Abstract
Purpose
Physical exercise has cardioprotective functions, which have been partly linked to high-density lipoprotein (HDL), and its functions. We studied the effects of endogenous oxidative stress, induced by acute exhaustive physical exercise, on concentration of oxidized HDL lipids.
Methods
Twenty-four male national top-level endurance runners, 12 middle-distance runners and 12 marathon runners performed a maximal run on a treadmill until exhaustion. We analyzed concentrations of oxidized HDL (oxHDLlipids) and LDL lipids (oxLDLlipids), serum antioxidant potential (TRAP), paraoxonase activity and malondialdehyde. Venous blood samples were taken before, immediately, 15 and 90 min after exercise.
Results
Immediately after the treadmill run the concentration of oxHDLlipids was increased by 24 % (p < 0.01). Simultaneously, the ratio of oxHDLlipids to oxLDLlipids increased by 55 % and the oxLDLlipids levels decreased by 19 % (p < 0.001), while serum malondialdehyde and TRAP increased by 54 % (p < 0.001) and 29 % (p < 0.01), respectively. After the 90 min recovery the concentration of oxHDLlipids was decreased towards the pre-exercise level, but that of oxLDLlipids remained decreased below pre-exercise values (p < 0.001). The change in oxLDLlipids after the run correlated positively with VO2max (r = 0.67, p < 0.001) and negatively with the change in paraoxonase activity (r = −0.47, p < 0.05).
Conclusions
We conclude that acute exhaustive physical exercise increased the concentration of oxHDLlipids and decreased that of oxLDLlipids and the ratio of oxLDLlipids to oxHDLlipids, which suggests that during physical exercise HDL has an active role in the removal of lipid peroxides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The function of serum lipoproteins is to transport lipids and lipid soluble material in the blood stream to and from the liver. Low-density lipoprotein (LDL) is the main transporter of cholesterol to the peripheral tissues and into arterial wall, while excess tissue cholesterol is returned to the liver by reverse cholesterol transport mediated by high-density lipoprotein (HDL) (Barter et al. 2007).
Oxidized lipids can have widespread effects on normal physiological conditions. In particular, oxidized LDL is considered to be the primary event leading to the initiation and progression of atherosclerosis (Parthasarathy et al. 1998; Steinberg and Witztum 2002), while HDL appears to be protective (Mackness et al. 2000). Reverse cholesterol transport is considered as the primary protective effect of HDL, but there is also evidence of anti-inflammatory, antioxidative and antithrombotic properties (Navab et al. 2011).
Exercise is a well-recognized model of physiological oxidative stress, but it can also positively influence risk factors associated with cardiovascular disease (Jenkins 1988; Mora et al. 2007; Swift et al. 2013). An important mechanism by which physical activity reduces the risk of cardiovascular disease is through regulating the concentration of plasma lipids (Monda et al. 2009; Wagganer et al. 2015). Exercise is known to decrease plasma triglyceride levels, and to increase concentrations of HDL cholesterol and apolipoprotein A-I (Leon and Sanchez 2001). Serum lipoproteins are known to contain variable amounts of lipid peroxides, and there is some evidence indicating that HDL may transport lipid oxidation products during physiological conditions (Ahotupa et al. 2010). Furthermore, it has been suggested that HDL does not only remove the excess cholesterol but also has a role in removal of lipid oxidation products (Shao and Heinecke 2009; Ahotupa et al. 2010).
Earlier studies have shown that acute prolonged physical exercise decreases oxidized LDL lipids (oxLDLlipids) levels (Vuorimaa et al. 2005; Välimäki et al. 2012). Furthermore, it has been suggested that acute strenuous physical activity might raise lipid oxidation products in HDL (Ahotupa et al. 2010). However, very limited amount of information exists on the transport of lipid peroxides by serum lipoproteins immediately following exercise and the concomitant recovery period. The aim of this study was to find out the effects of acute maximal treadmill run followed by 90 min recovery on the transport of lipid peroxides by serum HDL and LDL. Therefore, a group of trained middle-distance and marathon runners were recruited to study the effect of different training background on the responses to acute exercise.
Materials and methods
Subjects
Twenty-four male national top-level endurance runners, 12 middle-distance runners and 12 marathon runners volunteered for the study. All had been training and competing in middle and long distance running regularly for more than 5 years. The basic characteristics of the subjects are shown in Table 1. The study protocol was approved by local ethical committee (Hospital District of Southwest Finland: 91/1801/2015) and was performed in accordance with the Declaration of Helsinki on the use of human subjects. After provision of written and oral information regarding the possible risks and discomforts of the study, written informed consent was obtained from all participants included in the study.
Study design
The subjects performed a velocity-incremented continuous treadmill run (Telineyhtymä, Kotka, Finland) at 1 slope until exhaustion. The duration of each stage of the test was 2 min; the initial 2-min stage was run at a velocity of 10 km h−1 and thereafter the velocity was increased by 1 km h−1 for each consecutive stage until exhaustion. The whole duration of the treadmill run was 20–22 min, of which the last 6–8 min was performed in anaerobic level. Venous blood samples were taken before exercise, immediately, 15 and 90 min after exercise. On the test day, the subjects consumed a standardized, light breakfast 3 h before the exercise. The breakfast consisted of a cucumber sandwich, glass of juice, and a cup of coffee or tea (Table 2). The subjects were not allowed to take any supplements (like antioxidants) during the days preceding the study.
The maximal oxygen uptake was determined during the incremental VO2max test by an automated Oxygon Sigma gas analyzer (Mijnhard, The Netherlands), which was calibrated before each test according to the manufacture’s instructions. The calibration was regularly checked after the tests. Breath-by-breath metabolic data were averaged to 30-s intervals. VO2max was defined as the highest 60-s VO2 during the test. V max was the treadmill velocity at which the subject first attained VO2max (Billat et al. 1995). This was either the velocity of the last 2-min stage or the previous 2-min velocity, if VO2 was higher at this stage. If the value of VO2 at the two last stages was the same, the mean of these two velocities was accepted as V max (Lindsay et al. 1996). The reliability of this protocol has been confirmed by Billat et al. (1994, 1996).
Determination of oxidized lipoprotein lipids
Analysis of lipoprotein oxidized lipids was based on the determination of the baseline level of conjugated dienes in lipoprotein lipids (Ahotupa et al. 1998). The appearance of conjugated dienes has been commonly used as the index of oxidation in in vitro and ex vivo studies on LDL oxidation. Serum LDL was isolated by precipitation with buffered heparin (Ahotupa et al. 1998). Isolation of the HDL fraction from serum samples was based on phosphotungstic acid precipitation (Väisänen et al. 1992). Before the isolation of serum lipoproteins, serum samples (to which 1 mg/mL of EDTA were added) and precipitation reagents were allowed to equilibrate to room temperature. The isolation procedures were validated for the purpose, and did not affect the level of oxidized lipids (Ahotupa et al. 1998). Lipids were extracted from isolated lipoprotein samples (100 μL) by chloroform–methanol (2:1), dried under nitrogen, then redissolved in cyclohexane (1 mL), and analyzed spectrophotometrically at 234 nm. Absorbance units (difference A234–A300) were converted to molar units using the molar extinction coefficient 2.95 × 104 M−1 cm−1. Validation studies for the assay have ruled out interference by nonspecific substances, and shown that diene conjugation is a measure of oxidative LDL modification found in all LDL lipid classes. In addition to the specific absorption spectra at 234 nm, the presence of conjugated dienes has been verified by NMR studies (Vasankari et al. 2001). The coefficient of variation (CV) for within-assay precision for the determination of oxidized lipoprotein lipids was 4.4 %, and the CV for the between-assay precision was 4.5 %.
Determination of lipoprotein oxidation resistance in vitro
The oxidation resistance of lipoproteins was investigated in a model based on Cu2+-induced oxidation of HDL and LDL in vitro. Lipoproteins were isolated as described above. Lipoprotein concentrations in the incubations corresponded to 0.1 mL of serum/mL of the incubation volume. Oxidation of lipoprotein lipids was monitored by the formation of conjugated dienes, as described by Esterbauer et al. (1992).
Chemicals
Chloroform (p.a.), methanol (p.a.) and cyclohexane (Uvasol) were from E. Merck (Darmstadt, Germany), heparin from Lövens Kemiska Fabrik (Ballerup, Denmark). 5-Amino-2,3-dihydro-1,4-phthalazinedione (Luminol) was purchased from Bio-Orbit Ltd. (Turku, Finland), 2,2′azobis(2-amidinopropane) HCl (ABAP) from Polysciences Inc. (Warrington, PA, USA), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) from Aldrich Chemicals Co. (Milwaukee, WI, USA). Alkaline phosphatase was from Finnzymes (Espoo, Finland). All other reagents were from Sigma Chemicals Co. (St. Louis, MO, USA).
Other analytical procedures
Serum total peroxyl radical trapping antioxidant potential (TRAP) was estimated ex vivo by the potency of serum samples to resist ABAP-induced peroxidation (Ahotupa et al. 1996). Peroxyl radical trapping potential was assayed briefly as follows: 0.45 mL of 0.1 M sodium phosphate buffer, pH 7.4, containing 0.9 % NaCl, 0.02 mL of 120 mM linoleic acid, 0.05 mL of luminol (0.5 mg/mL), and serum samples were mixed in the cuvette. The assay was initiated by 0.05 mL of ABAP (83 mg/mL). Chemiluminescence in triplicate cuvettes at 37° was measured until a peak value for each sample was detected. Peroxyl radical trapping capacity was defined by the half-peak time point. Trolox served as a standard radical scavenger. Paraoxonase activity was determined using paraoxon (O,O-diethyl-O-p nitrophenylphosphate) as the substrate and measuring the increase in absorbance, due to the formation of 4-nitrophenol, at 412 nm (Harangi et al. 2004). Serum total cholesterol, LDL cholesterol and HDL cholesterol measurements were based on standard enzymatic methods and performed with commercial analytical kits (Roche Diagnostics, Mannhein, Germany). HDL cholesterol concentration was measured after phosphotungstic acid precipitation. Malondialdehyde concentration was measured as serum total (free and protein-bound) malondialdehyde as the 2,4-dinitrophenylhydrazine derivative by HPLC with 1,1,3,3-tetraethoxypropane as the standard (Pilz et al. 2000). The HPLC analyses were performed with a Shimadzu 10ADVP. Luna 3 µm reversed-phase column, 150 × 4.6 mm, was used, and the detection was based on UV detector operating at 307 nm. The eluent consisted of acetonitrile (55 %), H2O (44.8 %) and acetic acid (0.2 %), and the flow rate was 1.2 mL/min.
Statistical analyses
Statistical analyses were performed with SPSS for windows Statistical Software, version 18.0. The normality of the distribution was confirmed before statistical testing, and if needed individual variables was ln-transformed at every time point (oxLDLlipids, malondialdehyde, paraoxonase, the ratio of oxLDLlipids to LDL cholesterol, oxLDLlipids to HDL cholesterol and oxHDLlipids to oxLDLlipids). For all measured variables, there were no significant interactions for time x group (marathon vs. middle-distance runners). Therefore, all results are presented by combining the two groups of runners. Changes over time from the beginning of the trail were analyzed using general linear model analysis of variance (ANOVA) with repeated measurements. In case of significant time effect, the paired t test was used as a post hoc test using Bonferroni’s correction. The Pearson correlation coefficient was used to define the relationships between variables. A priori p value for statistical significance of 0.05 was used. The concentrations are expressed as mean ± SD.
Results
During the trial, both groups behaved similarly and there were no significant time x group response interactions in any measured variables (ANOVA). Time effect in ANOVA was statistically significant in oxidized HDL lipids (oxHDLlipids), oxidized LDL lipids (oxLDLlipids), malondialdehyde (MDA), paraoxonase, TRAP, the ratio of oxLDLlipids to LDL cholesterol, the ratio of oxLDLlipids to HDL cholesterol, ratio of oxHDLlipids to oxLDLlipids (p < 0.001, in all), LDL cholesterol, HDL cholesterol and the ratio of oxHDLlipids to HDL cholesterol (p < 0.05, each).
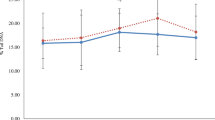
The concentration of both LDL and HDL cholesterol increased immediately after the treadmill run by 5 and 17 % (p < 0.02 and p < 0.01, respectively). Furthermore, after the treadmill run LDL cholesterol levels decreased by 7 % during the 90 min recovery period (p < 0.02), while the HDL cholesterol levels remained elevated by 10 % after the 15 min recovery period compared to the baseline levels (p < 0.001).
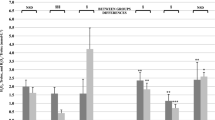
The concentration of the oxHDLlipids increased by 24 % (p < 0.01) immediately after the treadmill run, and remained elevated (29 %, p < 0.01) during 15 min recovery (Fig. 1). Most interestingly, the ratio of oxHDLlipids to oxLDLlipids increased by 55 % immediately after the treadmill run and after 15 min recovery by 71 % compared to before exercise levels (p < 0.001, both) (Table 3).
The treadmill run increased the concentration of serum MDA by 54 % (p < 0.001), but decreased that of ox-LDL by 19 % (p < 0.001). The treadmill run also decreased the ratio of oxLDLlipids to LDL cholesterol by 26 % (p < 0.01). After 90 min recovery period, MDA levels were decreased back to the pre-exercise level, but the concentration of oxLDLlipids remained decreased below pre-exercise levels (p < 0.001) (Fig. 2; Table 3).
Acute exercise did not statistically affect serum paraoxonase activity, but after 15 min recovery the activity was increased by 12 % compared to the pre-exercise levels (p < 0.05). However, from 15 to 90 min recovery period the activity was decreased back to the baseline levels (p < 0.01). Serum TRAP increased immediately after the exercise by 29 % (p < 0.01), and remained elevated during the 90 min recovery period (p < 0.01).
The resistance of lipoprotein particles to oxidation was estimated in an in vitro-model with Cu2+ as the oxidizing agent. We found that after a lag period of about 1.5 h LDL was readily oxidized, while HDL clearly showed higher oxidation resistance under the same pro-oxidant circumstances (Fig. 3).
Oxidation resistance of HDL and LDL in vitro. Serum lipoproteins were isolated as described in “Materials and methods”. Oxidation of the lipoproteins was induced by incubation (in a concentration corresponding to 0.1 mL of serum/mL incubation volume) with Cu2+ at +37 °C. Estimation of the oxidation resistance was based on appearance of conjugated dienes (abs 234 nm) during the 4 h incubation period
It is interesting to note that although both middle-distance and marathon runners demonstrated the same time and magnitude of effect describing the serum lipid oxidative response to exhaustive exercise, there was a significant between group difference or trend for a difference in serum concentrations of oxHDLlipids and the oxHDLlipids/HDL ratio, oxLDLlipids and the oxLDLlipids/LDL ratio, and TRAP mainly as a consequence of a higher baseline value for these variables in middle-distance runners than marathon runners (Figs. 1, 2; Table 3). Furthermore, at baseline, the concentration of oxLDLlipids correlated negatively with VO2max (r = −0.50, p < 0.05) and HDL cholesterol (r = −0.44, p < 0.05). The change in oxLDLlipids (from before value to immediately after value) correlated positively with VO2max (r = 0.67, p < 0.001) and negatively with the change in paraoxonase activity (r = −0.47, p < 0.05). Furthermore, before exercise concentration of serum TRAP correlated positively with BMI (r = 0.56, p < 0.05).
Discussion
Exercise training improves plasma lipid profile and diminishes the risk of coronary heart disease, and the beneficial effects have been attributed to effects on cholesterol transport functions of HDL and LDL (Powell et al. 1987; Durstine and Haskell 1994; Swift et al. 2013; Vasankari et al. 1998). In this study, we investigated the lipid peroxide transport function of serum lipoproteins during physiological oxidative stress, which was induced by an acute exhaustive run. We found that acute physical exercise substantially increased the concentration of oxidized HDL lipids (oxHDLlipids), while an opposite effect was seen in the concentration of oxidized LDL lipids (oxLDLlipids). This lipoprotein distribution differs markedly from that of exogenous lipid peroxides entering the body via food, when concentrations of lipid oxidation products in triglyceride-rich lipoproteins and LDL are rapidly increased and remain elevated for several hours, while only a moderate late increase in oxHDLlipids is seen (Ahotupa et al. 2010).
The exercise-induced increase in oxHDLlipids seen in this study is not likely due to peroxidation of the lipids in HDL particles, as no such increase was seen in oxLDLlipids despite the lower oxidation resistance of LDL (Fig. 3). Also, the significant increase in the ratio of oxHDLlipids to oxLDLlipids strengthens this assumption (Table 3). In conformity with this, Shao and Heinecke (2009) have concluded that it is unlikely that either HDL or LDL would be oxidized in plasma. It is also unlikely that oxidation products would be transferred directly from LDL to HDL in plasma, since the transfer of oxidation products between LDL and HDL appears to be too slow to affect their distribution in lipoproteins (Bowry et al. 1992). Instead, the elevation of oxHDLlipids in response to physiological oxidative stress could be indicative of the proposed lipid peroxide clearing function of HDL, where products of lipid oxidation are acquired by HDL at sites of their formation, and transported to liver for elimination (Shao and Heinecke 2009; Ahotupa et al. 2010).
In this study, treadmill running resulted in a decrease in oxLDLlipids concentration, which could not be explained by a decrease in LDL cholesterol, as indicated by the fact that at the same time the ratio of oxLDLlipids to LDL cholesterol decreased. This is in accordance with earlier studies where oxLDLlipids levels were reduced due to prolonged acute exercise, but no significant change was seen in LDL cholesterol (Vuorimaa et al. 2005; Välimäki et al. 2012). In line with the acute effects of exercise, a 10-month training program was found to reduce the level of oxLDLlipids and the ratio of oxLDLlipids to LDL cholesterol (Vasankari et al. 1998; Välimäki et al. 2012). The mechanism by which physical exercise decreases lipid oxidation products in LDL and protects it from oxidative changes is unsolved. One possible explanation could be the ability of HDL and the associated enzymes to protect LDL from oxidative changes (Mackness et al. 2000; Kontush et al. 2003). Paraoxonase is closely associated with circulating HDL, and suggested to be an anti-atherogenic and antioxidant element by decreasing the accumulation of lipid peroxidation products in LDL (Mackness et al. 1991; Kresanov et al. 2015). In this study, the acute exercise had only minor effects on paraoxonase activity, which was slightly elevated after 15 min recovery. However, the change of oxLDLlipids during the run associated with the change of paraoxonase. It has been reported that paraoxonase activity is high in well-trained athletes (Cakmak et al. 2010). It is therefore possible that even the small (3–12 %) increase in paraoxonase activity may explain lowering of the oxLDLlipids. In all, the present data is well in accordance with our earlier finding of the negative association between paraoxonase activity and oxLDLlipids (Kresanov et al. 2015).
A most striking finding of this study was that exhaustive exercise dramatically affected the oxHDLlipids/oxLDLlipids ratio (Table 3). This is indicative of a clear shift in direction of the transportation of lipid oxidation products in blood stream, and supports the idea of the lipid peroxide clearing and protective transport function of HDL (Shao and Heinecke 2009; Ahotupa et al. 2010).
Endurance runners are continuously exposed to various kinds of stress during sports training. Adaptations to stress occur on different levels: from adaptations on subcellular, cellular and tissue level, to adaptations of organs and the whole organism of an athlete (Mahoney and Tarnopolsky 2005; Coffey and Hawley 2007). Acute exhaustive exercise increases the cellular pro-oxidant states and can induce an increase of oxidative stress (Sies 1997; Bloomer 2008), which could lead to cell damage and inflammation. In this study, acute intense exercise promoted increase of serum malondialdehyde concentration, which could result from the production of reactive oxygen species/reactive nitrogen species. Physical training is known to upregulate adaptation mechanisms including also antioxidant protection (Fisher-Wellman and Bloomer 2009; Radak et al. 2013), and it is significant to understand that the production of pro-oxidants as a result of exercise serves as the stimulus to upregulate endogenous antioxidant mechanisms (Radak et al. 2008). The subjects in our study were well-trained, national level elite endurance runners, who are adjusted to regular training and have a long training history. This could have led to a well-functioning antioxidant protection and constitute a mechanism of defense for LDL oxidation (Brites et al. 2006). It is important to notice that the training history of subjects and intensity of the physical exercise may have affected results of this study.
Since antioxidant protection of the mammalian body consists of numerous antioxidative mechanisms, the functionality of the antioxidative defense as a whole cannot be assessed by measuring the concentration or activity of some few individual antioxidants only. We used the TRAP method to estimate the acute effect of maximal treadmill run and recovery on total antioxidant capacity. Serum concentrations of certain antioxidant compounds may significantly influence the serum TRAP value. In particular, the increased concentration of vitamin E (α-tocopherol) in serum raises the serum TRAP value markedly (Vasankari et al. 1997). In addition to vitamin E, vitamin C and uric acid are known to be significant contributors of serum TRAP (Kaur and Halliwell 1990; Kanter et al. 1993). In this study, the serum TRAP value increased during the treadmill run and the concomitant follow up period. This is in line with our earlier studies, where acute prolonged exercise increased serum TRAP value (Vasankari et al. 1997; Välimäki et al. 2012). Acute physical exercise is known to increase vitamin E (Pincemail et al. 1988) and uric acid in plasma (Svensson et al. 2002).
There are number of limitations that should be taken into consideration when interpreting these results. First, the study population consists of only top national athletes, and therefore, it is uncertain whether a similar response will occur in sedentary or less trained individuals. Second, it would be interesting to know if a woman would have similar response following an acute bout of exhaustive exercise (Enns and Tiidus 2010). Third, these results will only tell us how athletes react to acute exhaustive exercise, the longitudinal intervention data is lacking.
As we have mentioned earlier, in this study both marathon runners and middle-distance runners had similar responses immediately after exhaustive treadmill run despite of typical differences in training intensity, duration and form. Furthermore, if we compare pre-exercise levels between these groups, we notice that middle-distance runners have somewhat higher oxHDLlipids and oxLDLlipids levels and TRAP as a measure of antioxidant capacity. Due to the differences in resting values we did find significant between group effects and a trend for between group differences in these variables (Figs. 1, 2; Table 3). The higher level of TRAP in the middle-distance runners may indicate defense against oxidative stress is upregulated to a greater extent in the middle-distance runners possible due to greater oxidative stress imposed by that training form. Overall the group differences between the middle-distance and marathon runners may suggest evidence of how different types of training or training histories can affect endogenous antioxidant and lipid transportation mechanisms more chronically, even if the acute response to exercise is similar as we observed in this study (Magness 2014).
Conclusions
Despite the known positive effects of physical exercise in cardiovascular risk factors, the mechanisms by which exercise reduces atherogenic risk are not fully understood. In this study, we show that immediately after exhaustive treadmill run the concentration of oxHDLlipids increased and that of oxLDLlipids decreased. Most interestingly, the ratio of oxHDLlipids to oxLDLlipids increased dramatically. These findings may indicate that physiological oxidative stress, induced by exhaustive physical exercise, accelerated the transport of lipid oxidation products by HDL. The results further strengthen the view of the role of HDL in the clearance of noxious lipid peroxides, and the adaptive nature of this protective HDL function.
Abbreviations
- ABAB:
-
2,2′Azobis(2-amidinopropane)HCl
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- HCL:
-
Hydrochloride
- HDL:
-
High-density lipoproteins
- HLPC:
-
High-performance liquid chromatography
- LDL:
-
Low-density lipoproteins
- MDA:
-
Malondialdehyde
- oxHDLlipids:
-
Oxidized HDL lipids
- oxLDLlipids:
-
Oxidized LDL lipids
- TRAP:
-
Total peroxyl radical trapping antioxidant potential
- VO2max :
-
Maximal oxygen uptake
References
Ahotupa M, Ruutu M, Mäntylä E (1996) Simple methods of quantifying oxidation products and antioxidant potential of low density lipoproteins. Clin Biochem 29:139–144
Ahotupa M, Marniemi J, Lehtimäki T, Talvinen K, Raitakari OT, Vasankari T, Viikari J, Luoma J, Ylä-Herttuala S (1998) Baseline diene conjugation in LDL lipids as a direct measure of in vivo LDL oxidation. Clin Biochem 31:257–261
Ahotupa M, Suomela JP, Vuorimaa T, Vasankari T (2010) Lipoprotein-specific transport of circulating lipid peroxides. Ann Med 42:521–529
Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC (2007) HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357:1301–1310
Billat V, Renoux JC, Pinoteau J, Petit B, Koralsztein J-P (1994) Reproducibility of running time to exhaustion at VO2max in subelite runners. Med Sci Sports Exerc 26:254–257
Billat V, Renoux JC, Pinoteau J, Petit B, Koralsztein J-P (1995) Times to exhaustion at 90, 100 and 105 % of velocity at VO2max (maximal aerobic speed) and critical speed in elite long distance runners. Arch Physiol Biochem 103:129–135
Billat VL, Hill DW, Pinoteau J, Petit B, Koralsztein J-P (1996) Effect of protocol on determination of the velocity at VO2max and its time to exhaustion. Arch Physiol Biochem 104:313–321
Bloomer RJ (2008) Effect of exercise on oxidative stress biomarkers. Adv Clin Chem 46:1–50
Bowry VW, Stanley KK, Stocker R (1992) High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci USA 89:10316–10320
Brites F, ZagoV Verona J, Muzzio ML, Wikinski R, Schreier L (2006) HDL capacity to inhibit LDL oxidation in well-trained triathletes. Life Sci 78:3074–3081
Cakmak A, Zeyrek D, Atas A, Erel O (2010) Paraoxonase activity in athletic adolescents. Pediatr Exerc Sci 22:93–104
Coffey VG, Hawley JA (2007) The molecular bases of training adaptation. Sports Med 37:737–763
Durstine JL, Haskell WL (1994) Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sci Rev 22:477–521
Enns DL, Tiidus PM (2010) The influence of estrogen on skeletal muscle: sex matters. Sports Med 40:41–58
Esterbauer H, Gebicki J, Puhl H, Jurgens G (1992) The role of lipid peroxidation and antioxidants in oxidative modifications of LDL. Free Radic Biol Med 13:341–390
Fisher-Wellman K, Bloomer RJ (2009) Acute exercise and oxidative stress: a 30 years history. Dyn Med 8:1
Harangi M, Seres I, Varga Z, Emri G, Szilvassy Z, Paragh G, Remenyik E (2004) Atorvastatin effect on high-density lipoprotein-associated paraoxonase activity and oxidative DNA damage. Eur J Clin Pharmacol 60:685–691
Jenkins RR (1988) Free radical chemistry. Relationship to exercise. Sports Med 5:156–170
Kanter MM, Nolte LA, Holloczy JO (1993) Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol 74:965–969
Kaur H, Halliwell B (1990) Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem Biol Interact 73:235–247
Kontush A, Chantepie S, Chapman MJ (2003) Small dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol 23:1881–1888
Kresanov P, Vasankari T, Ahotupa M, Kaikkonen J, Juonala M, Kähönen M, Lehtimäki T, Viikari J, Raitakari OT (2015) Paraoxonase-1 and oxidized lipoprotein lipids. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 241:502–506
Leon AS, Sanchez OA (2001) Response of blood lipids to exercise training alone and combined with dietary intervention. Med Sci Sports Exerc 33:502–515
Lindsay FH, Hawley JA, Myburgh KH, Schomer HH, Noakes TD, Dennis SC (1996) Improved athletic performance in highly trained cyclists after interval training. Med Sci Sports Exerc 8:1427–1434
Mackness MI, Arrol S, Durrington PN (1991) Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett 29(286):152–154
Mackness MI, Durrington PN, Mackness B (2000) How HDL protects against the effects of lipid peroxidation. Curr Opin Lipidol 11:383–388
Magness S (2014) The science of running. In: Magness S (ed) Training for each event. Origin Press, USA, pp 253–284
Mahoney DJ, Tarnopolsky MA (2005) Understanding skeletal muscle adaptation to exercise training in humans: contributions from microarray studies. Phys Med Rehabil Clin N Am 16:859–873
Monda KL, Ballantyne CL, North KE (2009) Longitudinal impact of physical activity on lipid profiles in middle-aged adults: the Atherosclerosis Risk in Communities Study. J Lipid Res 50:1685–1691
Mora S, Cook N, Buring JE, Ridker PM, Lee IM (2007) Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116:2110–2118
Navab M, Reddy ST, Van Lenten BJ, Fogelman AM (2011) HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 8:222–232
Parthasarathy S, Santanam N, Auge N (1998) Oxidised low-density lipoprotein: a two-faced Janus in coronary artery disease? Biochem Pharmacol 56:279–284
Pilz J, Meineke I, Gleiter CH (2000) Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl 742:315–325
Pincemail J, Deby C, Camus G, Pirnay F, Bouchez R, Massaux L, Goutier T (1988) Tocopherol mobilization during intensive exercise. Eur J Appl Physiol 57:189–191
Powell KE, Thompson PD, Caspersen CJ, Kendrick JS (1987) Physical activity and the incidence of CHD. Annu Rev Public Health 8:253–287
Radak Z, Chung HY, Koltai E, Taylor AW, Goto S (2008) Exercise, oxidative stress and hormesis. Ageing Res Rev 7:34–42
Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M (2013) Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal 18:1208–1246
Shao B, Heinecke JW (2009) HDL, lipid peroxidation, and atherosclerosis. J Lipid Res 50:716–722
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Steinberg D, Witztum JL (2002) Is the oxidative modifications hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date reflect the hypothesis? Circulation 105:2107–2111
Svensson MB, Ekblom B, Cotgreave IA, Norman B, Sjöberg B, Ekblom O, Sjödin B, Sjödin A (2002) Adaptive stress response of glutathione and uric acid metabolism in man following controlled exercise and diet. Acta Physiol Scand 176:43–56
Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O’Keefe JH, Milani RV, Blair SN, Church TS (2013) Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J 77:281–292
Väisänen S, Gävert J, Julkunen A, Voutilainen E, Penttilä I (1992) Contents of apolipoprotein A-I, A-II and B of the human serum fractions for high-density and low-density lipoproteins prepared by common precipitation methods. Scand J Clin Lab Invest 52:853–862
Välimäki IA, Vuorimaa T, Ahotupa M, Kekkonen R, Korpela R, Vasankari T (2012) Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int J Sports Med 33:291–296
Vasankari TJ, Kujala UM, Vasankari TM, Vuorimaa T, Ahotupa M (1997) Effects of acute prolonged exercise on serum and LDL oxidation and antioxidant defences. Free Radic Biol Med 22:509–513
Vasankari TJ, Kujala UM, Vasankari TM, Ahotupa M (1998) Reduced oxidized LDL levels after 10-months exercise program. Med Sci Sports Exerc 30:1496–1501
Vasankari T, Ahotupa M, Toikka J, Mikkola J, Irjala K, Pasanen P, Neuvonen K, Raitakari O, Viikari J (2001) Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis 155:403–412
Vuorimaa T, Ahotupa M, Irjala K, Vasankari T (2005) Acute prolonged exercise reduces moderately oxidized LDL in healthy men. Int J Sports Med 26:420–425
Wagganer JD, Robison CE, Ackerman TA, Davis PG (2015) Effects of exercise accumulation on plasma lipids and lipoproteins. Appl Physiol Nutr Metab 40:441–447
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Välimäki, I.A., Vuorimaa, T., Ahotupa, M. et al. Strenuous physical exercise accelerates the lipid peroxide clearing transport by HDL. Eur J Appl Physiol 116, 1683–1691 (2016). https://doi.org/10.1007/s00421-016-3422-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3422-y