Abstract
Introduction
Exercise training is recommended for improving health and protecting against the development of metabolic and cardiovascular pathologies. Combined resistance and aerobic exercise training (CRAE) has been shown to provide unique benefits in older adults with cardiovascular diseases.
Purpose
We sought to determine the beneficial effects of CRAE in adolescent girls who are obese and hyperinsulinemic.
Methods
Forty adolescent girls who are obese (age 14.7 ± 1 years; BMI 30 ± 2) were randomly assigned to a “no exercise” (CON n = 20) or combined exercise group (EX n = 20). The EX group performed resistance and aerobic exercise for 12 weeks, 5 times per week. Exercise intensity was increased gradually, from 40 to 70% of heart rate reserve (HRR), every 4 weeks. The brachial-ankle pulse wave velocity (BaPWV), blood pressure (BP), heart rate (HR), blood leptin, adiponectin levels, and body composition were measured before and after the 12-week intervention.
Results
We observed that CRAE effectively reduced the body fat percentage, body weight, and waist circumference in the EX group (p < 0.05). After 12 weeks of training, subjects in the CRAE group maintained appropriate leptin and adiponectin levels and showed positive improvements of blood insulin, glucose, and insulin resistance parameters relative to baseline and to the CON group (p < 0.05).
Conclusion
CRAE is a useful therapeutic method to alleviate metabolic risk factors in adolescent girls who are obese and hyperinsulinemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the Centers for Disease Control and Prevention (CDC), the prevalence of obesity has more than doubled in children and quadrupled in adolescents during the past three decades. In 2012, more than one-third of children and adolescents were classified as overweight or obese (Ogden et al. 2014). Obesity predisposes individuals to cardiovascular complications, high blood pressure, high cholesterol, metabolic syndrome, and a general decline in quality of life. Moreover, children who are obese are more likely to be overweight or obese in adulthood, making them susceptible to several metabolic diseases such as cardiovascular diseases, stroke, and type 2 diabetes. Excess adiposity, especially visceral adiposity, has been established as a risk factor for the development of cardiovascular diseases (Despres 2012). Visceral fat promotes the secretion of free fatty acids and decreases insulin receptor sensitivity leading to the onset of metabolic dysregulation (Reaven and Chen 1988a, b). Lack of physical activity results in the initiation and progression of obesity and obesity-associated metabolic pathologies. Exercise training has been utilized to reduce obesity-associated risks of CVD (Park et al. 2016). Poehlman et al. reported that aerobic exercise training increases energy expenditure and promotes fat lipolysis (Poehlman and Horton 1989). Son et al. (2017) reported that combined resistance and endurance exercise training (CRAE) improves arterial stiffness, blood pressure, functional capacity, body composition, and blood nitrate/nitrite production in postmenopausal women with stage 1 hypertension (Son et al. 2017).
Studies in both animal and human models have looked at the benefits of CRAE. Sanches et al. (2015) compared the effects of resistance, aerobic, and combined exercise on cardiovascular autonomic control and mortality in diabetic ovariectomized mice. They observed that aerobic and combined exercise training promoted additional cardiovascular autonomic benefits compared to resistance training alone (Sanches et al. 2015). Santos et al. (2012) studied the effects of either resistance training or combined endurance and resistance training for 8 weeks, followed by 12 weeks of detraining, on body composition in adolescent girls. They concluded that combined resistance and endurance training does not negatively influence cardiorespiratory fitness and that the combined exercise modality is effective in improving general strength and cardiorespiratory fitness in healthy adolescent school girls. They also observed that 12 weeks of detraining was not sufficient to reduce the overall training effects (Santos et al. 2012). Marson et al (2016) performed a meta-analysis to assess the association of aerobic, resistance, and combined exercise training programs on insulin resistance, fasting glucose, and insulin levels in children and adolescents who are obese and overweight (Marson et al. 2016). The analysis revealed that exercise training in general was not associated with a reduction in fasting glucose; however, reduction in fasting insulin levels and HOMA IR were observed after aerobic exercise. Ha et al. (2012) conducted a study to determine the benefits of CRAE on the metabolic health of young adults. The study revealed that 12 weeks of CRAE could effectively reduce body fat percentage, waist circumference (WC), and systolic and diastolic blood pressure in young adults (Ha and So 2012). Ho et al. (2012) observed that 12 weeks of CRAE resulted in improvements in cardiovascular risk profile in participants who are obese and overweight, when compared to their respective sedentary controls. The study also showed that CRAE resulted in greater weight loss, fat loss, and improved cardiovascular fitness than aerobic or resistance training modalities alone and recommended CRAE for adults who are obese (Ho et al. 2012). In spite of these putative health benefits, the CRAE protocol still requires a standardized definition and its benefits in the context of improving metabolic parameters demand further investigation. Additionally, the benefit of this exercise modality in the context of improvement of metabolic parameters requires investigation. In our study, we sought to determine the benefits of CRAE in adolescent girls who are obese and hyperinsulinemic. We hypothesized that 12 weeks of CRAE training would improve insulin resistance, central adiposity, and cardiovascular health in hyperinsulinemic adolescent girls who are obese. Our results demonstrate that this exercise modality resulted in significant reductions in body weight and waist circumference, improved blood glucose levels and insulin sensitivity, and promoted healthy adiponectin and leptin levels. Adolescent girls who are obese also had a steady decline in their BMIs within 12 weeks of initiation of the regimen.
Methods
Participants
Forty girls who are obese (Tanner 2–3 stage) (age 14.7 ± 1 years) participated in the present study (Table 1). All participants were obese (BMI ≥ 30 kg/m2) with hyperinsulinemia (> 12.0 µU/ml) and abdominal obesity (waist > 80 cm). All participants were sedentary, defined as no regular exercise training or physical activity, and were not on a weight loss diet within the last 6 months. Exclusion criteria included hypertension, pregnancy, and chronic diseases. All participants and their parents signed a written informed consent approved by the Institutional Human Research Committee. All protocols were approved by the Institutional Review Board of the Public Institutional Review Board designated by Ministry of Health and Welfare (P02-201611-14-001), carried out in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants and their guardians prior to the study. This study was registered in a registry of clinical trial (clinical trial ID: NCT 03146026).
Study design
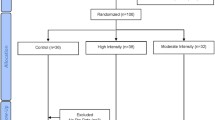
Participants were randomly assigned to an exercise training (EX; n = 20) or no exercise group (CON; n = 20) using a two-armed, parallel-design (Fig. 1). Participants were allocated using computer-generated blocks (ten randomized group assignments per block). Prior to and following the 12-week study periods, all participants reported to the laboratory in the morning to have blood samples drawn and body composition measured. Participants completed a maximal treadmill exercise test using the modified Bruce protocol at baseline and after the 12-week-period. Participants were reminded once a week to not make changes to their diet and physical activity habits during the study. Food logs were recorded to keep track of their regular dietary intake (~ 1921.7 kcal/day for both groups) and advised to make no changes to their diet and exercise habits during the study and were supervised by trained coaches every 2 days (Berkey et al. 2000; Rathnayake et al. 2014). The participants were supervised for the entire duration of the exercise protocol and did not have an energy expenditure goal. Participants in the CON group did not participate in any exercise protocol and were requested to maintain their regular lifestyle (i.e., dietary patterns) for the duration of the study. No drop out of participants was reported.
Blood sampling and analysis
Venous blood samples were obtained from an antecubital vein around 8:00 AM following an overnight fast both at baseline and following the 12-week study period. Blood glucose was measured using an automated analyzer (Toshiba 120-FR, Tokyo, Japan). Blood was centrifuged at 1000×g for 10 min at 4 °C and stored at − 80 °C for future analysis. Plasma insulin concentration was measured in duplicate using commercial radioimmunoassay kits (LincoResearch, St. Charles, MO, USA). Insulin resistance (IR) was estimated using the homeostasis model assessment of IR (HOMA IR) as previously described (Matthews et al. 1985). Plasma adiponectin was measured using commercial ELISA kits (B-Bridge International, San Jose, CA). Plasma leptin was determined by ELISA kits (Linco Research St. Charles, Missouri, USA). The sensitivity of the insulin, adiponectin, and leptin assays were 0.73 µU/ml, 25 pg/ml, and 0.5 ng/ml, respectively. The intra-assay and inter-assay coefficient of variation were 9.1 and 4.6%, 9.5 and 8.5%, and 6.2 and 8.3%, for insulin, adiponectin, and leptin, respectively.
Body composition
Height was assessed to the nearest 1.0 cm with bare feet and body weight was measured to the nearest 0.1 kg with the subject wearing light clothes. WC was measured at the midpoint between the lower rib and the iliac crest at the end of a normal expiration using a tape measure. BMI was calculated as weight (kg) divided by the square of height (m). Fat mass and fat-free mass were measured using a bioelectrical impedance meter (InBody 230, Biospace, Seoul, Korea) (Seo et al. 2012).
Exercise testing
Maximal heart rate (HRmax) was obtained at the end of a graded treadmill exercise test performed until volitional exhaustion. All maximal exercise criteria were met: (1) perceived exertion scores were ≥ 18 on a Borg scale (scale 6–20), (2) no change in HR with an increase in workload, and (3) volitional exhaustion, defined as request of the subject to stop the test.
Combined exercise training
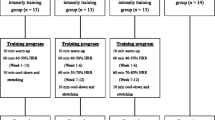
The CRAE program was performed for 60 min with 5 min of warm-up and cool-down per day, 5 times a week for 12 weeks. CRAE consisted of 20 min of various resistance band exercises (upper: seated rows, biceps curl, shoulder flexion, elbow flexion, pushup; lower: hip flexion, hip extension, calf raise, leg press, squat) and 30 min of treadmill walking for a total eleven exercises. The exercises were performed at a moderate intensity with 15–20 repetitions. The warm-up and cool-down consisted of static stretching. Exercise intensity was gradually increased from 40 to 50% heart rate reserve (HRR) in weeks 1–4, to 60–70% HRR in weeks 9–12. Each training session was fully supervised by the researchers. Every subject wore a heart rate monitor during the entire training session so as to maintain the correct training intensity (Son et al. 2017). The program is shown in Table 2.
Vascular function
A commercially available applanation tonometer (SphygmoCor, AtCor Medical Ltd, Sydney, Australia) was utilized with analysis software (version 8.0, SphygmoCor Cardiovascular Management Suite) for measurement of baPWV (m/s), an indicator of arterial stiffness. Two measurements were collected at each time point and averaged as previously described (Weber et al. 2004; Yambe et al. 2007).
Statistical analysis
All parameters were normally distributed as shown by the Kolmogorov–Smirnov test. Student’s t test was used for group comparisons at baseline. A two-way ANOVA with repeated measures [group (control and exercise) x time (before and after 12 weeks)] was used to determine the effects of CRAE and time on dependent variables. If a significant main effect or interaction was noted, paired t tests were used for post hoc comparisons. Since baPWV, a well-validated arterial stiffness measurement, was the major outcome for cardiovascular health, we selected baPWV to determine sample size. Based on a previous study, we estimated that a total of 40 women would enable 70% power with effect size of 0.17 to detect a 7% decrease in baPWV after the CRAE training (Figueroa et al. 2011). Analyses were performed using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL). Data are presented as mean ± SEM. Statistical significance was set at P < 0.05.
Results
Decline in leptin levels are observed upon CRAE training. Leptin is a hormone produced by adipocytes that can regulate satiety responses and thereby control food intake. Leptin is essential for energy homeostasis and its level depends on BMI and age. We observed that leptin levels were significantly reduced in EX versus CON. We also observed a significant increase in adiponectin levels in EX versus CON (p < 0.05) (Fig. 2).
CRAE training efficiently modulates adiponectin-to-leptin ratio. 12 weeks of exercise training caused a significant increase in the ratio of adiponectin to leptin, thereby suggesting improved metabolic status in EX versus CON (p < 0.05) (Fig. 3).
Exercise training prevents the onset of metabolic syndrome. CRAE training efficiently reduced body weight, body fat, and waist circumference, an indicator of central adiposity (p < 0.05). This is a significant observation because central adiposity has been implicated as the harbinger of several cardiovascular and metabolic abnormalities (Table 1). CRAE was also effective in reducing blood glucose, insulin, and HOMA IR in EX versus CON (p < 0.05) (Table 3). However, baPWV, a marker of arterial stiffness, was not changed after CRAE.
Discussion
The benefits of exercise and its importance in maintaining fitness and longevity has been the scope of many studies for several decades. Obesity occurring at younger ages is linked to pathological processes associated with cardiovascular diseases in later adulthood (Ewart et al. 1998; Omar et al. 2014). With the increase in childhood obesity and the reduction in the age of onset of metabolic diseases, several studies are refocusing research efforts to understand the molecular and cellular effects of exercise training. The primary focus of our study is to assess CRAE-mediated metabolic improvements and cardioprotective effects that occur in adolescent girls who are obese and hyperinsulinemic. We hypothesized that CRAE would result in the improvement of muscular strength in the participants during moderate intensity resistance training and that moderate intensity aerobic exercise would improve vascular function, along with normalization of potential negative effects of resistance exercise. As current results and previous data describe (Son et al. 2017), our proposed CRAE protocol is a useful exercise modality to improve muscular strength, central adiposity, and insulin resistance, thus making CRAE more useful than either aerobic or resistance training alone. We were able to assess several important endpoints in this study, pertaining to the overall health of the participants. One of the most interesting and beneficial outcomes of this training regimen is the significant reduction in central adiposity as measured by waist circumference (Table 1). Central adiposity is the accumulation of fat in the lower abdominal region and is associated with an increased risk of metabolic diseases, such as type 2 diabetes, inflammation, and CVD. Previously, Ali et al. (2014) observed that central adiposity is the most significant predictor of metabolic syndrome in children and adolescents. Besides the reduction in central adiposity, the exercise group also had a significant reduction in body fat percentage when compared to the control group. This is an important benefit of this exercise modality as excess body fat during adolescence has been known to promote greater health risks during adulthood and also can lead to poor pregnancy outcomes in young women.
The understanding of adipose tissue as an endocrine organ and its ability to regulate systemic metabolism underscores the importance of adiposity in CVD. As obesity sets in, the adipocytes enlarge and undergo cellular and functional changes that affect systemic metabolism. Free fatty acids and glycerol are released from the adipocytes which promote insulin resistance and inflammation. Additionally, insulin resistance has been shown to develop in muscle tissues that have been exposed to increasing levels of free fatty acids (Shulman 2004). In individuals who are obese, adipose tissue is the major determinant of plasma IL-6 levels. The expression of IL-6—a potent pro-inflammatory cytokine—increased almost ten-fold in adipose tissue from individuals who are obese, when compared to individuals who are lean and the expression was higher in the visceral adipose tissue than in the periphery (Fried et al. 1998). In children and adolescents, subcutaneous adiposity around the waist was the most significant covariate for HOMA IR, BMI percentile, and levels of triglyceride, LDL, and HDL (Omar et al. 2014). Our observation of significant reductions in body weight and WC in the exercise group vs. the control group is promising for continuing this exercise regimen as a permanent weight loss intervention in adolescents. However, the weight reduction in the EX group was greater than what we could realistically expect from CRAE training alone without diet modification. This result suggests that EX participants may not have maintained their normal diet as directed and may have reduced their caloric intake during training. This can be considered a limitation of the study.
Previously, our group observed that CRAE training reduced blood pressure, arterial stiffness, and insulin resistance in a different group of adolescent girls who are obese and have high blood pressure (Son et al. 2017a, b), therefore, we expected that CRAE training may have protective effects against increased central adiposity and insulin resistance-associated increases in arterial stiffness, which is a major risk factor for hypertension, atherosclerosis, and heart failure. However, baPWV, an indicator of arterial stiffness, was not changed after CRAE in the present study. This discrepancy may be related to the study participants, as our study participants were relatively young and normotensive. Also, 12 weeks of training may not be enough for inducing structural modification of vasculatures in this young population, and this warrants further study.
Other important indicators of metabolic dysregulation such as elevated blood glucose and blood insulin were normalized in the CRAE group (Table 3). Childhood obesity is associated with increased risk of insulin resistance. With the increase in insulin resistance (quantile of HOMA IR value) there is an increase in the onset of metabolic syndrome. In a study by Yin et al (2013), participants that received the highest quantile of HOMA IR were 60 times more likely to be classified with metabolic syndrome when compared to the lowest quantile group (Yin et al. 2013). In our study, the exercise-trained group had significant improvement of HOMA–IR when compared to baseline and when compared to sedentary controls (Table 3).
Another noteworthy finding of the study is the increase in the adiponectin-to-leptin ratio. Stylianou et al (2007) observed that in adolescents who are obese, the BMI, body fat percentage and HOMA IR positively correlated with leptin levels (Stylianou et al. 2007). Similarly, the results of the CASPIAN-III study of Iranian adolescents who are obese showed that leptin and insulin levels were higher in adolescents who are overweight than their counterparts with normal weight. Leptin levels were shown to positively correlate with age, fasting blood glucose, BMI, and insulin levels (Bahrami et al. 2014). Alikasifoglu et al (2009) showed that adiponectin is a marker of metabolic syndrome in adolescent subjects who are obese. The study showed that low density lipoprotein (LDL), triglycerides, insulin, and leptin levels were higher, whereas the high density lipoprotein (HDL) and adiponectin levels were lower in adolescents and children who are obese. However, they observed only a weak negative correlation between adiponectin levels and the severity of insulin resistance. They also observed that the mean serum adiponectin levels were lower in subjects with metabolic syndrome than in the subjects who are normal (Alikasifoglu et al. 2009). The present exercise regimen was effective in lowering leptin levels along with increasing the adiponectin levels (Fig. 2), thereby increasing the adiponectin-to-leptin ratio, which is a highly beneficial outcome in terms of improving overall metabolic health.
The present study sought to determine the benefits of a specific CRAE regimen on the metabolic health of adolescent girls who are obese and hyperinsulinemic. The observations herein show that exercise-trained adolescent girls had a significant reduction in body weight and BMI. These positive changes correlated with overall improvement in the metabolic milieu with reductions in plasma glucose, insulin, and leptin levels and an increase in adiponectin and adiponectin to leptin ratio. The HOMA IR, an index of insulin resistance and β-cell function, showed significant improvement in the trained group versus the sedentary controls. Our study is limited by the lack of resistance-only and aerobic-only exercise regimens, thus warranting further investigations with a focus on determining the efficacy of CRAE relative to resistance-only and aerobic-only exercise regimens in girls who are obese. Additionally, due to the relatively small sample size of this study, it is difficult to generalize the results to other girls who are obese. However, to the best of our knowledge, this current study is the first to report a significant improvement in the plasma adiponectin-to-leptin ratio in CRAE-trained adolescent girls. The exercise protocol was also effective in reducing WC and thereby central adiposity. Central adiposity is associated with increased inflammation and insulin resistance. Although our study did not assess the inflammatory profile of the study subjects, the insulin sensitivity was shown to be significantly improved in the exercise-trained group when compared to the controls or the trained group prior to beginning the exercise protocol (baseline). We recommend that the CRAE regimen be implemented to promote weight loss and improve metabolic fitness and cardiovascular health in adolescent girls who are obese and hyperinsulinemic.
Change history
05 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00421-020-04579-z
Abbreviations
- BaPWV:
-
Brachial-ankle pulse wave velocity
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CDC:
-
Centers for Disease Control and Prevention
- CVD:
-
Cardiovascular diseases
- CON:
-
No exercise group
- CRAE:
-
Combined resistance and aerobic exercise
- EX:
-
Exercise group
- HDL:
-
High density lipoprotein
- HOMA IR:
-
Homeostatic model assessment of insulin resistance
- HR:
-
Heart rate
- LDL:
-
Low density lipoprotein
- WC:
-
Waist circumference
- SEM:
-
The standard error of the mean
References
Alikasifoglu A, Gonc N, Ozon ZA, Sen Y, Kandemir N (2009) The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: adiponectin is a marker of metabolic syndrome. J Clin Res Pediatr Endocrinol 1:233–239
Bahrami E, Mirmoghtadaee P, Ardalan G, Zarkesh-Esfahani H, Tajaddini MH, Haghjooy-Javanmard S, Najafi H, Kelishadi R (2014) Insulin and leptin levels in overweight and normal-weight Iranian adolescents: The CASPIAN-III study. J Res Med Sci 19:387–390
Berkey CS, Rockett HR, Field AE, Gillman MW, Frazier AL, Camargo CA (2000) Jr. and Colditz GA. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 105:E56
Despres JP (2012) Body fat distribution and risk of cardiovascular disease: an update. Circulation 126:1301–1313
Ewart CK, Young DR, Hagberg JM (1998) Effects of school-based aerobic exercise on blood pressure in adolescent girls at risk for hypertension. Am J Public Health 88:949–951
Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH (2011) Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 18:980–984
Fried SK, Bunkin DA, Greenberg AS (1998) Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83:847–850
Ha CH, So WY (2012) Effects of combined exercise training on body composition and metabolic syndrome factors. Iran J Public Health 41:20–26
Ho SS, Dhaliwal SS, Hills AP, Pal S (2012) The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 12:704
Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF (2016) Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev Med 93:211–218
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311:806–814
Omar A, Cerjak D, Kent JW, James R, Blangero J, Zhang Y, Obesity (2014) Central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes 9(3):e58–e62
Park SY, Rossman MJ, Gifford JR, Bharath LP, Bauersachs J, Richardson RS, Abel ED, Symons JD, Riehle C (2016) Exercise training improves vascular mitochondrial function. Am J Physiol Heart Circ Physiol
Poehlman ET, Horton ES (1989) The impact of food intake and exercise on energy expenditure. Nutr Rev 47:129–137
Rathnayake KM, Roopasingam T, Wickramasighe VP (2014) Nutritional and behavioral determinants of adolescent obesity: a case-control study in Sri Lanka. BMC Public Health 14:1291
Reaven GM, Chen YD (1988a) Role of abnormal free fatty acid metabolism in the development of non-insulin-dependent diabetes mellitus. Am J Med 85:106–112
Reaven GM, Chen YD (1988b) Role of insulin in regulation of lipoprotein metabolism in diabetes. Diabetes Metab Rev 4:639–652
Sanches IC, Conti FF, Bernardes N, Brito JO, Galdini EG, Cavaglieri CR, Irigoyen MC, De AK (2015) Impact of combined exercise training on cardiovascular autonomic control and mortality in diabetic ovariectomized rats. J Appl Physiol (1985) 119:656–662
Santos AP, Marinho DA, Costa AM, Izquierdo M, Marques MC (2012) The effects of concurrent resistance and endurance training follow a detraining period in elementary school students. J Strength Cond Res 26:1708–1716
Seo DY, Lee S, Figueroa A, Kim HK, Baek YH, Kwak YS, Kim N, Choi TH, Rhee BD, Ko KS, Park BJ, Park SY, Han J (2012) Yoga training improves metabolic parameters in obese boys. Korean J Physiol Pharmacol 16:175–180
Shulman GI (2004) Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 19:183–190
Son WM, Sung KD, Bharath LP, Choi KJ, Park SY (2017a) Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens 39:546–552
Son WM, Sung KD, Cho JM, Park SY (2017b) Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause 24:262–268
Stylianou C, Galli-Tsinopoulou A, Farmakiotis D, Rousso I, Karamouzis M, Koliakos G, Nousia-Arvanitakis S (2007) Ghrelin and leptin levels in obese adolescents. Relationship with body fat and insulin resistance. Hormones (Athens) 6:295–303
Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B (2004) Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 109:184–189
Yambe M, Tomiyama H, Yamada J, Koji Y, Motobe K, Shiina K, Yamamoto Y, Yamashina A (2007) Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J Hypertens 25:87–93
Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, Mi J (2013) Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr 5:71
Acknowledgements
We thank Shakun Karki PhD for the drafted manuscript.
Author information
Authors and Affiliations
Contributions
LB: conducted experiments, analyzed data, wrote the manuscript. WC, JC, AS, AW: conducted experiments, analyzed data. TS: conducted experiments, analyzed data. SP: conducted experiments, analyzed data, wrote the manuscript.
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Bharath, L.P., Choi, W.W., Cho, Jm. et al. Combined resistance and aerobic exercise training reduces insulin resistance and central adiposity in adolescent girls who are obese: randomized clinical trial. Eur J Appl Physiol 118, 1653–1660 (2018). https://doi.org/10.1007/s00421-018-3898-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3898-8