Abstract
Purpose
The present study aimed to examine (1) the effect of task difficulty on unintended muscle activation (UIMA) levels in contralateral homologous muscle, (2) the difference between young and old adults in degree of UIMA with respect to task difficulty, and (3) temporal correlations between intended and contralateral unintended muscle activity at low frequency during unilateral intended force-matching tasks.
Methods
Twelve young (21.8 ± 2.4 years) and twelve old (69.9 ± 5.3 years) adult men performed steady isometric abductions with the left index finger at 20–80% of maximal voluntary contraction force. Two task difficulties were set by adjusting the spacing between two bars centered about the target force used for visual feedback on a monitor. The amplitude of surface electromyogram (aEMG) for both hands was calculated and normalized with respect to the maximal value. To determine if oscillations between intended and unintended muscle activities were correlated, cross-correlation function (CCF) of rectified EMG for both hands at low frequency was calculated for samples deemed adequate.
Results
The unintended aEMG (right hand) had significant main effects in task difficulty, age, and target force (all P < 0.05) without any interactions. Distinct significant peaks in CCF (0.38 on average, P < 0.05) with small time lags were present between rectified EMGs of intended and unintended muscles in 14 of the 17 samples.
Conclusions
The current results indicate that UIMA increases with greater task difficulty regardless of age, and temporal correlations exist between intended and contralateral unintended muscle activities at low frequency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During unilateral voluntary contractions, unintended muscle activity (UIMA) can be observed in contralateral homologous muscle (Gandevia et al. 1993; Zijdewind et al. 1998; Bodwell et al. 2003; Shinohara et al. 2003). Occurence of UIMA can be a factor depressing fine motor performance. For example, when playing piano with one hand whereas keeping the contralateral hand quiet on the keyboard due to edition of a music, occurence of UIMA may impair the fine piano performance by needless pressing a key with contralateral hand. Thus, understanding the relation between UIMA and physiological factors involved with the daily activities may provide new insight for assessing neuromuscular function of humans. As far, the degree of UIMA of healthy subjects depends on various factors strongly associated with day-to-day activities, e.g., contraction intensity (Todor and Lazarus 1986; Shinohara et al. 2003; Post et al. 2008), contraction speed (Bodwell et al. 2003), muscle contraction mode (Shinohara et al. 2003), and degree of muscle fatigue (Zijdewind and Kernell 2001; Shinohara et al. 2003; Post et al. 2008). In addition, another factor referred to as “task difficulty” is becoming of more note in recent daily activities which require performing precise and accurate motor movements, such as the hand dexterity needed when operating a smartphone or tablet with small screen. However, it is unclear to what extent UIMA is affected by task difficulty during motor tasks.
Although the origin of UIMA has yet to be confirmed, evidence suggests that spreading excitation to the contralateral motor cortex (i.e., primary motor area; M1) arising indirectly from the ipsilateral hemisphere via corpus callosum or directly from the higher centers (i.e., prefrontal area, premotor area, and supplementary motor area) may be the source of activity of healthy subjects (Carson 2005; Zijdewind et al. 2006). Furthermore, evidence obtained using transcranial magnetic stimulation (TMS) (Mayston et al. 1999) showed that UIMA was accompanied with simultaneous activation of crossed corticospinal pathways originating from both left and right motor cortices. A prior study adopting functional magnetic resonance imaging (fMRI) (Sterr et al. 2009) has reported that force-tracking tasks with greater task difficulty increased the cortical activity of the premotor area in both hemispheres when compared to a simple force-tracking task. Taken together, it is possible that UIMA increases with increasing task difficulty, due to increases in cortical excitability.
Older adults exhibit greater degree of UIMA than younger adults during unilateral finger-tapping (Bodwell et al. 2003) and force-matching tasks (Shinohara et al. 2003; Baliz et al. 2005). Compared with younger adults, older adults also require additional recruitment of cortical and subcortical areas when performing a motor task at a given contraction intensity to compensate for decreases in brain function accompanied with aging (Mattay et al. 2002). Based on this compensating theory, it is likely that older adults exhibit larger UIMA than younger adults when performing motor tasks of comparable task difficulty.
When activating multiple muscles, correlated oscillations can be found in the discharge times of pairs of motor units between synergistic muscles (De Luca and Erim 2002) and between antagonistic muscles (De Luca and Mambrito 1987) during unfatigued conditions. Correlated low-frequency oscillations (<5 Hz) in discharge rate between motor units are called “common drive” and suggested to originate from the supraspinal level (De Luca et al. 1982). As previously mentioned, UIMA of healthy subjects may be induced by spreading excitation via the corpus callosum or from higher centers in the brain to the contralateral motor cortex, which its descending pathways project to spinal motor neurons that innervate the contracting muscle (Mayston et al. 1999; Carson 2005; Zijdewind et al. 2006). Since the source of excitation that evokes the unintended contraction should be the same as the source of the intended contraction even if the final pathways are different, the presence of common drive can be expected. Only one study has attempted to elucidate this issue and showed no temporal correlation (flat cross-correlation histogram) between EMG from ipsilateral muscle pairs (Mayston et al. 1999). However, it should be noted that the same study did not employ rectified EMG but interference EMG, even though information regarding common drive cannot be obtained reliably from unrectified (interference) EMG at all, since low frequency (e.g., <5 Hz) signals are generally filtered out of interference EMG to eliminate unwanted noise at the amplifier. In contrast, low-frequency (<5 Hz) signals that represent common drive appear when the interference EMG is rectified (Yoshitake and Shinohara 2013a). Therefore, although the appropriateness of using rectified surface EMG for assessing neural oscillations has been debated, because rectification alters EMG power for a given frequency (Farina et al. 2004; Yao et al. 2007), calculation of the cross-correlation function of low-frequency rectified surface EMGs would be useful to evaluate, at least, the “presence” of common drive between muscles (Yoshitake and Shinohara 2013a; Yoshitake et al. 2017). Taken together, we were, therefore, motivated to examine the temporal correlations between intended and unintended muscle activities again by means of rectified EMG at low-frequency band.
The purpose of this study was to examine (1) the effect of task difficulty on the unintended contralateral muscle activity during force-matching tasks, (2) age-related differences in UIMA with respect to motor tasks of comparable task difficulty, and (3) temporal correlation in neural oscillations between intended and contralateral unintended contracting muscles. In the current study, the term of task difficulty is used as difficulty of motor task per se with same activation levels of the intended contracting muscle. It is of note that we did not focus on task complexity that is generally regarded as motor tasks simultaneously with different demanding functions as cognitive function. We hypothesized that (1) degree of UIMA was larger during motor tasks with greater task difficulty, (2) older adults exhibit larger UIMA than younger adults with respect to task difficulty, and (3) the correlated neural oscillations between intended and unintended contracting muscles is present.
Methods
Subjects
Twelve young (age, 21.8 ± 2.4 years; height, 169.5 ± 5.8 cm; body weight, 65.5 ± 9.2 kg; mean ± SD) and 12 old (age, 69.9 ± 5.3 years; height, 164.6 ± 4.7 cm; body weight, 62.6 ± 6.9 kg) adult men with no history of neuromuscular disorders participated in this study. All subjects were right-hand dominant as assessed by the Edinburgh Handedness Inventory (Oldfield 1971), and have not experienced specific long-term skilled activities by hands (the keyboard instruments, racket sports, etc.). Subjects were not aware of the purpose of this study or the phenomenon of the unintended contralateral activity at all throughout the experiments. This study was approved by the Ethics Committee of the National Institute of Fitness and Sports in Kanoya. All subjects gave informed consent before commencing with the experiments.
Experimental setup
A 23.6-inch computer display was located 1.5 m away from the subject at eye level and provided visual guidance for exerting force. The experimental setup was designed to allow subjects to perform isometric contractions equally with both hands. The shoulder joint was fixed at ~85° abducted position, the elbow joint was fixed at slightly flexed position, and the wrist was fixed at fully pronated position as subjects did not feel uncomfortable on the experimental device. The forearm was fixed with a vacuum foam pad to avoid the unwanted postural changes. The index finger was fixed by non-elastic tape to maintain the interphalangeal joint at full extension and the lateral surface of the index finger around the proximal interphalangeal joint was connected to a force transducer. The thumb was extended in a horizontal position and held with a brace. The other three fingers were flexed around a semicircular grip and fixed by a strap. The forehead was also restrained with a strap to prevent unwanted movements.
Experimental procedures
Subjects exerted an abduction force with the index finger by performing isometric contractions. First, to determine the maximal voluntary contraction force and corresponding maximal EMG amplitude, subjects exerted maximal voluntary contraction (MVC) unilaterally for both hands. After exerting a couple of submaximal contractions with a rest period of 3 min, subjects gradually increased abduction force from zero to maximum in ~3 s and then sustained maximal force for ~2 s. Subjects performed two MVC trials for each hand with at least 3-min rest between trials to avoid fatigue. Subjects performed additional trials if the differences in the peak force of the two MVC trials were more than 5%. The highest peak force in these trials was considered the MVC force.
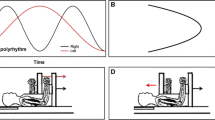
Second, to assess the unintended contralateral activity (UIMA), subjects performed unilateral force-matching tasks. Note that intended force-matching tasks were performed by the left hand (test hand) and subjects were not specially instructed with respect to right hand (Fig. 1c). Unintended muscle activation (UIMA) was assessed by surface EMG in the right hand. To specially examine the effect of task difficulty on UIMA during intended unilateral force-matching task in the current study, the background-target force should have been the same between tasks with different task difficulties (Fig. 1a, b). The background-target force for unilateral force-matching task by the left hand was set at 20, 40, 60, and 80% MVC force. Force feedback was given in the form of a cursor that moved across a computer monitor from left to right. As a result of several pilot experiments, we carefully chose the target range as ±2 and ±7% of each background-target force level for the difficult task (DIFF) and easy task (EASY), respectively. The target range was displayed as two horizontal bars centered about the required background-target force (Fig. 1a, b). The displacement between these bars on the display was identical across background-target force levels for each task. Subjects were instructed to adjust their exerted abduction force to the target in ~3 s and then maintained the exerted force within range of the two horizontal bars for 12 s for tasks ≤60% MVC and 6 s for task of 80% MVC, respectively. Subjects practiced performing the task for several seconds before commencing with the actual trial set and data collection. There was a rest period of at least 3 min between trials.
Drawing of experimental setup. The displayed screen in front of subjects during force-matching task for EASY (top left; a) and DIFF (top right; b) for visual guidance. The distance between upper and lower horizontal-bot bars in the screen represent the target range. Subjects performed isometric force-matching task with the left index finger and the unintended contralateral muscle activity (UIMA) was assessed by surface EMG detected from right hand (bottom; c)
Data acquisition
Exerted force levels in both hands were measured using custom-made devices containing load cells (LURA-SA1; Kyowa, Tokyo, Japan). Force was amplified and low-pass filtered (<100 Hz) with a direct-current amplifier (DPM-751A; Kyowa, Tokyo, Japan).
Surface electromyogram (EMG) was detected from the first dorsal interosseous muscle (FDI) of both hands with bipolar configuration (5-mm diameter, silver–silver chloride, and ~20-mm apart center-to-center). After the skin surface was shaved, rubbed with sandpaper, and cleaned with alcohol, an electrode was placed over the belly of the muscle, and the other electrode was attached to the skin over the base of the proximal phalanx of the index finger. A common electrode (5-mm diameter, silver–silver chloride) was placed on the styloid process of the ulna on the dorsal surface of the hand. The electrodes were connected to a differential amplifier (gain × 1000, MEG-6108; Nihon Kohden, Tokyo, Japan) with a bandwidth of 5–1000 Hz. Force and EMG were recorded at a sampling rate of 2000 Hz using a 16-bit A/D converter (Power Lab 16 s; ADInstruments, Sydney, Australia) and stored on a personal computer. The following analysis was performed using the custom-made software in MATLAB (R2011b, MathWork, Natick, USA).
Data analysis
In the MVC tasks, root mean square amplitude of EMG (aEMG) was determined over a 500-ms window centered on the time at which peak torque was attained (maximal aEMG) for each hand. In the force-matching tasks performed by the test (left) hand, aEMG in both hands were calculated over a 4-s window centered about the middle of the intended contractions. The aEMG was normalized with respect to maximal aEMG (% EMGmax) in each hand. In addition, coefficient of variation (CV) of force for the test hand was calculated to assess the degree of force fluctuation. To evaluate the difference in response of UIMA with respect to greater task difficulty across age and target force, percentage changes (from EASY to DIFF) in aEMG were also calculated.
To examine the extent of correlated rectified EMG oscillations at low frequency between the test and contralateral hands, we calculated the cross-correlation function (CCF) of rectified EMGs for FDI between the test and contralateral hands as described in our previous studies (Yoshitake et al. 2008, 2017; Yoshitake and Shinohara 2013a). UIMA was suggested to be of central origin (Zijdewind et al. 2006). Common drive would also involve supraspinal level (De Luca et al. 1982) and appear at low-frequency bands (<5 Hz) of rectified EMG (Yoshitake and Shinohara 2013b). Therefore, before calculating the CCF, EMG was full-wave rectified (rEMG) and then low-pass filtered with a cut-off frequency of 5 Hz using an eighth-order Butterworth filter with zero-phase lag and de-trended. From the CCF, a distinct peak above 95% confidence limit (CCF peak) was identified in the ±100 ms period between rEMGs of the test and contralateral hands (De Luca et al. 1982; Yoshitake et al. 2017). The time resolution of the calculation was 0.5 ms. Reliability or accuracy of CCF between rEMGs may be poor when EMG amplitude was relatively small, i.e., lower signal-to-noise ratio. Consequently, we only used data exceeding 10% EMGmax in the contralateral hand for calculating CCF in the current study.
Statistical analysis
MVC force was compared for each hand using an unpaired T test between young adults and old adults. In the force-matching task, CV of force and aEMG for the test (intended) hand and aEMG of the contralateral (unintended) hand were compared using a three-way (2 age groups × 2 task difficulty × 4 target forces) analysis of variance (ANOVA) with repeated measures. The changes in aEMG by task difficulty were compared using a two-way (2 age groups × 4 target forces) ANOVA with repeated measures. When appropriate, post hoc comparisons were performed with T test with Bonferroni correction. Statistical significance was set at P < 0.05. Effect sizes were calculated as partial eta squared (partial η 2) for all ANOVA outcomes and as eta squared (η 2) for all T tests outcomes. All data were analyzed using the SPSS software (SPSS Statistics 22; IBM, Tokyo, Japan). Descriptive data are expressed as mean ± SE in the figures and mean ± SD in the text and tables as preferred in our previous studies (Yoshitake et al. 2008; Yoshitake and Shinohara 2013b).
Results
MVC force for each hand was greater (ƞ 2 ≥ 0.41, P < 0.05) in young adults (right: 63.7 ± 17.0 N, left: 53.6 ± 22.1 N) than in old adults (51.6 ± 15.3 N, 32.6 ± 6.7 N). During force-matching tasks, CV of force for the test hand did not differ across task difficulty (F = 0.63, partial ƞ 2 < 0.01, P > 0.05), but did differ across age (F = 22.30, partial ƞ 2 = 0.02, P < 0.05) as well as target force (F = 41.45, partial ƞ 2 = 0.06, P < 0.05) without any interactions (Table 1). In addition, aEMG (expressed as % of EMGmax) for the test hand did not differ across task difficulty (F = 0.29, partial ƞ 2 < 0.01, P > 0.05) or age (F = 3.69, partial ƞ 2 < 0.01, P > 0.05), but did differ with respect to target force (F = 149.04, partial ƞ 2 = 0.72, P < 0.05) without any interactions (Table 1).
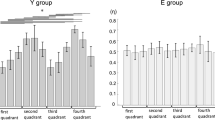
Unintended activity in the contralateral (right) muscle was almost present across all subjects (Fig. 2b) and across all target force levels except for the 20% MVC task. With respect to the aEMG of the contralateral (unintended) muscle, the three-way ANOVA showed main effects in all three factors: task difficulty (F = 4.01, partial ƞ 2 = 0.05, P < 0.05), age (F = 4.77, partial ƞ 2 = 0.07, P < 0.05), and target force (F = 12.54, partial ƞ 2 = 0.17, P < 0.05) without any interactions. Post hoc analysis revealed that (1) aEMG was larger during DIFF by 47.3% compared with EASY (ƞ 2 = 0.05, P < 0.05, Fig. 3b), (2) aEMG was 44.1% larger in old adults compared with young adults (ƞ 2 = 0.07, P < 0.05, Fig. 3c), and (3) aEMG during force-matching tasks at 80% MVC was largest compared with that at smaller target levels (ƞ 2 = 0.08, P < 0.05) and aEMG at 60% MVC was larger compared with that at 20% MVC (ƞ 2 = 0.06, P < 0.05) (Fig. 3d).
Averaged aEMG of the contralateral resting hand during the force-matching task with easy (EASY) and difficult (DIFF) task difficulty performed by young (Young) and old (Old) subjects by test hand at 20, 40, 60, and 80% of maximal voluntary contraction (MVC). a Averaged aEMG for each subject group as a function of contraction intensity of the test hand with different task difficulties. b Main effect of task difficulty on aEMG. Data are collapsed across contraction intensity and subject group. *P < 0.05 between EASY and DIFF. c Main effect of subject group on aEMG. Data are collapsed across contraction intensity and task difficulty. *P < 0.05 between young (Young) and old (Old) subjects. d Main effect of contraction intensity on aEMG. Data are collapsed across task difficulty and subject group. *P < 0.05 vs. 80% MVC. Data are shown as mean ± SE
With respect to the relative changes in aEMG by task difficulty, the two-way ANOVA with repeated measures (2 age groups × 4 target forces) did not show any significant differences (partial ƞ 2 < 0.01, P > 0.05).
As previously mentioned, we employed only data which exceeded 10% EMGmax of contralateral (unintended) muscle when calculating CCF between rEMGs. As a result, 17 sampled (5 EASY and 12 DIFF) data from 8 subjects (2 young adults and 6 old adults) were employed. Distinct significant peaks in CCF (0.38 on average, ranged from 0.18 to 0.56 across subjects, P < 0.05) between rEMGs with small time lags (28.12 ± 39.49 ms) were detected from 14 of the 17 samples, as shown in Fig. 4. It should be noted that the computed time lags were all positive (P < 0.05; compared with zero).
Discussion
The main findings of the present study were that unintended contralateral muscle activity (UIMA) was larger during unilateral motor tasks with greater task difficulty, and UIMA was larger for old adults compared with young adults. The aEMG of the unintended muscle had no significant interactions in all three factors: task difficulty, age, and target force. This implies that increases in UIMA during motor tasks with greater task difficulty are independent of age and contraction intensity. In addition, the current study found that the EMG oscillations at low-frequency bands between intended contractions and the associated unintended contractions were temporally correlated, indicating common origin of intended and unintended muscle activities during unilateral force-matching tasks.
Before giving a detailed account of these findings, we have to address the term “task difficulty”, which one might confuse with “task complexity” or with the findings of other related previous studies. UIMA was generally increased during motor tasks when a task different from the motor task was added (Bodwell et al. 2003; Baliz et al. 2005; Uttner et al. 2007). For example, UIMA increased during a finger-tapping task when mathematical task was added and performed simultaneously (Bodwell et al. 2003). In these cases, subjects performed tasks which differed in functionality, e.g., cognitive and motor. It should be of note that this condition has been called “task complexity” not “task difficulty”. On the other hand, when a mechanical disturbance stimulation was applied to a contracting limb during a force-matching task, UIMA increased compared to when a force-matching task was performed without any stimulation (Baliz et al. 2005). In this case, the larger muscle activation level may be required to compete with the mechanical disturbance stimulation. Bodwell et al. (2003) also showed that UIMA during tapping with faster rate was larger compared to that with slower rate. In this case, the muscle activation level was larger during faster tapping, because the firing rate of motor unit was increased with increasing muscle contraction velocity even when the load was same (Büdingen and Freund 1976). Since UIMA increases with muscle activation levels of the intended contracting muscle (Shinohara et al. 2003), the observed increases in UIMA in these studies should not mainly be due to greater task difficulty but simply due to increased muscle activation level of the intended contracting muscle (Baliz et al. 2005). In the current study, degree of UIMA was compared between conditions (EASY vs. DIFF) at the same EMG amplitude (Table 1) with no additional tasks. Therefore, we believe that experimental procedure in the current study is justified for examining the effect of task difficulty on unintended contralateral muscle activity during force-matching tasks and that, at least to our knowledge, this is the first study to examine the effect of task difficulty on UIMA.
In line with our first hypothesis, UIMA during motor task with greater task difficulty (DIFF) was larger compared with motor tasks with lesser task difficulty (EASY) (Fig. 3b). The origin of UIMA is suggested to result from spreading of increased excitation in the contralateral cerebral cortex or higher centers of the brain to the ipsilateral motor cortex (Mayston et al. 1999; Carson 2005; Zijdewind et al. 2006). Moreover, evidence obtained using fMRI (Sehm et al. 2010) has demonstrated that UIMA was correlated with activity in bilateral M1, supplementary motor area. Therefore, it is reasonable to consider that the amount of excitation in the ipsilateral motor cortex determines the degree of UIMA. Sterr et al. (2009) demonstrated by fMRI that unilateral force-tracking tasks with task difficulty increased cortical activity in the premotor area in both hemispheres compared with a simple force-tracking task. Moreover, Ehrsson et al. (2000) also found that precise grip force control by unilateral hand was associated with a larger bilateral neural network including supplementary motor area (SMA) and premotor areas. Since SMA and the premotor area, known as the higher center, play a role in planning upcoming motor tasks, larger excitation levels in the ipsilateral motor cortex which involves the higher center could be a mechanism responsible for enhancement of the UIMA during motor tasks that are greater in difficulty.
Compared with simple task (EASY), force-matching tasks which allow for less deviation from the target force (DIFF) would impose additional cognitive demands on subjects, because more attention or more effort will be required to control the force cursor more precisely. Greater attention or effort to perform the motor task accurately may be related to psychological stress associated with sympathetic nervous activity (Herman and Cullinan 1997). Recently, Buharin et al. (2013) demonstrated with a single pulse TMS that increases in sympathetic nerve activity enhanced motor-evoked potentials, indicating an increase in corticospinal excitability. Furthermore, Buharin et al. (2014) also demonstrated using paired pulse TMS that diminished intracortical inhibition occurred given an increase in sympathetic nerve activity. Taken together, attention- or effort-related increase in sympathetic nerve activity due to greater task difficulty may enhance the cortical excitability resulting in increases in UIMA. Further studies are needed to quantify the contribution of sympathetic nervous activity towards increases in UIMA.
In the current study, older adults exhibited larger UIMA compared with younger adults regardless of task difficulty. Older adults sometimes cannot fully activate their contracting muscle, even at maximal effort (Yue et al. 1999; Klass et al. 2007). Therefore, since intensity of the intended muscle contraction was determined based on relative terms (i.e., % of maximal voluntary contraction) in the current study, the target force exerted by older adults in this study would be relatively small compared to that exerted by younger adults. This indicates that UIMA was larger in older adults than in younger adults, even though the intentionally exerted force was relatively smaller in older adults compared with younger adults in the current study. This further confirms that UIMA was larger in older adults when compared to younger adults.
Though it is unclear what causes larger UIMA in older adults than in younger adults from the current study, there are two possible mechanisms. One is additional cortical activation during the tasks performed by older adults. It has been reported that older adults required additional recruitment of cortical and subcortical areas to compensate for a decrease of brain function accompanied by aging when they performed motor tasks at a given contraction intensity (Mattay et al. 2002). Calautti et al. (2001) demonstrated that the cortical activity in higher centers of the brain (i.e., premotor and supplementary motor area) during motor task was larger in older adults than in younger adults. These observations suggest that the greater UIMA observed in older adults than in younger adults may be interpreted as a response to heightened functional demands placed on the aging brain. Another possible mechanism is a decrease in interhemispheric inhibition in older adults during motor task. During unilateral hand movements, transcallosal inhibition generally occurs to suppress unintended contralateral activity through the transcallosal pathways, i.e., corpus callosum (Meyer et al. 1995, 1998). The interhemispheric inhibition decreases with advancing age, which may be due to an accompanied decrease in size or demyelination of the corpus callosum (Allen et al. 1991; Weis et al. 1993; Sullivan et al. 2002; Suganthy et al. 2003; Salat et al. 2005). Taking these observations into account, it is assumed that the greater UIMA observed in older adults during unilateral motor tasks is due to functional changes in the corpus callosum, resulting in decreases in interhemispheric inhibition.
Positive CCF peak was demonstrated in rectified surface EMG < 3 Hz (Fig. 4). This is an evidence of the presence of common drive component between the rectified EMGs of intended contracting and contralateral homologous muscles during unilateral force-matching tasks. As inconsistent results with the current study, Mayston et al. (1999) showed no temporal correlation (flat cross-correlation histogram) between “interference (unrectified)” EMGs obtained from left and right FDI during steady unilateral contractions. However, it should be noted that oscillation information in the low-frequency range (<5 Hz) cannot be obtained from unrectified EMG at all, because low-frequency signals are generally high-pass filtered out at more than 5 Hz to eliminate unwanted noise or movement artifacts. In contrast, low-frequency common oscillations in motor unit discharge rate (common drive) were reflected in the low-frequency range of “rectified” EMG (<5 Hz) (Negro et al. 2009; Yoshitake and Shinohara 2013b). Therefore, for studying the component of common drive via surface EMG, rectification as conducted in the current study is required.
Low-frequency oscillations in the instantaneous discharge rate of single motor units are well reflected in low-frequency rectified surface EMG (Yoshitake and Shinohara 2013b). The positive CCF peak and small positive time lag values shown in the current study reveal that the common drive is certainly present between homologous muscles during unilateral force-matching tasks, even though EMG activity of a muscle unintentionally appeared. Common drive has been suggested to be of central origin (De Luca et al. 1982). Therefore, the presence of positive CCF peak would further support the possibility that UIMA is induced by spreading excitation to the contralateral motor cortex arising from the ipsilateral hemisphere via the corpus callosum or higher centers of the brain. The time lag between rEMGs of intended and unintended muscles was positive indicating that intended muscle activity preceded associated unintended muscle activity. If excitation in the ipsilateral hemisphere (M1), which also induces UIMA, is from the higher center, there should be no time lag between EMG in the left and right homologous muscles. The time lag (28.12 ± 39.49 ms) in the current study showed a similar range of interhemispheric transfer time (~16 ms) (Barnett and Corballis 2005). This supports the possibility that UIMA is induced by spreading excitation to the contralateral motor cortex arising indirectly from the ipsilateral hemisphere via the corpus callosum (Zijdewind et al. 2006) rather than directly from the higher center. In the current study, however, EMG data adopted for CCF analysis, being 10% EMGmax and more, were very limited. Further studies are needed to examine this issue in more detail.
The UIMA observed in this study was relatively smaller than that reported in previous studies (Shinohara et al. 2003; Post et al. 2008). This is partly due to that UIMA is smaller during isometric steady contractions, conducted in the current study, compared to dynamic contractions, e.g., eccentric, concentric, and phasic contraction (Bodwell et al. 2003; Shinohara et al. 2003). We adopted isometric steady contractions to examine the temporal correlation at low frequency between intended and unintended muscle activities, because (1) the long data length with small signal trends is needed to perform the cross-correlation analysis and (2) intended phasic muscle activation accompanies phasic unintended muscle activity in the contralateral limb, being due to different physiological mechanisms from those in steady muscle contractions (Mayston et al. 1999; Giovannelli et al. 2006). In addition, the analysis duration adopted here would also contribute to the magnitude of the observed UIMA. We adopted at least 4-s window for analysis, whereas previous studies calculated UIMA for only a 0.5-s window (Shinohara et al. 2003; Post et al. 2008).
The current study establishes the relevance for examining the relation between degree of UIMA during intended muscle contractions and task difficulty, whereas most studies on UIMA are focused on the contraction intensity or muscle fatigue (Todor and Lazarus 1986; Zijdewind et al. 1998; Arányi and Rösler 2002; Shinohara et al. 2003). The present findings should lay the foundation for future exploration into adaptation in unilateral motor tasks of high task difficulty and potential effects on the brain due to unilateral motor control training and task difficulty. For example, assessing the degree of UIMA would be useful for quantifying adaptation in the motor cortex when the unilateral motor control training is adapted for older adults to help improve their ability to perform precise motor movements without expensive measuring apparatus. For example, when UIMA is reduced following intervention periods due to adaptation, then either task difficulty or the task itself could be altered to perhaps incite further improvements in motor function. The motor performance, e.g., “unilateral” maximal voluntary muscle contraction force or power, has been shown to be improved after the strength training by the “contralateral” homologous muscle, which so called “cross education” (Carroll et al. 2006). The increase in the motor performance is mainly due to improvement of neural factors (Goodwill et al. 2012), including motor cortex excitability. The findings obtained here suggest that the procedures adopted in the current study would be applicable to clarify how induced cross education in motor performance can be associated with UIMA. Moreover, the imaged training without actual motor performance sometimes improves the motor performance (Yue and Cole 1992; Zijdewind et al. 2003). Taking this into account together with the current results, it seems that, for severe stroke patients who can hardly contract their muscle voluntary, training regimen consisted of imaged contractions with task difficulty would be a modality for regaining their voluntary motor performances. In the future, further studies are encouraged to elucidate these aspects.
In conclusion, unilateral motor tasks of greater task difficulty increased unintended muscle activity in the contralateral homologous muscle. These unintended contralateral activities were larger in older adults compared with younger adults. Finally, temporal correlations at low frequency of rectified EMGs were present between the intended and unintended contralateral muscles.
Abbreviations
- ANOVA:
-
Analysis of variance
- CCF:
-
Cross-correlation function
- DIFF:
-
Difficult task
- EASY:
-
Easy task
- EMG:
-
Electromyogram
- FDI:
-
First dorsal interosseous muscle
- MVC:
-
Maximal voluntary contraction
- UIMA:
-
Unintended muscle activity
References
Allen S, Richey F, Chai YM, Gorski A (1991) Sex differences in the corpus callosum of the living human being. J Neurosci 11:933–942
Arányi Z, Rösler KM (2002) Effort-induced mirror movements: a study of transcallosal inhibition in humans. Exp Brain Res 145:76–82. doi:10.1007/s00221-002-1101-1
Baliz Y, Armatas C, Farrow M et al (2005) The influence of attention and age on the occurrence of mirror movements. J Int Neuropsychol Soc 11:855–862. doi:10.1017/S1355617705051003
Barnett KJ, Corballis MC (2005) Speeded right-to-left information transfer: the result of speeded transmission in right-hemisphere axons? Neurosci Lett 380:88–92. doi:10.1016/j.neulet.2005.01.025
Bodwell JA, Mahurin RK, Waddle S et al (2003) Age and features of movement influence motor overflow. J Am Geriatr Soc 51:1735–1739. doi:10.1046/j.1532-5415.2003.51557.x
Büdingen JH, Freund HJ (1976) The relationship between the rate of rise of isometric tension and motor unit recruitment in a human forearm muscle. Pflügers Arch Eur J Physiol 362:61–67. doi:10.1007/BF00588682
Buharin VE, Butler AJ, Rajendra JK, Shinohara M (2013) Enhanced corticospinal excitability with physiologically heightened sympathetic nerve activity. J Appl Physiol 114:429–435. doi:10.1152/japplphysiol.01586.2011
Buharin VE, Butler AJ, Shinohara M (2014) Motor cortical disinhibition with baroreceptor unloading induced by orthostatic stress. J Neurophysiol 111:2656–2664. doi:10.1152/jn.00778.2013
Calautti C, Serrati C, Baron JC (2001) Effects of age on brain activation during auditory-cued thumb- to-index opposition-a positron emission tomography study. Stroke 32:139–146. doi:10.1161/01.STR.32.1.139
Carroll TJ, Herbert RD, Munn J et al (2006) Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol 101:1514–1522. doi:10.1152/japplphysiol.00531.2006
Carson RG (2005) Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Rev 49:641–662. doi:10.1016/j.brainresrev.2005.03.005
De Luca CJ, Erim Z (2002) Common drive in motor units of a synergistic muscle pair. J Neurophysiol 87:2200–2204. doi:10.1152/jn.00793.2001
De Luca CJ, Mambrito B (1987) Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol 58:525–542. doi:10.1152/jn.00348.2014
De Luca CJ, LeFever RS, McCue MP, Xenakis AP (1982) Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329:129–142
Ehrsson HH, Fagergren A, Jonsson T et al (2000) Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83:528–536
Farina D, Merletti R, Enoka RM (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96:1486–1495. doi:10.1152/japplphysiol.01070.2003
Gandevia BYSC, Macefieldt VG, Gorman RB, Burke D (1993) Motoneuronal output and gradation of effort in attempts to contract acutely paralysed leg muscles in man. J Physiol 471:411–427
Giovannelli F, Borgheresi A, Balestrieri F et al (2006) Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp Brain Res 175:633–640. doi:10.1007/s00221-006-0581-9
Goodwill AM, Pearce AJ, Kidgell DJ (2012) Corticomotor plasticity following unilateral strength training. Muscle Nerve 46:384–393. doi:10.1002/mus.23316
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo- pituitary-adrenocortical axis. Trends Neurosci 20:78–84. doi:10.1016/S0166-2236(96)10069-2
Klass M, Baudry S, Duchateau J (2007) Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol 100:543–551. doi:10.1007/s00421-006-0205-x
Mattay V, Fera F, Tessitore A et al (2002) Neurophysiological correlates of age-related changes in human. Neurology 58:630–635. doi:10.1212/WNL.58.4.630
Mayston MJ, Harrison LM, Stephens JA (1999) A neurophysiological study of mirror movements in adults and children. Ann Neurol 45:583–594. doi:10.1002/1531-8249(199905)45:5<583:AID-ANA6>3.0.CO;2-W
Meyer B, Roricht S, von Einsiedel HG et al (1995) Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118:429–440
Meyer B, Roricht S, Woiciechowsky C (1998) Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43:360–369
Negro F, Holobar A, Farina D (2009) Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol 587:5925–5938. doi:10.1113/jphysiol.2009.178509
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. doi:10.1016/0028-3932(71)90067-4
Post M, Bayrak S, Kernell D, Zijdewind I (2008) Contralateral muscle activity and fatigue in the human first dorsal interosseous muscle. J Appl Physiol 105:70–82. doi:10.1152/japplphysiol.01298.2007
Salat DH, Tuch DS, Greve DN et al (2005) Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26:1215–1227. doi:10.1016/j.neurobiolaging.2004.09.017
Sehm B, Perez MA, Xu B et al (2010) Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20:34–45. doi:10.1093/cercor/bhp075
Shinohara M, Keenan KG, Enoka RM (2003) Contralateral activity in a homologous hand muscle during voluntary contractions is greater in old adults. J Appl Physiol 94:966–974. doi:10.1152/japplphysiol.00836.2002
Sterr A, Shen S, Kranczioch C et al (2009) fMRI effects of task demand and feedback accuracy on grip force tracking. Neurosci Lett 457:61–65. doi:10.1016/j.neulet.2009.04.013
Suganthy J, Raghuram L, Antonisamy B et al (2003) Gender-and age-related differences in the morphology of the corpus callosum. Clin Anat 403:396–403. doi:10.1002/ca.10161
Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE (2002) Differential rates of regional brain change in callosal and ventricular size: a 4-year longitudinal MRI study of elderly men. Cereb Cortex 12:438–445
Todor JI, Lazarus JA (1986) Exertion level and the intensity of associated movements. Dev Med Child Neurol 28:205–212. doi:10.1111/j.1469-8749.1986.tb03856.x
Uttner I, Kraft E, Nowak DA, Müller F, Philipp J, Zierdt AHJ (2007) Mirror movements and the role of handedness: isometric grip forces changes. Mot Control 11:16–28
Weis S, Kimbacher M, Wenger E, Neuhold A (1993) Morphometric analysis of the corpus callosum using MR: correlation of measurements with aging in healthy individuals. Am J Neuroradiol 14:637–645
Yao B, Salenius S, Yue GH et al (2007) Effects of surface EMG rectification on power and coherence analyses: an EEG and MEG study. J Neurosci Methods 159:215–223. doi:10.1016/j.jneumeth.2006.07.008
Yoshitake Y, Shinohara M (2013a) Low-frequency component of rectified EMG is temporally correlated with force and instantaneous rate of force fluctuations during steady contractions. Muscle Nerve 47:577–584. doi:10.1002/mus.23628
Yoshitake Y, Shinohara M (2013b) Oscillations in motor unit discharge are reflected in the low-frequency component of rectified surface EMG and the rate of change in force. Exp Brain Res 231:267–276. doi:10.1007/s00221-013-3689-8
Yoshitake Y, Masani K, Shinohara M (2008) Laser-detected lateral muscle displacement is correlated with force fluctuations during voluntary contractions in humans. J Neurosci Methods 173:271–278. doi:10.1016/j.jneumeth.2008.06.022
Yoshitake Y, Kanehisa H, Shinohara M (2017) Correlated EMG oscillations between antagonists during cocontraction in men. Med Sci Sports Exerc 49:538–548. doi:10.1249/MSS.0000000000001117
Yue G, Cole KJ (1992) Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67:1114–1123
Yue GH, Ranganathan VK, Siemionow V et al (1999) Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol Ser A 54:M249–M253
Zijdewind I, Kernell D (2001) Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol 85:1907–1913
Zijdewind I, Zwarts MJ, Kernell D (1998) Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett 253:41–44. doi:10.1016/S0304-3940(98)00609-0
Zijdewind I, Toering ST, Bessem B et al (2003) Effects of imagery motor training on torque production of ankle plantar flexor muscles. Muscle Nerve 28:168–173. doi:10.1002/mus.10406
Zijdewind I, Butler J, Gandevia S, Taylor J (2006) The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res 175:526–535. doi:10.1007/s00221-006-0570-z
Acknowledgements
The authors would like to thank Dr. Naokazu Miyamoto (National Institute of Fitness and Sports in Kanoya) for helpful suggestions. We thank Mr. Garrett Jones (National Institute of Fitness and Sports in Kanoya) for help proofreading the manuscript and improving the software for data analysis. We thank Mr. Shobu Kurohara (National Institute of Fitness and Sports in Kanoya) for assistance with the experiment. This study was funded, in part, by JSPS KAKENHI Grant Number JP16H03222 (Grant-in-Aid for Scientific Research B) to YY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors acknowledge no conflict of interest.
Additional information
Communicated by Bénédicte Schepens.
Rights and permissions
About this article
Cite this article
Watanabe, H., Kanehisa, H. & Yoshitake, Y. Unintended activity in homologous muscle during intended unilateral contractions increases with greater task difficulty. Eur J Appl Physiol 117, 2009–2019 (2017). https://doi.org/10.1007/s00421-017-3689-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3689-7