Abstract
Purpose
To examine if ad libitum drinking will adequately support hydration during exertional heat stress.
Methods
Ten endurance-trained runners ran for 2 h at 60% of maximum oxygen uptake under different conditions. Participants drank water ad libitum during separate trials at mean ambient temperatures of 22 °C, 30 °C and 35 °C. Participants also completed three trials at a mean ambient temperature of 35 °C while drinking water ad libitum in all trials, and with consumption of programmed glucose or whey protein hydrolysate solutions to maintain euhydration in two of these trials. Heart rate, oxygen uptake, rectal temperature, perceived effort, and thermal sensation were monitored, and nude body mass, hemoglobin, hematocrit, and plasma osmolality were measured before and after exercise. Water and mass balance equations were used to calculate hydration-related variables.
Results
Participants adjusted their ad libitum water intake so that the same decrease in body mass (1.1–1.2 kg) and same decrease in body water (0.8–0.9 kg) were observed across the range of ambient temperatures which yielded significant differences (p < .001) in sweat loss. Overall, water intake and total water gain replaced 57% and 66% of the water loss, respectively. The loss in body mass and body water associated with ad libitum drinking resulted in no alteration in physiological and psychophysiological variables compared with the condition when hydration was nearly fully maintained (0.3 L body water deficit) relative to pre-exercise status from programmed drinking.
Conclusions
Ad libitum drinking is an appropriate strategy for supporting hydration during running for 2 h duration under hot conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is continued disagreement about whether or not hydration can be adequately maintained by “drinking to the dictates of thirst” (Armstrong et al. 2016a, b; Hoffman et al. 2016b, c), which has been suggested to be interchangeable with “ad libitum drinking” (Armstrong et al. 2014). However, once recognizing that body mass loss does not fully and directly equate to body water loss (Cheuvront and Kenefick 2017; Cheuvront and Montain 2017; Hoffman et al. 2018; Maughan et al. 2007), current evidence suggests that ad libitum drinking, even during prolonged exercise under hot conditions, generally provides for adequate hydration (Costa et al. 2013, 2014; Dempster et al. 2013; Hoffman and Stuempfle 2014, 2016; Nolte et al. 2011; Tam et al. 2011).
Ad libitum drinking has been shown to attenuate thermal and circulatory strain (Armstrong et al. 1997). Most studies have also demonstrated that ad libitum drinking during exercise does not impair performance compared with higher volumes of fluid intake (de Melo-Marins et al. 2018; Dion et al. 2013; Dugas et al. 2009; Lee et al. 2014; Lopez et al. 2016), though this finding is not consistent (Bardis et al. 2017) and hydration guidelines commonly indicate that ad libitum drinking is an inadequate hydration strategy during prolonged exercise (Hoffman et al. 2016a).
The present work further explores the effect of ad libitum drinking on plasma osmolality, change in plasma volume and body water balance from 2-h running trials that were originally performed to study gastrointestinal perturbations and symptoms (Snipe et al. 2017, 2018a, b). Analysis 1 of the present work, compares these hydration variables with ad libitum water intake during exercise across a range of ambient temperature conditions. Analysis 2 compares hydration variables and physiological and psychophysiological responses for ad libitum water intake with that for programmed fluid consumption resulting in nearly complete replacement of exercise-associated body water losses during the prolonged exercise in a hot ambient temperature.
Methods
Participants
Study participants were endurance-trained runners living in and around Melbourne, Victoria, Australia, where the studies were performed. All experimental procedures were conducted during the cooler seasonal periods (ambient temperatures consistently ≤ 20 °C). Analysis 1 had ten subjects (six men and four women) with mean (± SD) age of 31 ± 6 years, nude body mass of 66.3 ± 10.5 kg, height of 1.71 ± 0.10 m, and maximum oxygen uptake (\(\dot {V}{{\text{O}}_{{\text{2max}}}}\)) of 55 ± 8 mL/kg/min. Analysis 2 had ten subjects (six men and four women) with mean (± SD) age of 32 ± 5 years, nude body mass of 65.5 ± 12.6 kg, height of 1.72 ± 0.10 m, and \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) of 55 ± 7 mL/kg/min. Eight individuals (five men and three women) participated in trials for both analyses. The subjects and the researchers at the time of data collection were unaware that the data would be used for analysis of hydration-related variables. The study was approved by the local ethics committee and all participants provided written informed consent.
Experimental procedures
One week before the first experimental trial, height and nude body mass were measured and \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) (Vmax Encore Metabolic Cart; Carefusion, San Diego, CA, USA) was determined by a continuous incremental exercise test to volitional exhaustion on a motorized treadmill (Forma Run 500; Technogym, Seattle, WA, USA) as previously described (Costa et al. 2009). Running speed to generate approximately 60% \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) at 1% gradient was extrapolated and then verified from individual relationships between oxygen uptake (\(\dot {V}{{\text{O}}_{\text{2}}}\)) and speed.
Trials for analysis 1 and analysis 2 were conducted in a randomized order with each trial separated by at least 1 week. All trials consisted of 2 h of running on a motorized treadmill at the previously determined speed eliciting 60% of \(\dot {V}{{\text{O}}_{{\text{2max}}}}\), initiated at the same time of day (0900 h). Trials were performed in an environmental chamber with temperature and relative humidity recorded at 10-min intervals during the exercise. For analysis 1, mean (± SD) ambient temperatures and relative humidities were 22.2 ± 1.2 °C and 44 ± 6%, 30.2 ± 0.9 °C and 35 ± 7%, and 35.2 ± 2.1 °C and 26 ± 4% for the three trials. Analysis 2 conditions were the same at 35.4 ± 2.2 °C and 26 ± 4%, 35.5 ± 1.4 °C and 27 ± 6%, and 35.5 ± 2.2 °C and 28 ± 5% for the three trials. These conditions translate to mean wet bulb globe temperatures (WBGTs) of 17 °C, 23 °C and 26 °C. For perspective, it has been advised that an increased risk for exertional heat illnesses, such as heat exhaustion and heatstroke, begins at WBGTs of 18 °C and exercise should be discontinued when WBGTs are above 28 °C (American College of Sports Medicine et al. 2007a).
As part of the gastrointestinal studies reported elsewhere (Snipe et al. 2017, 2018a, b), participants were provided a low-fermentable oligo-, di-, monosaccharide, and polyol (FODMAP) diet, inclusive of 35 ml/kg/day of water, for the 24-h period before each experimental trial as previously described (Snipe et al. 2017, 2018a, b), and were free to consume additional water as desired, but refrained from consuming alcohol or caffeinated beverages during this time. They also refrained from strenuous exercise for 48 h before each trial. Two hours before each trial (0700 h), subjects consumed a standardized low FODMAP breakfast with 400 ml of water. They were asked to void before a nude body mass measurement was made and recorded to the nearest 0.1 kg (Seca 515 MBCA; Seca Group, Hamburg, Germany). During voiding, subjects provided a mid-flow urine sample into a 30 ml universal tube. After seated rest for 5–10 min in a 20 °C room, blood was collected from the subject by venipuncture from an antecubital vein into a vacutainer (6 mL, 1.5 IU/mL heparin). Subjects then inserted a thermocouple 12 cm beyond the external anal sphincter (Grant REC soft insertion probe thermocouple; Grant 2010 Squirrel data logger, Shepreth, UK).
In analysis 1, participants were provided water and advised to drink ad libitum. In analysis 2, subjects drank water ad libitum in one trial. In the other trials as part of the gastrointestinal studies reported elsewhere (Snipe et al. 2017, 2018a, b), they were provided either an in-house formulated flavored glucose solution (255 kJ of which protein = 0 g, carbohydrate = 15.0 g, and fat = 0 g, 6.0% mass/volume; Glucodin, Valeant, Laval, Que., Canada) or an in-house formulated flavored whey protein hydrolysate solution (255 kJ of which protein = 14.8 g, carbohydrate = 0.1 g, and fat = 0.1 g, 6.4% mass/volume; Tatua HWP406, Morrinsville, New Zealand) immediately pre-exercise and every 20 min during the 2-h run for a total of ~ 1400 ml, with additional water provided to be consumed ad libitum. The water for ad libitum consumption was provided in a 750-ml insulated opaque sports bottle with high-flow bite valve (Podium Big Chill .75L – Race Edition, Camelbak, Petaluma, CA, USA) that was placed directly in front of the subject so it could be easily reached while on the treadmill. The bottle was checked every 20 min and refilled when emptied or at the request of the subject. In all trials, 100 ml of water with 5 g lactulose (Duphalac; Abbott Biologicals, Olst, Netherlands) and 1 g L rhamnose (MP Biomedicals; LLC, Solon, USA) was consumed at 90 min into exercise as part of the gastrointestinal studies reported elsewhere (Snipe et al. 2017, 2018a, b). Fluid temperature was measured (Acurite, Lake Geneva, WI, USA) and maintained at 22 °C. Subjects were allowed to stop briefly to urinate during the run when necessary, but a full 2 h of running was still completed. Urine mass was measured to the nearest g (digital precision APTK-461 scales).
Heart rate (Polar Electro, Kempele, Finland), rating of perceived exertion (RPE) (Borg 1982), thermal comfort rating (13-point Likert-type scale with 7 indicative of comfortable, 10 indicative of hot, and 13 indicative of unbearably hot, adapted from Hollies and Goldman 1977), thirst rating (10-point Likert-type scale with 0 indicative of no thirst, 5 indicative of moderate thirst, and 10 indicative of extremely high thirst) (Miall et al. 2018), and rectal temperature were recorded every 10 min while the subjects were running. Breath-by-breath indirect calorimetry (Vmax Encore Metabolic Cart, CaseFusion-BD, Franklin Lakes, NJ, USA) was used to determine \(\dot {V}{{\text{O}}_2}\), carbon dioxide production (\(\dot {V}{\text{C}}{{\text{O}}_2}\)), and respiratory quotient for 5 min continuously every 20 min during the run. Total water intake was also determined during each trial through measurements to the nearest g (digital precision APTK-461 scales).
Immediately after exercise, nude body mass was again measured on the same scale after towel drying, and a blood sample was collected from the subject after seated rest in a 20 °C room, mirroring pre-exercise procedures. Blood samples were analyzed for whole-blood hemoglobin and hematocrit for determination of change in plasma volume (Dill and Costill 1974). Hemoglobin was determined in duplicate using lithium heparin blood samples (HemoCue Hb 201, HemoCue AB, Angelholm, Sweden) and hematocrit was determined in triplicate by capillary method (Maughan et al. 2001) using lithium heparin blood samples and a micro-hematocrit reader. Aliquots of plasma in heparin (50 µl) were used to determine plasma osmolality in duplicate [coefficient of variation (CV): 2.7%], by freezepoint osmometry (Osmomat 030, Gonotec, Berlin, Germany). In the 8 of 60 cases when pre-exercise plasma osmolality was over 300 mOsmol/kg, pre-exercise urine-specific gravity and urine osmolality were determined and verified to be below 1.020 and 600 mOsmol/kg, respectively, to assure subjects were euhydrated at the start of exercise (Armstrong 2007; Armstrong et al. 1994).
Calculations
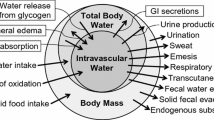
Body water balance
Body water balance (Δbody water) is accounted for by a basic equation that includes water gain (Wgain) and water loss (Wloss) as follows (Consolazio et al. 1963):
where,
and
In the context of this study, no participant required defecation between the pre-exercise and post-exercise body mass measurements and we considered fecal mass and fecal water content to be unchanged, so Wfeces can be removed from the equation. Additionally, no solid food was consumed, so Wfood can also be removed. Thus, the remaining components include water consumed in drink (Wdrink), water generated from fuel oxidation (Wmet), and water lost in urine (Wurine), through sweat (Wskin) and through respiration (Wresp), yielding:
W drink and Wurine were measured in this study, while the other variables were calculated as outlined below.
Metabolic water
Total carbohydrate and fat oxidation (g/min) were calculated from the \(\dot {V}{{\text{O}}_2}\) and \(\dot {V}{\text{C}}{{\text{O}}_{\text{2}}}\) (L/min) determined during each measurement period using nonprotein respiratory quotient values in accordance with Péronnet and Massicotte (1991) such that:
W met was then calculated as the sum of metabolic water from carbohydrate oxidation and the metabolic water from fat oxidation, with 0.60 and 1.13 being the proportional mass of water generated from oxidation of carbohydrate and fat, respectively (Maughan et al. 2007). The effect of protein oxidation was considered negligible under the study conditions and was, therefore, ignored.
Respiratory water
W resp was determined after calculating the rate of evaporative water loss in expired air (\({\dot {m}_{\text{e}}}\), g/min), from \(\dot {V}{{\text{O}}_2}\) (L/min) and the ambient water vapor pressure (Pa, mmHg) (Mitchell et al. 1972):
Sweat
W skin determination was based on a mass balance equation accounting for the mass exchange of all liquids, solids and gases between a subject and the environment (Consolazio et al. 1963):
As noted above, we can exclude Wfeces and Wfood in the context of this study. Thus, solving for Wskin yields:
The change in body mass (Δbody mass), Wdrink and Wurine were measured, and Wresp was calculated as described above. The mass of solids taken in (solidsin) was the sum of solid mass in the dual sugar solution for all trials plus the solid mass in the programmed glucose and whey protein hydrolysate solutions when taken. The mass of solids lost (solidsout) was considered to be zero. The change in mass due to gas exchange (gasesin − gasesout) was calculated with consideration that oxygen inhalation and carbon dioxide exhalation were 1.07 and 1.47 g per g of carbohydrate oxidized, and 2.88 and 2.75 g per g of fat oxidized (Maughan et al. 2007).
Assumptions of equivalence were accepted for 1 kg equating to 1 L for water, sweat and urine in all calculations (Cheuvront and Kenefick 2017).
Statistical analysis
Power calculations were based on previously reported impact of exertional heat stress on markers of cardiovascular and thermoregulatory strain (Snipe et al. 2018a, b; Snipe and Costa 2018). This resulted in an effect size (Cohen’s d) of 1.04, 2.86, 3.39 and 2.73 for rectal temperature, heart rate, thermal comfort rating and RPE, respectively. Using standard alpha (0.025) and beta (0.95) values, the current cohort size (n = 10) provides sufficient power for adequate statistical precision to detect the magnitude of effect on physiological and psychophysiological responses.
Comparisons of pre-exercise to post-exercise hydration-related variables and rectal temperature at the cessation of running among the two sets of three conditions were made with one-way repeated measures analysis of variance and Tukey–Kramer post hoc tests since most of the data were found to be normally distributed by the D’Agostino-Pearson normality test. Environmental, physiological and psychophysiological measurements made at multiple time points during each trial were compared with two-way (condition x time) repeated measures analysis of variance. For examination of the relationships between ad libitum water intake and total water gain with total water loss, mean slopes from individual linear regressions forced to pass through the origin were determined. Significance was set at p ≤ .05.
Results
Analysis 1
The mean (± SD) running speed for trials in analysis 1 was 10.4 ± 0.8 km/h. Mean (± SD) heart rates (overall trial values 146 ± 15 beats/min at 22 °C, 155 ± 14 beats/min at 30 °C, and 163 ± 16 beats/min at 35 °C; p = .038), RPEs (11 ± 1 at 22 °C, 12 ± 2 at 30 °C, and 14 ± 2 at 35 °C; p = .011), and thermal comfort ratings (8 ± 1 at 22 °C, 9 ± 1 at 30 °C, and 10 ± 1 at 35 °C; p = .0016) during running, and rectal temperature at the cessation of running (38.4 ± 0.4 °C at 22 °C, 38.8 ± 0.6 °C at 30 °C, and 39.6 ± 0.7 °C at 35 °C; p < .0001) were significantly affected by ambient temperature, confirming findings reported previously (Snipe et al. 2018a, b). Thirst rating showed a small (p = .041) time effect, but no condition (p = .074) or interaction (p = .82) effect and mean values remained well below “moderate thirst” throughout the exercise (Fig. 1).
Thirst ratings during 2 h of running at 60% \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) at three different ambient temperatures while drinking water ad libitum. Brackets represent 1 SD and are shown in only one direction for two conditions to preserve clarity. Statistical analysis revealed a small time effect (p = .046) but no condition or interaction effects
Comparisons of pertinent hydration variables for ad libitum drinking of water across the three different ambient temperature conditions are shown in Table 1. Across the wide ambient temperature range, mean measured body mass change did not differ, ranging from − 1.7% to − 1.9%. Plasma volume change and urine output were also not different among conditions. Significantly different (p < .0001) sweat losses among the three conditions were partially offset by significantly different (p < .0001) water intakes so that mean body water loss amounted to 0.8–0.9 L across all three conditions. Mean post-exercise plasma osmolalities of 294–295 mOsmol/kg did not differ (p = .058) from pre-exercise values of 292–293 mOsmol/kg or across temperature conditions (p = .94).
Individual relationships for water intake and total water gain with total water loss are shown in Fig. 2. Mean (± SD) slopes of 0.57 ± 0.07 and 0.66 ± 0.09 indicate that water intake and total water gain replaced on average 57% and 66% of the water loss, respectively.
Individual linear regressions for ad libitum water intake (top) and total water gain (bottom) with total water loss during 2 h of running at 60% \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) and mean ambient temperatures of 22 °C, 30 °C and 35 °C. Each line extends across the range of values for the given subject. The dotted lines are the lines of equality. The mean (± SD) slopes of the linear regressions forced to pass through the origin were 0.57 ± 0.07 and 0.66 ± 0.09, respectively
Analysis 2
The mean (± SD) running speed for trials in analysis 2 was 10.4 ± 0.7 km/h. Mean (± SD) heart rates (overall trial values 160 ± 17 beats/min for ad libitum water, 155 ± 15 beats/min for programmed glucose solution, and 154 ± 14 beats/min for programmed whey protein hydrolysate solution; p = .61), RPEs (14 ± 2 for ad libitum water, 13 ± 2 for programmed glucose solution, and 13 ± 2 for programmed whey protein hydrolysate solution; p = .65), and thermal comfort ratings (9 ± 1 for all conditions; p = .94) during running, and rectal temperature at the cessation of running (39.2 ± 0.8 °C for ad libitum water, 38.9 ± 0.7 °C for programmed glucose solution, and 39.0 ± 0.7 °C for programmed whey protein hydrolysate solution; p = .19) did not differ among conditions, confirming findings reported previously (Snipe et al. 2017). Thirst rating showed no time (p = .17) or condition (p = .20) effect, but there was a significant (p = .017) interaction effect suggesting that thirst followed a different pattern with the ad libitum water condition compared with the other conditions, but mean values remained well below “moderate thirst” (Fig. 3).
Thirst ratings during 2 h of running at 60% \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) and 35 °C while drinking water ad libitum (Water ad lib) or drinking either a programmed glucose solution (GLUC + ad lib water) or whey protein hydrolysate solution (WPH + ad lib water) and drinking water ad libitum. Brackets represent 1 SD and are shown in only one direction for two conditions for clarity. Statistical analysis revealed no time or condition effects, but there was a significant (p = .011) interaction effect
Comparisons of pertinent hydration variables for ad libitum drinking of water with and without programmed intake of a glucose or whey protein hydrolysate solution are shown in Table 2. The mean measured body mass change of -1.6% for the ad libitum drinking condition was significantly different (p < .01) from the small changes of − 0.7% with the glucose condition and − 0.6% for the whey protein hydrolysate condition. The glucose and whey protein hydrolysate conditions were also associated with minimal decrease in plasma volume and body water, which were significantly more (p < .01) for the ad libitum water condition. These effects were due to a significantly greater (p < .05) water intake with the glucose and whey protein hydrolysate conditions, while urine output remained the same. A small (0.2 L) but significantly greater (p < .05) sweat loss occurred with the ad libitum condition compared with the other conditions. Mean post-exercise plasma osmolalities of 284–292 mOsmol/kg did not differ (p = .33) from pre-exercise values of 285–291 mOsmol/kg or across conditions (p = .45).
Discussion
This work reveals that during 2 h of running at a moderate intensity (1) ad libitum water intake was adjusted so that hydration status at exercise completion was comparable across a wide range of ambient temperatures causing meaningful differences in sweat loss, and (2) consumption of programmed glucose or whey protein hydrolysate solutions along with ad libitum water yielding nearly complete maintenance of euhydration throughout the exertional heat stress resulted in no improvement in physiological and psychophysiological responses compared with ad libitum water intake that yielded a mean body mass loss of 1.6%.
Ad libitum drinking is commonly considered to be an inadequate strategy for maintaining euhydration during prolonged exercise according to much of the scientific literature (Armstrong et al. 2016a, b; Bardis et al. 2017; Kenefick 2018), in professional sports, exercise nutrition and dietetic practice, and in most resources accessed by the public (Hoffman et al. 2016a). A recent publication concluded that circumstances where drinking to thirst may be sufficient “include activities or competitions of < 1–2 h of duration, that are of lower exercise intensity, and that take place in cool or temperate environments” but otherwise, “a tailored programed drinking strategy will need to be employed to avoid potential thermoregulatory, cardiovascular, and exercise performance impairment (2% body mass loss)” (Kenefick 2018). Such inferences seem to be largely based upon the acceptance of hydration guidelines recommending that no more than 2% of body mass should be lost during exercise without regard for exercise duration (American College of Sports Medicine et al. 2007a, b; American Dietetic Association et al. 2009; Casa et al. 2000, 2005; Kreider et al. 2010; McDermott et al. 2017; Thomas et al. 2016). It is not unusual for those drinking in accordance to thirst during prolonged exercise to drop below this 2% body mass margin (Cheuvront and Haymes 2001; Dion et al. 2013; Dugas et al. 2009; Hoffman et al. 2013; Lopez et al. 2016; Noakes et al. 2005), so this may be used as further evidence for the inadequacy of ad libitum drinking. But, it is important to recognize the relevance of mass loss from endogenous fuel utilization and water generation from fuel oxidation in prolonged activities due to the additive effect of these factors that are relatively trivial during short periods of exercise. In fact, for the present 2-h exercise trials, our calculations demonstrate that roughly 0.3 kg (~ 0.5% body mass) loss was required to maintain a constant total body water pool.
The present work demonstrates the capability of humans to use thirst to adjust water intake in response to the extent of water loss during exercise. Across a range of mean sweat loss from 1.7 to 2.5 L, water intake was adjusted so that mean body mass loss was limited to 1.6–1.9% at the end of the 2 h of running in different ambient temperature conditions. An innate ability to increase water intake to support exercise circumstances where water losses are greater is not a new finding. Previous work examined hydration status during ad libitum drinking among women running 30 km at a mean intensity of 71% \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) across WBGTs of 12 to 25 °C (Cheuvront and Haymes 2001). In that study, it was found that the women finished with similar mean body mass losses of 2.4–2.8% across the different temperature conditions by drinking more under the hottest condition. That study also demonstrated that ad libitum water intake replaced 63–73% of sweat losses, whereas the present study found that, on average, 57% of the water loss was replaced by water intake and 66% was replaced through the combination of water intake and generation of water during fuel oxidation.
Since water losses are not fully replaced through ad libitum water intake or even through the combination of ad libitum water intake and the production of metabolic water, some might consider this supporting evidence for the early belief that ad libitum drinking is inadequate to support fluid needs during exercise (Dill et al. 1933; Greenleaf and Sargent 1965). On the other hand, the present work demonstrates that there was no negative effect on plasma osmolality, heart rate, rectal temperature, RPE and thermal comfort rating during moderate intensity running from a 1.6% body mass loss (and calculated 0.7 L deficit in body water) compared with a 0.6–0.7% body mass loss (and calculated 0.3 L deficit in body water). Since the fluid deficits from ad libitum water intake were not associated with evidence for physiological or psychophysiological impairment, the findings could reflect the presence of internal cues that stimulate adequate drinking to limit the water deficit by the end of exercise to a magnitude that will not adversely affect exercise responses.
It is appropriate to note the controversy about the fate of water generated during fuel oxidation. Some have suggested that endogenous sources of water generation play an important role in offsetting the extent of fluid needs during exercise (Hoffman et al. 2018; Maughan et al. 2007; Nolte et al. 2011; Pastene et al. 1996; Rogers et al. 1997). In contrast, others believe it is best to consider this water as being noncontributory to hydration status even though they may acknowledge that there should be some mass loss during prolonged exercise (Cheuvront and Montain 2017). In this work, we considered the water generated during fuel oxidation to become available to support the intravascular volume. This metabolic water amounted to 0.21–0.22 L, representing an additional 13–22% volume of water beyond that consumed during ad libitum drinking, so it is relevant in hydration considerations. As previously noted (Hoffman et al. 2018), it is reasoned that this water generated within the cell will be distributed throughout the body water pool according to local osmotic, oncotic and hydrostatic gradients. Since we do not anticipate a large increase in intracellular osmolality in active muscles due to the accumulation of glycolytic intermediates and end products during modest intensity exercise, such exercise should cause relatively little disturbance to the osmolality of tissues that would promote retention of the water within the cells (Lundvall et al. 1969). Thus, it seems reasonable to consider this water as being available to contribute to the intravascular fluid compartment. In contrast, we ignored all water that might be liberated in the oxidation of glycogen based upon recent evidence suggesting that most of the water associated with glycogen may be osmotically active (King et al. 2018).
Avoiding significant hypohydration during 2 h of running is not very difficult. In fact, no water intake would have resulted in 3.2–4.4% body mass loss for the exercise trials examined here, assuming sweat rate and urinary output was unchanged, which may overestimate the actual loss that would have occurred since hypohydration lowers sweating rate for a given core temperature (Sawka et al. 2001). To nearly maintain euhydration while exercising at 35 °C, a mean of slightly less than 1 L/h (nearly 2 L total in 2 h) of water was taken in. And to hydrate adequately to avoid evidence of alterations in exercise responses, a mean of less than 850 ml/h of water was consumed. These fluid intakes are well within the tolerable range for most individuals (Lambert et al. 2008). On the other hand, it would be reasonable to question if athletes would voluntarily take in these volumes of fluid over longer bouts of exercise, such as the 8–30 h that might be required to complete a long course triathlon, or 100-km to 161-km ultramarathon event. Furthermore, if replacing only two-thirds of the water losses during 2 h of exercise via ad libitum drinking, it is legitimate to wonder if an increasing water deficit might develop during longer bouts of exercise. In this regard, prior field studies have observed that ad libitum drinking during long durations of exercise can be effective at maintaining adequate hydration even under hot conditions (Costa et al. 2013, 2014; Dempster et al. 2013; Hoffman and Stuempfle 2014, 2016; Nolte et al. 2011; Tam et al. 2011). In fact, studies at 161-km ultramarathons have shown that ad libitum drinking is associated with a fairly stable body mass after the initial 48 km even though it should actually continue to decrease to maintain euhydration (Hoffman and Stuempfle 2014, 2016).
It is interesting that the mean total programmed water consumed in the glucose and whey protein hydrolysate conditions was approximately 1.5 L, compared with the consumption of a mean of approximately 1.7 L of water in the ad libitum condition. The greater total water consumption in the programmed drinking conditions compared with the ad libitum condition resulted from the subjects choosing to drink a mean of 0.4–0.5 L of additional water beyond the programmed fluid intake. Participants reported that this water intake was not in response to thirst or interest in drinking, but was rather consumed to rinse and cleanse the mouth of the prior consumed nutrient rich solution. Future studies examining ad libitum drinking should consider this factor when selecting the fluids to be consumed. Furthermore, this phenomenon could be used to encourage additional fluid intake when necessary and might partially account for the observed effectiveness of ad libitum drinking in field studies involving prolonged exercise.
We acknowledge some limitations to generalization of the present findings, namely that the exercise only lasted 2 h, which limited the potential extent of hypohydration. For this reason, the present data do not allow us to make conclusions about the adequacy of ad libitum drinking to support hydration during longer bouts of exercise where it is possible that greater water deficits could develop as noted above. Thus, it would be valuable to examine hydration variables in the laboratory, as was done here, for longer bouts of exercise. The findings are also specific for exercise situations in which fluid is freely available during exercise, which is often the case with prolonged running and cycling because water can be carried by the athlete, but is not the case with other activities, especially team sports. Another study limitation is that the exercise bouts did not include a period of maximum or near maximum exertion to adequately test for performance limitations from the water deficit of 0.7 L with the ad libitum drinking condition. On the other hand, performance at a high intensity during long periods of exercise has little practical relevance for many endurance and ultra-endurance activities. Furthermore, several prior studies have suggested that high intensity exercise is not impaired with ad libitum drinking compared with greater intake during exercise lasting ~ 90 min to over 2 h (Dion et al. 2013; Dugas et al. 2009; Lee et al. 2014; Lopez et al. 2016). A minor limitation was that body mass measurements were to the nearest 0.1 kg which limited the calculated sweat loss and body water change to the nearest 0.1 L. Finally, we cannot exclude the possibility that ad libitum drinking behavior might have been influenced by the request for a thirst rating every 10 min and the 5-min \(\dot {V}{{\text{O}}_2}\) measurements every 20 min during the runs. We believe the former was unlikely to have had an important effect since a prior study of similar exercise duration that did not assess thirst rating (Lee et al. 2014) found even less decrease in body mass with self-selected drinking than the present work, and it also seems unlikely that the request of thirst ratings would have altered fluid intake for athletes who are already attentive to internal cues. In terms of the latter issue, we believe the inability to drink during these short time periods would have been offset by a transient increase in water intake once allowed to drink.
Conclusion
In conclusion, this work demonstrates that during 2 h of running exercise in which water was readily accessible, ad libitum water intake was adjusted so that plasma osmolality and plasma volume were preserved within physiologically acceptable limits across a wide range of ambient temperatures resulting in large differences in sweat loss. Furthermore, ad libitum drinking was found to have no adverse effects on physiological and psychophysiological variables compared with a condition when hydration was essentially fully maintained through programmed drinking. Thus, this work is consistent with the prior findings that ad libitum drinking can be an appropriate hydration strategy during two hours of continuous running, even under hot conditions.
Abbreviations
- Pa :
-
Ambient water vapor pressure
- \(\dot {V}{\text{C}}{{\text{O}}_{\text{2}}}\) :
-
Carbon dioxide production
- Δbody mass:
-
Change in body mass
- Δbody water:
-
Change in body water
- gasesin—gasesout :
-
Change in mass due to gas exchange
- FODMAP:
-
Low-fermentable oligo-, di-, monosaccharide, and polyol
- Solidsout :
-
Mass of solids lost
- Solidsin :
-
Mass of solids taken in
- \(\dot {V}{{\text{O}}_{{\text{2max}}}}\) :
-
Maximum oxygen uptake
- \(\dot {V}{{\text{O}}_2}\) :
-
Oxygen uptake
- \({\dot {m}_e}\) :
-
Rate of evaporative water loss in expired air
- RPE:
-
Rating of perceived exertion
- W gain :
-
Total water gain
- W loss :
-
Total water lost
- W food :
-
Water consumed in food
- W drink :
-
Water consumed in drink
- W met :
-
Water generated from fuel oxidation
- W feces :
-
Water lost in feces
- W skin :
-
Water lost in sweat
- W urine :
-
Water lost in urine
- W resp :
-
Water lost through respiration
- WBGT:
-
Wet bulb globe temperature
References
American College of Sports Medicine, Armstrong LE, Casa DJ, Millard-Stafford M, Moran DS, Pyne SW, Roberts WO (2007a) American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc 39(3):556–572. https://doi.org/10.1249/MSS.0b013e31802fa199
American College of Sports Medicine, Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS (2007b) American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39(2):377–390. https://doi.org/10.1249/mss.0b013e31802ca597
American Dietetic Association; Dietitians of Canada; American College of Sports Medicine, Rodriguez NR, Di Marco NM, Langley S (2009) American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc 41(3):709–731. https://doi.org/10.1249/MSS.0b013e31890eb86
Armstrong LE (2007) Assessing hydration status: the elusive gold standard. J Am Coll Nutr 26(5 Suppl):575S–584S
Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, Riebe D (1994) Urinary indices of hydration status. Int J Sport Nutr 4(3):265–279
Armstrong LE, Maresh CM, Gabaree CV, Hoffman JR, Kavouras SA, Kenefick RW, Castellani JW, Ahlquist LE (1997) Thermal and circulatory responses during exercise: effects of hypohydration, dehydration, and water intake. J Appl Physiol 82(6):2028–2035. https://doi.org/10.1152/jappl.1997.82.6.2028
Armstrong LE, Johnson EC, Kunces LJ, Ganio MS, Judelson DA, Kupchak BR, Vingren JL, Munoz CX, Huggins RA, Hydren JR, Moyen NE, Williamson KH (2014) Drinking to thirst versus drinking ad libitum during road cycling. J Athl Train 49(5):624–631. https://doi.org/10.4085/1062-6050-49.3.85
Armstrong LE, Johnson EC, Bergeron MF (2016a) COUNTERVIEW: Is Drinking to thirst adequate to appropriately maintain hydration status during prolonged endurance exercise? No. Wilderness Environ Med 27(2):195–198. https://doi.org/10.1016/j.wem.2016.03.002
Armstrong LE, Johnson EC, Bergeron MF (2016b) REBUTTAL from “No”. Wilderness Environ Med 27(2):200–202. https://doi.org/10.1016/j.wem.2016.04.005
Bardis CN, Kavouras SA, Adams JD, Geladas ND, Panagiotakos DB, Sidossis LS (2017) Prescribed drinking leads to better cycling performance than ad libitum drinking. Med Sci Sports Exerc 49(6):1244–1251. https://doi.org/10.1249/MSS.0000000000001202
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BS, Roberts WO, Stone JA (2000) National Athletic Trainers’ Association position statement: fluid replacement for athletes. J Athl Train 35(2):212–224
Casa DJ, Clarkson PM, Roberts WO (2005) American College of Sports Medicine roundtable on hydration and physical activity: consensus statements. Curr Sports Med Rep 4(3):115–127
Cheuvront SN, Haymes EM (2001) Ad libitum fluid intakes and thermoregulatory responses of female distance runners in three environments. J Sports Sci 19(11):845–854. https://doi.org/10.1080/026404101753113796
Cheuvront SN, Kenefick RW (2017) CORP: Improving the status quo for measuring whole body sweat losses. J Appl Physiol 123(3):632–636. https://doi.org/10.1152/japplphysiol.00433.2017
Cheuvront SN, Montain SJ (2017) Myths and methodologies: making sense of exercise mass and water balance. Exp Physiol 102(9):1047–1053. https://doi.org/10.1113/EP086284
Consolazio CF, Johnson RE, Pecora LJ (1963) Chap. 8. The computation of metabolic balances. In: Physiological measurements of metabolic functions in man. McGraw-Hill Book Company, New York, pp 313–339
Costa RJ, Oliver SJ, Laing SJ, Waiters R, Bilzon JL, Walsh NP (2009) Influence of timing of postexercise carbohydrate-protein ingestion on selected immune indices. Int J Sport Nutr Exerc Metab 19(4):366–384
Costa RJ, Teixeira A, Rama L, Swancott AJ, Hardy LD, Lee B, Camões-Costa V, Gill S, Waterman JP, Freeth EC, Barrett E, Hankey J, Marczak S, Valero-Burgos E, Scheer V, Murray A, Thake CD (2013) Water and sodium intake habits and status of ultra-endurance runners during a multi-stage ultra-marathon conducted in a hot ambient environment: an observational field based study. Nutr J 12:13. https://doi.org/10.1186/1475-2891-12-13
Costa RJ, Gill SK, Hankey J, Wright A, Marczak S (2014) Perturbed energy balance and hydration status in ultra-endurance runners during a 24 h ultra-marathon. Br J Nutr 112(3):428–437. https://doi.org/10.1017/S0007114514000907
de Melo-Marins D, Souza-Silva AA, da Silva-Santos GLL, Freire-Júnior FA, Lee JKW, Laitano O (2018) Personalized hydration strategy attenuates the rise in heart rate and in skin temperature without altering cycling capacity in the heat. Front Nutr 5:22. https://doi.org/10.3389/fnut.2018.00022
Dempster S, Britton R, Murray A, Costa RJ (2013) Case study: Nutrition and hydration status during 4,254 km of running over 78 consecutive days. Int J Sport Nutr Exerc Metab 23(5):533–541
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248. https://doi.org/10.1152/jappl.1974.37.2.247
Dill DB, Bock AV, Edwards HT (1933) Mechanisms for dissipating heat in man and dog. Am J Physiol 104(1):36–43. https://doi.org/10.1152/ajplegacy.1933.104.1.36
Dion T, Savoie FA, Asselin A, Gariepy C, Goulet ED (2013) Half-marathon running performance is not improved by a rate of fluid intake above that dictated by thirst sensation in trained distance runners. Eur J Appl Physiol 113(12):301130–301120. https://doi.org/10.1007/s00421-013-2730-8
Dugas JP, Oosthuizen U, Tucker R, Noakes TD (2009) Rates of fluid ingestion alter pacing but not thermoregulatory responses during prolonged exercise in hot and humid conditions with appropriate convective cooling. Eur J Appl Physiol 105(1):69–80. https://doi.org/10.1007/s00421-008-0876-6
Greenleaf JE, Sargent F (1965) Voluntary dehydration in man. J Appl Physiol 20(4):719–724. https://doi.org/10.1152/jappl.1965.20.4.719
Hoffman MD, Stuempfle KJ (2014) Hydration strategies, weight change and performance in a 161 km ultramarathon. Res Sports Med 22(3):213–225. https://doi.org/10.1080/15438627.2014.915838
Hoffman MD, Stuempfle KJ (2016) Is sodium supplementation necessary to avoid dehydration during prolonged exercise in the heat? J Strength Cond Res 30(3):615–620. https://doi.org/10.1519/JSC.0000000000001138
Hoffman MD, Hew-Butler T, Stuempfle KJ (2013) Exercise-associated hyponatremia and hydration status in 161-km ultramarathoners. Med Sci Sports Exerc 45(4):784–791. https://doi.org/10.1249/MSS.0b013e31827985a8
Hoffman MD, Bross TL, Hamilton RT (2016a) Are we being drowned by overhydration advice on the Internet? Phys Sportsmed 44(4):343–348. https://doi.org/10.1080/00913847.2016.1222853
Hoffman MD, Cotter JD, Goulet ÉD, Laursen PB (2016b) VIEW: is drinking to thirst adequate to appropriately maintain hydration status during prolonged endurance exercise? Yes. Wilderness Environ Med 27(2):192–195. https://doi.org/10.1016/j.wem.2016.03.003
Hoffman MD, Cotter JD, Goulet ÉD, Laursen PB (2016c) REBUTTAL from “Yes”. Wilderness Environ Med 27(2):198–200. https://doi.org/10.1016/j.wem.2016.04.004
Hoffman MD, Goulet EDB, Maughan RJ (2018) Considerations in the use of body mass change to estimate change in hydration status during a 161-kilometer ultramarathon running competition. Sports Med 48(2):243–250. https://doi.org/10.1007/s40279-017-0782-3
Hollies NRS, Goldman RF (1977) Psychological scaling in comfort assessment. In: Clothing comfort: Interaction of thermal, ventilation, construction, and assessment factors. Ann Arbor Science Publishers, Mich., pp 107–120
Kenefick RW (2018) Drinking strategies: planned drinking versus drinking to thirst. Sports Med 48(Suppl 1):31–37. https://doi.org/10.1007/s40279-017-0844-6
King RFGJ, Jones B, O’Hara JP (2018) The availability of water associated with glycogen during dehydration: a reservoir or raindrop? Eur J Appl Physiol 118(2):283–290. https://doi.org/10.1007/s00421-017-3768-9
Kreider RB, Wilborn CD, Taylor L, Campbell B, Almada AL, Collins R, Cooke M, Earnest CP, Greenwood M, Kalman DS, Kerksick CM, Kleiner SM, Leutholtz B, Lopez H, Lowery LM, Mendel R, Smith A, Spano M, Wildman R, Willoughby DS, Ziegenfuss TN, Antonio J (2010) ISSN exercise and sport nutrition review: research and recommendations. J Int Soc Sports Nutr 7:7. https://doi.org/10.1186/1550-2783-7-7
Lambert GP, Lang J, Bull A, Eckerson J, Lanspa S, O’Brien J (2008) Fluid tolerance while running: effect of repeated trials. Int J Sports Med 29(11):878–882. https://doi.org/10.1055/s-2008-1038620
Lee MJ, Hammond KM, Vasdev A, Poole KL, Impey SG, Close GL, Morton JP (2014) Self-selecting fluid intake while maintaining high carbohydrate availability does not impair half-marathon performance. Int J Sports Med 35(14):1216–1222. https://doi.org/10.1055/s-0034-1375635
Lopez RM, Casa DJ, Jensen KA, Stearns RL, DeMartini JK, Pagnotta KD, Roti MW, Armstrong LE, Maresh CM (2016) Comparison of two fluid replacement protocols during a 20-km trail running race in the heat. J Strength Cond Res 30(9):2609–2616. https://doi.org/10.1519/JSC.0000000000001359
Lundvall J, Mellander S, White T (1969) Hyperosmolality and vasodilatation in human skeletal muscle. Acta Physiol Scand 77(1):224–233. https://doi.org/10.1111/j.1748-1716.1969.tb04566.x
Maughan RJ, Leipers JB, Greaves M (2001) Haematology. In: Eston RG, Reilly T (eds) Kinanthropometry and exercise physiology laboratory manual: tests, procedures and data. Exercise physiology. Routledge, Oxon, pp 99–116
Maughan RJ, Shirreffs SM, Leiper JB (2007) Errors in the estimation of hydration status from changes in body mass. J Sports Sci 25(7):797–804. https://doi.org/10.1080/02640410600875143
McDermott BP, Anderson SA, Armstrong LE, Casa DJ, Cheuvront SN, Cooper L, Kenney WL, O’Connor FG, Roberts WO (2017) National Athletic Trainers’ Association position statement: Fluid replacement for the physically active. J Athl Train 52(9):877–895. https://doi.org/10.4085/1062-6050-52.9.02
Miall A, Khoo A, Rauch C, Snipe RMJ, Camões-Costa VL, Gibson PR, Costa RJS (2018) Two weeks of repetitive gut-challenge reduce exercise-associated gastrointestinal symptoms and malabsorption. Scand J Med Sci Sports 28(2):630–640. https://doi.org/10.1111/sms.12912
Mitchell JW, Nadel ER, Stolwijk JA (1972) Respiratory weight losses during exercise. J Appl Physiol 32(4):474–476. https://doi.org/10.1152/jappl.1972.32.4.474
Noakes TD, Sharwood K, Speedy D, Hew T, Reid S, Dugas J, Almond C, Wharam P, Weschler L (2005) Three independent biological mechanisms cause exercise-associated hyponatremia: evidence from 2135 weighed competitive athletic performances. Proc Natl Acad Sci USA 102(51):18550–18555. https://doi.org/10.1073/pnas.0509096102
Nolte HW, Noakes TD, van Vuuren B (2011) Protection of total body water content and absence of hyperthermia despite 2% body mass loss (‘voluntary dehydration’) in soldiers drinking ad libitum during prolonged exercise in cool environmental conditions. Br J Sports Med 45(14):1106–11012. https://doi.org/10.1136/bjsm.2010.075507
Pastene J, Germain M, Allevard AM, Gharib C, Lacour JR (1996) Water balance during and after marathon running. Eur J Appl Physiol Occup Physiol 73(1–2):49–55
Péronnet F, Massicotte D (1991) Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16(1):23–29
Rogers G, Goodman C, Rosen C (1997) Water budget during ultra-endurance exercise. Med Sci Sports Exerc 29(11):1477–1481
Sawka MN, Montain SJ, Latzka WA (2001) Hydration effects on thermoregulation and performance in the heat. Comp Biochem Physiol A Mol Integr Physiol 128(4):679–690
Snipe RMJ, Costa RJS (2018) Does the temperature of water ingested during exertional-heat stress influence gastrointestinal injury, symptoms, and systemic inflammatory profile? J Sci Med Sport 21(8):771–776
Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS (2017) Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl Physiol Nutr Metab 42(12):1283–1292. https://doi.org/10.1139/apnm-2017-0361
Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS (2018a) The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur J Appl Physiol 118(2):389–400. https://doi.org/10.1007/s00421-017-3781-z
Snipe RMJ, Khoo A, Kitic CM, Gibson PR, Costa RJS (2018b) The impact of mild heat stress during prolonged running on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profiles. Int J Sports Med. https://doi.org/10.1055/s-0043-122742. (Epub ahead of print)
Tam N, Nolte HW, Noakes TD (2011) Changes in total body water content during running races of 21.1 km and 56 km in athletes drinking ad libitum. Clin J Sport Med 21(3):218–225. https://doi.org/10.1097/JSM.0b013e31820eb8d7
Thomas DT, Erdman KA, Burke LM (2016) American College of Sports Medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc 48(3):543–568. https://doi.org/10.1249/MSS.0000000000000852
Acknowledgements
The authors would like to thank all the participants that volunteered to take part in this study, and the Monash University Sports Dietetic Research Team and collaborators who supported various aspects of data and sample collection and analysis. We also thank Dr. Eric Goulet for critical review of an early draft of our manuscript. The study was supported by Monash University, Faculty of Medicine, Nursing and Health Sciences, Faculty Strategic Grant Scheme, SGS15-0128. This material is also the result of work supported with resources and the use of facilities at the VA Northern California Health Care System. The contents reported here do not represent the views of the Department of Veterans Affairs or the United States Government.
Funding
The study was supported by Monash University, Faculty of Medicine, Nursing and Health Sciences, Faculty Strategic Grant Scheme, SGS15-0128. This material is also the result of work supported with resources and the use of facilities at the VA Northern California Health Care System.
Author information
Authors and Affiliations
Contributions
RJSC and RMJS conceived and designed the original studies, and conducted the experiments from which the paper evolved. MDH conceptualized the present analysis, analyzed the data for this paper, and drafted the manuscript. All authors contributed to the revision of the initial manuscript, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Narihiko Kondo.
Rights and permissions
About this article
Cite this article
Hoffman, M.D., Snipe, R.M.J. & Costa, R.J.S. Ad libitum drinking adequately supports hydration during 2 h of running in different ambient temperatures. Eur J Appl Physiol 118, 2687–2697 (2018). https://doi.org/10.1007/s00421-018-3996-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3996-7