Abstract
Purpose
Strenuous exercise induces inflammation and muscle damage. Turmeric (Curcuma longa L.) is a widely used spice that exhibits potent anti-inflammatory response and appears to decrease indirect markers of muscle damage. A randomized, double-blind, placebo-controlled trial was conducted to evaluate the effects of Curcuma longa L. extract (CLE) on inflammation and muscle damage after a half-marathon race.
Methods
Twenty-eight healthy, normal-weight men were randomly assigned to one of two groups: (1) CLE (3 capsules per day, 500 mg each); or (2) placebo (PLA, 3 capsules per day, 500 mg of microcrystalline cellulose). Participants received the intervention for 4 weeks and immediately before and after the half-marathon race. Creatine kinase, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, myoglobin, interleukins 6 and 10 were assessed at baseline, immediately before, after, and at 2, 24, and 48 h after the half-marathon race.
Results
The half-marathon race increased markers of inflammation and muscle damage. A greater increase in interleukin-10 was observed in the CLE group immediately after the competition compared to the PLA group (7.54 ± 1.45 vs 5.25 ± 0.59 pg/mL; p < 0.05; d = 0.55). Myoglobin concentration was lower 2 h after the race in participants from the CLE group compared to the PLA group (62.10 ± 8.26 vs 107.85 ± 18.45 ng/mL; p = 0.01; d = 0.86).
Conclusion
Curcuma longa L. extract supplementation leads to an increase in IL-10 and decreased myoglobin in recreational male runners after a half-marathon race.
Trial registration number
U1111-1179-6335, February 13, 2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strenuous exercise causes muscle damage, which is characterized by sarcolemma disruption, swelling or disruption of the sarcotubular system, distortion of the myofibril contractile components, cytoskeletal damage, and extracellular myofiber matrix abnormalities (Friden and Lieber 1992). As a consequence of strenuous exercise, individuals can experience impaired neuromuscular function, reduced range of motion, and muscle soreness (Howatson and van Someren 2008). There are still controversies on redox homeostasis in response to exercise (Nikolaidis et al. 2013; Margaritelis et al. 2019). Adaptations to oxidative stress and consequent attenuation of muscle damage may occur after repeated exercise in trained individuals, independently of the age (Nikolaidis et al. 2013). However, high-intensity exercise may induce greater oxidative stress, inflammation, and muscle damage (González-Bartholin et al. 2019). An increase in inflammation and muscle damage, characterized by elevated activities of lactate dehydrogenase (LDH), creatine kinase (CK), and aspartate aminotransferase (AST), was observed after a half-marathon race (Briviba et al. 2005; Lippi et al. 2011, 2008).
Strategies able to attenuate the exacerbated inflammatory response caused by strenuous exercise are becoming increasingly popular. Among them, nutritional supplements with antioxidant and anti-inflammatory properties have been widely consumed for this purpose (Howatson et al. 2010; Howatson and van Someren 2008; Trombold et al. 2010). Among the nutritional compounds able to positively impact inflammation, curcuminoids have recently emerged. These naturally occurring polyphenols are extracted from the rhizome of the Curcuma longa L., an herb commonly known as turmeric root (Kiuchi et al. 1993; Zhou et al. 2011). Among the curcuminoids found in this herb, the three main active compounds are curcumin, demethoxycurcumin, and bisdemethoxycurcumin (Kiuchi et al. 1993).
A recent review described that curcuminoids can positively impact several conditions, such as atherosclerosis, cardiac hypertrophy, hypertension, ischemia/reperfusion injury, and diabetic cardiovascular complications due to its anti-inflammatory properties (Li et al. 2019). This effect may be mediated via Toll-like receptors inhibition, a major family of pattern recognition receptors that are key in the response and regulation of both innate and adaptive immunity (Boozari et al. 2019). Besides its use in sickness, curcuminoids have been shown to positively impact healthy, exercising individuals. After eccentric exercise, curcumin was shown to attenuate inflammation by reducing interleukin-8 (Tanabe et al. 2019b), and to prevent muscle damage by down-regulating nuclear factor kappa B (NF-kB) activity (Sahin et al. 2016). This compound has also been shown to reduced pain associated with delayed-onset muscle soreness in men after a heavy eccentric exercise session, which positively impacted participants’ recovery of muscle performance (Nicol et al. 2015). Another study showed that curcumin attenuated CK activity and accelerated recovery of maximal voluntary contraction in men after a single session of maximal isokinetic eccentric exercise (Tanabe et al. 2015). In these studies, curcumin was supplemented for a short period of time and in isolated form (Nicol et al. 2015; Tanabe et al. 2015). Thus, considering that curcuminoids present cumulative and synergistic properties (Ahmed and Gilani 2014; Kiuchi et al. 1993), Curcuma longa L. extract (CLE) might result in greater benefits. To the authors’ knowledge, no research evaluated the effects of CLE supplementation on exercise-induced inflammation and muscle damage in a recreational population of runners. Therefore, we conducted an applied study to investigate the effect of chronic CLE supplementation on cytokines and indirect markers of muscle damage in healthy men after a half-marathon race.
Methods
Participants
The study protocol was reviewed and approved by the Ethics Committee of the Federal University of Goiás (number 421.008), and all experiments were performed in accordance with all institutional and governmental guidelines and regulations. Written informed consent was obtained from all participants. This clinical trial was registered at ensaiosclinicos.gov.br (number U1111-1179-6335). Eligible participants were male amateur runners with a normal body mass index (> 18.5 and < 24.9 kg/m2), aged between 25 and 50 years, who expressed their willingness to participate in this research. Potential participants were not included in the study if they (1) were on weight loss diet/medication, (2) were diagnosed with any acute or chronic disease, (3) were taking medication that could affect the immune system, (4) were injured, (5) were smokers, or (6) had a history of chronic alcoholism.
A total of 40 participants were screened for participation; 36 met the inclusion criteria and were randomly allocated to the CLE (n = 18) and placebo (PLA; n = 18) groups. Eight participants withdrew for personal reasons (n = 4 in each group). The reasons were surgery (PLA, n = 1), gastritis (PLA, n = 1), use of anti-inflammatory drugs (PLA, n = 1), which did not comply with protocol (PLA, n = 1 and CLE, n = 2), muscle injury (CLE, n = 1), and motorcycle accident (CLE, n = 1). Therefore, 14 participants from the CLE group and 14 from PLA group completed the study and were included in the analyses.
Study design, randomization, and intervention
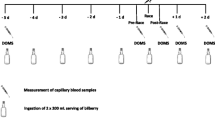
This study was a randomized, double-blind, placebo-controlled clinical trial. Participants ingested three opaque capsules per day containing CLE (500 mg each) or PLA (microcrystalline cellulose, 500 mg each). Curcuma Longa L. powder (1.5 g) was analyzed by high-performance liquid chromatography (HPLC) equipped with photodiode array detector and showed 64.5 mg of the major curcuminoids (i.e., 33.0 mg of curcumin, 16.5 mg of demethoxycurcumin, and 15.0 mg of bisdemethoxycurcumin). The total amount of curcuminoids was inferred by the standard addition method (Sakkiadi et al. 2007). Briefly, a six-point calibration curve of turmeric powder solutions was spiked with a known amount of curcumin analytical grade. HPLC–UV detector was set at a common wavelength (420 nm), according to spectral similarities between curcumin and their methoxylated derivatives (demethoxycurcumin and bisdemethoxycurcumin) (Poudel et al. 2019). The dosage was established after a previous crossover, double-blind, placebo-controlled pilot study performed by our research group (results not published). This pilot study showed no differences in total antioxidant capacity and curcumin concentration between 1.5, 3, and 6 g of CLE. Two capsules were ingested with lunch and one with dinner for 29 days before the half-marathon race, and immediately before the competition, when participants were fed a standardized breakfast and consumed three capsules of CLE. These recommendations were provided to increase the bioavailability of curcuminoids. Participants were randomly stratified by age, finishing time of their last half-marathon race, and dietary intake of legumes, vegetables, fruits, and nuts, with a 1:1 allocation (www.randomization.com). Randomization was performed by a member of the study team not involved in the assessment of outcomes. Study participants and members of the study team involved in the collection of data and analysis of the outcomes were blinded to the treatment condition. Adherence to treatment was assessed by weekly phone calls and by counting empty packs returned to the research unit. Participants were instructed to contact the research team if they felt any discomfort during the intervention period. Participants’ training volume was controlled before the competition. Participants were oriented to avoid running more than 16 km in one session, to keep the exercise intensity at 70–85% of the heart rate, and to rest 2 days before the competition. The experimental design is shown in Fig. 1 of the supplementary material.
Dietary intake
Participants were instructed to maintain their habitual dietary intake during the intervention period. Thirty minutes before the half-marathon race, participants were fed a standardized breakfast composed of one banana, one slice of white toast, two slices of cheese, and 200 mL of maltodextrin (total: 277 kcal, 50 g of carbohydrate, 6.86 g of protein, and 5.52 g of fat) (Kerksick et al. 2018). None of the participants reported gastrointestinal symptoms due to breakfast. During the half-marathon race, participants received two sachets of Carb-Up® (Probiotica, Sao Paulo, Brazil), which contained maltodextrin, fructose syrup, and waxy maize (20 g of carbohydrate per sachet). Dietary intake was assessed at baseline and after the intervention period using 24-h dietary recalls and dietary records, including 2 week days and 1 weekend day. Dietary data were converted from household measures to grams and milliliters to enable the chemical analysis of food consumption by the software Avanutri® (Avanutri Equipamentos de Avaliação Ltda, Rio de Janeiro, Brazil). Energy intake, protein, carbohydrate, fat, vitamins, and minerals were analyzed.
Anthropometric assessment and body composition
Participants were weighed, measured, and had their body composition assessed at baseline. The weighing was performed on a digital scale (Filizola, Brazil) accurate to 0.1 kg, installed on a flat, firm, and smooth surface, and away from the wall. Height was measured with a wall-fixed stadiometer (Model Standard, Sanny®) that graduated to 0.1 cm. The values were recorded immediately after the measures, without rounding. All measurements were collected with participants in bare feet and wearing light clothing. Participants’ fat mass (FM) and lean mass (LM) were assessed by dual-energy X-ray absorptiometry (DXA) using a General Electric Lunar Prodigy scanner (Encore 2011 software, version 13.60, General Electric Medical Systems Lunar, Madison, EUA). The coefficient of variation (CV) for the DXA tests of LM and FM was 0.75% and 1.03%, respectively.
Half-marathon race
After 29 days of intervention, all participants performed a half-marathon race (21 km) to induce muscle damage. The race took place in Goiania (GO, Brazil) and started at 7:00 a.m. The average temperature during the competition was 29 ± 2 °C and the wind speed was 16 km/h. The average inclination of the route was 2.4%, with a maximum inclination of 12.9% uphill and 12.8% downhill.
Biochemical analysis
Blood samples were drawn into vacuum tubes containing K2EDTA from the antecubital vein of each participant’s arm. Plasma samples were separated from whole blood by centrifugation at 3500 rpm for 10 min at 4 °C (Combate, C.E.L.M) and frozen at − 80 °C until analysis. CK, LDH, alanine aminotransferase (ALT), and AST concentrations were determined by automated enzymatic methods on a Selectra 2 Merck®, using kits from Labtest Diagnóstica SA, with sensitivity of 7.449 U/L, 0.054 mg/dL, 1.913 U/L, and 1.751 U/L, respectively. Myoglobin, IL-6, and IL-10 concentrations were quantified by ELISA using a SpectraMax plus 384 Absorbance Microplate Reader microplate reader (San Diego, California, USA) with a filter of 450 nm. Reagent kits were Calbiotech® Myoglobin ELISA kit with sensitivity of 25 ng/mL, Ebioscience® IL-6, and IL-10 ELISA kits with sensitivities of 1.6 pg/mL and 2.3 pg/mL, respectively.
Free curcumin and its hydrolyzed conjugated forms were determined using a complete validated HPLC-fluorescence method based on Schiborr et al. (2010) and carried out in accordance with official guidelines (Kadian et al. 2016). Chromatographic separation was carried out in an Infinity 1260 LC system (Agilent, USA) using a C18 Luna® column (150 × 4.0 mm, 3 μm) protected by a TM Gemini guard column (4.0 × 3.0 mm, Phenomenex, USA). The mobile phase consisted of a gradient elution of acetonitrile (ACN)/acetic acid (AcOH) pH 3.2, as described: 45% ACN (0–8 min), 60% ACN (8.05–15 min), and 45% ACN (15.05–20 min). The fluorescence detector was programmed to switch wavelengths to 429/529 nm and 285/529 nm for curcumin and internal standard, respectively. Briefly, the method was validated using an eight-point calibration curve ranging from 44.0 to 261.0 ng/mL (Antunes et al. 2020). A linear regression equation (y = 0.000883 ± 0.01669 + 0.00378 x ± 0.00008, where y is the peak area and x is the concentration) was set showing correlation coefficient (R2) > 0.98 and highly significant F value (p < 0.01). Accuracy was expressed by relative error (RE) < 15% and an intra- and inter-day precision showed relative standard deviation (RSD) < 5.6%. The limit of quantification (LOQ) was found to be 44.0 ng/mL for curcumin.
Curcumin was determined in plasma samples after enzymatic treatment. Three time points were considered for this analysis: before and after 29 days of supplementation and 2 h after the half-marathon race. Shortly before HPLC injection, an enzymatic hydrolysis of conjugates (Asai and Miyazawa 2000) was performed in borosilicate tubes with β-Glucuronidase aliquots (50 μL) from Helix pomatia type HP-2 (Sigma-Aldrich, Germany), followed by a deproteination (AcoEt:MeOH) procedure. The extraction residue was reconstituted (ACN, 100 μL) and injected (10 μL) into the HPLC-fluorescence detector system. Chromatographic analysis showed very good peak symmetry (A < 1.1) and separation efficiency for curcumin and its internal standard (N = 6330 and 54,674, respectively).
Statistical analysis
The sample size was estimated a priori using the G* Power software version 3.1.7. Based on a reduction in IL-6 levels (effect size, d = 1.2) after exercise in a group supplemented with curcumin (Davis et al. 2007), a sample size of 12 participants would be needed in each group to give 80% power to detect a significant difference between CLE and placebo (with a two-sided type 1 error of 5%). Data distribution was evaluated by the Kolmogorov–Smirnov test. Homogeneity of variance was verified with Levene’s test. Results were expressed as mean ± standard error (SE). One-way analysis of variance (ANOVA) was performed to compare curcumin concentration across the time in the CLE group. A 2 × 6 [group (PLA, CLE) × time (baseline, immediately before, after, and at 2, 24 and 48 h after the half-marathon race pre, post, 24 h, 48 h, 72 h, and 96 h)] analysis of covariance (ANCOVA), with baseline scores entered as the covariate, was used to analyze all variables. When a significant interaction was found, Duncan’s method for multiple comparisons was used. A Fisher’s exact test was used to determine any differences in the proportion of participants who correctly guessed their supplements. Macronutrient dietary intake and micronutrient dietary intake were adjusted for energy and baseline values (when not homocedastic at baseline). Effect sizes were calculated using Cohen’s d formula and classified as small (d = 0.2), medium (d = 0.5), and large (d = 0.8) (Cohen 1988). All statistical analyses were performed using SPSS (IBM) version 21, with p ≤ 0.05 considered significant.
Results
Baseline characteristics
There were no differences between groups in any of the baseline characteristics (Table 1).
Exercise training, dietary intake, side effects, and blinding
The volume of training before the competition did not differ between groups (PLA 158 ± 3.71; CLE 162 ± 2.99 AU; p > 0.05; Table 1). Dietary intake also did not differ between groups (p > 0.05; Table 2). No side effects were reported during the intervention period. At the conclusion of the study, 3 (21.4%) and 4 (28.6%) participants from the PLA and CLE groups correctly identified the supplement ingested (p = 0.66).
Plasma curcumin concentration
Plasma curcumin was not detectable in participants from the PLA group and varied with time in participants from the CLE group. Plasma curcumin concentration increased immediately after the half-marathon race in the CLE group (8.3 ± 5.6 vs 83.0 ± 7.2 ng/mL, p < 0.01), and 2 h after the competition it dropped to 39.1 ± 10.1 ng/mL (p < 0.01; Fig. 1).
Inflammatory markers
A significant group × time interaction was found for IL-10. Participants from the CLE group showed higher IL-10 concentration immediately after the race compared to the PLA group (p = 0.04; d = 0.55; Fig. 2a). This marker remained higher 2 h after the competition when compared to pre-race concentration in the CLE group (4.43 ± 0.75 vs 2.60 ± 0.35 pg/mL; p = 0.01; Fig. 2a). As regards to IL-6, both groups followed a similar pattern characterized by a remarkable increase immediately after the half-marathon race compared to pre-race (PLA 4.29 ± 1.56 pg/mL; CLE 3.26 ± 1.32 pg/mL; p < 0.05; Fig. 2b).
Effect of the half-marathon race and Curcuma Longa L. extract supplementation on interleukin-10 (IL-10) (a) and interleukin-6 (IL-6) (b). *Indicates statistical difference between groups. †Indicates statistical difference compared to M0 and pre-competition in the same group. ‡Indicates statistical difference compared to all moments in the same group. CLECurcuma Longa L. extract group, M0 before supplementation, PLA placebo group, Pre before the half-marathon race, Post immediately after the half-marathon race, 2 h post 2 h after the half-marathon race, 48 h post 48 h after the half-marathon race
Muscle damage indices
The ANCOVA revealed a significant group × time interaction for myoglobin concentration. As shown in Fig. 3, 2 h after the half-marathon race, the CLE group exhibited a lower concentration of myoglobin than the PLA group (CLE 62.10 ± 8.26 vs. PLA 107.85 ± 18.45 ng/mL, p = 0.01; d = 0.86; Fig. 3). Only time interaction was observed for CK, AST, ALT, and LDH activities. In both groups, participants had increased activities of CK (PLA 348.08 ± 121.49 U/L; CLE 557.62 ± 135.36 U/L; p < 0.05; Fig. 4a) and AST (PLA 15.69 ± 3.75 U/L; CLE 23.80 ± 5.30 U/L; p < 0.05; Fig. 4c) 24 h after the half-marathon race, when compared to pre-race values. The same was observed in LDH activity immediately after the competition compared to pre-race (p < 0.01; Fig. 4b). Although it was not observed differences between groups, LDH activity returned to pre-race value in the CLE group after 24 h (24 h post 465.28 ± 17.26 U/L; pre 440.08 ± 26.91 U/L, p = 0.34; Fig. 4b), but not in the PLA (24 h post 503.54 ± 53.98 U/L; pre 460.64 ± 53.61 U/L, p = 0.02; Fig. 4b). ALT activity increased immediately after the race (p < 0.01) without difference between groups (PLA 29.21 ± 1.71 U/L; CLE 27.38 ± 1.83 U/L; p = 0.24, Fig. 4d). The half-marathon race time was 111.57 ± 24.99 min for participants in the CLE group and 103.92 ± 17.95 min for those assigned to the PLA group (p > 0.05).
Effect of the half-marathon race and Curcuma longa L. extract supplementation on myoglobin concentrations. *Indicates statistical difference between groups. †Indicates statistical difference compared to M0, pre, and immediately after the half-marathon race competition in the same group. CLECurcuma Longa L. extract group, M0 baseline, before supplementation, PLA placebo group, Pre before the half-marathon race, Post immediately after the half-marathon race, 2 h post 2 h after the half-marathon race, 48 h post 48 h after the half-marathon race
Effect of the half-marathon race and Curcuma longa L. extract supplementation on creatine kinase (CK) (a), lactate dehydrogenase (LDH) (b), aspartate aminotransferase (AST) (c), and alanine aminotransferase (ALT) (d). †Indicates statistical difference compared to M0 and pre-competition in the same group. ‡Indicates statistical difference compared to all moments in the same group. #Indicates statistical difference compared to pre in the same group. CLECurcuma Longa L. extract group, M0 baseline, before supplementation, PLA placebo group, Pre before the half-marathon race, Post immediately after the half-marathon race, 2 h post 2 h after the half-marathon race, 48 h post 48 h after the half-marathon race
Discussion
This study demonstrated that chronic supplementation of CLE increased IL-10 and decreased myoglobin after a half-marathon race in healthy, normal-weight men. To the authors’ knowledge, this was the first randomized, double-blind, placebo-controlled applied trial to evaluate the chronic effects of CLE supplementation on exercise-induced inflammation and muscle damage in a recreational population of runners.
In this study, participants of both groups experienced an increase in IL-6 concentration immediately after the half-marathon race (Barros et al. 2017), revealing no effect of chronic CLE supplementation on this parameter. Similarly, in athletes who performed 2 h of cycling, acute curcumin supplementation had no effect on IL-6 concentration (Sciberras et al. 2015). On the other hand, a study with rats showed that curcumin was able to reduce concentrations of TNF-α and IL-6 after an eccentric exercise, reflecting improved muscle recovery (Davis et al. 2007). In chronic inflammatory diseases, curcumin appears to be effective in reducing IL-6 concentration (Cho et al. 2007); however, the baseline IL-6 level is significantly higher, suggesting that the anti-inflammatory effect of curcumin might depend on the intensity of the inflammatory process. In response to muscle contraction, IL-6 acts as a precursor for the synthesis and release of other anti-inflammatory markers and myokines, such as IL-10, which gives IL-6 a more restorative than inflammatory function (Gleeson et al. 2011; Petersen and Pedersen 2005).
IL-10 is an anti-inflammatory cytokine that acts by suppressing the activation and function of immune cells after an initial inflammatory response is established (Mosser and Zhang 2008). Increased IL-10 concentration can accelerate the inflammatory recovery process (Peake et al. 2017), and its deregulation can impact the development of several inflammatory diseases (Dagdeviren et al. 2016; Gleeson et al. 2011). Curcumin has the ability to induce the expression and production of IL-10, which partly explains its anti-inflammatory effects26. In this study, participants assigned to the CLE group had higher IL-10 levels immediately after the half-marathon race when compared to participants from the PLA group. This result might be explained by the effect of curcumin on IL-10 release (Mollazadeh et al. 2017). In accordance with this finding, studies with mice showed that curcumin was able to increase IL-10 production and to inhibit leukocyte recruitment and IL-1β, TNF-α, and NF-kB activation (Fattori et al. 2015; Sahin et al. 2016). Although strenuous exercise increases inflammation1, Ostrowski et al. (1999) demonstrated that the release of pro-inflammatory cytokines is balanced by IL-10 production after a marathon race, which might positively impact the recovery process for exercising individuals. On the other hand, clinical trials that evaluated the acute supplementation with curcumin before exercise did not find a positive impact on inflammation (Drobnic et al. 2014; Nicol et al. 2015; Sciberras et al. 2015). The inconsistent results might be associated with the length of intervention (acute vs chronic supplementation). Therefore, future studies are needed to better understand the acute and chronic mechanisms related to the anti-inflammatory responses generated by CLE supplementation.
The exercise protocol used for inducing muscle damage in participants of this study was efficient, as reflected by increased response of CK, LDH, myoglobin, and AST, which is consistent with the other studies (Lippi et al. 2008, 2011). Muscle damage may be associated with altered membrane permeability and cell disruption, resulting in plasma protein extravasation (Brancaccio et al. 2007). Therefore, it is characterized by an increase in intramuscular proteins in blood, temporary decrease in force production, increased muscle soreness, and swelling of the involved muscle groups (Howatson and van Someren 2008). Our participants showed a similar half-marathon race end time to their previously reported end times, suggesting a similar physical effort.
In this study, participants from the CLE group experienced lower myoglobin concentration than the PLA group, which indicates reduced muscle damage. Myoglobin is one of the intramuscular proteins used to monitor muscle damage or increased muscle membrane permeability; however, its peak is early due to the size of the molecule (Driessen-Kletter et al. 1990). In accordance with findings from this clinical trial, experimental studies demonstrated that curcumin supplementation was effective in reducing myoglobin concentration after induction of physical stress (Boz et al. 2014; Davis et al. 2007). Moreover, studies have shown that curcumin supplementation reduced muscle soreness immediately (Basham et al. 2019), 24 h (Nicol et al. 2015), and 3 days (Tanabe et al. 2019b) after exercise in healthy men. On the other hand, a commercially available curcumin supplement did not reduce muscle damage after an acute muscle injury induced by eccentric continuous exercise (Drobnic et al. 2014). The inconsistency among studies might be explained by the amount of curcumin present in the supplements and the time which it was offered to participants. It was already demonstrated that a high concentration of antioxidants induced pro-oxidant activity (Rahal et al. 2014). Supporting this finding, an experimental study showed that, in a concentration-dependent manner, curcumin can exhibit both anti- and pro-oxidant activities (Li et al. 2019). Interestingly, two papers recently published by the same research group showed that lower dosage of curcumin (180 mg) for a longer period of time (i.e., 7 days) attenuated the exercise-induced acute inflammation, muscle soreness, and damage in healthy men (Tanabe et al. 2019a, b).
The increased AST and ALT activities following strenuous exercise may indicate liver and skeletal muscle damage (Jastrzebski et al. 2015). For this reason, studies have investigated the activity of liver enzymes as a marker of muscle damage (Jastrzebski et al. 2015; Lippi et al. 2011). In rats, curcumin was shown to reduce liver damage via inhibition of NF-kB, reducing inflammation in the hepatic microcirculation (Fan et al. 2014). However, only experimental studies and models of hepatic steatosis could verify the effects of curcumin on liver function, and these results cannot be extrapolated to humans. In this study, CLE supplementation did not reduce AST and ALT activity after the half-marathon race. These findings suggest that CLE has no effect on acute injury or no effect on the activity of hepatic transaminases in humans.
Limitations of this study include (1) the short time frame to assess the changes in biomarkers after the half-marathon race (48 h); (2) the inclusion of a specific population group (healthy, normal-weight men); and (3) the performance of only one type of physical activity (half-marathon race). To assess a more comprehensive long-term recovery, 72–96 h of assessment after the race would be needed. Moreover, further research would be needed to investigate the effects of CLE supplementation in other population groups (e.g., women, older adults, and individuals with obesity) performing different types of physical activity (e.g., resistance or aerobic exercise training and other sports). Important advantages of this study are the ecological experimental approach, the chronic supplementation with turmeric extract, and the study design. In conclusion, 4 weeks of CLE supplementation leads to an increase in IL-10 and decreased myoglobin in recreational male runners after a half-marathon race. Future research should investigate the real impact of CLE on inflammatory response after a strenuous exercise.
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AST:
-
Aspartate aminotransferase
- CV:
-
Coefficient of variation
- CK:
-
Creatine kinase
- CLE:
-
Curcuma longa L. Extract
- DXA:
-
Dual-energy X-ray absorptiometry
- FM:
-
Fat mass
- LDH:
-
Lactate dehydrogenase
- LM:
-
Lean mass
- NF-kB:
-
Nuclear factor kappa B
- PLA:
-
Placebo
- VAS:
-
Visual analog scale
References
Ahmed T, Gilani AH (2014) Therapeutic potential of turmeric in Alzheimer’s disease: curcumin or curcuminoids? Phytother Res 28:517–525. https://doi.org/10.1002/ptr.5030
Antunes AH, Faria FR, Mota JF, Santiago MF, Kogawa AC, Rezende KR (2020) Bioanalytical method by HPLC-FLD for curcumin analysis in supplemented athletes. Saudi Pharm J. https://doi.org/10.1016/j.jsps.2020.03.012
Asai A, Miyazawa T (2000) Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci 67:2785–2793
Barros ES, Nascimento DC, Prestes J, Nobrega OT, Cordova C, Sousa F, Boullosa DA (2017) Acute and chronic effects of endurance running on inflammatory markers: a systematic review. Front Physiol 8:779. https://doi.org/10.3389/fphys.2017.00779
Basham SA, Waldman HS, Krings BM, Lamberth J, Smith JW, McAllister MJ (2019) Effect of curcumin supplementation on exercise-induced oxidative stress, inflammation, muscle damage, and muscle soreness. J Diet Suppl. https://doi.org/10.1080/19390211.2019.1604604
Boozari M, Butler AE, Sahebkar A (2019) Impact of curcumin on toll-like receptors. J Cell Physiol 234:12471–12482. https://doi.org/10.1002/jcp.28103
Boz I, Belviranli M, Okudan N (2014) Curcumin modulates muscle damage but not oxidative stress and antioxidant defense following eccentric exercise in rats. Int J Vitam Nutr Res 84:163–172. https://doi.org/10.1024/0300-9831/a000203
Brancaccio P, Maffulli N, Limongelli FM (2007) Creatine kinase monitoring in sport medicine. Br Med Bull 81–82:209–230. https://doi.org/10.1093/bmb/ldm014
Briviba K et al (2005) A half-marathon and a marathon run induce oxidative DNA damage, reduce antioxidant capacity to protect DNA against damage and modify immune function in hobby runners. Redox Rep 10:325–331. https://doi.org/10.1179/135100005x83716
Cho JW, Lee KS, Kim CW (2007) Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets. Int J Mol Med 19:469–474κ
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, United States of America
Dagdeviren S et al (2016) Altered interleukin-10 signaling in skeletal muscle regulates obesity-mediated inflammation and insulin resistance. Mol Cell Biol 36:2956–2966. https://doi.org/10.1128/mcb.00181-16
Davis JM et al (2007) Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol 292:R2168–2173. https://doi.org/10.1152/ajpregu.00858.2006
Driessen-Kletter MF, Amelink GJ, Bar PR, van Gijn J (1990) Myoglobin is a sensitive marker of increased muscle membrane vulnerability. J Neurol 237:234–238
Drobnic F et al (2014) Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva(R)): a randomised, placebo-controlled trial. J Int Soc Sports Nutr 11:31. https://doi.org/10.1186/1550-2783-11-31
Fan Z et al (2014) The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model. Oxid Med Cell Longev 2014:191624. https://doi.org/10.1155/2014/191624
Fattori V et al (2015) Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-κB activation. Inflamm Res 64:993–1003. https://doi.org/10.1007/s00011-015-0885-y
Friden J, Lieber RL (1992) Structural and mechanical basis of exercise-induced muscle injury. Med Sci Sports Exerc 24:521–530
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11:607–615. https://doi.org/10.1038/nri3041
González-Bartholin R, Mackay K, Valladares D, Zbinden-Foncea H, Nosaka K, Peñailillo L (2019) Changes in oxidative stress, inflammation and muscle damage markers following eccentric versus concentric cycling in older adults. Eur J Appl Physiol 119:2301–2312. https://doi.org/10.1007/s00421-019-04213-7
Howatson G, van Someren KA (2008) The prevention and treatment of exercise-induced muscle damage. Sports Med 38:483–503
Howatson G et al (2010) Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports 20:843–852. https://doi.org/10.1111/j.1600-0838.2009.01005.x
Jastrzebski Z, Zychowska M, Radziminski L, Konieczna A, Kortas J (2015) Damage to liver and skeletal muscles in marathon runners during a 100 km run with regard to age and running speed. J Hum Kinet 45:93–102. https://doi.org/10.1515/hukin-2015-0010
Kadian N, Raju KSR, Rashid M, Malik MY, Taneja I, Wahajuddin M (2016) Comparative assessment of bioanalytical method validation guidelines for pharmaceutical industry. J Pharm Biomed Anal 126:83–97. https://doi.org/10.1016/j.jpba.2016.03.052
Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, Collins R, Cooke M, Davis JN, Galvan E, Greenwood M, Lowery LM, Wildman R, Antonio J, Kreider RB (2018) ISSN exercise and sports nutrition review update: research and recommendations. J Int Soc Sports Nutr 15:38. https://doi.org/10.1186/s12970-018-0242-y
Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y (1993) Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 41:1640–1643. https://doi.org/10.1248/cpb.41.1640
Li C et al (2019) Curcuminoids: implication for inflammation and oxidative stress in cardiovascular diseases. Phytother Res 33:1302–1317. https://doi.org/10.1002/ptr.6324
Lippi G et al (2008) Acute variation of biochemical markers of muscle damage following a 21-km, half-marathon run. Scand J Clin Lab Inv 68:667–672. https://doi.org/10.1080/00365510802126844
Lippi G, Schena F, Montagnana M, Salvagno GL, Banfi G, Guidi GC (2011) Significant variation of traditional markers of liver injury after a half-marathon run. Eur J Int Med 22:e36–38. https://doi.org/10.1016/j.ejim.2011.02.007
Margaritelis NV, Theodorou AA, Kyparos A, Nikolaidis MG, Paschalis V (2019) Effect of body composition on redox homeostasis at rest and in response to exercise: the case of underfat women. J Sports Sci 37:1630–1637. https://doi.org/10.1080/02640414.2019.1578450
Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A (2017) Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2017.1358139
Mosser DM, Zhang X (2008) Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226:205–218. https://doi.org/10.1111/j.1600-065X.2008.00706.x
Nicol LM, Rowlands DS, Fazakerly R, Kellett J (2015) Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur J Appl Physiol 115:1769–1777. https://doi.org/10.1007/s00421-015-3152-6
Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA, Panayiotou G, Grivas GV, Zafeiridis A, Dipla K, Vrabas IS (2013) Aging is not a barrier to muscle and redox adaptations: applying the repeated eccentric exercise model. Exp Gerontol 48:734–743. https://doi.org/10.1016/j.exger.2013.04.009
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515:287–291. https://doi.org/10.1111/j.1469-7793.1999.287ad.x
Peake JM, Neubauer O, Della Gatta PA, Nosaka K (2017) Muscle damage and inflammation during recovery from exercise. J Appl Physiol 122:559–570. https://doi.org/10.1152/japplphysiol.00971.2016(Bethesda, Md : 1985)
Petersen AM, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol 98:1154–1162. https://doi.org/10.1152/japplphysiol.00164.2004(Bethesda, Md : 1985)
Poudel A, Pandey J, Lee HK (2019) Geographical discrimination in curcuminoids content of turmeric assessed by rapid UPLC-DAD validated analytical method. Molecules. https://doi.org/10.3390/molecules24091805
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res Int 2014:761264. https://doi.org/10.1155/2014/761264
Sahin K, Pala R, Tuzcu M, Ozdemir O, Orhan C, Sahin N, Juturu V (2016) Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: an in vivo model. J Inflamm Res 9:147–154. https://doi.org/10.2147/jir.s110873κ
Sakkiadi AV, Georgiou CA, Haroutounian SA (2007) A standard addition method to assay the concentration of biologically interesting polyphenols in grape berries by reversed-phase HPLC. Molecules 12:2259–2269
Schiborr C, Eckert GP, Rimbach G, Frank J (2010) A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem 397:1917–1925. https://doi.org/10.1007/s00216-010-3719-3
Sciberras JN, Galloway SD, Fenech A, Grech G, Farrugia C, Duca D, Mifsud J (2015) The effect of turmeric (Curcumin) supplementation on cytokine and inflammatory marker responses following 2 hours of endurance cycling. J Int Soc Sports Nutr 12:5. https://doi.org/10.1186/s12970-014-0066-3
Tanabe Y et al (2015) Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin. Eur J Appl Physiol 115:1949–1957. https://doi.org/10.1007/s00421-015-3170-4
Tanabe Y, Chino K, Ohnishi T, Ozawa H, Sagayama H, Maeda S, Takahashi H (2019a) Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation. Scand J Med Sci Sports 29:524–534. https://doi.org/10.1111/sms.13373
Tanabe Y, Chino K, Sagayama H, Lee HJ, Ozawa H, Maeda S, Takahashi H (2019b) Effective timing of curcumin ingestion to attenuate eccentric exercise-induced muscle soreness in men. J Nutr Sci Vitaminol (Tokyo) 65:82–89. https://doi.org/10.3177/jnsv.65.82
Trombold JR, Barnes JN, Critchley L, Coyle EF (2010) Ellagitannin consumption improves strength recovery 2–3 days after eccentric exercise. Med Sci Sports Exerc 42:493–498. https://doi.org/10.1249/MSS.0b013e3181b64edd
Zhou H, Beevers CS, Huang S (2011) The targets of curcumin. Curr Drug Targets 12:332–347
Acknowledgements
The authors would like to thank Alcides Correia de Moraes Júnior, Alexandre Albuquerque, Marina Monteiro Celestino, Anna Paula Oliveira Gomes, Monallisa Alves Ferreira, Patricia Borges Botelho, Ronyson Camilo Soares, and Tatyanne Letícia Nogueira Gomes for their assistance during data collection.
Funding
The study was supported by the National Council for Scientific and Technological Development (CNPq), Brazil (484023/2013-6). Joao Felipe Mota has been financially supported by CNPq. (305082/2019-1).
Author information
Authors and Affiliations
Contributions
JFM and MSA designed research; FRF and ACG conducted research; FRF, ACG, KRR, AA, CLPO, GDP, BMA, FL, and JFM participated in acquisition and/or the analysis of data; FRF and ACG wrote the paper; all authors participated in the interpretation of the reported results and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Michalis G Nikolaidis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faria, F.R., Gomes, A.C., Antunes, A. et al. Effects of turmeric extract supplementation on inflammation and muscle damage after a half-marathon race: a randomized, double-blind, placebo-controlled trial. Eur J Appl Physiol 120, 1531–1540 (2020). https://doi.org/10.1007/s00421-020-04385-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04385-7