Abstract
The purpose of this study was to examine the acute hormonal and muscular responses to a strenuous strength loading [bilateral leg press (LP) 10 × 10 1RM] followed by loading-specific active (AR, n = 7, LP 10 × 10 × 30% 1RM) or passive (PR, n = 11, seated) recovery. The subjects were men age: 26 ± 4 years, height: 174 ± 8 cm, body mass: 75 ± 13 kg. After control measurements, experimental measurements were conducted at pre- and post-loading as well as post-recovery and next morning. A significantly higher absolute concentration (p < 0.05) of serum luteinizing hormone (LH) was observed in AR than PR at next morning while no differences were observed in serum testosterone (T), cortisol (C) or sex hormone binding globulin (SHBG). Significant differences in relative hormonal responses to the loading were observed at next morning with greater responses observed in AR than in PR in terms of LH, and T (p < 0.05). Maximal bilateral isometric force (MVC) and countermovement jump height (CMJ) decreased significantly (p < 0.001) from the control measurements in both AR and PR but returned to control levels by next morning. No between-group differences were observed in mean absolute or relative changes in MVC or CMJ. From a hormonal perspective, the present AR method appears to have had some favorable effects following the strenuous strength loading; however, acute decreases in muscular force production did not significantly differ between groups. These results provide insight into the development of training programs that may help to support the performance of individuals involved in strenuous tasks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute physical loading and recovery influence hormonal and neuromuscular responses, which subsequently affect long-term adaptations to training. Significant loading of the muscles, which occurs with heavy resistance training or lifting induces significant acute decreases in force production capacity (Häkkinen 1994; Häkkinen et al. 1998; Linnamo et al. 1998). This type of loading may trigger physiological cascades that play a key role in strength development and muscle hypertrophy as well as overall recovery (Cumming et al. 1986; Kraemer et al. 1990). In order to optimally perform subsequent training sessions or strenuous tasks, recovery from a strength training session or other physical stress is of high importance. Many methods for recovery have been developed or discovered (Barnett 2006) including passive recovery methods that require lying prone or remaining seated in order to reduce metabolic demand (Carter et al. 2002; Crisafulli et al. 2003), or active recovery methods including upright standing (Coffey et al. 2004), walking and aerobic running (Castagna et al. 2008), or submaximal cycling (Andersson et al. 2008) that are suggested to facilitate recovery via mechanisms such as increased blood flow and substrate clearance.
A heavy lifting session often increases serum levels of serum testosterone (T), growth hormone (GH), insulin-like growth factor I (IGF-I), and cortisol (C) (Häkkinen and Pakarinen 1993a; Kraemer et al. 1995), yet during 2 days of recovery after a strenuous strength training session, decreased levels of T have been observed (Häkkinen and Pakarinen 1993a). Resting levels of androgenic hormones like T have been associated with changes in strength and muscle mass while other hormones have also been observed to play a key role in the trainability of strength and muscle mass (Cumming et al. 1986; Häkkinen et al. 1988; Kraemer et al. 1990) via the growth and protein synthesis that these hormones stimulate (Kraemer and Rogol 2008; Kraemer and Ratamess 2005). Some studies suggest that these transient increases in anabolic hormones are not related to muscle protein synthesis, but that local mechanisms are responsible for the signaling that influences subsequent muscle hypertrophy (West et al. 2009). It is, however, important to remember that serum hormone concentrations are influenced by the availability of hormone transporters and receptors and that hormones bound to, e.g., sex hormone binding globulin (SHBG), which has a specific “high-affinity” binding site for transporting sex hormones such as T are not immediately available for use. Furthermore, luteinizing hormone (LH) is a precursor to sex hormones, like T, that is produced by the testes in men (Kraemer and Rogol 2008). A decrease in LH may indicate a subsequent or concurrent decrease in secretion of T, however, as LH is released in a pulsatile fashion, there are a number of factors that can influence the pulsatility of this hormone.
The primary human glucocorticoid, cortisol, is produced by the adrenal cortex, and promotes protein breakdown and glucose production while stimulating fat metabolism (Kraemer and Rogol 2008) in addition to assisting with blood pressure maintenance, cardiovascular function, and inflammatory response (Brooks and Carter 2013). In adequate concentrations cortisol has been shown to aid in recovery from training (Nindl et al. 2001). High concentrations of cortisol relative to testosterone have been reported to interfere with the anabolic processes that promote muscle hypertrophy, which may in turn negatively affect strength development (Daly et al. 2005; Häkkinen et al. 1985; Izquierdo et al. 2004) and could ultimately be a marker of acute overtraining (Adlercreutz et al. 1986; Häkkinen et al. 1985; Stone et al. 1991). Over a more prolonged period of training without adequate rest and recovery cortisol concentrations may, however, become significantly decreased as well (Brooks and Carter 2013).
Monitoring hormonal concentrations over the course of a strenuous training session and subsequent recovery may help us to elucidate mechanisms of recovery and determine what recovery methods are the most favorable under specific loading and nutritional conditions. The purpose of this study was to examine the acute hormonal and muscular responses to a strenuous hypertrophic strength loading session followed by active or passive recovery modalities. The loading was intended to simulate a heavy lifting task that puts substantial strain on the lower extremities while the recovery modalities were designed to be simple and task-specific. We hypothesize that active recovery will be the more effective recovery modality because of the potential for increased blood flow and substrate clearance. We do, however, acknowledge that several studies have shown that passive recovery is more effective or that no differences between active and passive recovery exist. For example, during repeated sprinting, the use of passive recovery is advisable during competition in order to limit fatigue as a consequence of repeated high-intensity exercise (Castagna et al. 2008) while active recovery was shown to have no effects on the recovery pattern of neuromuscular and biochemical parameters after a female soccer match (Andersson et al. 2008). Unique to this study design is the longer follow-up and morning after loading measurements.
Methods
Subjects
Subjects included 18 physically active men who participated voluntarily in the study. The experimental group was divided into a passive recovery group (PR, n = 11, mean ± SD, age: 25.7 ± 3.9 years, height: 181.0 ± 7.7 cm, body mass: 78.4 ± 10.9, body mass index: 23.9 ± 2.4, VO2max 60.8 ± 4.9 ml kg−1 min−1) and an active recovery group (AR, n = 7 age: 25.4 ± 3.1 years, height: 180.1 ± 7.7 cm, body mass: 80.5 ± 15.5, body mass index: 24.7 ± 3.4, VO2max 59.7 ± 10.2 ml kg−1 min−1). No significant differences were observed between physical characteristics of the groups. Subjects were asked to avoid alcohol, caffeine, and tobacco for at least 4 h prior to each testing session and not to participate in strenuous exercise on the loading day or the day before. Likewise, the subjects were in a fasted state on the morning of each testing day.
Ethics statement
Ethical approval of both methodology and consent procedures was granted by the University of Jyväskylä Ethical Committee, the Central Finland Health Care District and the Surgeon General of the Finnish Defence Forces. The study was conducted according to the provisions of the most recent Declaration of Helsinki. All subjects received written and oral information about the study design and measurement procedures and the possible risks and benefits of participation in the study were thoroughly explained prior to the signing of an informed consent document.
Study design
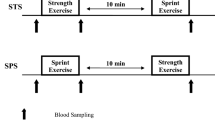
A familiarization session was completed before the loading day in order to minimize any learning effects. The study did not include a separate control group, because each subject acted as their own control prior to the strength loading. On the loading day, force measurements of the lower extremities were performed pre-, mid-, and post-loading as well as after either active or passive recovery. Force measurements were also completed the morning after the loading to evaluate neuromuscular recovery. These same time points were used for blood sample collection. The study design is shown in Fig. 1.
Strength loading protocol
The strength loading protocol included 10 × 10 × 70% one repetition maximum (RM) with 2 min of rest between sets that started at approximately the same time of day (11.00) for all subjects and lasted for ~ 60 min. A break of 10 min between the 5th and the 6th set allowed for additional measurements (data not presented). Loads were adjusted such that each subject was able to perform 10 repetitions during each set of the loading protocol. If the load became too heavy, the subject was provided some assistance by trained laboratory personnel. The range of motion for each repetition was from a knee angle of ~ 60° to a knee angle of ~ 180° (full extension). The strength loading protocol has previously been described (Ahtiainen et al. 2015).
Recovery protocols
Active and passive recovery started after force and blood measurements were performed after the loading protocol. All subjects in the AR group followed an identical recovery procedure of 10 × 10 × 30% 1RM leg press with 5 min of passive rest between sets, lasting ~ 60 min. The subjects in the PR group remained seated in a chair for 60 min. These subjects were permitted to read, listen to music or work on the computer and were only allowed to walk to and from the bathroom if necessary.
Maximal isometric force
An electromechanical isometric leg extension device (horizontal leg press, designed and manufactured by Biology of Physical Activity, Faculty of Sport and Health Sciences at the University of Jyväskylä, Finland) was used to measure maximal isometric bilateral strength (MVC) of the lower extremities. The subjects’ knee angle was 107° measured using the greater trochanter, lateral tibiofemoral joint space and lateral malleolus as reference points, while the hip angle was 110°. For reference, full extension of the leg is considered to be 180°. Subjects were instructed to produce force “as fast and as hard as possible” for approximately 3 s. The arms were kept crossed over the chest. In total, five submaximal warm-up trials (3 × ~ 50% and 2 × ~ 85%) were performed before the maximal performance. Subjects were verbally encouraged. The warm-up trials were used only before the morning measurements (i.e., control and morning time points) while subsequent MVCs were performed without warm-up. Subjects performed at least three MVCs (Häkkinen et al. 1998) with ~ 1 min of rest between repetitions. If the maximum force produced during the last trial was greater than 5% compared to the previous trial, an additional trial was performed. Force data were collected at a sampling frequency of 2000 Hz, and then filtered with a 20 Hz low pass filter. Force data were analyzed using customized scripts (Signal 4.04, CED, UK). The best performance trial, in terms of maximal force, was used for statistical analysis. Reliability of MVC has previously been reported to be r = 0.98, CV = 4.1% (Viitasalo et al. 1980) while CMJ’s reliability has reported as r = 0.95, CV = 4–5% (Viitasalo et al. unpublished data) and used consistently in reports from our lab (Bosco et al. 1982).
Countermovement jump
A force platform (Biology of Physical Activity, Faculty of Sport and Health Sciences at the University of Jyväskylä, Finland) was used to measure maximal dynamic explosive force by countermovement jump (CMJ) height (Komi and Bosco 1978). Subjects were instructed to stand with their feet approximately hip-width apart with their hands on their hips. They were then instructed to perform a quick and explosive countermovement jump on verbal command so that knee angle for the jump was no less than 90 degrees. Force data were collected and analyzed by computer software (Signal 2.14, CED, Cambridge, UK), which used the equation h = I 2 × 2 g−2 to calculate jump height from impulse (I = impulse, g = gravity and m = mass of participant).
Blood samples and serum hormones
Venous blood samples were collected from the antecubital vein after 12 h of fasting between 07:00–08:00 on all three testing days. Blood samples were collected using sterile needles into serum tubes (Venosafe, Terumo Medical Co., Leuven, Belgium) by a qualified lab technician who reviewed analyses of the basic blood count (Sysmex KX-21N, Kobe, Japan) to check for abnormalities prior to further analysis. Whole blood was centrifuged at 2500g (Megafuge 1.0R, Heraeus, Germany) for 10 min after which serum was removed and stored at − 80 °C until further analysis. Blood samples were used for determination of serum testosterone (T), free testosterone (FT), luteinizing hormone (LH), cortisol (C), and sex hormone binding globulin (SHBG). Analyses were performed using chemical luminescence techniques (Immunlite 1000, DCP Diagnostics Corporation, Los Angeles, California, USA) and hormone-specific immunoassay kits (Siemens, New York, NY, USA). The sensitivity of T, LH, C and SHBG assays were: 0.5 nmol l−1, 0.1 ml U l−1, 5.5, and 5.5 nmol l−1, respectively. The intra-assay coefficients of variation for T, LH, C and SHBG were: 5.7, 5.8, 4.6, and 2.4%, respectively. The average of two fasting blood samples (from the familiarization session and the morning of the experiment) were used to determine control values. The testosterone–cortisol ratio (T/C ratio) was calculated as [T (nmol l−1)/C (nmol l−1) × 100] while the free androgen index (FAI) was calculated as [T (nmol l−1) × 100/SHBG (nmol l−1)]. Although we recognize that loading-induced changes in plasma volume shift may influence hormonal concentrations (Kargotich et al. 1998), plasma volume changes were not used to correct hormone concentrations in the present study. We believe that the interpretation of the changes in these hormones is related to biological responses that are dependent on an increase/decrease in interaction with receptors that is possible regardless of plasma volume shift (Kraemer and Ratamess 2005).
Blood lactate
Blood lactate was analyzed from blood samples using a Biosen S_line Lab + lactate analyzer (EKF Diagnostic, Magdeburg, Germany).
Nutrition
Loading day caloric intake was restricted for all subjects. Subjects were given an energy bar (commercially available: 700 kJ/170 kcal, proteins 7 g, carbohydrates 21 g, fats 2.5 g) following fasting blood measurements and approximately 3 h prior to the start of the loading protocol. The energy bar was accompanied by 0.5 L of water. A second energy bar and 0.5 L of water was consumed approximately 3 h after the loading. Otherwise subjects were allowed to drink a limited amount of water ad libitum.
Statistical methods
Standard statistical methods were used for calculation of means and standard deviation (SD). Group differences and group-by-loading interaction were analyzed by a repeated measures analysis using mixed models and an unstructured covariance matrix. Groups were compared with a Bonferroni post hoc analysis in a mixed models analysis when appropriate. The criterion for significance was set at *p < 0.05, **p < 0.01 and ***p < 0.001. Statistical analysis was completed with PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Results
Serum LH concentrations were statistically unaltered through control, pre-loading, post-loading, post-recovery and morning measurements (Fig. 2), however, at the morning measurement following the combined loading and recovery sessions, a significantly higher absolute concentration (p < 0.05) of serum LH was observed in the AR group while the relative response of LH in the AR group was also significantly greater than that of the PR group (+ 26% in AR vs. − 22% in PR, p < 0.05).
Serum T concentrations decreased significantly from control to pre-loading in both AR and PR groups (p < 0.05), while a significant difference in absolute mean T concentrations was observed between AR and PR post-loading (p < 0.05), although no significant differences were observed in relative T response from pre-loading to post-loading and even post-recovery (Fig. 3). A significant difference in mean relative T response was, however, observed at morning (+ 8% in AR vs − 28% in PR compared to control, p < 0.01) in which absolute T concentrations of the PR group were significantly lower (p < 0.01) than at control and T concentrations had returned to control in AR.
Mean (± SD) serum testosterone absolute changes as columns and relative changes as lines (mean ± SD). For absolute values within-group differences from control are marked as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. Significant difference between groups for absolute and relative changes ǂ p < 0.05 and ǂǂ p < 0.01. AR = 7 and PR = 11
Blood lactate concentrations increased significantly in both AR and PR groups at post-loading (p < 0.001) and returned to resting values at post-recovery (Table 1). No differences in relative blood lactate response were observed.
Serum C concentrations increased significantly from control to post-loading in both AR and PR groups (38% in AR and 35% in PR, p < 0.01, Table 1) and remained significantly elevated (34%, p < 0.05) in the AR group at Post-Recovery. At Post-Recovery in PR, the serum C concentrations were slightly, but insignificantly elevated (18%, n.s.) in PR. At morning, C concentrations had returned to control. No differences in relative C responses were observed.
Serum SHBG concentrations increased significantly from control to post-loading in both groups (9% in AR and 12% in PR, p < 0.05, Table 1) and returned to control by post-recovery. No differences in relative serum SHBG responses were observed.
Testosterone/cortisol ratio (T/C ratio) decreased significantly between control and post-loading (p < 0.05 in AR and p < 0.001 in PR). By morning T/C ratio had returned to control in AR, but remained significantly lower in PR (p < 0.05) although no significant between-group differences were observed.
Free androgen index (FAI) decreased significantly in PR from control to pre-loading (p < 0.01) before returning to control levels at post-loading. Another significant decrease in FAI was observed from control to post-recovery in PR (p < 0.05); however, FAI returned to control levels by morning and no statistically significant differences between AR and PR were observed at any time point.
Maximal bilateral isometric force (MVC) decreased significantly (− 54% in AR and − 46% in PR, p < 0.001) from the control measurements following the 10 × 10 × 70% 1RM bilateral leg press loading protocol in both AR and PR groups (Table 2). By post-recovery, MVC of the PR group had returned to the same level as control measurements whereas MVC of the AR group remained significantly reduced (− 39%, p < 0.01). No between-group differences were observed in mean absolute or relative changes in MVC over the entire experimental period. Likewise, CMJ height decreased significantly from control to post-loading (− 44% in AR and − 28% in PR, p < 0.001) in both active and passive recovery groups (AR and PR, respectively, Table 2). A mixed model analysis with the control as a covariate indicated that CMJ in the PR group was still significantly (p < 0.05) lower than control at morning while jumping height in the AR group had returned to control levels; however, no significant differences between groups were observed.
Discussion
The primary findings of the present study were that following the combined experimental strength loading and recovery protocol, both absolute and relative LH concentrations next morning in the PR group were observed to be significantly lower than those of the AR group, although statistically both groups were similar to control. In addition, relative serum T concentrations in the AR group next morning were significantly higher than what was observed in the PR group, although absolute concentrations at next morning were similar between groups and only PR showed significantly lower concentrations of serum T in comparison to control. Both groups showed, as expected, drastic decreases in MVC post-loading, however, no between-group differences were observed in MVC next morning when strength levels had recovered to control levels.
LH is a precursor to secretion of sex hormones like T (Kraemer and Rogol 2008), thus a decrease in LH may indicate a subsequent or concurrent decrease in secretion of T; however, as LH is released in a pulsatile fashion and various factors can influence the pulsatility of this hormone, the present methodology does not allow us to conclude with certainty that the decrease in LH is of physiological significance. In addition, the magnitude of the difference in LH concentrations between AR and PR is relatively small thus calling into question whether or not this statistical difference is of biological/physiological significance. In the present study, a decrease in absolute T concentrations the morning after the present strength loading was observed in PR. In absolute terms these concentrations were similar; however, in relative terms, these concentrations were significantly lower in PR than in AR. Decreased T may indicate physiological stress (Häkkinen and Pakarinen 1993b) or suggest a protein catabolic state (Adlercreutz et al. 1986). Other sources postulate that decreased testosterone points to usage of the hormone in target tissues (or uptake by non-target tissues), for example, muscle protein synthesis or increased hepatic clearance (Cadoux-Hudson et al. 1985). Other sources (West et al. 2009) suggest that the transient changes in anabolic hormones are not, in fact, related to muscle protein synthesis and that other local factors are of greater importance. The initial decrease in serum testosterone observed between control and pre-loading measurements may be attributed to circadian rhythms as previous studies have shown a substantial decrease in morning testosterone concentrations between 04:00 and 08:00 and a more gradual daily decrease from 08:00 until 22:00 (Kraemer et al. 2001). Blood samples were collected between 07:00 and 8:00, which suggests that serum testosterone concentrations are still decreasing before the pre-loading samples were taken at 11.00.
While LH and T production were limited or usage/clearance was increased, serum C concentrations increased significantly from control to post-loading in both AR and PR groups. The group performing AR maintained increased C concentrations post-recovery (although not statistically significantly different from the PR group). AR, in contrast to PR, maintained increased systemic blood flow, which may have influenced the time-course of the peak in C. Due the relatively infrequent nature of our blood sampling procedures, we cannot be sure when the actual C peaks for AR and PR occurred. We can only speculate that the C peak came somewhere between post-loading and post-recovery in PR. An increase in C is often postulated to be indicative of the overall stress or load induced by the combined strength loading and recovery protocol. This could, in turn, suggest that AR is counter-productive to increasing/maintaining lean body mass (Kraemer and Ratamess 2005) due to its nature as a “catabolic” stress hormone (Adlercreutz et al. 1986). However, this increase in C could, in fact, be a positive response as an increase in C has been significantly correlated with positive changes in lean body mass over a prolonged period in situations where free testosterone was not (West and Phillips 2012). The return of C levels to control by morning further supports the idea that the effects of the loading were not prolonged and the loading itself was not exceptionally stressful. It should also be noted that C is also affected by diurnal variations (Häkkinen and Pakarinen 1995; Kanaley et al. 2001), thus this data should be interpreted with caution although control and next morning blood samples were collected at the same time of day to minimize the possible influence of these circadian rhythms. The T/C ratio can also be used as an indicator of anabolic/catabolic metabolism of the muscle (Adlercreutz et al. 1986). In the present study, the clear decrease in T/C ratio by post-loading in both groups may indicate the stress of the loading. T/C ratio values at morning in AR had returned to control while in PR values were still significantly lower than at control, which may further support the idea that active recovery is more effective than passive recovery. Interestingly, although FAI fluctuated significantly in PR, no significant differences were observed between groups.
It is important to remember that serum hormone concentrations are influenced by the availability of hormone transporters and receptors. SHBG was significantly higher at post-loading than at control in both AR and PR; however, this change may not be physiologically significant as the binding affinity of SHBG does not appear to be associated with exercise (Fahrner and Hackney 1998).
Finally, an acute decrease in MVC and CMJ capabilities was expected following the strenuous hypertrophic 10 × 10 × 70% 1RM strength loading. No differences were observed between groups in terms of fatigue induced by the strength loading at post-loading, making the differences in hormonal responses all the more interesting. The decreases in MVC and CMJ were accompanied by expected significant increases in blood lactate concentrations. Strenuous strength training sessions are known to induce neuromuscular fatigue characterized by a decrease in muscle activation and force production abilities (Häkkinen 1994; Häkkinen et al. 1998; Linnamo et al. 1998), which is related to the decrease in pH of the working muscles (via lactate production) and subsequent impairment of calcium (Ca2+) release that disturbs excitation–contraction coupling (Bosco et al. 2000). The AR and PR methods appeared to affect muscular performance and recovery similarly as no differences in groups were observed in absolute or relative terms at post-recovery or morning.
While hormonal responses to loadings may be influenced by time of day and nutrition, in the present study the comparison of fasting serum hormonal concentrations examined from the mornings before and after the present strenuous strength loading seems to show the greatest differences with regard to the effects of AR and PR. These results are the best indicators of the effectiveness of the present recovery strategies. As always, changes in serum levels of hormones may not entirely reflect the processes occurring at a local skeletal level, thus, these results should be interpreted with caution.
Conclusions
The results of this study provide valuable insight into the development of training programs that may help to support the performance and health of individuals involved in strenuous tasks. From a hormonal perspective, the combined responses of LH, T, and C may indicate that the present active recovery method had favorable effects following the strenuous strength loading, although the acute decreases in muscular force production did not significantly differ between groups and these small differences in hormonal responses may or may not be of biological significance. Whether or not these acute responses would then lead to limited strength gains over several weeks or months of strength training needs to be examined in the future.
Abbreviations
- AR:
-
Active recovery
- C:
-
Cortisol
- CMJ:
-
Countermovement jump
- GH:
-
Growth hormone
- IGF-1:
-
Insulin-like growth factor I
- LH:
-
Luteinizing hormone
- MVC:
-
Maximal bilateral isometric force
- PR:
-
Passive recovery
- SD:
-
Standard deviation
- SHBG:
-
Sex hormone binding globulin
- T:
-
Testosterone
References
Adlercreutz H, Harkonen M, Kuoppasalmi K et al (1986) Effect of training on plasma anabolic and catabolic steroid hormones and their response during physical exercise. Int J Sports Med 7:27–28
Ahtiainen JP, Walker S, Silvennoinen M et al (2015) Exercise type and volume alter signaling pathways regulating skeletal muscle glucose uptake and protein synthesis. Eur J Appl Physiol 115:1835–1845. https://doi.org/10.1007/s00421-015-3155-3
Andersson HM, Raastad T, Nilsson J, Paulsen G, Garthe I, Kadi F (2008) Neuromuscular fatigue and recovery in elite female soccer: effects of active recovery. Med Sci Sports Exercise 40:372–380
Barnett A (2006) Using recovery modalities between training sessions in elite athletes. Sports Med 36:781–796
Bosco C, Viitasalo J, Komi P, Luhtanen P (1982) Combined effect of elastic energy and myoelectrical potentiation during stretch-shortening cycle exercise. Acta Physiol 114:557–565
Bosco C, Colli R, Bonomi R, von Duvillard SP, Viru A (2000) Monitoring strength training: neuromuscular and hormonal profile. Med Sci Sports Exerc 32:202–208
Brooks K, Carter J (2013) Overtraining, Exercise, and Adrenal Insufficiency. J Nov Physiother 3:11717
Cadoux-Hudson TA, Few JD, Imms FJ (1985) The effect of exercise on the production and clearance of testosterone in well trained young men. Eur J Appl Physiol Occup Physiol 54:321–325
Carter R, Wilson TE, Watenpaugh DE, Smith ML, Crandall CG (2002) Effects of mode of exercise recovery on thermoregulatory and cardiovascular responses. J Appl Physiol 93:1918–1924
Castagna C, Abt G, Manzi V, Annino G, Padua E, D’Ottavio S (2008) Effect of recovery mode on repeated sprint ability in young basketball players. J Strength Condition Res 22:923–929
Coffey V, Leveritt M, Gill N (2004) Effect of recovery modality on 4-hour repeated treadmill running performance and changes in physiological variables. J Sci Med Sport 7:1–10
Crisafulli A, Orru V, Melis F, Tocco F, Concu A (2003) Hemodynamics during active and passive recovery from a single bout of supramaximal exercise. Eur J Appl Physiol 89:209–216
Cumming DC, Brunsting LA, Strich G, Ries AL, Rebar RW (1986) Reproductive hormone increases in response to acute exercise in men. Med Sci Sports Exerc 18:369–373
Daly W, Seegers CA, Rubin DA, Dobridge JD, Hackney AC (2005) Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur J Appl Physiol 93:375–380. https://doi.org/10.1007/s00421-004-1223-1
Fahrner CL, Hackney AC (1998) Effects of endurance exercise on free testosterone concentration and the binding affinity of sex hormone binding globulin (SHBG). Int J Sports Med 19:12–15. https://doi.org/10.1055/s-2007-971872
Häkkinen K (1994) Neuromuscular adaptation during strength training, aging, detraining, and immobilization. Crit Rev Phys Rehab Med 6:161–161
Häkkinen K, Pakarinen A (1993a) Acute hormonal responses to two different fatiguing heavy-resistance protocols in male athletes. J Appl Physiol 74:882–887
Häkkinen K, Pakarinen A (1993b) Muscle strength and serum testosterone, cortisol and SHBG concentrations in middle-aged and elderly men and women. Acta Physiol Scand 148:199–207. https://doi.org/10.1111/j.1748-1716.1993.tb09549.x
Häkkinen K, Pakarinen A (1995) Acute hormonal responses to heavy resistance exercise in men and women at different ages. Int J Sports Med 16:507–513. https://doi.org/10.1055/s-2007-973045
Häkkinen K, Pakarinen A, Alén M, Komi P (1985) Serum hormones during prolonged training of neuromuscular performance. Eur J Appl Physiol 53:287–293. https://doi.org/10.1007/BF00422840
Häkkinen K, Pakarinen A, Alén M, Kauhanen H, Komi PV (1988) Daily hormonal and neuromuscular responses to intensive strength training in 1 week. Int J Sports Med 9:422–428. https://doi.org/10.1055/s-2007-1025044
Häkkinen K, Kallinen M, Izquierdo M et al (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349
Izquierdo M, Ibanez J, Häkkinen K, Kraemer WJ, Ruesta M, Gorostiaga EM (2004) Maximal strength and power, muscle mass, endurance and serum hormones in weightlifters and road cyclists. J Sports Sci 22:465–478. https://doi.org/10.1080/02640410410001675342
Kanaley JA, Weltman JY, Pieper KS, Weltman A, Hartman ML (2001) Cortisol and Growth Hormone Responses to Exercise at Different Times of Day. J Clin Endocrinol Metab 86:2881–2889. https://doi.org/10.1210/jc.86.6.2881
Kargotich S, Goodman C, Keast D, Morton AR (1998) The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med 26:101–117
Komi PV, Bosco C (1978) Utilization of stored elastic energy in leg extensor muscles by men and women. Med Sci Sports 10:261–265
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Kraemer WJ, Rogol AD (2008) The encyclopaedia of sports medicine an IOC medical commission publication, the endocrine system in sports and exercise. Wiley-Blackwell
Kraemer WJ, Marchitelli L, Gordon SE et al (1990) Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol 69:1442–1450
Kraemer WJ, Aguilera BA, Terada M et al (1995) Responses of IGF-I to endogenous increases in growth hormone after heavy-resistance exercise. J Appl Physiol 79:1310–1315
Kraemer WJ, Loebel CC, Volek JS et al (2001) The effect of heavy resistance exercise on the circadian rhythm of salivary testosterone in men. Eur J Appl Physiol 84:13–18
Linnamo V, Häkkinen K, Komi PV (1998) Neuromuscular fatigue and recovery in maximal compared to explosive strength loading. Eur J Appl Physiol 77:176–181. https://doi.org/10.1007/s004210050317
Nindl BC, Kraemer WJ, Deaver DR et al (2001) LH secretion and testosterone concentrations are blunted after resistance exercise in men. J Appl Physiol 91:1251–1258
Stone M, Keith R, Kearney J, Fleck S, Wilson G, Triplett N (1991) Overtraining: a review of the signs, symptoms and possible causes. J Strength Condition Res 5:35–50
Viitasalo JT, Saukkonen S, Komi PV (1980) Reproducibility of measurements of selected neuromuscular performance variables in man. Electromyogr Clin Neurophysiol 20:487–501
West DW, Phillips SM (2012) Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Eur J Appl Physiol 112:2693–2702. https://doi.org/10.1007/s00421-011-2246-z
West DW, Kujbida GW, Moore DR et al (2009) Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587:5239–5247. https://doi.org/10.1113/jphysiol.2009.177220
Acknowledgements
The authors wish to thank the technical staff of the University of Jyväskylä Faculty of Sport and Health Sciences Biology of Physical Activity group for their irreplaceable expertise and assistance in completing this project. We also wish to thank our hard working student researchers and the subjects who volunteered to participate in this study. We are thankful for funding from the Biology of Physical Activity group and the Finnish Defence Forces. Sheila S. Gagnon is supported in part by Western University’s Bone and Joint Institute and the Collaborative Training Program in Musculoskeletal Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Taipale, R.S., Kyröläinen, H., Gagnon, S.S. et al. Active and passive recovery influence responses of luteinizing hormone and testosterone to a fatiguing strength loading. Eur J Appl Physiol 118, 123–131 (2018). https://doi.org/10.1007/s00421-017-3753-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3753-3