, Bistrian BR, Evans WJ, Gervino E, Blackburn GL (1983) The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32:769–776
Pickering C (2018) Letter to the editor. Metabolism 83:e1
Pickering C, Grgic J (2019) Caffeine and exercise: what next? Sport Med 49:1007–1030
Pitsiladis YP, Smith I, Maughan RJ (1999) Increased fat availability enhances the capacity of trained individuals to perform prolonged exercise. Med Sci Sport Exerc 31:1570–1579
Podlogar T, Free B, Wallis GA (2020) High rates of fat oxidation are maintained after the sleep low approach despite delayed carbohydrate feeding during exercise. Eur J Sport Sci 28:1–11. https://doi.org/10.1080/17461391.2020.1730447
Poffé C, Ramaekers M, Bogaerts S, Hespel P (2020) Bicarbonate unlocks the ergogenic action of ketone monoester intake in endurance exercise. Med Sci Sport Exerc. https://doi.org/10.1249/MSS.0000000000002467
Powley TL, Phillips RJ (2004) Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol Behav 82:69–74
Prins PJ, Koutnik AP, D’Agostino DP, Rogers CQ, Seibert JF, Breckenridge JA, Jackson DS, Ryan EJ, Buxton JD, Ault DL (2020) Effects of an exogenous ketone supplement on five-kilometer running performance. J Hum Kinet 72:115–127
Pugh JN, Wagenmakers AJM, Doran DA, Fleming SC, Fielding BA, Morton JP, Close GL (2020) Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: a randomized, double-blind, placebo-controlled crossover trial. Am J Physiol Endocrinol Metab 318:E504–E513
Ravussin E, Bogardus C, Scheidegger K, LaGrange B, Horton ED, Horton ES (1986) Effect of elevated FFA on carbohydrate and lipid oxidation during prolonged exercise in humans. J Appl Physiol 60:893–900
Rehrer N, Wagenmakers A, Beckers E, Halliday D, Leiper J, Brouns F, Maughan R, Westerterp K, Saris W (1992) Gastric emptying, absorption, and carbohydrate oxidation during prolonged exercise. J Appl Physiol 72:468–475
Richards JC, Racine ML, Hearon CM, Kunkel M, Luckasen GJ, Larson DG, Allen JD, Dinenno FA (2018) Acute ingestion of dietary nitrate increases muscle blood flow via local vasodilation during handgrip exercise in young adults. Physiol Rep 6:e13572
Richter EA, Garetto LP, Goodman MN, Ruderman NB (1982) Muscle glucose metabolism following exercise in the rat. Increased sensitivity to insulin. J Clin Invest 69:785–793
Riddell MC, Bar-Or O, Hollidge-Horvat M, Schwarcz HP, Heigenhauser GJF (2000) Glucose ingestion and substrate utilization during exercise in boys with IDDM. J Appl Physiol 88:1239–1246
Riddell M, Bar-Or O, Wilk B, Parolin M, Heigenhauser G (2001) Substrate utilization during exercise with glucose and glucose plus fructose ingestion in boys ages 10–14 yr. J Appl Physiol 90:903–911
Riddell MC, Partington SL, Stupka N, Armstrong D, Rennie C, Tarnopolsky MA (2003) Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance-trained athletes. Int J Sport Nutr Exerc Metab 13:407–421
Riddell MC, Scott SN, Fournier PA, Colberg SR, Gallen IW, Moser O, Stettler C, Yardley JE, Zaharieva DP, Adolfsson P, Bracken RM (2020) The competitive athlete with type 1 diabetes. Diabetologia 63:1475–1490
Riis S, Møller AB, Dollerup O, Høffner L, Jessen N, Madsen K (2019) Acute and sustained effects of a periodized carbohydrate intake using the sleep-low model in endurance-trained males. Scand J Med Sci Sport 29:1866–1880
Roberts MD, Lockwood C, Dalbo VJ, Volek J, Kerksick CM (2011) Ingestion of a high-molecular-weight hydrothermally modified waxy maize starch alters metabolic responses to prolonged exercise in trained cyclists. Nutrition 27:659–665
Roberts JD, Tarpey MD, Kass LS, Tarpey RJ, Roberts MG (2014) Assessing a commercially available sports drink on exogenous carbohydrate oxidation, fluid delivery and sustained exercise performance. J Int Soc Sports Nutr 11:8
Robitaille M, Dubé MC, Weisnagel SJ, Prud’homme D, Massicotte D, Péronnet F, Lavoie C (2007) Substrate source utilization during moderate intensity exercise with glucose ingestion in Type 1 diabetic patients. J Appl Physiol 103:119–124
Rodger S, Plews D, Laursen P, Driller M (2017) Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycl 6:26–31
Rodriguez NR, Di Marco NM, Langley S (2009) American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sport Exerc 41:709–731
Romano-Ely BC, Todd MK, Saunders MJ, Laurent TS (2006) Effect of an isocaloric carbohydrate-protein-antioxidant drink on cycling performance. Med Sci Sport Exerc 38:1608–1616
Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265:E380–E391
Romijn JA, Coyle EF, Sidossis LS, Zhang XJ, Wolfe RR (1995) Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J Appl Physiol 79:1939–1945
Rosset R, Egli L, Lecoultre V (2017) Glucose–fructose ingestion and exercise performance: the gastrointestinal tract and beyond. Eur J Sport Sci 17:874–884
Roux-Mallouf T, Laurent J, Besset D, Marillier M, Larribaut J, Belaidi E, Corne C, Doutreleau S, Verges S (2019) Effects of acute nitric oxide precursor intake on peripheral and central fatigue during knee extensions in healthy men. Exp Physiol 104:1100–1114
Rowell LB, Brengelmann GL, Blackmon JR, Twiss RD, Kusumi F (1968) Splanchnic blood flow and metabolism in heat-stressed man. J Appl Physiol 24:475–484
Rowlands DS, Clarke J (2011) Lower oxidation of a high molecular weight glucose polymer vs glucose during cycling. Appl Physiol Nutr Metab 306:298–306
Rowlands DS, Hopkins WG (2002a) Effect of high-fat, high-carbohydrate, and high-protein meals on metabolism and performance during endurance cycling. Int J Sport Nutr Exerc Metab 12:318–335
Rowlands DS, Hopkins WG (2002b) Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism 51:678–690
Rowlands D, Houltham S (2017) Multiple-transportable carbohydrate effect on long-distance triathlon performance. Med Sci Sport Exerc 49:1734–1744
Rowlands DS, Wadsworth DP (2012) No effect of protein coingestion on exogenous glucose oxidation during exercise. Med Sci Sport Exerc 44:701–708
Rowlands DS, Wallis GA, Shaw C, Jentjens RLPG, Jeukendrup AE (2005) Glucose polymer molecular weight does not affect exogenous carbohydrate oxidation. Med Sci Sport Exerc 37:1510–1516
Rowlands DS, Swift M, Ros M, Green JG (2012) Composite versus single transportable carbohydrate solution enhances race and laboratory cycling performance. Appl Physiol Nutr Metab 37:425–436
Rowlands DS, Houltham S, Musa-Veloso K, Brown F, Paulionis L, Bailey D (2015) Fructose-glucose composite carbohydrates and endurance performance: critical review and future perspectives. Sport Med 45:1561–1576
Ruby BC, Coggan AR, Zderic TW (2002) Gender differences in glucose kinetics and substrate oxidation during exercise near the lactate threshold. J Appl Physiol 92:1125–1132
Russell RR, Taegtmeyer H (1991) Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest 87:384–390
Satabin P, Portero P, Defer G, Bricout J, Guezennec CY (1987) Metabolic and hormonal responses to lipid and carbohydrate diets during exercise in man. Med Sci Sport Exerc 19:218–223
Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL (1995) Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9:651–658
Saunders MJ (2011) Carbohydrate-protein intake and recovery from endurance exercise: Is chocolate milk the answer? Curr Sport Med Rep 10:203–210
Saunders MJ, Luden ND (2012) Macronutrient intake during endurance activity to optimize performance. In: Nutrient timing: Metabolic optimization for health, performance, and recovery. Taylor & Francis, pp 119–138
Saunders MJ, Kane MD, Todd MK (2004) Effects of a carbohydrate-protein beverage on cycling endurance and muscle damage. Med Sci Sport Exerc 36:1233–1238
Saunders MJ, Luden ND, Herrick JE (2007) Consumption of an oral carbohydrate-protein gel improves cycling endurance and prevents postexercise muscle damage. J Strength Cond Res 21:678–684
Saunders MJ, Moore RW, Kies AK, Luden ND, Pratt CA (2009) Carbohydrate and protein hydrolysate coingestions improvement of late-exercise time-trial performance. Int J Sport Nutr Exerc Metab 19:136–149
Saunders MJ, Luden ND, DeWitt CR, Gross MC, Rios AD (2018) Protein supplementation during or following a marathon run influences post-exercise recovery. Nutrients 10:333
Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS (2007) American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sport Exerc 39:377–390
Schroer AB, Saunders MJ, Baur DA, Womack CJ, Luden ND (2014) Cycling time trial performance may be impaired by whey protein and l-alanine intake during prolonged exercise. Int J Sport Nutr Exerc Metab 24:507–515
Scott BE, Laursen PB, James LJ, Boxer B, Chandler Z, Lam E, Gascoyne T, Messenger J, Mears SA (2019) The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport 22:702–706
Scott S, Kempf P, Bally L, Stettler C (2019) Carbohydrate intake in the context of exercise in people with type 1 diabetes. Nutrients 11:3017
Scott SN, Anderson L, Morton JP, Wagenmakers AJM, Riddell MC (2019) Carbohydrate restriction in type 1 diabetes: a realistic therapy for improved glycaemic control and athletic performance? Nutrients 11:1022
Senefeld JW, Wiggins CC, Regimbal RJ, Dominelli PB, Baker SE, Joyner MJ (2020) Ergogenic effect of nitrate supplementation: a systematic review and meta-analysis. Med Sci Sports Exerc 52:2250–2261
Shaw DM, Merien F, Braakhuis A, Plews D, Laursen P, Dulson DK (2019) The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab 29:466–473
Shearer J, Graham TE (2014) Performance effects and metabolic consequences of caffeine and caffeinated energy drink consumption on glucose disposal. Nutr Rev 72:121–136
Shi X, Osterberg KL, Petrie H, Stofan JR, Murray R (2017) Effect of different osmolalities, CHO types, and [CHO] on gastric emptying in humans. Med Sci Sport Exerc 49:1015–1021
Skinner TL, Desbrow BEN, Arapova J, Schaumberg MA, Osborne J, Grant GD, Anoopkumar-Dukie S, Leveritt MD (2019) Women experience the same ergogenic response to caffeine as men. Med Sci Sport Exerc 51:1195–1202
Slivka D, Hailes W, Cuddy J, Ruby B (2008) Caffeine and carbohydrate supplementation during exercise when in negative energy balance: effects on performance, metabolism, and salivary cortisol. Appl Physiol Nutr Metab 33:1079–1085
Smith J, Zachwieja JJ, Péronnet F, Passe DH, Massicotte D, Lavoie C, Pascoe DD (2010) Fuel selection and cycling endurance performance with ingestion of [13C]glucose: evidence for a carbohydrate dose response. J Appl Physiol 108:1520–1529
Smith J, Pascoe DD, Passe DH, Ruby BC, Stewart LK, Baker LB, Zachwieja JJ (2013) Curvilinear dose-response relationship of carbohydrate (0–120 g·h-1) and performance. Med Sci Sport Exerc 45:336–341
Southward K, Rutherfurd-Markwick KJ, Ali A (2018) The effect of acute caffeine ingestion on endurance performance: a systematic review and meta-analysis. Sport Med 48:1913–1928
Souza DB, Del Coso J, Casonatto J, Polito MD (2017) Acute effects of caffeine-containing energy drinks on physical performance: a systematic review and meta-analysis. Eur J Nutr 56:13–27
Sriamornsak P (2011) Application of pectin in oral drug delivery. Expert Opin Drug Deliv 8:1009–1023
Stannard SR, Thompson MW, Brand-Miller JC (2000) The effect of glycemic index on plasma glucose and lactate levels during incremental exercise. Int J Sport Nutr Exerc Metab 10:51–61
Stellingwerff T, Cox GR (2014) Systematic review: carbohydrate supplementation on exercise performance or capacity of varying durations. Appl Physiol Nutr Metab 14:1–14
Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, Burke LM (2006) Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab 290:E380–E388
Stellingwerff T, Boon H, Gijsen AP, Stegen JH, Kuipers H, van Loon LJ (2007) Carbohydrate supplementation during prolonged cycling exercise spares muscle glycogen but does not affect intramyocellular lipid use. Pflugers Arch 454:635–647
Stephens FB, Roig M, Armstrong G, Greenhaff PL (2008) Post-exercise ingestion of a unique, high molecular weight glucose polymer solution improves performance during a subsequent bout of cycling exercise. J Sport Sci 26:149–154
Stepto NK, Carey AL, Staudacher HM, Cummings NK, Burke LM, Hawley JA (2002) Effect of short-term fat adaptation on high-intensity training. Med Sci Sport Exerc 34:449–455
Stevenson E, Williams C, Biscoe H (2005) The metabolic responses to high carbohydrate meals with different glycemic indices consumed during recovery from prolonged strenuous exercise. Int J Sport Nutr Exerc Metab 15:291–307
Stevenson EJE, Astbury NM, Simpson EJ, Taylor MA, Macdonald IA (2009) Fat oxidation during exercise and satiety during recovery are increased following a low-glycemic index breakfast in sedentary women. J Nutr 139:890–897
Stocks B, Betts JA, McGawley K (2016) Effects of carbohydrate dose and frequency on metabolism, gastrointestinal discomfort, and cross-country skiing performance. Scand J Med Sci Sport 26:1100–1108
Sutehall S, Muniz-Pardos B, Bosch AN, Di Gianfrancesco A, Pitsiladis YP (2018) Sports drinks on the edge of a new era. Curr Sports Med Rep 17:112–116
Sutehall S, Galloway SDR, Bosch A, Pitsiladis Y (2020) Addition of an alginate hydrogel to a carbohydrate beverage enhances gastric emptying. Med Sci Sport Exerc 52:1785–1792
Tarnopolsky MA (1994) Caffeine and endurance performance. Sport Med Eval Res Exerc Sci Sport Med 18:109–125
Tarnopolsky MA (2000) Gender differences in substrate metabolism during endurance exercise. Can J Appl Physiol 25:312–327
Tarnopolsky MA, Atkinson SA, Phillips SM, MacDougall JD (1995) Carbohydrate loading and metabolism during exercise in men and women. J Appl Physiol 78:1360–1368
Tarnopolsky MA, Zawada C, Richmond LB, Carter S, Shearer J, Graham T, Phillips SM (2001) Gender differences in carbohydrate loading are related to energy intake. J Appl Physiol 91:225–230
Tarpey MD, Roberts JD, Kass LS, Tarpey RJ, Roberts MG (2013) The ingestion of protein with a maltodextrin and fructose beverage on substrate utilisation and exercise performance. Appl Physiol Nutr Metab 38:1245–1253
Taylor C, Higham D, Close GL, Morton JP (2011) The effect of adding caffeine to postexercise carbohydrate feeding on subsequent high-intensity interval-running capacity compared with carbohydrate alone. Int J Sport Nutr Exerc Metab 21:410–416
Temesi J, Johnson NA, Raymond J, Burdon CA (2011) Carbohydrate ingestion during endurance exercise improves performance in adults. J Nutr 141:890–897
ter Steege RWF, Kolkman JJ (2012) Review article: the pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment Pharm Ther 35:516–528
Thomas DE, Brotherhood JR, Brand JC (1991) Carbohydrate feeding before exercise: effect of glycemic index. Int J Sport Med 12:180–186
Thomas DT, Erdman KA, Burke LM (2016) Nutrition and athletic performance. Med Sci Sport Exerc 48:543–568
Thomson ABR, Keelan M, Thiesen A, Clandinin MT, Ropeleski M, Wild GE (2001) Small bowel review: normal physiology part 1. Dig Dis Sci 46:2567–2587
Thong FSL, Derave W, Kiens B, Graham TE, Ursø B, Wojtaszewski JFP, Hansen BF, Richter EA (2002) Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes 51:583–590
Thorburn MS, Vistisen B, Thorp RM, Rockell MJ, Jeukendrup AE, Xu X, Rowlands DS (2006) Attenuated gastric distress but no benefit to performance with adaptation to octanoate-rich esterified oils in well-trained male cyclists. J Appl Physiol 101:1733–1743
Thorburn MS, Vistisen B, Thorp RM, Rockell MJ, Jeukendrup AE, Xu X, Rowlands DS (2007) No attenuation of gastric distress or benefit to performance with adaptation to octanoate-rich esterified oils in female cyclists. Eur J Sport Sci 7:179–192
Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JFP, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ (1999) Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol Endocrinol Metab 277(4 Pt):E733–E741
Tremblay J, Peronnet F, Massicotte D, Lavoie C (2010) Carbohydrate supplementation and sex differences in fuel selection during exercise. Med Sci Sport Exerc 42:1314–1323
Trenell MI, Stevenson E, Stockmann K, Brand-Miller J (2008) Effect of high and low glycaemic index recovery diets on intramuscular lipid oxidation during aerobic exercise. Br J Nutr 99:326–332
Trexler ET, Keith DS, Lucero AA, Stoner L, Schwartz TA, Persky AM, Ryan ED, Smith-Ryan AE (2019) Effects of citrulline malate and beetroot juice supplementation on energy metabolism and blood flow during submaximal resistance exercise. J Diet Suppl 17:698–717
Triplett D, Doyle JA, Rupp JC, Benardot D (2010) An isocaloric glucose-fructose beverage’s effect on simulated 100-km cycling performance compared with a glucose-only beverage. Int J Sport Nutr Exerc Metab 20:122–131
Trommelen J, Fuchs CJ, Beelen M, Lenaerts K, Jeukendrup AE, Cermak NM, Van Loon LJC (2017) Fructose and sucrose intake increase exogenous carbohydrate oxidation during exercise. Nutrients 9:167
Truswell AS, Seach JM, Thorburn AW (1988) Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr 48:1424–1430
Valentine RJ, Saunders MJ, Todd MK, St Laurent TG (2008) Influence of carbohydrate-protein beverage on cycling endurance and indices of muscle disruption. Int J Sport Nutr Exerc Metab 18:363–378
Van De Walle GP, Vukovich MD (2018) The effect of nitrate supplementation on exercise tolerance and performance: a systematic review and meta-analysis. J Strength Cond Res 32:1796–1808
van Essen M, Gibala MJ (2006) Failure of protein to improve time trial performance when added to a sports drink. Med Sci Sport Exerc 38:1476–1483
van Loon LJ, Saris WH, Kruijshoop M, Wagenmakers AJ (2000) Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am J Clin Nutr 72:106–111
Van Loon LJC (2014) Is there a need for protein ingestion during exercise? Sport Med 44:S105–S111
Van Hall G, MacLean DA, Saltin B, Wagenmakers AJM (1996) Mechanisms of activation of muscle branched-chain α-keto acid dehydrogenase during exercise in man. J Physiol 494:899–905
Van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM (2001) The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536:295–304
Van Nieuwenhoven MA, Brummer RJM, Brouns F (2000) Gastrointestinal function during exercise: comparison of water, sports drink, and sports drink with caffeine. J Appl Physiol 89:1079–1085
Van Zyl CG, Lambert EV, Hawley JA, Noakes TD, Dennis SC (1996) Effects of medium-chain triglyceride ingestion on fuel metabolism and cycling performance. J Appl Physiol 80:2217–2225
Vandenbogaerde TJ, Hopkins WG (2011) Effects of acute carbohydrate supplementation on endurance performance: a meta-analysis. Sport Med 41:773–792
Veech RL (2004) The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fat Acids 70:309–319
Venables MC, Brouns F, Jeukendrup AE (2008) Oxidation of maltose and trehalose during prolonged moderate-intensity exercise. Med Sci Sport Exerc 40:1653–1659
Vist GE, Maughan RJ (1995) The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J Physiol 486(Pt 2):523–531
Vogt S, Heinrich L, Schumacher YO, Großhauser M, Blum A, König D, Berg A, Schmid A (2005) Energy intake and energy expenditure of elite cyclists during preseason training. Int J Sport Med 26:701–706
Vukovich MD, Costill DL, Hickey MS, Trappe SW, Cole KJ, Fink WJ (1993) Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. J Appl Physiol 75:1513–1518
Waldman HS, Basham SA, Price FG, Smith JW, Chander H, Knight AC, Krings BM, McAllister MJ (2018) Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab 43:711–717
Walker JL, Heigenhauser GJF, Hultman E, Spriet LL (2000) Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J Appl Physiol 88:2151–2158
Wallis GA, Rowlands DS, Shaw C, Jentjens RLPG, Jeukendrup AE (2005) Oxidation of combined ingestion of maltodextrins and fructose during exercise. Med Sci Sport Exerc 37:426–432
Wallis GA, Dawson R, Achten J, Webber J, Jeukendrup AE (2006) Metabolic response to carbohydrate ingestion during exercise in males and females. Am J Physiol Endocrinol Metab 290:E708–E715
Wanders AJ, Jonathan MC, Van Den Borne JJGC, Mars M, Schols HA, Feskens EJM, De Graaf C (2013) The effects of bulking, viscous and gel-forming dietary fibres on satiation. Br J Nutr 109:1330–1337
Watson P, Shirreffs SM, Maughan RJ (2004) The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 93:306–314
Wee SL, Williams C, Gray S, Horabin J (1999) Influence of high and low glycemic index meals on endurance running capacity. Med Sci Sport Exerc 31:393–399
Welch IM, Bruce C, Hill SE, Read NW (1987) Duodenal and ileal lipid suppresses postprandial blood glucose and insulin responses in man: possible implications for the dietary management of diabetes mellitus. Clin Sci 72:209–216
West DJ, Morton RD, Stephens JW, Bain SC, Kilduff LP, Luzio S, Still R, Bracken RM (2011) Isomaltulose improves postexercise glycemia by reducing CHO oxidation in T1DM. Med Sci Sports Exerc 43:204–210
Whitley HA, Humphreys SM, Campbell IT, Keegan MA, Jayanetti TD, Sperry DA, MacLaren DP, Reilly T, Frayn KN (1998) Metabolic and performance responses during endurance exercise after high-fat and high-carbohydrate meals. J Appl Physiol 85:418–424
Williams M, Raven PB, Fogt DL, Ivy JL (2003) Effects of recovery beverages on glycogen restoration and endurance exercise performance. J Strength Cond Res 17:12–19
Wilson PB, Ingraham SJ (2015) Glucose-fructose likely improves gastrointestinal comfort and endurance running performance relative to glucose-only. Scand J Med Sci Sports 25:e613–e620
Wolever T, Jenkins D (1986) The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 43:167–172
Yeo SE, Jentjens RLPG, Wallis GA, Jeukendrup AE (2005) Caffeine increases exogenous carbohydrate oxidation during exercise. J Appl Physiol 99:844–850
Yeo WK, Paton CD, Garnham AP, Burke LM, Carey AL, Hawley JA (2008) Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J Appl Physiol 105:1462–1470
Yeo WK, Carey AL, Burke L, Spriet LL, Hawley JA (2011) Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab 36:12–22
Zderic TW, Schenk S, Davidson CJ, Byerley LO, Coyle EF (2004) Manipulation of dietary carbohydrate and muscle glycogen affects glucose uptake during exercise when fat oxidation is impaired by β-adrenergic blockade. Am J Physiol Endocrinol Metab 287:E1195–E1201
Zorzano A, Palacín M, Gumà A (2005) Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand 183:43–58
Zuhl MN, Lanphere KR, Kravitz L, Mermier CM, Schneider S, Dokladny K, Moseley PL (2014) Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol 116:183–191
Zuntz N, Loeb W (1894) Über die Bedeutung der verschiedene Nährstoff als Energiequelle der Muskelkraft. Arch Anat Physiol 18:541–543
Acknowledgements
The authors would like to thank Katie Baur for reviewing this manuscript and providing comments. The authors are also grateful to Dr. William Wightman, for creating the artwork used in Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and drafting of this review. Daniel Baur was primarily responsible for drafting the following sections: abstract, introduction, conclusions, and sections on modified carbohydrate, hydrogels, caffeine, lipids, nitrate, diets, specials populations, and tables. Michael Saunders drafted the section on multiple transportable carbohydrates, protein, and the figure. Both authors provided edits and comments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Communicated by Michael Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baur, D.A., Saunders, M.J. Carbohydrate supplementation: a critical review of recent innovations. Eur J Appl Physiol 121, 23–66 (2021). https://doi.org/10.1007/s00421-020-04534-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04534-y