Abstract
Purpose
It is important to know how thermal sensation is affected by normal aging under conditions that elevate core body temperature for the prevention of heat-related illness in older people. We assessed whether thermal sensation under conditions of normothermia (NT) and mild hyperthermia (HT) is lowered in older adults.
Methods
Seventeen younger (23 ± 3 years) and 12 older (71 ± 3 years) healthy men underwent measurements of the cold and warmth detection thresholds ( ± 0.1 °C/s) of their chest and forearm skin, and whole body warmth perception under NT (esophageal temperature, T es, ~36.5 °C) and HT (T es, ~37.3 °C; lower legs immersed in 42 °C water) conditions.
Results
Warmth detection threshold at the forearm was increased in older compared with younger participants under both NT (P = 0.006) and HT (P = 0.004) conditions. In contrast, cold detection threshold at the forearm was decreased in older compared with younger participants under NT (P = 0.001) but not HT (P = 0.16). Mild hyperthermia decreased cold detection threshold at forearm in younger participants (P = 0.001) only. There were no effects of age and condition on warmth and cold detection thresholds at chest. Whole body warmth perception increased during HT compared with NT in both groups (both, P < 0.001), and older participants had lower values than the younger group under NT (P = 0.001) and HT (P = 0.051).
Conclusions
Skin warmth detection thresholds at forearm and whole body warmth perception under NT and HT and skin cold detection thresholds at forearm under NT deteriorated with aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aged individuals, even in the absence of overt disease, are more susceptible to heat-related illness during heat waves than their younger counterparts (Robine et al. 2008; Semenza et al. 1999). Previous studies examining the physiological factors that may contribute to the age-related increase in the susceptibility of healthy older adults to heat-related illness have demonstrated that older adults have diminished heat tolerance (Kenney and Munce 2003) and attenuated behavioral (e.g., adjusting ambient temperature) (Natsume et al. 1992; Van Someren et al. 2002) and autonomic thermoregulatory responses (i.e., increase in skin blood flow and eccrine sweating in response to increased body temperatures) (Inoue et al. 1991; Inoue and Shibasaki 1996; Kenney et al. 1997) relative to younger adults.
Importantly, a decline in thermal sensation is thought to be one of the causes of the age-associated decrease in thermoregulatory responses (Dufour and Candas 2007; Guergova and Dufour 2011; Natsume et al. 1992; Taylor et al. 1995). The results of previous studies examining age-related changes in thermal perception have varied with the methodology used, including the body region(s) assessed, the types of measures used, and whether the experimental stimulus was heat or cold (Guergova and Dufour 2011). However, experiencing less discomfort during whole body exposure to warm or cool environments (Collins et al. 1981; Natsume et al. 1992; Taylor et al. 1995) and an increased (deteriorated) skin threshold for detecting innocuous thermal stimuli with the detection thresholds (Fowler et al. 1987; Jamal et al. 1985; Kenshalo 1986; Stevens and Choo 1998) or with the method of limits technique (Tochihara et al. 2011; Lin et al. 2005), are generally observed in older compared with younger individuals (Guergova and Dufour 2011). Additionally, the skin threshold for detection of thermal stimuli has been shown to increase more in the extremities than in other regions of the body (Stevens and Choo 1998; Tochihara et al. 2011), following a distal–proximal pattern with aging (Guergova and Dufour 2011). The pathogenesis of heat-related illness is an increased core body temperature under various conditions; therefore, for the prevention of this illness, it is more important to know how thermal sensation is affected by aging under conditions that elevate the core body temperature. To our knowledge, no previous study has reported the effects of normal aging on the threshold of detection of innocuous thermal stimuli during hyperthermia.
The purpose of the present study was to compare the skin surface thresholds for perception of heat and cold at the extremity (forearm) and the torso (chest), as well as whole body warmth perception, between younger and older adult participants with mildly increased core temperatures (mild hyperthermia, HT) and under normothermic (NT) conditions. We hypothesized that older participants would have lower thermal detection threshold especially in the extremity and less sensitive whole body warmth perception than younger participants during both HT and NT.
Methods
Participants
The study procedure was approved by the Institutional Review Board of Osaka City University Graduate School of Medicine (No. 2711) and conformed to the standards set by the Declaration of Helsinki. The experimental protocol was fully explained and 17 healthy young male volunteers [age, 22.8 ± 3.2 years; height, 175 ± 7 cm; body weight, 64.7 ± 6.7 kg (mean ± SD)] and 12 healthy older male volunteers [age, 71.2 ± 3.0 years; height, 166 ± 6 cm; body weight, 61.4 ± 3.9 kg (mean ± SD)] gave their written, informed consent before participating in this study. All participants were non-smokers and had no overt history of cardiovascular, metabolic, pulmonary disease, or neurological disorders. All experiments were performed during the cool seasons (months other than July, August, and September) in Japan. Only male participants were included in this study as previous research has reported gender difference in thermal sensation (Gerrett et al. 2014; Golja et al. 2003).
Experimental protocol
The participants were instructed to refrain from consuming beverages containing caffeine or alcohol, and to avoid vigorous exercise for at least 24 h before arriving at the laboratory. In addition, they were required to refrain from consuming any food for at least 2 h after having a light breakfast. To avoid dehydration, they drank more than 500 ml of water at least 1 h before arriving at the laboratory. The participants arrived at the laboratory between 06:00 and 09:30, and were familiarized with the procedures for measuring noticeable increases and decreases in skin temperature (skin warmth and cold detection thresholds). They then voided urine, were weighed in the nude, and donned short pants. Prior to entering the climatic chamber (TBR-6W2S2L2M; ESPEC Co., Osaka, Japan), an esophageal thermistor was inserted through their external nares to measure esophageal temperature (T es) and thermistor probes were applied to the skin surfaces to measure skin temperature at five sites as described below. The participants sat for 20 min in the climatic chamber on a reclining chair with the backrest at a 70° angle while other measurement devices for cardiovascular and thermoregulatory variables were applied. Participants were familiarized with the measurements of skin warmth and cold detection thresholds and of whole body warmth perception. Baseline data were then collected for 10 min at an ambient temperature of 28.0 ± 0.1 °C and relative humidity of 40 ± 1 % as a thermoneutral condition according to previous studies using the same passive heating protocol (Suzuki et al. 2014; Takamata et al. 1997) and also skin thermal detection thresholds (Tochihara et al. 2011). These conditions were maintained in the climatic chamber for the duration of the experiment.
Participants then underwent the cardiovascular measurements in sitting and supine positions for purposes other than for the present study. After confirming that physiological variables returned to baseline values and were stable for 5 min, cardiovascular and thermoregulatory data were collected for 5 min with the participants in a sitting position in NT conditions. Then, whole body warmth perception was measured, the skin warmth and cold detection thresholds were determined (warmth before cold, and chest before forearm), and whole body warmth perception was measured again. The participants then remained sitting and placed their lower legs in 42 °C water. The temperature of water during 20–40 min heating was controlled between 40 and 42 °C to increase T es by 0.7–0.9 °C after 40 min of heating for both groups. During the measurement of HT, the temperature of water was kept at a constant level. After 40 min, they underwent the same measurements in the same order during HT conditions. We used immersion of the lower legs in hot water to passively heat the participants and avoid elevating the skin temperature at the threshold measurement sites.
Measurements
Cardiovascular responses
To compare cardiovascular responses between younger and older participants, we measured heart rate (HR), systolic (SBP) and diastolic blood pressures (DBP). HR was monitored using a bedside electrocardiogram monitor (BSM-7201; Nihon Kohden Co., Tokyo, Japan). SBP and DBP were measured every 5 min by auscultation using a pressure cuff (STBP-780, Colin, Komaki, Japan) applied to the left upper arm at the level of the heart. Pulse pressure (PP) was calculated as SBP − DBP. Mean blood pressure (MBP) was calculated as DBP + PP/3.
T es and skin temperatures
T es was measured with an esophageal thermistor inserted into a polyethylene tube (LT-ST08-11; Gram Co, Saitama, Japan), the tip of which was advanced into the esophagus to a distance equal to one-fourth of the participant’s standing height. Skin surface temperatures were measured at five sites using thermistors (LT-ST08-12; Gram Co) placed on the right side of the chest (T chest), upper arm (T arm), forearm (T forearm), thigh (T thigh) and lower leg (T leg). Mean skin temperature (T sk) was calculated as 0.3 × (T chest + T arm) + 0.2 × (T thigh + T leg), as previously described (Ramanathan 1964).
Skin blood flow and sweat rate
To compare autonomic heat dissipative responses between younger and older participants, we measured skin blood flow and sweat rate. Skin blood flow (SkBF) was measured using laser-Doppler flowmetry (LDF type ALF-21D; Advance, Tokyo, Japan) at the forearm (SkBFforearm) and chest (SkBFchest). The laser probe was placed externally 1 cm away from the thermistor for T forearm and T chest, while avoiding superficial veins. Sweat rate (SR) was measured by the ventilated capsule method (SKN-2000; Nishizawa Electronic Measuring Instruments, Nagano, Japan) at the forearm (SRforearm) and chest (SRchest). A 0.785-cm2 capsule was placed proximal to the laser probe at each site. The air flow rate of the capsule was 300–600 ml.min−1.
Measurement of skin warmth and cold detection thresholds (method of limits)
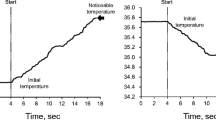
Skin warmth and cold detection thresholds were measured on the right (dominant side of all participants except one young participant) forearm and chest using a skin sensation threshold measurement device consisting of a Peltier thermo-electronic probe and a push-button switch (Intercross-200, Intercross, Tokyo, Japan) based on the method used in previous studies (Kawano et al. 2009; Tochihara et al. 2011). All of the measurements were performed by an experienced investigator (R.T.) for all participants. The surface area of the thermo-electronic probe in contact with the skin was 25 × 25 mm (6.25 cm2 of area). The thermo-electronic probe was applied and fixed to the skin surface by the hand of the investigator with a minimal pressure and the measurement was started when heat flux was maintained at ± 30 W/m2 for 4 s; this confirmed that the temperature at the probe was equal to the surface temperature of the skin. This temperature was defined as the “Initial temperature”. The probe was set to warm and cool at 0.1 °C/s. All participants were instructed to push the switch the moment they felt a “slightly warm” or “slightly cool” sensation. This temperature was defined as “Noticeable temperature.” We calculated the warmth and cold detection thresholds using the following equation:
Between the measurements, the thermo-electronic probe was removed, and we waited for several minutes until the temperature of the thermo-electronic probe and of the skin site had returned to the initial value of each trial. In the previous studies (Kawano et al. 2009; Tochihara et al. 2011), measurements were repeated twice and the average of the two measurements was determined. In the present study, the warmth and cold detection thresholds at each skin site were measured 3 times and the average value of the two closest measurements was calculated and reported. The typical errors for the measurement of skin warmth and cold detection thresholds at the chest during mild hyperthermia, obtained from multiple analyses of 17 datasets, were 0.19 and 0.14 °C (coefficients of variation of 14.3 and 10.1 %, respectively).
Measurement of whole body warmth perception
Whole body warmth perception was determined using a 100-mm visual analog scale from “no warm sensation” at zero to “extremely warm sensation” at 100 (Strigo et al. 2000). An examiner moved a pen from zero to 100 and marked where participants nodded to indicate their whole body warmth perception at that time. These measurements were taken before and after the warmth and cold detection threshold measurements at individual skin sites. The average values of the whole body warmth perception measurements were then calculated and reported. The typical error for the whole body warmth perception measurements during mild hyperthermia, obtained from multiple analyses of 17 datasets, was 3.4 (coefficients of variation of 7.9 %).
Data analysis
Data on T es, skin temperature, SkBFforearm, SkBFchest, SRforearm, and SRchest were collected using a 16-channel computerized data acquisition system (Intercross-310; Intercross Co, Tokyo, Japan: and MP 150 and BIOPAC Systems Inc.; Goleta, CA, USA) and stored on a laboratory computer. Cutaneous vascular conductance at the forearm (%CVCforearm) and chest (%CVCchest) was calculated as SkBFforearm and SkBFchest divided by MBP, respectively, and were expressed as the relative changes from the baseline. The average data collected over 5 min was reported for each variable.
Statistics
The effects of body temperature (condition, NT vs. HT) and aging (age, younger vs. older) on each variable were tested using a two-way repeated-measures analysis of variance (ANOVA). Subsequent post hoc tests to determine significant differences in each pairwise comparison were performed using the Tukey’s test. The null hypothesis was rejected at P < 0.05. Values are expressed as mean ± SEM.
Results
Cardiovascular responses are shown in Table 1. We found effects of age on DBP (P = 0.004), MBP (P = 0.041), and PP (P = 0.027); and effects of condition on HR (P < 0.001) and DBP (P = 0.006). HR increased in HT compared with NT in both groups (both, P < 0.001). SBP, MBP, and PP remained unchanged during heat stress in both groups, while DBP decreased in HT compared with NT only in the older group (P = 0.013). Age did not affect HR or SBP. In both NT and HT conditions, DBP was lower in the older than in the younger group (NT, P = 0.021; HT, P = 0.002); this was also true of MBP in HT conditions (P = 0.006). During NT, PP was higher in the older than in the younger group (P = 0.003). We found an interaction between condition (NT vs. HT) and age (younger vs. older) on HR, SBP, MBP, and PP (P = 0.013–0.045), indicating that the response in these variables with passive heating was different between groups.
Table 2 shows the body temperatures and thermoregulatory responses of the participants. We found effects of age on T sk (P = 0.025), T forearm (P = 0.029), and %CVCforearm (P = 0.006); and effects of condition on all variables (all, P < 0.001) except T forearm (P = 0.89). T es and T sk increased in HT compared with NT in both groups (all, P < 0.001). T chest was lower during HT than NT in both groups (younger P = 0.013; older P < 0.001). Heat stress did not affect Tforearm in either group. %CVC and SR at both sites increased with heating in both groups (all, P < 0.001 except %CVCforearm in the older group, P = 0.06). Age did not affect T es and T chest. T sk was lower in the older than in the younger group in both NT and HT (P = 0.06 and P = 0.15, respectively). Tforearm during HT was higher in the older than in the younger group (P = 0.032). %CVCforearm during HT was lower in the older than in the younger group (P < 0.001). We found an interaction between condition (NT vs. HT) and age (younger vs. older) on T es, %CVCforearm, and SRchest (P = 0.002–P = 0.02), indicating that the increase in Tes with passive heating was greater while the increase in %CVCforearm and SRchest was attenuated with aging.
Figure 1 shows the skin warmth detection threshold. We found no effect of condition (chest, P = 0.52; forearm, P = 0.74) nor interaction between condition and age (chest, P = 0.96; forearm, P = 0.87) at either site. We found effects of age at forearm (P = 0.002) but not at chest (P = 0.08). The warmth detection threshold at forearm was higher (less sensitive detection) in the older than in the younger group in both NT (younger 0.63 ± 0.10 °C; older 2.04 ± 0.59 °C; P = 0.006) and HT (younger 0.66 ± 0.08 °C; older 2.15 ± 0.53 °C; P = 0.004) conditions.
Figure 2 shows the skin cold detection threshold. We found effects of age (P = 0.011) and condition (P = 0.017) at forearm, while not at chest (P = 0.12 and P = 0.08, respectively). The cold detection threshold at forearm was lower (less sensitive detection) in the older than in the younger group in NT (younger −0.29 ± 0.06 °C; older −1.28 ± 0.32 °C; P = 0.001) while not in HT (younger −0.89 ± 0.16 °C; older −1.29 ± 0.29 °C; P = 0.16). Interestingly, in contrast to warmth detection, the cold detection threshold at forearm was lower (less sensitive detection) in HT than NT in the younger group (P < 0.001) while not in the older group (P = 0.93). We found an interaction between condition (NT vs. HT) and age (younger vs. old) at the forearm (P = 0.023) but not at the chest (P = 0.13), indicating that the response in the cold detection threshold at forearm with passive heating was different between groups.
Figure 3 shows the whole body warmth perception. We found effects of age (P = 0.004) and condition (P < 0.001), while there was no interaction (P = 0.16). Whole body warmth perception was increased by heat stress in both groups (both, P < 0.001). Most importantly, the sensation of warmth was lower in the older than in the younger group in both NT (younger 39.6 ± 5.0; older 20.3 ± 0.7; P = 0.001) and HT (younger 68.9 ± 2.9; older 57.7 ± 4.6; P = 0.051) conditions.
Discussion
The major findings of this study are: (1) the warmth detection threshold at forearm was increased in older adults compared with younger adults during both NT and HT; (2) the cold detection threshold at forearm was decreased in older adults compared with younger adults during NT; (3) decreasing of the cold detection threshold at forearm during HT was observed in younger but not in older participants; (4) these observations were not detected at chest; (5) whole body warmth perception was lower in older adults than in younger adults during both NT and HT. These findings may suggest that warmth but not cold detection on the skin surface at the extremity as well as whole body warmth perception during HT is deteriorated by normal aging in a manner similar to that observed during NT.
The thermoregulatory command center in the preoptic area (POA) controls autonomic and behavioral thermoregulatory responses based on input signals from thermo-sensitive neurons in the POA and from the temperature receptors in the skin and other deep body tissues (Egan et al. 2005; Morrison and Nakamura 2011; Nagashima 2006; Simon et al. 1986). Therefore, the lowered thermal sensation observed with aging is thought to be one of the causes of the age-associated decrease in thermoregulatory responses (Dufour and Candas 2007; Guergova and Dufour 2011; Natsume et al. 1992; Taylor et al. 1995; Van Someren et al. 2002) and, therefore, thought to be associated with the increased prevalence of heat-related illness in aged individuals (Robine et al. 2008; Semenza et al. 1999). Considering the pathogenesis of the heat-related illness, it is worthy knowing how thermal sensation is affected by normal aging under conditions that elevate the core body temperatures. In the present study, as shown in Figs. 1 and 2, we demonstrated for the first time that the skin warmth and cold detection threshold at forearm were deteriorated under NT while the skin warmth but not cold detection threshold at forearm was lowered under HT condition with aging.
Several previous studies have reported less sensitive skin detection of innocuous warm and cold stimuli in older participants with the detection thresholds (Fowler et al. 1987; Jamal et al. 1985; Kenshalo 1986; Stevens and Choo 1998) or with the method of limits (Tochihara et al. 2011; Lin et al. 2005), although all of these studies were conducted under normothermic conditions. Jamal et al. (1985) observed age-related increases in both warm and cold detection thresholds in the ankle, and a similar increase in the cold threshold in the wrist using a thermode (an initial skin temperature was 34–35 °C) in a quiet room at a constant air temperature of 22 °C. Dufour and Candas (2007) also found increased thresholds for warmth detection on the hand, foot, and calf in older adults (age >60 years old) relative to middle-aged (40-50 years old) and younger adults (20–30 years old) in a thermoneutral dry environment (air and wall temperature at 28 °C with a dew point temperature of 14 °C) and also in a hot humid environment (air and wall temperature at 40 °C with a dew point temperature of 25 °C) though skin and sublingual temperatures in young adults were lower than in the other two groups. Thus, our results support previously published observations and provide further information on the change in thermal detection and perception that occurs in younger and older adults during hyperthermia.
In addition, the age-related decrease in detection of warmth and cold has been found to be more apparent in certain body regions, particularly the extremities (Stevens and Choo 1998; Tochihara et al. 2011), and thus follows a distal–proximal pattern with aging (Guergova and Dufour 2011). Recently, Tochihara et al. (2011) demonstrated that the cutaneous warm thresholds for the hand, shin, and foot were higher for the older than for the younger adults while the remaining body parts showed no age difference in a thermoneutral (28 °C) and in a cool environment (22 °C). Our findings confirmed previous observations by showing more remarkable deterioration in the warmth and cold detection thresholds with aging at the forearm than at the chest in thermoneutral environment both under NT and HT conditions (Figs. 1 and 2).
The major factors underlying the lowered ability of the skin to detect warmth and cold sensation that occurs with aging are local structural changes in the skin (Dufour and Candas 2007), including age-related lowering in thermoreceptor density (Chang et al. 2004; Goransson et al. 2004; Panoutsopoulou et al. 2009) and in the superficial vascular network (especially the capillary loops of the papillary dermis) which could lower the functionality of temperature receptors (Balin and Pratt 1989; Cerimele et al. 1990; Montagna and Carlisle 1979; Smith 1989). In addition, age-related changes in the peripheral nervous system, particularly fiber loss and decreased conduction velocity (Guergova and Dufour 2011) may be another factor. The sensation of warmth is transmitted by unmyelinated C fibers and the sensation of cold is transmitted by myelinated Aδ fibers and unmyelinated C fibers (Campero et al. 2001; Palmer et al. 2000). Indeed, unmyelinated (Ceballos et al. 1999; Nakayama et al. 1998) and myelinated (Nakayama et al. 1998; Robertson et al. 1993) fiber densities decrease with aging, although the free nerve endings associated with thermal sensation appear to remain intact in older humans (Montagna and Carlisle 1979). In addition, there were several abnormalities involved in myelinated fibers, including the smaller size of those fibers in the aged individuals (Verdu et al. 2000). Indeed, it has been reported that the reduction in skin innervations and fiber densities of peripheral nerves can decrease the peripheral input of heat impulses (Chao et al. 2007).
As shown in Fig. 3, whole body thermal perception was attenuated in older adults relative to the younger participants during HT and NT. Recently, Schlader et al. (2015) used a water-perfusion suit to show that whole body thermal perception was comparable between younger and older individuals both during NT and HT under controlled internal and mean skin temperatures. The whole body sensation of warmth assessed in the present study is basically influenced by information from the skin and body core temperatures (Frank et al. 1999; Gagge et al. 1967, 1969; Hall and Klemm 1969). In the present study, the temperature of water during 20–40 min heating was controlled between 40 and 42 °C to increase T es by 0.7–0.9 °C for both groups after 40 min of heating and we confirmed that T es both in NT and HT conditions were comparable between the younger and the older participants, and that delta increase in T sk with passive heating was also comparable between groups while T sk in NT and HT conditions were lower in the older than in the younger participants as shown in Table 2. The lower T sk in the older relative to those of the younger participants may explain the lower sensation of whole body warmth experienced by the older participants in the present study. The attenuated increase in skin blood flow observed at the forearm in the older compared with the younger participants (Table 2) is thought to be associated with the lowered mean skin temperature in the older participants. Further studies are required to elucidate the mechanisms of the lower sensation of whole body warmth with aging.
Although a method for quantitative assessment of the thermoregulatory response behavior in humans has not yet been established, thermal perception in the whole body can be used to estimate the driving force for the behavioral thermoregulatory response (Nagashima 2006; Tokizawa et al. 2010). Therefore, the attenuation of whole body warmth found in the older participants (Fig. 3) suggests a lower driving force for the behavioral thermoregulatory response than in the younger participants during mild hyperthermia. In addition, the cold detection threshold, but not the warmth detection threshold, was decreased (less sensitive detection) during hyperthermia in the younger participants but not in the older participants (as shown in Figs. 1, 2). The greater attenuation of the skin cold detection threshold during hyperthermia might enhance whole body warmth perception and act as the driving force for the behavioral response in the younger adults. The observed decrease in cold detection by the skin during mild hyperthermia could be caused by decreased firing of cold-responsive neurons when the core body temperature is elevated (Inoue and Murakami 1976); this appears to disappear with aging. Collectively, aging is associated with decreased thermal sensations in the whole body and at specific skin sites during mild hyperthermia.
We also observed age effects on CVC at the forearm and also an interaction on CVC at the forearm and SR at the chest (Table 2), indicating that autonomic thermoregulatory responses during hyperthermia were attenuated with normal aging as has been suggested in previous studies (Inoue et al. 1991; Inoue and Shibasaki 1996; Kenney et al. 1997). In some other species, augmented behavioral thermoregulatory responses have been observed to compensate for the aging-associated decline in autonomic thermoregulation (Aujard et al. 2006). However, the present results coupled with previous observations indicate that compensatory behavioral responses would not be expected in older adults during hyperthermia. This is an important physiological factor that may contribute to the increased susceptibility of healthy older adults to heat-related illness.
Limitations
There are many confounding factors involved in thermal perception studies (Guergova and Dufour 2011). Previous studies that assessed age-related changes in thermal sensation reported varied and inconsistent results depending on their methodology. The body region(s) assessed, types of measures used, and the types of stimuli (warm, cold) (Guergova and Dufour 2011) all affected their results. Therefore, the present observations may not be supported by different methodology. Thermal stimuli applied to the skin evoke not only thermal perception, but also cutaneous vasoconstriction and vasodilation (Kenney and Munce 2003). Thus, changes in autonomic thermoregulatory responses that occur with aging may affect thermal sensation itself. In addition, there are several methodological limitations in the present study. We used the fixed protocol for the measurement of the skin warmth and cold sensation threshold, thereby leaving a possible order or learning effects on the measurement. The thermo-electronic probe for the measurement was applied and fixed to the skin surface by the hand of the investigator, therefore, we did not monitor nor regulate the pressure of the probe application, which might have an effect on the results. The other limitation is the evaluation of whole body warmth perception. In the present experimental setting, the participants did not themselves mark their sensation on the scale because they could not move both their arms to measure blood pressure at left arm and skin blood flow and sweat rate at right arm. In addition, a few participants reported 0 in VAS in NT condition although no participant claimed cold. The VAS scale we used might force the participants into a category or make incorrect assumptions on their thermal perception. It is though that if we used self-reporting and a scale that could also evaluate the cold perception such as a bipolar scale, the evaluation of whole body warmth perception might be more sensitive and the results of it might be different. Finally, several participants had small increase in SR and CVC relative to baseline values even in NT condition at the thermoneutral environment (Suzuki et al. 2014; Takamata et al. 1997; Tochihara et al. 2011), which might have an effect on the observations.
In conclusion, skin warmth detection thresholds at forearm and whole body warmth perception deteriorated with aging during both normothermia and mild hyperthermia. In contrast, skin cold detection thresholds at forearm deteriorated with aging during normothermia. These mechanisms are thought to be associated with the increased susceptibility of healthy older adults to heat-related illness.
Abbreviations
- NT:
-
Normothermia
- HT:
-
Mild hyperthermia
- T es :
-
Esophageal temperature
- T sk :
-
Skin temperature
- HR:
-
Heart rate
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- PP:
-
Pulse pressure
- MBP:
-
Mean blood pressure
- SkBF:
-
Skin blood flow
- SR:
-
Sweat rate
- CVC:
-
Cutaneous vascular conductance
References
Aujard F, Seguy M, Terrien J, Botalla R, Blanc S, Perret M (2006) Behavioral thermoregulation in a non human primate: effects of age and photoperiod on temperature selection. Exp Gerontol 41(8):784–792. doi:10.1016/j.exger.2006.06.001
Balin AK, Pratt LA (1989) Physiological consequences of human skin aging. Cutis 43(5):431–436
Campero M, Serra J, Bostock H, Ochoa JL (2001) Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol 535(Pt 3):855–865
Ceballos D, Cuadras J, Verdu E, Navarro X (1999) Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat 195(Pt 4):563–576
Cerimele D, Celleno L, Serri F (1990) Physiological changes in ageing skin. Br J Dermatol 122(Suppl 35):13–20
Chang YC, Lin WM, Hsieh ST (2004) Effects of aging on human skin innervation. NeuroReport 15(1):149–153
Chao CC, Hsieh ST, Chiu MJ, Tseng MT, Chang YC (2007) Effects of aging on countsct heat-evoked potentials: the physiological assessment of thermal perception. Muscle Nerve 36:30–38
Collins KJ, Exton-Smith AN, Dore C (1981) Urban hypothermia: preferred temperature and thermal perception in old age. BMJ 282(6259):175–177
Dufour A, Candas V (2007) Ageing and thermal responses during passive heat exposure: sweating and sensory aspects. Eur J Appl Physiol 100(1):19–26. doi:10.1007/s00421-007-0396-9
Egan GF, Johnson J, Farrell M, McAllen R, Zamarripa F, McKinley MJ, Lancaster J, Denton D, Fox PT (2005) Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc Natl Acad Sci U S A 102(14):5262–5267. doi:10.1073/pnas.0409753102
Fowler CJ, Carroll MB, Burns D, Howe N, Robinson K (1987) A portable system for measuring cutaneous thresholds for warming and cooling. J Neurol Neurosurg Psychiatry 50(9):1211–1215
Frank SM, Raja SN, Bulcao CF, Goldstein DS (1999) Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J Appl Physiol 86(5):1588–1593
Gagge AP, Stolwijk JA, Hardy JD (1967) Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res 1(1):1–20
Gagge AP, Stolwijk JA, Saltin B (1969) Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res 2(3):209–229
Gerrett N, Ouzzahra Y, Coleby S, Hobbs S, Redortier B, Voelcker T, Havenith G (2014) Thermal sensitivity to warmth during rest and exercise: a sex comparison. Eur J Appl Physiol 114:1451–1462
Golja P, Tipton MJ, Mekjavic IB (2003) Cutaneous thermal threshold-the reproducibility of their measurements and the effects of gender. J Therm Biol 28:341–346
Goransson LG, Mellgren SI, Lindal S, Omdal R (2004) The effect of age and gender on epidermal nerve fiber density. Neurology 62(5):774–777
Guergova S, Dufour A (2011) Thermal sensitivity in the elderly: a review. Ageing Research Reviews 10(1):80–92. doi:10.1016/j.arr.2010.04.009
Hall JF Jr, Klemm FK (1969) Thermal comfort in disparate thermal environments. J Appl Physiol 27(5):601–606
Inoue S, Murakami N (1976) Unit responses in the medulla oblongata of rabbit to changes in local and cutaneous temperature. J Physiol 259(2):339–356
Inoue Y, Nakao M, Araki T, Murakami H (1991) Regional differences in the sweating responses of older and younger men. J Appl Physiol 71(6):2453–2459
Inoue Y, Shibasaki M (1996) Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating. Eur J Appl Physiol Occup Physiol 74(1–2):78–84
Jamal GA, Hansen S, Weir AI, Ballantyne JP (1985) An improved automated method for the measurement of thermal thresholds. 1. Normal subjects. J Neurol Neurosurg Psychiatry 48(4):354–360
Kawano T, Kabasawa Y, Ashikawa S, Sato Y, Jinno S, Omura K (2009) Accuracy and reliability of thermal threshold measurement in the chin using heat flux technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108(4):500–504. doi:10.1016/j.tripleo.2009.05.034
Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA (1997) Decreased active vasodilator sensitivity in aged skin. Am J Physiol 272(4 Pt 2):H1609–H1614
Kenney WL, Munce TA (2003) Invited review: aging and human temperature regulation. J Appl Physiol 95(6):2598–2603. doi:10.1152/japplphysiol.00202.2003
Kenshalo DR Sr (1986) Somesthetic sensitivity in young and elderly humans. J Gerontol 41(6):732–742
Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST (2005) influence of ageing on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Syst 10:269–281
Montagna W, Carlisle K (1979) Structural changes in aging human skin. J Invest Dermatol 73(1):47–53
Morrison SF, Nakamura K (2011) Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 16:74–104
Nagashima K (2006) Central mechanisms for thermoregulation in a hot environment. Ind Health 44(3):359–367
Nakayama H, Noda K, Hotta H, Ohsawa H, Hosoya Y (1998) Effects of aging on numbers, sizes and conduction velocities of myelinated and unmyelinated fibers of the pelvic nerve in rats. J Auton Nerv Syst 69(2–3):148–155
Natsume K, Ogawa T, Sugenoya J, Ohnishi N, Imai K (1992) Preferred ambient temperature for old and young men in summer and winter. Int J Biometeorol 36(1):1–4
Palmer ST, Martin DJ, Steedman WM, Ravey J (2000) C- and Adelta-fibre mediated thermal perception: response to rate of temperature change using method of limits. Somatosens Mot Res 17(4):325–333
Panoutsopoulou IG, Wendelschafer-Crabb G, Hodges JS, Kennedy WR (2009) Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology 72(14):1205–1210. doi:10.1212/01.wnl.0000340984.74563.1c
Ramanathan NL (1964) A New Weighting System for Mean Surface Temperature of the Human Body. J Appl Physiol 19:531–533
Robertson A, Day B, Pollock M, Collier P (1993) The neuropathy of elderly mice. Acta Neuropathol 86(2):163–171
Robine JM, Cheung SL, Le Roy S, Van Oyen H, Griffiths C, Michel JP, Herrmann FR (2008) Death toll exceeded 70,000 in Europe during the summer of 2003. CR Biol 331(2):171–178. doi:10.1016/j.crvi.2007.12.001
Schlader ZJ, Gagnon D, Adams A, Rivas E, Cullum CM, Crandall CG (2015) Cognitive and perceptual responses during passive heat stress in younger and older adults. Am J Physiol Regul Integr Comp Physiol 308(10):R847–R854. doi:10.1152/ajpregu.00010.2015
Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR (1999) Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16(4):269–277
Simon E, Pierau FK, Taylor DC (1986) Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev 66(2):235–300
Smith L (1989) Histopathologic characteristics and ultrastructure of aging skin. Cutis 43(5):414–424
Stevens JC, Choo KK (1998) Temperature sensitivity of the body surface over the life span. Somatosens Mot Res 15(1):13–28
Strigo IA, Carli F, Bushnell MC (2000) Effect of ambient temperature on human pain and temperature perception. Anesthesiology 92(3):699–707
Suzuki A, Okazaki K, Imai D, Takeda R, Naghavi N, Yokoyama H, Miyagawa T (2014) Thermoregulatory responses are attenuated after fructose but not glucose intake. Med Sci Sports Exerc 46(7):1452–1461. doi:10.1249/MSS.0000000000000233
Takamata A, Nagashima K, Nose H, Morimoto T (1997) Osmoregulatory inhibition of thermally induced cutaneous vasodilation in passively heated humans. Am J Physiol 273:R197–R204
Taylor NA, Allsopp NK, Parkes DG (1995) Preferred room temperature of young vs aged males: the influence of thermal sensation, thermal comfort, and affect. J Gerontol A Biol Sci Med Sci 50(4):M216–M221
Tochihara Y, Kumamoto T, Lee JY, Hashiguchi N (2011) Age-related differences in cutaneous warm thresholds of human males in thermoneutral and cool environments. J Therm Biol 36:105–111
Tokizawa K, Yasuhara S, Nakamura M, Uchida Y, Crawshaw LI, Nagashima K (2010) Mild hypohydration induced by exercise in the heat attenuates autonomic thermoregulatory responses to the heat, but not thermal pleasantness in humans. Physiol Behav 100(4):340–345. doi:10.1016/j.physbeh.2010.03.008
Van Someren EJ, Raymann RJ, Scherder EJ, Daanen HA, Swaab DF (2002) Circadian and age-related modulation of thermoreception and temperature regulation: mechanisms and functional implications. Ageing Research Reviews 1(4):721–778
Verdu E, Ceballos D, Vilches JJ, Navarro X (2000) Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst 5:191–208
Acknowledgments
We are very grateful to the volunteers who participated in this study. We also thank Dr. Takaaki Okumoto from our laboratory, and Dr. Shinya Matsumura from the department of Sport Science and Medical Science, Osaka University of Health and Sport Science, for useful comments and suggestions regarding this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Funding
This work was supported by a Grant-in-Aid for Young Scientists (A 23689014) and Challenging Exploratory Research (25560372; to K. Okazaki) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Additional information
Communicated by George Havenith.
Rights and permissions
About this article
Cite this article
Takeda, R., Imai, D., Suzuki, A. et al. Lower thermal sensation in normothermic and mildly hyperthermic older adults. Eur J Appl Physiol 116, 975–984 (2016). https://doi.org/10.1007/s00421-016-3364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3364-4