Abstract
Objective
Little is known about the impact of exercise training on conduit artery wall thickness in type 2 diabetes. We examined the local and systemic impact of exercise training on superficial femoral (SFA), brachial (BA), and carotid artery (CA) wall thickness in type 2 diabetes patients and controls.
Methods
Twenty patients with type 2 diabetes and 10 age- and sex-matched controls performed an 8-week training study involving lower limb-based combined aerobic and resistance exercise training. We examined the SFA to study the local effect of exercise, and also the systemic impact of lower limb-based exercise training on peripheral (i.e. BA) and central (i.e. CA) arteries. Wall thickness (WT), diameter and wall:lumen(W:L)-ratios were examined using automated edge detection of ultrasound images.
Results
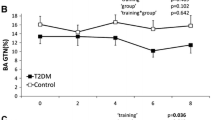
Exercise training did not alter SFA or CA diameter in type 2 diabetes or controls (all P > 0.05). BA diameter was increased after training in type 2 diabetes, but not in controls. Exercise training decreased WT and W:L ratio in the SFA and BA, but not in CA in type 2 diabetes. Training did not alter WT or W:L ratio in controls (P > 0.05).
Conclusion
Lower limb-dominant exercise training causes remodelling of peripheral arteries, supplying active and inactive vascular beds, but not central arteries in type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise training is an important adjunct to medical therapy in type 2 diabetes which is associated with improved glucose homeostasis (Marwick et al. 2009; Wei et al. 2000). In addition, regular exercise training has strong and independent cardioprotective effects, leading to lower cardiovascular morbidity and mortality in type 2 diabetes (Church et al. 2004). These effects of exercise training may, at least partly, be mediated through the direct effect of exercise on the arterial wall (Green et al. 2008; Joyner and Green 2009). Indeed, exercise training improves vascular function measured as flow-mediated dilation in peripheral vessels in type 2 diabetes, most likely contributing to the cardioprotective effects of exercise training (Thijssen et al. 2010; Maiorana et al. 2001; Xiang and Wang 2004).
Little attention has been paid to the impact of exercise training in type 2 diabetes on arterial wall thickness (WT); a surrogate measure of atherosclerosis (de Groot et al. 2004; Lorenz et al. 2007), which has been shown to be increased in subjects with type 2 diabetes (Metcalf et al. 2000; Wagenknecht et al. 1997, 1998). Cross-sectional studies in healthy subjects demonstrated that athletes have a generally lower artery wall thickness compared to sedentary controls (Rowley et al. 2011, 2012; Maiorana et al. 2011). A recent study in healthy young men confirmed the presence of a generalised decrease in arterial wall thickness after 8-week cycling exercise training (3 sessions per week) on both the carotid and the superficial femoral artery (Thijssen et al. 2013). However, the effects of exercise training on wall thickness may be different in subjects with increased cardiovascular risk, such as type 2 diabetes. Endurance-trained post-menopausal women show a smaller wall thickness in peripheral [i.e. superficial femoral artery (SFA)], but not the carotid artery (CA), compared to sedentary controls (Moreau et al. 2002). These findings raise the possibility that exercise training may induce site-specific changes in wall thickness, with predominance in peripheral vessels, especially in subjects with a priori increased cardiovascular risk such as type 2 diabetes.
Reduced wall thickness of the carotid artery (O’Leary et al. 1999), but also of peripheral vessels such as the brachial artery (BA) (Sorensen et al. 1997; Suessenbacher et al. 2013) and SFA (Paul et al. 2005), is associated with reduced risk of cardiovascular events. Exercise training-induced remodelling in wall thickness may, therefore, have prognostic implications. Evidence indicates an inverse relationship between physical fitness and carotid intima-media thickness in type 2 diabetes (Jae et al. 2012), whilst a recent study provided evidence that aerobic exercise training attenuates CA wall thickness (Kadoglou et al. 2013). However, relatively little is known about the potential impact of exercise training on wall thickness of peripheral arteries in type 2 diabetes, and whether changes in peripheral arteries are in agreement with central conduit artery wall thickness. We, therefore, examined the impact of 8 weeks of supervised exercise training on wall thickness in peripheral (SFA and BA) and central (CA) vessels in type 2 diabetes and controls. By performance of lower limb-dominant exercise, we can distinguish between the impact of exercise on peripheral arteries supplying active (i.e. SFA) versus inactive (i.e. BA) areas. We hypothesise that training would result in a generalised decrease in wall thickness in controls, and in a decrease in wall thickness of peripheral, but not central arteries in type 2 diabetes.
Methods
Subjects
Twenty sedentary (i.e. no regular physical exercise) men with type 2 diabetes (>2 years since diagnosis, 59 ± 6 years of age) and 10 sedentary, age-matched men (58 ± 7 years) without diabetes were recruited. Insulin resistance in the group with diabetes was 5.2 ± 3.8 10/%S, calculated via the Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) (Matthews et al. 1985) as \({\text{HOMA}} - {\text{IR}} = \left( {{\text{glucose}}\left( {{\text{mmol}}/{\text{L}}} \right)\; \times \;{\text{insulin}}\left( {{\text{mmol}}/{\text{L}}} \right)} \right)/ 2 2. 5\). Exclusion criteria included overt cardiovascular disease (previously diagnosed by a physician), smoking in the last 15 years, type I diabetes mellitus, age <40 or >65, and diabetes-related manifest vascular complications, neuropathy. The study procedures were approved by the medical ethical committee of the Radboud University Nijmegen Medical Centre and adhered to the Declaration of Helsinki. All subjects gave written informed consent before participation in this study.
Experimental measures
Subjects were allocated to 8-week lower limb-dominant exercise training (3 exercise sessions per week). Each exercise session was supervised by a well-trained researcher and consisted of a 5-min warming-up, followed by a circuit of resistance exercises (leg press, calf raise, leg curl, leg extension, lower back, abdominal crunch, 3 series of 12 repetitions each, with 1 min of rest between sets within each exercise) interspersed with aerobic activities (e.g. cycling, running) (Maiorana et al. 2002; Watts et al. 2004). The total protocol was as follows: 5 min warming-up (cycling), 5 min of cycling, leg curl, leg extension, 5 min of running, lower back, abdominal crunch, 5 min of cycling, leg press, calf raise, 5 min of running. The total duration of a training session was approximately 60 min. A heart rate monitor (Polar Electro Oy, Kempele, Finland) was used to continuously monitor heart rate during the aerobic exercise and heart rate was maintained at 70–75 % of heart rate reserve. The intensity level for each of the resistance exercises was set at a level which enables the participant to complete the three series of 12 repetitions. Intensity was increased each week under the guidance of the trainers. If a participant missed a supervised exercise session, an extra session was planned in the same or following week, so that each participant performed a total of 24 supervised sessions (100 % compliance). Assessments were performed before and after 8-week combined resistance and aerobic exercise training. A subset of our type 2 diabetes participants (n = 8) also received a dual endothelin-receptor blocker (Bosentan, Actelion Pharmaceuticals) during the first 4 weeks representing 62.5 mg taken orally twice per day, followed by 4 weeks of 125 mg twice daily (Monfredi et al. 2011). A priori we expected no impact of this intervention on arterial wall thickness and, to increase the power of our study, we have combined these subjects with type 2 diabetes patients who performed exercise training only (n = 12). However, to acknowledge any possible impact of Bosentan on our outcome parameters, we perform an extra statistical analysis in our type 2 diabetes group with ‘Bosentan’ as an additional factor. All subjects followed the same exercise training protocol.
All studies were conducted in a quiet, temperature-controlled environment and each visit for a given subject was performed at the same time of day. Subjects were asked to fast for >4 h, abstain from alcohol and caffeine for 16 h, and not to perform any exercise for 24 h before testing to exclude variation in resting tone, as this can importantly influence wall thickness (Thijssen et al. 2011b).
After reporting to the laboratory, anthropometrics were measured, and a venous blood sample was taken to determine glucose and insulin (for calculating HOMA-IR), cholesterol, HDL, LDL, and triglycerides. Subsequently, subjects were positioned in the supine position on a comfortable bed. After a rest period of at least 15 min, blood pressure was measured twice using a manual sphygmomanometer, and the mean blood pressure was used for analysis. This was followed by assessment of baseline diameter and wall thickness at rest of the SFA, BA and CA by a trained sonographer. A 10-MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (T3000, Terason, Burlington, MA, USA) was used to image the artery lumen (the area from the intima to lumen border on the near wall to the lumen to intima border on the far wall) and far wall thickness (WT). Clearly demarcated intimal-medial boundaries were obtained via perpendicular incidence imaging in relation to the orientation of the vessel. Images were optimised using contrast controls on the ultrasound machine, which was consistently maintained between arteries for each individual (Potter et al. 2008). Ultrasound parameters were set to optimise longitudinal B-mode images of the lumen/arterial wall interface. Diameter and WT were collected from three distinct angles and data were recorded for at least 10 s at a sampling frequency of 30 Hz. Values for lumen and WT were calculated as the average of all frames and all angles for each sample.
Assessment of the CA was performed with the subject supine and the neck slightly extended to allow scanning of the artery. Images for the CA were made on the proximal 1.5 cm straight portion of this artery. This was followed by assessment of the SFA diameter and wall thickness, which was performed in the proximal third of the thigh, at least 3 cm distal from the bifurcation. Assessment of the BA was performed on the distal 1/3rd of the upper arm. The repeated measurements were made on the same segment of the artery.
On a subsequent day, subjects performed an incremental cycle exercise test to examine maximal workload and peak oxygen consumption. Data from this test were used to determine maximal heart rate, which was used to calculate work load during exercise training. Each subject performed an incremental maximal exercise test on a cycling ergometer (Lode, Excalibur, Groningen, The Netherlands) before and after the training program. These tests started at a power output of 10 W for 1 min and power output increased by 10 W/min until exhaustion. Subjects were instructed to maintain a cadence of between 60 and 80 rpm during the test. We continuously recorded oxygen consumption (VO2, in ml O2/kg per min), ventilation (Ve, in l/min), respiratory quotient (RQ) (Oxycon IV, Jaeger, Germany) and heart rate (HR, in bpm). Furthermore, we measured blood lactate levels (mmol/l) using Accutrend® Lactate (Roche Diagnostics GmbH, type 3012522, Mannheim, Germany) before and 2 min after finishing the maximal exercise test. For a test to be classified as successful, at least 3 out of the following 4 criteria had to be met: clinical signs of exhaustion of the participant, respiratory quotient ≥1.10, finishing within 10 beats of the maximum predicted heart rate (=220-age), and flattening of VO2 uptake curve (≤110 ml increase during the last minute) (Balady et al. 2010).
Analysis of conduit artery diameter and wall thickness
Post-test analysis of the CA, BA and SFA was performed by a researcher who was blinded to the subject identity and timing of the assessment. Furthermore, we used custom-designed edge-detection and wall-tracking software which is largely independent of investigator bias (Potter et al. 2007, 2008). Briefly, the echo signal was encoded in real time and stored as a DICOM file using an IMAQ-PCI-1407 card. Subsequent software analysis of this data was performed at 30 Hz using an icon-based graphical programming language and toolkit (LabVIEW™ 6.02, National Instruments, Austin, TX, USA). The initial phase of image analysis involved the identification of regions of interest (ROI) on the first frame of every individual study. The ROI enables automated calibration for diameters on the B-mode image. A ROI was then drawn around the optimal area of the B-mode image and within this ROI a pixel-density algorithm automatically identified the angle-corrected near and far wall e-lines for every pixel column within the ROI. The same algorithm also identifies the far wall media–adventitia interface. Detection of the near and far wall lumen edges and the far wall media–adventitia interface is performed on every frame. We have shown that the diameter measurements using this semi-automated software possess an intra-observer CV of 5.1 % (Potter et al. 2007). Furthermore, our method of WT assessment is closely correlated with a “phantom” artery system (Potter et al. 2008). To correct for differences in diameter, we also calculated the wall-to-lumen ratio (W:L).
Statistics
Statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA). Two-way repeated measures ANOVA in both groups were used to examine whether the effect of the 8-week lower limb-dominant exercise training (‘training’; 0 versus 8 weeks) on arterial diameter, wall thickness and wall-to-lumen ratio differed between the arteries (‘artery’; BA, SFA, versus CA). Pre-training values were compared between groups using unpaired t tests. According to guidelines, we adopted the least significant difference approach to multiple comparisons (Rothman 1990; Perneger 1998). All data are reported as mean (±SD) and statistical significance was assumed at P < 0.05.
Results
At baseline, participants with type 2 diabetes demonstrated significantly higher weight, BMI, glucose, insulin and HOMA-IR, and lower cholesterol, low-density lipoprotein, peak oxygen uptake and peak workload compared to their age-matched controls (Table 1). We found no significant differences between groups in (systolic and diastolic) blood pressure and triglycerides. Across the 8-week exercise training period, there were no significant changes in weight, body mass index, systolic blood pressure, HDL, triglycerides, glucose, insulin, and HOMA-IR in either type 2 diabetes or controls (Table 1). We found a significant main effect of training for peak oxygen uptake, peak workload, diastolic blood pressure, total cholesterol, and low-density lipoprotein, whilst these changes after the 8-week exercise training were comparable between type 2 diabetes and controls (Table 1). The group with type 2 diabetes significantly used more metformin, sulfonylurea, and statin users compared with the control group (Table 1).
Diameter
Prior to exercise training, we found no differences between type 2 diabetes and controls in baseline diameter of the BA, SFA and CA. A two-way ANOVA in both groups revealed a difference in diameter between vessels in type 2 diabetes and controls (P < 0.001, Table 2), as well as a significant training × vessel interaction in type 2 diabetes (P = 0.023, Table 2). Subsequent post hoc testing revealed that training significantly increased BA diameter in type 2 diabetes, but not in controls (Table 2).
Wall thickness
Before exercise training, participants with type 2 diabetes demonstrated a larger artery wall thickness than controls in the SFA but not BA and CA. A two-way ANOVA in both groups revealed an effect of training on wall thickness in type 2 diabetes, which was comparable between arteries (Table 2), but no effect of training on wall thickness in controls. Furthermore, we found a difference in wall thickness between vessels in type 2 diabetes and controls (P < 0.001, Table 2).
W:L ratio
Before training, participants with type 2 diabetes demonstrated a significantly larger W:L ratio than controls in the SFA and BA, but not CA (Table 2). A two-way ANOVA in type 2 diabetes revealed that the effect of exercise training on W:L ratio differed between the 3 vessels (Table 2). Post hoc analysis showed a significant decrease in W:L ratio in the BA and SFA, but not in the CA in type 2 diabetes (Table 2). In controls, the two-way ANOVA revealed no effect of exercise training on conduit artery W:L ratio.
Bosentan
We performed a separate analysis to examine whether the effect of exercise training differed between type 2 diabetes patients with and without Bosentan. Separate two-way ANOVA revealed a significant decline in WT and wall:lumen ratio in the T2DM patients with Bosentan, which was similar across the three vessels (Table 3). In contrast, the training effect in the placebo group on the wall:lumen ratio and diameter differed between arteries (Table 3). Whilst no changes in CA and SFA were found after training in the subgroup on placebo, a decline in brachial artery wall:lumen ratio was found (Table 3).
Discussion
The aim of this study was to examine whether exercise training in type 2 diabetes leads to site-specific adaptations on wall thickness in peripheral arteries supplying active (i.e. SFA) versus inactive (i.e. BA) areas, and central (CA) vessels. Our results suggest that 8 weeks of combined aerobic (70–75 % of maximal heart rate) and resistance (3 series of 12 repetitions) lower limb-based exercise training are associated with a significant decrease in wall-to-lumen ratio in both the SFA and BA, but not in the CA. These findings suggest that this type of exercise training in type 2 diabetes leads to a site-specific adaptation in arterial wall-to-lumen ratio, with systemic remodelling of peripheral vessels supplying the active and inactive regions, but no adaptation of central arterial wall-to-lumen ratio.
To our knowledge, this study is the first to investigate the effect of exercise training on wall thickness in peripheral and central conduit arteries in type 2 diabetes patients. We found that 8 weeks of exercise training in type 2 diabetes is associated with a significant decrease in arterial wall thickness of peripheral arteries (i.e. SFA and BA). The ability of exercise training to decrease peripheral artery wall thickness is in line with some (Thijssen et al. 2011a, 2013; Green et al. 2010), but not all (Thijssen et al. 2007), previous studies which examined remodelling of peripheral conduit arteries after exercise training in healthy volunteers. An important observation in our study is that we found similar decreases in wall thickness of the BA and SFA, whilst subjects performed lower limb-dominant exercise training only. This, together with the increase in BA lumen diameter we observed, strongly supports the presence of systemic adaptations in wall thickness after exercise training in type 2 diabetes. Interestingly, a recent study from Maiorana et al. also reported a significant decrease in BA WT after a similar exercise training protocol in patients with heart failure. This suggests that exercise training, in healthy subjects and those with increased cardiovascular risk, has a systemic effect on peripheral conduit artery wall thickness (Maiorana et al. 2011).
We found no improvements in the CA after exercise training both in type 2 diabetes and in controls, which is in marked contrast to a recent exercise training study from Kadaglou. One potential explanation for this discrepancy is the absence of pre-training differences in CA WT between patients with type 2 diabetes and controls, as a higher pre-training CA WT would allow for a larger decline. For example, Kadaglou et al. (2013) found a decrease in CA WT after training in T2DM patients who demonstrate a much higher CA WT at baseline than in our study (±0.8 versus ±0.6 mm). Another explanation is the duration of the study, which was markedly longer in the study by Kadaglou et al. compared to our study. Furthermore, Kadaglou et al. only found an effect on CA WT after aerobic exercise training, and not after combined exercise training. Therefore, the type of exercise may importantly impact the effect on WT. Other previous cross-sectional and longitudinal studies in healthy controls and subjects with cardiovascular risk/disease found that exercise training is associated with a decrease in CA WT [see review (Thijssen et al. 2012)]. Also, studies that specifically examined type 2 diabetes patients (Kim et al. 2006; Watarai et al. 1999) reported an association between higher physical activity levels and smaller CA WT. This suggests that the ability of exercise training to decrease WT is not exclusively related to a priori thickening of the arterial wall. Future studies should look into specific differences between exercise training durations and modalities in type 2 diabetes.
Alternatively, the site-specific changes observed in our study may relate to a larger adaptive response of peripheral vessels for wall thickness compared to the carotid artery. In line with this, Moreau and colleagues found that exercise training in post-menopausal women was associated with a generally lower wall thickness of the SFA, but not the CA (Moreau et al. 2002). In a previous study in older men, we also found no effect of 8-week exercise training on CA WT (Thijssen et al. 2007). These site-specific adaptations may relate to the characteristics of the wall, contributing to distinct vascular adaptations to stimuli between arteries (Green et al. 2011; Thijssen et al. 2011c). Future studies should further examine the potential differences in adaptation between arteries, and especially the time it takes for central and peripheral arteries to adapt in response to exercise training. Taken together, our data endorse the concept that exercise training leads to systemic adaptation of conduit artery wall thickness in type 2 diabetes patients, but the effects of exercise training have a site-specific effect on peripheral versus central conduit arteries.
The finding of a site-specific adaptation in artery wall thickness renders the question regarding the mechanisms involved that relate to adaptation in artery wall thickness. When looking at functional as opposed to structural outcome measures, a large number of previous studies in animals (Langille et al. 1989; Langille and O’Donnell 1986) and humans (Tinken et al. 2010; Rowley et al. 2011) have identified shear stress as the key stimulus for localised adaptation in conduit arteries. For example, recent studies in humans found that adaptation in BA function as measured by flow-mediated dilation in response to 8-week exercise training (Birk et al. 2012; Tinken et al. 2010) or heat exposure (Naylor et al. 2011) is abolished in the contra-lateral arm when the increases in shear rate are mitigated by subdiastolic cuff inflation. Interestingly, when a similar design was used to examine the importance of the shear rate stimulus for adaptation in wall thickness, the abolished shear stimulus did not prevent the decreases in BA WT across 8 weeks of handgrip training (Thijssen et al. 2011a). This suggests that shear stress may not represent the principle stimulus for adaptations in wall thickness. Alternatively, systemic hemodynamic stimuli, such as cyclic transmural pressure changes, have been suggested to explain the impact of exercise training on WT (Newcomer et al. 2011). Our study provides further evidence for the importance of systemic stimuli to induce changes in wall thickness, as we found a decrease in BA WT after lower limb-dominant exercise training. Studies, largely performed in animals, found that such stimuli are associated with remodelling and anti-atherogenic effects [e.g. eNOS-expression/activity (Awolesi et al. 1995), EDHF-synthase expression (Quilley et al. 1997)]. Alternative systemic mechanisms may relate to novel markers, such as endothelial progenitor cells (EPCs) and miRNA (Thijssen et al. 2010). Other potential systemic, non-hemodynamic factors that may contribute to our findings relate to changes in muscle sympathetic nerve activity, vasoconstrictor tone, antioxidant level or inflammation [see review (Newcomer et al. 2011)].
Clinical relevance
Previous studies have suggested that arterial wall thickness represents a surrogate measure for (the development of) atherosclerosis. More specifically, several studies have demonstrated a strong and independent predictive capacity for the CA WT for future cardiovascular and cerebrovascular events (Lorenz et al. 2007). Not surprisingly, studies have also found predictive capacity of the SFA (Paul et al. 2005) and BA (Sorensen et al. 1997; Suessenbacher et al. 2013) WT for future development of cardiovascular events. Our findings, therefore, have important clinical relevance. The significant decline in peripheral artery wall thickness may contribute, at least partly, to the cardioprotective effects of exercise training in patients with type 2 diabetes.
Previous studies from our group (Thijssen et al. 2011a, 2013) have shown that 8 weeks of exercise training in young healthy controls resulted in decreases in WT, whilst the current study did not reproduce this finding in the control group. A possible explanation for this discrepancy could be that a higher physical fitness in the younger subjects in Thijssen’s study led to a higher absolute workload during the exercise bouts. The absolute lower workload in our study may not have been sufficient to elicit peripheral adaptations in controls. Also, as suggested by Maiorana et al. (2011), the type of exercise training may partly explain the discrepancies, as the previous studies employed continuous cycling (Thijssen et al. 2013) and handgrip (Thijssen et al. 2011a) exercise, as opposed to combined aerobic and strength training in the current study.
It is important to emphasise that some of the type 2 diabetes subjects ingested Bosentan. Before training we did not expect any impact of Bosentan on wall thickness and/or that Bosentan would alter the impact of exercise training on wall thickness in type 2 diabetes. However, a post hoc analysis between type 2 diabetes patients with and without Bosentan revealed a significant difference in the impact of exercise training on BA, SFA and CA wall thickness between type 2 diabetes patients with and without Bosentan. Interestingly, type 2 diabetes patients using Bosentan did not demonstrate the site-specific change in diameter and wall:lumen ratio as observed in the pooled data set or in the type 2 diabetes subjects using placebo (Table 3). This suggests that Bosentan induces a systemic change in diameter and wall:lumen ratio, possibly by influencing the vascular tone that, subsequently, can alter diameter and wall:lumen ratio (Thijssen et al. 2011b). This systemic effect is not present in the placebo group and disappears when combining both data sets. Taken together, Bosentan may have contributed somewhat to the effects of exercise training on diameter and wall:lumen ratio. However, since these effects are likely to be systemic, the use of Bosentan does not alter the main findings of our study, in that exercise training can lead to site-specific adaptations in wall:lumen ratio.
Limitations
The strengths of our study relate to the highly controlled exercise training, inclusion of a control group, within-subject comparisons, the use of semi-automated and validated analysis software and the blinded analysis of our data. A potential limitation of our study is the a priori differences between type 2 diabetes subjects and controls in physical fitness. The type 2 diabetes patients were less fit at baseline, meaning that the window of opportunity for improvement in this group was larger than in controls. Another possible limitation is that we did not record WT under complete relaxation, effectively eliminating the influence of resting tone on WT (Thijssen et al. 2011b). Our results may, therefore, relate to a lower vascular tone after training. However, observation of a similar WT in the carotid artery after exercise training suggests that (systemic) changes in vascular tone unlikely explain the main finding of our study. Another limitation is that even though subjects were instructed to minimise the gripping of handlebars, we cannot fully rule out the possibility that subjects were gripping handlebars during some of the resistance exercises. This may have impacted our results, especially the increase in BA diameter may relate to a higher activity level of upper limbs as anticipated (Rowley et al. 2011). A final limitation is the difference between groups concerning medication use, which is difficult to control for statistically or methodologically. Therefore, we cannot rule out the possibility that our results are influenced by the systemic effects of the medication interacting with exercise training in patients with type 2 diabetes mellitus.
In conclusion, we found that an 8-week exercise training program in patients with type 2 diabetes mellitus is associated with site-specific decreases in WT. As training involved lower limb-dominant exercise, the significant decrease in WT of both the SFA and BA suggests the presence of generalised adaptation in the peripheral arterial wall in arteries that supply active and non-active regions, respectively. However, the absence of changes in the CA suggests that the effects of exercise training on WT differ between peripheral and central vessels. Whether this site-specific difference relates to the a priori differences in WT between groups or to site-specific sensitivity in adaptation remains to be elucidated. Based on the prognostic information of conduit artery WT for future cerebro- and cardiovascular events, the site-specific decreases in arterial WT may contribute to the beneficial impacts of exercise training on cerebro-and cardiovascular events in type 2 diabetes.
Abbreviations
- BA:
-
Brachial artery
- CA:
-
Carotid artery
- CV:
-
Coefficient of variation
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SE:
-
Standard error
- SFA:
-
Superficial femoral artery
References
Awolesi MA, Sessa WC, Sumpio BE (1995) Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Investig 96(3):1449–1454. doi:10.1172/JCI118181
Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, American Heart Association Exercise CR, Prevention Committee of the Council on Clinical Cardiology, Council on Epidemiology Prevention, Council on Peripheral Vascular Disease, Interdisciplinary Council on Quality of C, Outcomes Research (2010) Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122(2):191–225. doi:10.1161/CIR.0b013e3181e52e69
Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ (2012) Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol 112(10):1653–1658. doi:10.1152/japplphysiol.01489.2011
Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN (2004) Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 27(1):83–88
de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, Kastelein JJ (2004) Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation 109(23 Suppl 1):III33–III38. doi:10.1161/01.CIR.0000131516.65699.ba
Green DJ, O’Driscoll G, Joyner MJ, Cable NT (2008) Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 105(2):766–768. doi:10.1152/japplphysiol.01028.2007
Green DJ, Swart A, Exterkate A, Naylor LH, Black MA, Cable NT, Thijssen DH (2010) Impact of age, sex and exercise on brachial and popliteal artery remodelling in humans. Atherosclerosis 210(2):525–530. doi:10.1016/j.atherosclerosis.2010.01.048
Green DJ, Spence A, Halliwill JR, Cable NT, Thijssen DH (2011) Exercise and vascular adaptation in asymptomatic humans. Exp Physiol 96(2):57–70. doi:10.1113/expphysiol.2009.048694
Jae SY, Heffernan K, Fernhall B, Choi YH (2012) Cardiorespiratory fitness and carotid artery intima media thickness in men with type 2 diabetes. J Phys Act Health 9(4):549–553
Joyner MJ, Green DJ (2009) Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587(Pt 23):5551–5558. doi:10.1113/jphysiol.2009.179432
Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS (2013) The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med 30(2):e41–e50. doi:10.1111/dme.12055
Kim SH, Lee SJ, Kang ES, Kang S, Hur KY, Lee HJ, Ahn CW, Cha BS, Yoo JS, Lee HC (2006) Effects of lifestyle modification on metabolic parameters and carotid intima-media thickness in patients with type 2 diabetes mellitus. Metab Clin Exp 55(8):1053–1059. doi:10.1016/j.metabol.2006.03.017
Langille BL, O’Donnell F (1986) Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231(4736):405–407
Langille BL, Bendeck MP, Keeley FW (1989) Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol 256(4 Pt 2):H931–H939
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115(4):459–467. doi:10.1161/CIRCULATIONAHA.106.628875
Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D (2001) The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38(3):860–866
Maiorana A, O’Driscoll G, Goodman C, Taylor R, Green D (2002) Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract 56(2):115–123
Maiorana AJ, Naylor LH, Exterkate A, Swart A, Thijssen DH, Lam K, O’Driscoll G, Green DJ (2011) The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension 57(1):56–62. doi:10.1161/HYPERTENSIONAHA.110.163022
Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, Blumenthal RS, Philippides G, Rocchini A, Council on Clinical Cardiology AHAECR, Prevention Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Nutrition PA, Metabolism, Interdisciplinary Council on Quality of Care, Outcomes Research (2009) Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119(25):3244–3262. doi:10.1161/CIRCULATIONAHA.109.192521
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Metcalf PA, Folsom AR, Davis CE, Wu KK, Heiss G (2000) Haemostasis and carotid artery wall thickness in non-insulin dependent diabetes mellitus. Diabetes Res Clin Pract 47(1):25–35
Monfredi O, Griffiths L, Clarke B, Mahadevan VS (2011) Efficacy and safety of bosentan for pulmonary arterial hypertension in adults with congenital heart disease. Am J Cardiol 108(10):1483–1488. doi:10.1016/j.amjcard.2011.07.006
Moreau KL, Donato AJ, Seals DR, Dinenno FA, Blackett SD, Hoetzer GL, Desouza CA, Tanaka H (2002) Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol 283(4):H1409–H1417. doi:10.1152/ajpheart.00035.2002
Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ (2011) Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300(2):H664–H669. doi:10.1152/ajpheart.00985.2010
Newcomer SC, Thijssen DH, Green DJ (2011) Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. J Appl Physiol 111(1):311–320. doi:10.1152/japplphysiol.00033.2011
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr (1999) Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 340(1):14–22. doi:10.1056/NEJM199901073400103
Paul TK, Srinivasan SR, Chen W, Li S, Bond MG, Tang R, Berenson GS (2005) Impact of multiple cardiovascular risk factors on femoral artery intima-media thickness in asymptomatic young adults (the Bogalusa Heart Study). Am J Cardiol 95(4):469–473. doi:10.1016/j.amjcard.2004.10.013
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316(7139):1236–1238
Potter K, Green DJ, Reed CJ, Woodman RJ, Watts GF, McQuillan BM, Burke V, Hankey GJ, Arnolda LF (2007) Carotid intima-medial thickness measured on multiple ultrasound frames: evaluation of a DICOM-based software system. Cardiovasc Ultrasound 5:29. doi:10.1186/1476-7120-5-29
Potter K, Reed CJ, Green DJ, Hankey GJ, Arnolda LF (2008) Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound 6:6. doi:10.1186/1476-7120-6-6
Quilley J, Fulton D, McGiff JC (1997) Hyperpolarizing factors. Biochem Pharmacol 54(10):1059–1070
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1:43–46
Rowley NJ, Dawson EA, Birk GK, Cable NT, George K, Whyte G, Thijssen DH, Green DJ (2011) Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol 110(5):1190–1195. doi:10.1152/japplphysiol.01371.2010
Rowley NJ, Dawson EA, Hopman MT, George K, Whyte GP, Thijssen DH, Green DJ (2012) Conduit diameter and wall remodelling in elite athletes and spinal cord injury. Med Sci Sports Exerc 44(5):844–849. doi:10.1249/MSS.0b013e31823f6887
Sorensen KE, Kristensen IB, Celermajer DS (1997) Atherosclerosis in the human brachial artery. J Am Coll Cardiol 29(2):318–322
Suessenbacher A, Dorler J, Wunder J, Hohenwarter F, Alber HF, Pachinger O, Frick M (2013) Comparison of brachial artery wall thickness versus endothelial function to predict late cardiovascular events in patients undergoing elective coronary angiography. Am J Cardiol 111(5):671–675
Thijssen DH, de Groot PC, Smits P, Hopman MT (2007) Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 190(3):221–228. doi:10.1111/j.1748-1716.2007.01685.x
Thijssen DH, Maiorana AJ, O’Driscoll G, Cable NT, Hopman MT, Green DJ (2010) Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108(5):845–875. doi:10.1007/s00421-009-1260-x
Thijssen DH, Dawson EA, van den Munckhof IC, Tinken TM, den Drijver E, Hopkins N, Cable NT, Green DJ (2011a) Exercise-mediated changes in conduit artery wall thickness in humans: role of shear stress. Am J Physiol Heart Circ Physiol 301(1):H241–H246. doi:10.1152/ajpheart.00170.2011
Thijssen DH, Scholten RR, van den Munckhof IC, Benda N, Green DJ, Hopman MT (2011b) Acute change in vascular tone alters intima-media thickness. Hypertension 58(2):240–246. doi:10.1161/HYPERTENSIONAHA.111.173583
Thijssen DH, Willems L, van den Munckhof I, Scholten R, Hopman MT, Dawson EA, Atkinson G, Cable NT, Green DJ (2011c) Impact of wall thickness on conduit artery function in humans: is there a “Folkow” effect? Atherosclerosis 217(2):415–419
Thijssen DH, Cable NT, Green DJ (2012) Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond) 122(7):311–322. doi:10.1042/CS20110469
Thijssen DH, Dawson EA, van den Munckhof I, Birk GK, Cable NT, Green DJ (2013) Local and systemic effects of leg cycling training on arterial wall thickness in healthy humans. Atherosclerosis 229(2):282–286
Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ (2010) Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55(2):312–318. doi:10.1161/HYPERTENSIONAHA.109.146282
Wagenknecht LE, D’Agostino R Jr, Savage PJ, O’Leary DH, Saad MF, Haffner SM (1997) Duration of diabetes and carotid wall thickness. The Insulin Resistance Atherosclerosis Study (IRAS). Stroke 28(5):999–1005
Wagenknecht LE, D’Agostino RB Jr, Haffner SM, Savage PJ, Rewers M (1998) Impaired glucose tolerance, type 2 diabetes, and carotid wall thickness: the Insulin Resistance Atherosclerosis Study. Diabetes Care 21(11):1812–1818
Watarai T, Yamasaki Y, Ikeda M, Kubota M, Kodama M, Tsujino T, Kishimoto M, Kawamori R, Hori M (1999) Insulin resistance contributes to carotid arterial wall thickness in patients with non-insulin-dependent-diabetes mellitus. Endocr J 46(5):629–638
Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O’Driscoll G, Green DJ (2004) Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol 43(10):1823–1827. doi:10.1016/j.jacc.2004.01.032
Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN (2000) Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132(8):605–611
Xiang GD, Wang YL (2004) Regular aerobic exercise training improves endothelium-dependent arterial dilation in patients with impaired fasting glucose. Diabetes Care 27(3):801–802
Acknowledgments
We acknowledge the help of David de Munnik (Department of Physiology, Radboud University Nijmegen Medical Centre, The Netherlands) with the analysis of the data and supervision of the training sessions. DHJT is recipient of the E. Dekker stipend (Netherlands Heart Foundation, 2009T064) and the Actelion Endothelin Research Award (2009 046).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Massimo Pagani.
Rights and permissions
About this article
Cite this article
Schreuder, T.H.A., Van Den Munckhof, I., Poelkens, F. et al. Combined aerobic and resistance exercise training decreases peripheral but not central artery wall thickness in subjects with type 2 diabetes. Eur J Appl Physiol 115, 317–326 (2015). https://doi.org/10.1007/s00421-014-3016-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-014-3016-5