Abstract
Purpose
Long-term training influence on athletes’ immune cell response to acute exercise has been poorly studied, despite the complexity of both chronic and acute adaptations induced by training. The purpose of the study is to study the influence of a 4-month swimming training cycle on the immune cell response to a high-intensity training session, during 24 h of recovery, considering sex, maturity, and age group.
Methods
Forty-three swimmers (16 females, 14.4 ± 1.1 years; 27 males, 16.2 ± 2.0) performed a standardized high-intensity session, after the main competition of the first (M1), and second (M2) macrocycles. Blood samples were collected before (Pre), immediately after (Post), 2 h after (Post2h) and 24 h after (Post24h) exercise. Haemogram and lymphocytes subsets were assessed by an automatic cell counter and by flow cytometry, respectively. Subjects were grouped according to sex, competitive age groups, or pubertal Tanner stages. Results express the percentage of relative differences from Pre to Post, Post2h and Post24h. Upper respiratory symptoms (URS) and training load were quantified.
Results
At M2, we observed smaller increases of leukocytes (M1: 14.0 ± 36.3/M2: 2.33 ± 23.0%) and neutrophils (M1: 57.1 ± 71.6/M2: 38.9 ± 49.9%) at Post; and less efficient recoveries of total lymphocytes (M1: − 22.0 ± 20.1/M2: − 30.0 ± 18.6%) and CD19+ (M1: 4.09 ± 31.1/M2: − 19.1 ± 24.4%) at Post2h. At Post2h, the increment of CD4+/CD8+ was smaller in youth (M1: 21.5 ± 16.0/M2: 9.23 ± 21.4%), and bigger in seniors (M1: 3.68 ± 9.21/M2: 23.2 ± 15.0%); and at Post24h late pubertal swimmers’ CD16+56+ recovered less efficiently (M1: − 0.66 ± 34.6/M2: − 20.5 ± 34.2%).
Conclusions
The training cycle induced an attenuated immune change immediately after exercise and a less efficient recovery of total lymphocytes, involving an accentuated CD19+ decrease. The concomitant higher URS frequency suggests a potential immune depression and a longer interval of susceptibility to infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At the time of important competitions, an healthy immunological, metabolic, hormonal, circulatory and respiratory condition along with an optimized functional capacity is needed (Hellard et al. 2013). This optimal functionality allows the athlete to achieve the best performance, and is usually the result of an adequate balance between training loads and recovery throughout the different phases of the periodization of a training season (Mujika et al. 1995). However, in endurance sports, such as swimming, during the cycles of high training volume and intensity that include consecutive training sessions with little recovery time in between, athletes may experience a temporary diminished performance concomitant with an immunodepression state (Rama et al. 2013; Gleeson 2007).
Exercise immunology literature is consistent regarding post-exercise cell counts (leucocytosis, neutrophilia, monocytosis, and lymphocytosis) after a single bout of high-intensity swimming (Kargotich et al. 1997; Morgado et al. 2014, 2016) or other endurance sports (Zhang et al. 2006; Natale et al. 2003; Yamada et al. 2000; McCarthy et al. 1992, 1991). The higher circulating number of these cells suggests an acute overall increased surveillance of both innate and acquired cellular immunity, thus conferring a temporary improved host defence that has been associated with the “fight or flight” response (Walsh et al. 2011).
During initial recovery period, swimming studies report leucocytosis and neutrophilia (Ferrer et al. 2009; Kargotich et al. 1997), while monocytes recover to baseline levels and lymphocytes decline (Kargotich et al. 1997). A delayed leucocytosis and neutrophilia has been reported at 1 h after cycling (Gabriel et al. 1992; Green et al. 2003) and at 2 h after running (Gabriel et al. 1992; Yamada et al. 2000). It was also observed 3 h into recovery after different types of cycling tasks (McCarthy et al. 1991, 1992; Natale et al. 2003) and after a resistance exercise circuit (Natale et al. 2003).
Regarding the effect of long-term swimming seasons (lasting from 3 to 7 months) at rest, decreased values of CD56+ NK cells (Rama et al. 2013; Gleeson et al. 1995, 2000), neutrophils and monocytes (Morgado et al. 2012) were observed. Therefore, it appears that intense training over long periods affect the number and function of innate and acquired immune cells at rest, possibly contributing to upraise the risk of infection (Walsh et al. 2011).
However, the acute (Kargotich et al. 1997; Morgado et al. 2014, 2016; Tauler et al. 2008) and chronic (Gleeson et al. 1995; Morgado et al. 2012; Mujika et al. 1996b; Rama et al. 2013; Teixeira et al. 2014) immune systemic changes in response to swimming have been addressed separately. A more integrated approach is desirable, since the acute and chronic immune cell changes can influence the potential of the swimmer to respond properly to high-intensity training sessions throughout the course of the swimming season. This ability is indispensable to acquire positive adaptations and ultimately to guarantee training attendance. To our knowledge, the influence of the long-term training on the acute immune cell changes to a swimming session has not yet been studied.
Thus, this study aimed to investigate the influence of a 4-month training cycle of a swimming season over the immune cell response to a high-intensity swimming training session integrated in the normal training process, during a 24-h recovery period, whilst controlling for the effects of sex, maturity, and age group. In athletes, we expect to grasp the cumulative effects of the training process.
Methods
Participants
Forty-three swimmers, 16 females (14.4 ± 1.1 years.), and 27 males (16.2 ± 2.0 years.), undertaking 15–18 h of pool training and 4–7 h of dryland training per week, volunteered to participate in this study.
After receiving detailed information about the aim of the study and the possible risks of the investigation, either the subjects or their parents, as appropriate, provided written informed consent to participate. All procedures were approved by the Ethics Committee of the Faculty of Human Kinetics of the University of Lisbon and were conducted in accordance with the Declaration of Helsinki for human studies (World Medical Association 2008).
During the period of observation athletes were asked not to take dietary supplements, nor any kind of medication other than that prescribed for episodes of acute illness.
The swimmers had different competitive swimming backgrounds (5.5 ± 0.3 mean years of competitive experience with a range of 4–11 years) and were included into different age groups according to the regulation of the National Swimming Federation and the Ligue Européene de Natation (youth: n = 26, 13–14 years in females and 14–15 years in males; juniors: n = 10, 15–16 years in females and 16–17 years in males; seniors: n = 7, ≥ 17 years in females and ≥ 18 years in males) or into different maturity groups (late pubertal: n = 28 years; mature: n = 15 years).
Study design
This study used an observational design with a follow-up of the second macrocycle of a swimming winter training season lasting 17 weeks. Swimmers followed the training program set by the coaches.
The evaluation of the swimmers was made at two moments: M1 (after the main competition of the first macrocycle) and M2 (after the main competition of the second macrocycle).
At each moment of evaluation, swimmers performed a standardized high-intensity swimming session designed by experienced coaches and framed in the training periodization. Immunological profile was scrutinized before (Pre), immediately after (Post), 2 h after (Post2h) and 24 h after (Post24h) the training sessions at the two moments of evaluation. Data collected also included subjects’ chronological age, body composition measurements, and an indicator of biological maturity (pubertal Tanner stages). Athletes were instructed not to consume anything but water after 10 p.m. of the preceding day, to have a minimum of 8 h rest before testing, and not to perform extenuating exercise in the previous 24 h. To standardize pre-exercise food intake and to avoid extending the duration of their fasted state, participants consumed a sandwich with butter and a juice after the body composition measurements and the resting blood sample collection. The experimental sessions took place between 6:30 and 10 a.m.
Throughout the follow-up season the incidence of upper respiratory symptoms (URS) was monitored weekly and training load and mean intensity of all scheduled swimming sessions were quantified. The characteristics of the training regimens and competition schedules were not modified by the present study in anyway nor any swimmer suffered from major injury or sickness preventing them from training for more than 1 day.
Body composition measurements
Stature and body mass were measured after wakening in the fasted state wearing a bathing suit without shoes. Stature was measured to the nearest 0.1 cm (Siber-Hegner anthropometric kit). Body mass and fat mass percentage (%FM) were assessed using Bioelectrical Impedance Analysis (TANITA BC-601 body composition scale monitor) with a measuring current of 50 kHz, 100 μA. Body mass index (BMI) was calculated as body mass (BM; kg) divided by the square of the stature (m). Fat mass (FM) was calculated according to the formula: FM (kg) = BM × %FM/100. Free fat mass (FFM) was calculated according to the formula: FFM (kg) = BM − (BM × %FM/100).
Maturity: tanner stages
After receiving detailed instructions, the participants self-assessed their degree of genital organ, breast, and pubic hair development using a questionnaire (Tanner 1962) accompanied by figures and were then grouped according to pubertal stage. Stage 1 corresponds to the pre-adolescent state; stages 2, 3, and 4 correspond to the sexual maturation stages, respectively, early, mid, and late pubertal states; and stage 5 corresponds to mature state (Tanner 1962). This method has been shown to be valid and reproducible for the evaluation of maturity in studies addressing the immune response to exercise in adolescents (Boas et al. 1996; Timmons et al. 2006).
Female subjects were asked to point out the days of menstruation.
Swimming training season
The observed 4-month training cycle was the second macrocycle of a swimming competitive season (Fig. 1). This macrocycle started with a development period characterized by an increasing training volume, intensity, and frequency that lasted until the end of the specific preparatory subphase reaching the peak of training load of the season by week 23. Afterwards the training load was progressively reduced preparing for the National Championships.
Periodization of the swimming winter training competitive season in which the 4-month training macrocycle is incorporated and schedule of the two moments of evaluation.  , Dryland training;
, Dryland training;  , technique training; continuous line, volume; dotted line, intensity; dashed line, peaking. M1, 1st moment of evaluation; M2, 2nd moment of evaluation; TRANS., transition; GENERAL PREP., general preparatory; SPECIFIC PREP., specific preparatory; PRE COMP., pre-competitive; Peaking 1–5, peaking index, where 1 is peaking at 100%, 2 at 90%, 3 at 80%, 4 at 70% and 5 at 60%; 10–100%, peaking percentage
, technique training; continuous line, volume; dotted line, intensity; dashed line, peaking. M1, 1st moment of evaluation; M2, 2nd moment of evaluation; TRANS., transition; GENERAL PREP., general preparatory; SPECIFIC PREP., specific preparatory; PRE COMP., pre-competitive; Peaking 1–5, peaking index, where 1 is peaking at 100%, 2 at 90%, 3 at 80%, 4 at 70% and 5 at 60%; 10–100%, peaking percentage
Quantification of the training load
Training load of each training session was assessed by quantifying the volume (total amount of metres swum) and arbitrary units of load (AUL) based on the work of Mujika et al. (1995, 1996a), Rama et al. (2013) and Morgado et al. (2012).
To quantify the AULs a stress index scale was established in reference to the theoretical values of blood lactate accumulation usually associated with the different training zones: I—warm-up and recovery, II—endurance 1, III—endurance 2, IV—endurance 3, V—lactate tolerance, VI—lactate production and VII—sprint. The volume accomplished in each training zone was quantified (mI, mII, mIII, mIV, mV, mVI and mVII). The magnitude of the load (AUL) was obtained from the ratio between the weighed volume, calculated by adding the volumes swum in each training zone multiplied by the respective index (1, 2, 3, 4, 6, 8, 10) as suggested by Rama et al. (2013), and the total volume was effectively completed, according to the formula:
This was performed for all season sessions of the competitive season.
The weekly load was characterized by the sum of the load of all the training sessions of the week and the comparison between the two moments of evaluation was performed based on the mean of the weekly training load of the whole macrocycle and of the 4 weeks prior to each moment.
Standardized swimming training session
The swimming session that was performed to evaluate the acute effect of exercise on the immune system started with a 1500 m standardized warm-up lasting 30–35 min. This was followed by a high-intensity main task that lasted 50 min and a 500 m recovery task (8 min of duration). The main task was designed to induce maximal lactate accumulation and had a total distance of 1000–1200 m, depending on the age group considered. For the youth group the main task consisted of two sets of four repetitions of 75 m front crawl on a 5-min cycle, with 10 min of active recovery between sets. This reduction of distance aimed to elicit an effort situated at the same maximal lactate accumulation zone of intensity, thereby levelling the physiologic impact between groups. The swimmers were instructed to perform each repetition at 90–95% of 100 m freestyle personal best race time. The session designed for juniors and seniors was identical to the latter, except with repetitions of 100 m.
To determine the mean effort intensity percentage (%) in relation to the personal best at the 100 m freestyle race the times of each repetition were registered.
A heart rate (HR) monitor (Polar RS800CX™, Kempele, Finland) was used during each training session to calculate percentage of estimated maximal heart rate.
Immune system parameters
Peripheral venous blood samples were collected via standard procedures before (Pre, between 6:00–6:30 a.m. in the fasted state), immediately after (Post), 2 h after (Post 2 h) and 24 h after (Post 24 h) each swimming training sessions. Venous blood was collected into tubes containing EDTA for assessment of hemogram and leukogram and for counting of total lymphocytes and subsets (CD3+, total T lymphocytes cells; CD4+, T helper cells; CD8+, T cytotoxic cells, CD16+56+, natural killer (NK) cells; and CD19+, B cells). Hemogram and leukogram were performed in an automated haematology analyzer (Coulter LH 750, Beckman) which produced information about the following parameters: haemoglobin concentration (g dL−1), hematocrit (%); and counts of white blood cells, namely leukocytes, neutrophils, monocytes, and eosinophils. Total lymphocytes and subsets were counted by flow cytometry (FACS Calibur, BD Biosciences), using the commercial kits from BD Biosciences (BD multitest IMK kit). Results were expressed as number of cells·109 L−1 for leukogram parameters and as number of cells·µL−1 for total lymphocytes and subsets. Plasma variation was calculated and Post, Post 2 h and Post 24 h values were corrected for plasma volume variation (Dill and Costill 1974).
Upper respiratory symptoms
Athletes self-reported upper respiratory symptoms (URS) using daily log books as described in Rama and colleagues (Rama et al. 2013). The list of symptoms includes headache, fever, ear pain, chills, runny or blocked nose, pharyngitis/tonsillitis, bronquitis, asthma, phlegm, cough, conjunctivitis, itchy, watery eyes, nausea/vomiting, and diarrhoea. According to Bishop (2006), one episode was defined as the repetition of more than two symptoms on at least two consecutive days, and a new episode was considered after a minimum interval of 10 days following the previous one. Additionally, all swimmers were asked to indicate the medication they were on.
Statistical analysis
The statistical analyses were performed using the software IBM SPSS Statistics (version 21; IBM Corp., Armonk, N.Y., USA) and the R software (version 2.15.1; R Core Team), both for Windows, with a significance level of 5%.
Descriptive statistics, including means and standard deviation (mean ± SD) were performed for all outcome measurements.
To have values that reflect the acute change of the immune parameters at each moments of evaluation, the percentage of relative differences from Pre to Post, Pre to Post 2 h and Pre to Post 24 h were calculated according to the formula:
where RV is the percentage of relative difference, X is the final value and Y is the starting value.
Normality of the outcome variables was analysed using the Shapiro–Wilk test. To verify if participants were within the “clinically normal” values associated with each variable, the one-sample t test was used to compare group means with the upper or lower limits of the reference interval (INSA 2011; Lewis et al. 2006).
It was evaluated if sex, maturity, and swimming age group, influenced the effect of training on the immune response to the swimming session using nonparametric mixed-design ANOVAs. The within-subject factor was the moment of evaluation (two levels: M1 and M2), which is referred as the effect of training, and the subjects’ factors were the aforementioned influential variables. The nonparametric mixed-design ANOVA has an ANOVA-type statistic (ATS) for each effect, and also a modified ANOVA-type statistic (MATS) for the subject’s factor. The option for the nonparametric approach was due to the violation of the assumptions of parametric mixed ANOVA, namely the normality of the dependent variables in each factor’s level, homogeneity of variances or sphericity. This nonparametric analysis was performed with the nparLD package (Noguchi et al. 2012) from the R software. Subsequent analyses were performed according to procedures adopted previously (Morgado et al. 2016, 2017).
To analyse the influence of training over the acute immune response to exercise, independently of any of the factors tested, nonparametric Wilcoxon test was used.
Heart rate comparison between groups at each moment of evaluation was assessed using parametric one-way Anova test and the correspondent non-parametric test of Kruskal–Wallis when normality was not assumed.
Results
The participant’s characteristics, including demographics and body composition related variables, are presented in Table 1.
No subject reported stages 1, 2 or 3 of the Tanner classification. Swimmers’ physical characteristics slightly changed over the training macrocycle, reflecting little increases in stature, body mass, and FFM.
The main sets of the swimming training sessions were accomplished at the requested high intensity in relation to their personal best time at the 100 m freestyle race: 92.3 ± 4.7% at M1, and 93.4 ± 7.2% at M2.
Neither the absolute maximal heart rate achieved nor the percentage of the expected maximal heart rate were significantly different between swimming age groups or between maturation groups at any of the two moments (Table 2).
The baseline immune profile of the participant was within the reference interval associated for with each variable at both moments of evaluation (Lewis et al. 2006). Plasma volume variation according to Dill and Costill (1974) from M1 to M2 was 5.80 ± 8.37% (t = − 4.541, p = 0.000).
Seasonal training workload
Training load characterization of the whole macrocycle and of the 4 weeks before each moment of evaluation is presented in Table 3.
The load score of the 4 weeks prior to M2 was lower, and the volume and load score of the whole macrocycle that preceded each moment of evaluation were higher at M2, than at M1.
Influence of training on the acute immune cell changes in response to exercise
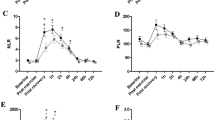
Considering the effects of the 4-month training cycle on the response to the training session, at M2, the increase of leukocytes and neutrophils from Pre to Post was smaller (Fig. 2a, b) and the decrease of total lymphocytes (Fig. 2c) and the magnitude of variation of CD19+ (Fig. 2d) from Pre to Post 2 h were bigger. The effects on CD16+56+ from Pre to Post 24 h were dependent on maturity [F(1, ∞) = 4.470, p = 0.035] with a bigger negative variation at M2 in late pubertal swimmers (Fig. 2e) and the changes of CD4+/CD8+ ratio from Pre to Post 2 h were dependent on swimming age group [F(1.881, ∞) = 10.847, p = 0.000] with a more accentuated increase of CD4+/CD8+ ratio in seniors and less accentuated increase in youth at M2 (Fig. 2f).
Mean ± SD of the relative difference (%) values of the leukocytes (a), neutrophils (b), total lymphocytes (c) and subsets CD19+ (d), CD16+56+ (e), and CD4+/CD8+ ratio (f) cell changes in response to a standardized high-intensity swimming training session performed at the beginning (M1) and at the end (M2) of a 4-month swimming training cycle. Open square, M1; filled square M2; asterisk, different from M1
Upper respiratory symptoms
The number of episodes of URS was monitored weekly (Fig. 3).
When comparing the two moments of evaluation, during the 4 weeks before M2 there was a higher frequency of URS episodes than along the 4 weeks prior to M1.
Discussion
In the present investigation, to understand the influence of training over the acute immune changes in response to intense prolonged exercise, a representative high-intensity swimming training session was performed after the main competition of the first macrocycle and after the main competition of the second macrocycle, of a 4-month swimming training cycle.
To replicate investigations and to compare results, it has been suggested that if significant plasma volume changes are expected to influence the results, either the corrected or both the measured and corrected data should be presented (Kargotich et al. 1998). Additionally, resting and/or pre-exercise blood samples taken over the course of a training season should adopt consistent sampling procedures by enforcing a standard sampling posture and resting interval for each sample.
Bearing in mind all the physiological mechanisms that can affect cell quantification (McMurray 1983; Nielsen et al. 1984; Kargotich et al. 1998) and function of the immune system, in our study the comparisons between the magnitude of response of the immune parameters to the training sessions at the two moments was made by calculating relative differences in percentage, based on the post-exercise values corrected for plasma volume variation. Thus, as we are not comparing absolute values we may argue that our results were not affected by plasmatic volume changes.
At the end of the training cycle, the lower increase of leukocytes and neutrophils from Pre to Post suggests an attenuated acute response, maybe resulting from a smaller recruitment of cells from the reservoirs or marginated pool of cells (Kruger and Mooren 2014). Furthermore, the higher magnitude of the decrease from Pre to Post2h of total lymphocytes and CD19+ subset suggests a less efficient recovery of the acquired immunity (in particular CD19+ lymphocytes) in the first 2 h after the intense training session, and a longer interval of immune susceptibility to infection than at the beginning of the macrocycle.
The idea of a potential immune depression and a longer interval of immune susceptibility to infection were considered in this investigation. Although the training load intensity of the 4 weeks prior to each evaluation decreased marginally from M1 to M2, the training load of the whole macrocycle preceding M2 was higher than M1. Concomitantly, the number of URS tended to be higher at M2 than at M1. This evidence suggests a potential immune depression, and we can speculate that this may result from the cumulative effects of the swimming training loads. Throughout adolescence, especially during puberty, the physiological levels of some hormones (e.g. catecholamines, cortisol, growth hormone, oestrogen, and testosterone) in association with a differential effect of these hormones and cytokines on lymphocyte subsets (Nemet and Eliakim 2010; Steensberg et al. 2001) may also influence the exercise-induced immune changes and, therefore, should be considered. Timmons et al. (2006) have reported that when exposed to equal exercise conditions, alterations in some immune cellular and humoral components were smaller in pre- and early-pubertal boys (10 years) versus adult men (22 years) and recovery from strenuous exercise was faster in children than in the adults (Timmons et al. 2004). Although these authors have not investigated the influence of long-term training on the acute response to exercise, these results highlight the importance of controlling for age-related variables, especially during adolescence. We still have to consider seasonal variations of infectious agents (Fig. 3). In addition, the difficulty of CD16+56+ to recover in 24 h in late pubertal swimmers, and the lower magnitude of change of the CD4+/CD8+ ratio during the early recovery in the youth group, suggests that the younger athletes’ immune changes were more sensitive to the influence of long-term training.
Considering the studies that control URS frequency, the information provided by participants lacked confirmation by a physician and/or laboratory analyses (Cox et al. 2008; Spence et al. 2007). Consequently, it is possible that the outcomes could have misled the conclusions, probably tending to overestimate the frequency of infections in athletes (Gleeson et al. 2004; Hellard et al. 2015); hence, it is difficult to infer between inflammation and infection (Cox et al. 2008; Spence et al. 2007). To our knowledge only another investigation conducted in Portuguese swimmers (Rama et al. 2013) studied the URS occurrence with laboratory cells count confirmation. The authors (Rama et al. 2013) reported a higher URS frequency during the hardest training phases, which is in accordance with our data (Fig. 3), despite the lack of statistical inference. Therefore, one can state that the results obtained in our investigation reinforce those obtained by Rama et al. (2013).
The reduction in the cell trafficking and cell proliferation and/or increased cell death responses (Kruger and Mooren 2014) could have relied upon the long-term adaptations of the adherence of cells to the endothelium and their redistribution amongst organs or compartments, and of the physiological responses to acute exercise, namely cardiac output, shear stress, and blood flow to working muscle, and improved ability to counteract pH and temperature changes (Adams et al. 2011). Moreover, training might have influenced catecholamines, and cortisol concentrations and their regulation of lymphocyte subset redistribution (McCarthy et al. 1991; Mignini et al. 2008). During the post-exercise recovery period cortisol acts as conditioner of the entry of lymphocytes into the circulation contributing to their return to lymphoid compartments (Nieman 1994), and regulates both lymphopenia and neutrophilia (McCarthy et al. 1991; Mignini et al. 2008). Although the overall long-term training effects of cortisol over resting lymphocytes remains unclear, and herein was not evaluated, we may speculate that cortisol’s levels may partially explain the less efficient recovery change of total lymphocytes and CD19+ subset to the swimming session at the end of the cycle.
It is commonly accepted that training load increments in well-trained athletes undertaking periods of elevated training volume and intensity can lead to the stimulation of adaptive mechanisms related to metabolic and hormonal circulatory and respiratory responses that can compromise performance and, therefore, induce an impaired immune status, including falls in the number and activity of T and B cells (Lancaster et al. 2004; Walsh et al. 2011). This conjuncture can contribute to elevate the risk of infection; nevertheless, the literature suggest that this situation may be reversible by a tapering or recovering period (Gleeson and Bishop 2005; Walsh et al. 2011). Hence, coaches and athletes ought to implement intervention and behavioural strategies during taper periods to contribute to maintain health conditions, preventing the onset of fatigue and associated diminished performance, thus helping to avoid illness and reaching the peak performance at competitions. Also, athletes should take special precautions during the first hours after intense training sessions.
Conclusions
In the present investigation, at the end of the training macrocycle, the lower magnitude of the immediate leukocytosis and neutrophilia followed by the more prolonged recovery of total lymphocytes and B cells changes in response to the swimming session appears to dictate a general attenuated acute immune change. This change seemed even more attenuated in the younger athletes, reflected by the difficulty of CD16+56+ to recover in 24 h in late pubertal swimmers, and by the lower magnitude of the CD4+/CD8+ ratio change during the early recovery in the youth group. Concurrently, there was a higher URS frequency, which reinforces the idea of a potential immune depression and a longer interval of immune susceptibility to infection. It appears that the cumulative effects of the swimming training loads induced an overall reduction of the ability of the immune system to respond to intense training sessions, especially in the younger athletes, which makes effective resting time more valuable.
Abbreviations
- %FM:
-
Fat mass percentage
- ANOVA:
-
Analysis of variance
- ATS:
-
ANOVA-type statistic
- AUL:
-
Arbitrary units of load
- BM:
-
Body mass
- BMI:
-
Body mass index
- CD:
-
Cluster of differentiation
- CIPER:
-
Interdisciplinary center for the study of human performance
- EDTA:
-
Ethylenediaminetetraacetic acid
- FFM:
-
Free fat mass
- HR:
-
Heart rate
- INSA:
-
National Health Institute Doutor Ricardo Jorge
- M1:
-
First moment of evaluation
- M2:
-
Second moment of evaluation
- MATS:
-
Modified ANOVA-type statistic
- NK:
-
Natural killer
- Post:
-
Immediately after exercise
- Post 24h:
-
24 h after exercise
- Post 2h:
-
2 h after exercise
- Pre:
-
Before exercise
- RBC:
-
Red blood cells
- SD:
-
Standard deviation
- URS:
-
Upper respiratory symptoms
- WBC:
-
White blood cells
References
Adams GR, Zaldivar FP, Nance DM, Kodesh E, Radom-Aizik S, Cooper DM (2011) Exercise and leukocyte interchange among central circulation, lung, spleen, and muscle. Brain Behav Immun 25(4):658–666. https://doi.org/10.1016/j.bbi.2011.01.002
Bishop NC (2006) Acute exercise and acquired immune function. In: Gleeson M (ed) Immune function in sports and exercise. Churchill Livingstone Elsevier, Edinburgh, pp 91–113
Boas SR, Joswiak ML, Nixon PA, Kurland G, O’Connor MJ, Bufalino K, Orenstein DM, Whiteside TL (1996) Effects of anaerobic exercise on the immune system in eight- to seventeen-year-old trained and untrained boys. J Pediatr 129(6):846–855
Cox AJ, Gleeson M, Pyne DB, Callister R, Hopkins WG, Fricker PA (2008) Clinical and laboratory evaluation of upper respiratory symptoms in elite athletes. Clin J Sport Med 18(5):438–445. https://doi.org/10.1097/JSM.0b013e318181e50100042752-200809000-00012
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37(2):247–248
Ferrer MD, Tauler P, Sureda A, Tur JA, Pons A (2009) Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J Sports Sci 27(1):49–58. https://doi.org/10.1080/02640410802409683
Gabriel H, Schwarz L, Steffens G, Kindermann W (1992) Immunoregulatory hormones, circulating leucocyte and lymphocyte subpopulations before and after endurance exercise of different intensities. Int J Sports Med 13(5):359–366. https://doi.org/10.1055/s-2007-1021281
Gleeson M (2007) Immune function in sport and exercise. J Appl Physiol 103(2):693–699. https://doi.org/10.1152/japplphysiol.00008.2007
Gleeson M, Bishop NC (2005) The T cell and NK cell immune response to exercise. Ann Transplant 10(4):43–48
Gleeson M, McDonald WA, Cripps AW, Pyne DB, Clancy RL, Fricker PA (1995) The effect on immunity of long-term intensive training in elite swimmers. Clin Exp Immunol 102(1):210–216
Gleeson M, McDonald WA, Pyne DB, Clancy RL, Cripps AW, Francis JL, Fricker PA (2000) Immune status and respiratory illness for elite swimmers during a 12-week training cycle. Int J Sports Med 21(4):302–307
Gleeson M, Pyne DB, Callister R (2004) The missing links in exercise effects on mucosal immunity. Exerc Immunol Rev 10:107–128
Green KJ, Rowbottom DG, Mackinnon LT (2003) Acute exercise and T-lymphocyte expression of the early activation marker CD69. Med Sci Sports Exerc 35(4):582–588. https://doi.org/10.1249/01.MSS.0000058361.82096.26
Hellard P, Avalos M, Hausswirth C, Pyne D, Toussaint JF, Mujika I (2013) Identifying Optimal Overload and Taper in Elite Swimmers over Time. J Sports Sci Med 12(4):668–678
Hellard P, Avalos M, Guimaraes F, Toussaint JF, Pyne DB (2015) Training-related risk of common illnesses in elite swimmers over a 4-yr period. Med Sci Sports Exerc 47(4):698–707. https://doi.org/10.1249/MSS.0000000000000461
INSA INdSDRJ, IP (2011) Boletim de Análises Clínicas–Valores Referência Subpopulações Linfocitárias. INSA, Portugal
Kargotich S, Keast D, Goodman C, Crawford GP, Morton AR (1997) The influence of blood volume changes on leucocyte and lymphocyte subpopulations in elite swimmers following interval training of varying intensities. Int J Sports Med 18(5):373–380. https://doi.org/10.1055/s-2007-972649
Kargotich S, Goodman C, Keast D, Morton AR (1998) The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med 26(2):101–117
Kruger K, Mooren FC (2014) Exercise-induced leukocyte apoptosis. Exerc Immunol Rev 20:117–134
Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M (2004) Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev 10:91–106
Lewis SM, Bain BJ, Bates I (2006) Dacie and Lewis practical haematology, 10 edn. Churchill Livingstone Elsevier, Philadelphia
McCarthy DA, Grant M, Marbut M, Watling M, Wade AJ, Macdonald I, Nicholson S, Melsom RD, Perry JD (1991) Brief exercise induces an immediate and a delayed leucocytosis. Br J Sports Med 25(4):191–195. https://doi.org/10.1136/bjsm.25.4.191
McCarthy DA, Macdonald I, Grant M, Marbut M, Watling M, Nicholson S, Deeks JJ, Wade AJ, Perry JD (1992) Studies on the immediate and delayed leucocytosis elicited by brief (30-min) strenuous exercise. Eur J Appl Physiol Occup Physiol 64(6):513–517. https://doi.org/10.1007/BF00843760
McMurray RG (1983) Plasma volume changes during submaximal swimming. Eur J Appl Physiol Occup Physiol 51(3):347–356
Mignini F, Traini E, Tomassoni D, Vitali M, Streccioni V (2008) Leucocyte subset redistribution in a human model of physical stress. Clin Exp Hypertens 30(8):720–731. https://doi.org/10.1080/07420520802572333
Morgado JM, Rama L, Silva I, de Jesus Inacio M, Henriques A, Laranjeira P, Pedreiro S, Rosado F, Alves F, Gleeson M, Pais ML, Paiva A, Teixeira AM (2012) Cytokine production by monocytes, neutrophils, and dendritic cells is hampered by long-term intensive training in elite swimmers. Eur J Appl Physiol 112(2):471–482. https://doi.org/10.1007/s00421-011-1966-4
Morgado JP, Monteiro CP, Matias CN, Alves F, Pessoa P, Reis J, Martins F, Seixas T, Laires MJ (2014) Sex-based effects on immune changes induced by a maximal incremental exercise test in well-trained swimmers. J Sports Sci Med 13(3):708–714
Morgado JP, Monteiro CP, Teles J, Reis JF, Matias C, Seixas MT, Alvim MG, Bourbon M, Laires MJ, Alves F (2016) Immune cell changes in response to a swimming training session during a 24-h recovery period. Appl Physiol Nutr Metab. https://doi.org/10.1139/apnm-2015-0488
Morgado JP, Matias CN, Monteiro CP, Alves F, Reis JF, Santos DA, Silva AM, Martins F, Seixas MT, Rocha-Pereira P, Sardinha LB, Laires MJ (2017) Comparison of immunohematological profile between endurance- and power-oriented elite athletes. Appl Physiol Nutr Metab 42(3):257–262. https://doi.org/10.1139/apnm-2016-0435
Mujika I, Chatard JC, Busso T, Geyssant A, Barale F, Lacoste L (1995) Effects of training on performance in competitive swimming. Can J Appl Physiol 20(4):395–406
Mujika I, Busso T, Lacoste L, Barale F, Geyssant A, Chatard JC (1996a) Modeled responses to training and taper in competitive swimmers. Med Sci Sports Exerc 28(2):251–258
Mujika I, Chatard JC, Geyssant A (1996b) Effects of training and taper on blood leucocyte populations in competitive swimmers: relationships with cortisol and performance. Int J Sports Med 17(3):213–217. https://doi.org/10.1055/s-2007-972834
Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, Shephard RJ (2003) Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J 121(1):9–14. https://doi.org/10.1590/S1516-31802003000100003
Nemet D, Eliakim A (2010) Growth hormone-insulin-like growth factor-1 and inflammatory response to a single exercise bout in children and adolescents. Med Sport Sci 55:141–155. https://doi.org/10.1159/000321978
Nielsen B, Sjogaard G, Bonde-Petersen F (1984) Cardiovascular, hormonal and body fluid changes during prolonged exercise. Eur J Appl Physiol Occup Physiol 53(1):63–70
Nieman DC (1994) Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc 26(2):128–139
Noguchi K, Gel YR, Brunner E, Konietschke F (2012) nparLD: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 50(12):1–23
Rama L, Teixeira AM, Matos A, Borges G, Henriques A, Gleeson M, Pedreiro S, Filaire E, Alves F, Paiva A (2013) Changes in natural killer cell subpopulations over a winter training season in elite swimmers. Eur J Appl Physiol 113(4):859–868. https://doi.org/10.1007/s00421-012-2490-x
Spence L, Brown WJ, Pyne DB, Nissen MD, Sloots TP, McCormack JG, Locke AS, Fricker PA (2007) Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc 39(4):577–586. https://doi.org/10.1249/mss.0b013e31802e851a
Steensberg A, Toft AD, Schjerling P, Halkjaer-Kristensen J, Pedersen BK (2001) Plasma interleukin-6 during strenuous exercise: role of epinephrine. Am J Physiol Cell Physiol 281(3):C1001–C1004
Tanner JM (1962) Growth at Adolescence. Blackwell, Oxford
Tauler P, Ferrer MD, Romaguera D, Sureda A, Aguilo A, Tur J, Pons A (2008) Antioxidant response and oxidative damage induced by a swimming session: influence of gender. J Sports Sci 26(12):1303–1311. https://doi.org/10.1080/02640410801974992
Teixeira AM, Rama L, Carvalho HM, Borges G, Carvalheiro T, Gleeson M, Alves F, Trindade H, Paiva A (2014) Changes in naive and memory T-cells in elite swimmers during a winter training season. Brain Behav Immun 39:186–193. https://doi.org/10.1016/j.bbi.2014.01.002
Timmons BW, Tarnopolsky MA, Bar-Or O (2004) Immune responses to strenuous exercise and carbohydrate intake in boys and men. Pediatr Res 56(2):227–234. https://doi.org/10.1203/01.PDR.0000132852.29770.C5
Timmons BW, Tarnopolsky MA, Snider DP, Bar-Or O (2006) Immunological changes in response to exercise: influence of age, puberty, and gender. Med Sci Sports Exerc 38(2):293–304. https://doi.org/10.1249/01.mss.0000183479.90501.a0
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P (2011) Position statement. Part one: immune function and exercise. Exerc Immunol Rev 17:6–63
World Medical Association (2008) Declaration of Helsinki—ethical principles for medical research involving human subjects. WMJ 54 (4):122–125
Yamada M, Suzuki K, Kudo S, Totsuka M, Simoyama T, Nakaji S, Sugawara K (2000) Effect of exhaustive exercise on human neutrophils in athletes. Luminescence 15 (1):15–20
Zhang X, Matsuo K, Farmawati A, Higashi Y, Ogawa K, Nagata K, Nagatomi R (2006) Exhaustive exercise induces differential changes in serum granulysin and circulating number of natural killer cells. Tohoku J Exp Med 210(2):117–124. https://doi.org/10.1620/tjem.210.117
Acknowledgements
We would like to express our gratitude to the athletes for their time and effort, swimming teams for making both their infrastructures and specialized coaches and staff available for the study. We also thank Maria T. Seixas, Marta Alvim and Mafalda Bourbon from Instituto Nacional de Saúde Dr. Ricardo Jorge for their help in the assessment of the biochemical parameters. José Morgado, and Catarina N. Matias and Joana Reis were supported by a scholarship from the Portuguese Foundation for Science and Technology (SFRH/BD/48211/2008, and SFRH/BD/61520/2009 and SFRH/BPD/84315/2012, respectively) and the study was financed by the Interdisciplinary Center for the Study of Human Performance (CIPER). The results of this study are actual and real, presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Morgado, J.P., Monteiro, C.P., Matias, C.N. et al. Long-term swimming training modifies acute immune cell response to a high-intensity session. Eur J Appl Physiol 118, 573–583 (2018). https://doi.org/10.1007/s00421-017-3777-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3777-8