Abstract
Purpose
The health risk of chronic exposure to radionuclides includes changes in the genome (e.g., chromosomal aberrations and micronuclei) that increase chromosomal instability. There are also other phenomena, which seem to appear more frequently in metaphases of exposed persons (such as premature centromere division). The aim of this study was to discover whether or not there is correlation between incidence of named cytogenetic changes in persons occupationally exposed to radionuclides in comparison with unexposed control group, and if significant correlation is determined, can premature centromere division be consider as a biomarker of radiation exposure?
Methods
The exposed group comprised 50 individuals occupationally exposed to radionuclides. The reference control group consisted of 40 unexposed individuals. Chromosomal aberrations, micronuclei and premature centromere division were analyzed according to a standard International Atomic Energy Agency protocol. Statistical analyses were performed using SPSS 17.0 statistics.
Results
The means for analyzed cytogenetic changes were significantly higher in the exposed group. Positive correlation between them was found in exposed group. Premature centromere division parameter PCD5-10 was selected as particularly suitable for separating groups (exposed/unexposed).
Conclusions
Identification of other phenomena related to radionuclide exposure, beside well known, may clarify recent problems in radiobiology concerning the biological response to low doses of ionizing radiation and its consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The health risk of chronic occupational exposure to low doses of ionizing radiation (IR) includes changes in the genome that increase chromosomal instability as one of the main forces driving the onset and progression of carcinogenesis (Hoeijmakers 2001; Miller et al. 2008; Morgan 2003; Mothersill and Seymour 2004; Ullrich and Ponnaiya 1998). Genome instability induced by low doses of radiation manifests as changes, such as aneuploidy, micronuclei, chromosomal aberrations, oxidative stress and increased apoptosis incidence (Miller et al. 2008; Engin et al. 2005). Several cytogenetic studies performed on peripheral blood lymphocytes (PBL) from hospital workers exposed to low doses of IR during the course of their employment revealed an enhanced baseline of chromosomal aberrations (CAs) as well as micronuclei (MNs) compared to control populations (Ballardin et al. 2007; Barquinero et al. 1993; Bigatti et al. 1988; Bonassi et al. 1997; Bouraoui et al. 2013; Jovičić et al. 2009; Maffei et al. 2002; Ropolo et al. 2012; Saberi et al. 2013; Sari-Minodier et al. 2007; Thierens et al. 2000; Touil et al. 2002).
Besides them, there are other phenomena visible on cytogenetic level, which seem to appear more frequently in metaphases of persons exposed to IR than in unexposed groups. One of them, certainly, is premature centromere division (PCD) (Jovičić et al. 2010), discovered in peripheral lymphocytes, characterized by chromatids already separated in metaphase of the cell cycle. Centromere dysfunction presented by premature centromere splitting is caused by disorder in spatial and temporal regulation of mitosis, which finally leads to aneuploidy and genomic instability (Litmanovic et al. 1998; Vig and Wodnicki 1974). This phenomenon was predominantly studied as consequence of exposure to genotoxic chemicals (Major et al. 1999).
However, the prevalence of PCD in persons occupationally exposed to ionizing radiation has not been studied thoroughly. Among exposed workers, those who perform nuclear medicine and interventional procedures may receive higher doses and are the subjects of concern (Sari-Minodier et al. 2007).
Regarding all the aforementioned, the aim of this study was to examine the relationship between PCD prevalence in exposed and control group and then to discover whether or not there is correlation between the prevalence of CAs, MN with the prevalence of PCDs in persons occupationally exposed to IR, in nuclear medicine department.
If there is significant correlation between those three phenomena in the exposed subjects, can we then consider PCD as a biomarker of radiation exposure and what are the cutoff values of PCD parameters that are sensitive and specific enough to classify an examined subject to the exposed or unexposed group?
Materials and methods
Subjects
The exposed group comprised 50 individuals (29 females and 21 males; average age 45.24 years, age range 27–59 years) occupationally exposed (daily continual exposure ranged from 5 to 35 years) to radionuclides (Y90 and I131). The reference group consisted of 40 individuals (20 females and 20 males; average age 44.40 years, age range, 34–60 years) who had never been occupationally exposed to IR or chemical mutagens in their workplaces. Among exposed persons, 26 subjects (12 females and 14 males) were regular smokers—smoking between 15 and 20 cigarettes per day, up to 20 years consecutively (16.14 years average)—and 24 were not (17 female and seven male subjects). In the control group, 17 subjects (eight females and nine males) were regular smokers—smoking between 15 and 20 cigarettes per day, up to 20 years consecutively (14.28 years average)—and 23 were not (12 female and 11 male subjects). No subject reported medicinal treatment (including exposure to diagnostic X-rays) over 6 months before blood sampling. The characteristics of the study population regarding gender (male or female), age, working experience (WE), occupational exposure time (DOE—duration of occupational exposure in years), smoking habit, use of protective measurements and effective doses (external exposure) for the preceding and last 5 years in mSv (ED1 and ED5, respectively) are listed in Table 1.
Personal dosimeters were used for estimation of the external exposure. The amounts of IR absorbed by the subjects’ bodies were measured with thermo-luminescent dosimeters (TLD) once a month during occupational exposure time and expressed in mSv as the mean of annual effective doses for the period of exposure. Routine personal dosimetry was performed using an automatic TLD reader (Harsaw Model 6600 with LiF:Mg Ti cards; Thermo Scientific, 81 Wyman Street, Waltham, MA 02454). The glow curve was used for sophisticated data processing with Harsaw CGCD (computerized glow curve deconvolution) software (German et al. 2000).
The controls matched the exposed subjects in gender, age and smoking habits.
Detailed information on the occupational and medical history of all examined subjects was obtained by completing a targeted questionnaire. This included demographic data, smoking, alcohol intake and use of medication, use of protective measurements and duration of exposure to radiation or chemicals. Only subjects who had not been exposed to any mutagen except for IR (exposed group) or any mutagen at all (control group) were included in the analysis. The participants signed written informed consent, and study protocol was reviewed and approved by the Serbian Institute of Occupational Health (SIOH) ethical committee. Examinations were performed in accordance with the ethical standards of Helsinki Declaration of 1975 as revised in 2013.
Chromosomal aberrations, premature centromere division and micronuclei
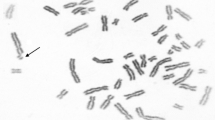
Chromosomal aberrations and PCD in PBL were analyzed according to a standard protocol (IAEA 2001). Lymphocyte cultures were prepared by adding whole blood to PB-MAX™ Karyotyping Medium (Gibco). Lymphocytes were incubated in vitro for 48 h at 37 °C. During the last 2 h of incubation, colchicine (0.05 μg/ml; Sigma-Aldrich, 3050 Spruce St., St. Louis, MO 63103) was added to the medium. The cells were exposed to hypotonic solution (20 min) by stepwise addition of 0.075 M KCl followed by fixation (3 × 20 min) with cold methanol/acetic acid (3:1). Fixed cells were spread on slides and dried over a flame. The slides were aged for the next 5–7 days. Giemsa-stained slides were coded and scored blind under a light microscope.
For the cytokinesis block micronucleus (CBMN) test, lymphocytes were cultivated the same way as for classical cytogenetic analysis (IAEA 2001). After incubation for 44 h, 0.1 ml of cytochalasin B (Sigma-Aldrich, 3050 Spruce St., St. Louis, MO 63103) solution in DMSO (Sigma-Aldrich, 3050 Spruce St., St. Louis, MO 63103) (final concentration of 3 μg/ml) was added and cultivation continued for another 24 h. The cultures were then treated with 0.9 % NaCl solution, followed by cold hypotonic solution to lyse red blood cells. The supernatant was removed and replaced with fixative, consisting of methanol/acetic acid (3:1) with 1 % formaldehyde. After incubation for 20 min at room temperature, the cells were washed with four changes in fixative without formaldehyde. The cells were then resuspended gently and the suspension dropped onto clean glass slides and allowed to dry. Cells were stained using 10 % Giemsa in distilled water.
Cytogenetic analysis
Chromosomal aberration parameters: chromatid (CB) and isochromatid (iCB) breaks, acentrics (ACE), dicentrics (DIC) and ring chromosomes, as well as three parameters of PCDs, were evaluated for at least 200 metaphase cells, while five parameters of micronuclei (MN) were evaluated for at least 1000 binucleated cells. PCD was diagnosed when separation between sister chromatids was equal to or more than the thickness of the chromatid (Madan et al. 1987; Ruskovsky et al. 2003). Three PCD parameters were included in the analysis: tPCD (number of metaphases per individual with 11 or more chromosomes with separated chromatids), PCD1–5 (number of metaphases per individual with 1–5 chromosomes with PCD) and PCD5–10 (number of metaphases per individual with 6–10 chromosomes with PCD). Five MN parameters were included in the analysis: total number micronuclei (ΣMN), frequency of binuclear (BN) cells with one, two, three and four MN (MN1, MN2, MN3 and MN4, respectively).
Statistical analysis
The incidence was estimated for several predictor variables including IR exposure (control group vs. exposed group), gender (men vs. women), smoking habit (smokers vs. non-smokers), age and working experience (WE). The control and exposed groups were coded as “control” and “exposed,” respectively, gender qualifications were coded as “m” for male and “f” for female, and smoking habits were coded as binary (0 or 1). Age, WE and effective doses were used as continuous predictor variables.
The data for CAs, PCDs and MNi were tested for normal dispersion. Parameters PCD5–10, ΣMN and MN1 fitted a normal distribution curve (p > 0.05). The frequencies of total aberrant cells (tAC), DIC, ring chromosomes, ACE, CB and iCB, tPCD, PCD1–5, MN2, MN3 and MN4 showed significant differences from normal distribution (p < 0.05).
A pretest—independent sample t test—was performed to determine the possible influence of gender and smoking habit on numeric variables. Then, the effects of those nonparametric predictor variables (IR exposure, gender qualification and smoking habit) on differences in incidence of CAs, PCDs and MNi were tested by an independent sample t test for parameters with normal distribution and the Mann–Whitney U test for parameters with nonparametric distribution.
Correlations between continuous predictor variables (age, WE, ED1 and ED5) and CAs, PCDs and MNi parameters were calculated as Pearson’s correlations for variables with normal distribution and Spearman rank correlations for nonparametric variables.
Multinomial logistic regression was performed in order to predict affiliation to the control or exposed group for all numerical variables (tAC, ACE, DIC, CB, iCB, total PCD, PCD1–5, PCD5–10, ΣMN, MN1, MN2, MN3 and MN4) as predictor variables. The criterion for classification to the control or exposed group was the Wald coefficient value and its significance. A probability p < 0.05 was considered statistically significant in each analysis.
Diagnostic qualities of applied cytogenetic tests were analyzed by determining contingency test parameters (sensitivity, specificity and cutoff value), while graphical analysis is presented by a receiver operating characteristic (ROC) curve, used in medicine to determine cutoff values for clinical/diagnostic tests, and ROC space, known as AUC (area under curve), as an important measure of the accuracy of the clinical test. Uncertainties for all measures of accuracy (including AUC) were expressed as 95 % confidence limits. The analyses were performed using SPSS 17.0 statistics (SPSS Inc.).
Results
A total of 18,000 metaphase cells were scored for CAs and PCDs: 8000 in the control group and 10,000 in the exposed group (200 per individual). A total of 90,000 binucleated cells were analyzed for MN by the CBMN test: 40,000 in the control group and 50,000 in the exposed group (1000 per individual). The results are presented in Table 2. In comparison with the control group, incidence of cells with aberrations/micronuclei was increased by twofold for PCD1–5, ΣMN and CBs, by threefold for iCBs and PCD5–10, by fivefold for DICs, by sevenfold for ACE and by 8.5-fold for tPCD. No cells with ring chromosomes were found in the control group, but in the exposed group, the ratio was 1 cell with a ring per 2500 cells.
The means for CAs, PCDs and MNi were significantly higher in the exposed group, as illustrated in Fig. 1, which gives the values of all CAs and PCDs per 200 analyzed metaphases of each examined individual.
Gender, age and smoking habit were without influence on numerical variables, in both control and exposed groups, except for parameters of CBMN assay, which mean values were higher in females, smokers and subjects older than 45 years.
In order to test PCDs as a new genotoxic endpoint, correlations between CAs, MNi and PCDs were examined with the Spearman rank test on the whole sample. There were positive linear correlations between CAs and PCDs for the DIC–tPCD, DIC–PCD5–10, ACE–tPCD, ACE–PCD1–5, CB–tPCD and CB–PCD5–10 variable pairs, which indicated that an increase in incidence of one variable was coupled with an increase in incidence of the other. For PCDs and MNi, the only correlation was between MN4 and tPCD.
Duration of occupational exposure was positively correlated with total number of aberrant cells (tAC) and acentric fragments (ACE)—regarding parameters of CAs and total number of micronuclei ƩMN and parameters of micronuclei distribution MN1 and MN2. Furthermore, positive correlation was found between duration of occupational exposure (DOE) and all PCD parameters. However, this was not the case for physical dosimetry data (ED1 and ED5) and PCDs, or other analyzed cytogenetic parameters (CAs and MNi). Table 3 presents significant correlations for all listed parameters and for the exposed group only.
A logistic regression test was performed in order to predict affiliation of subjects to the control or exposed group for all numerical variables (tACs, ACE, DIC, CB, iCB, total PCD, PCD1–5, PCD5–10, ΣMN, MN1, MN2, MN3 and MN4) as predictor variables. The criterion for classification to the control or exposed group was a Wald coefficient value higher than 1.
ROC analysis was applied in order to investigate ability of all analyzed (particularly PCD) parameters to separate groups, since it is used in medicine as a fundamental tool for diagnostic test evaluation, by determination a cutoff value for a clinical/diagnostic test. Among the listed variables, PCD5–10 was found to be the best. From ROC analysis optimum value of 3, 50 cells (per 200 cells examined), with 6–10 chromosomes with PCD, as the cutoff value was selected. The sensitivity of this value is 75.0 %, and the specificity (the ability to separate exposed from unexposed individuals) is 87.5 %. The area under the curve (AUC) is 90.8 % for this value. Figure 2 shows the ROC analysis for PCD5–10, while in Fig. 3, ROC analysis for three parameters with the highest Wald coefficient values (PCD5–10, tAC and ΣMN) can be seen.
Discussion
Occupational exposure is an especially delicate area of research, where effects of chronic exposure to low doses play an important role in consideration of the biological response to IR (Hoeijmakers 2001; Jovičić et al. 2009; Miller et al. 2008; Mothersill and Seymour 2004). It is therefore, always useful to investigate new biomarkers visible on the first, cytogenetic level, which can indicate effect of genotoxic exposure. Information on radiation effects can be considered in the context of cancer risk (Preston 2005), since there is recent evidence indicating that low doses of radiation exert both suppression and induction of neoplastic transformation (Ko et al. 2006).
The results presented in this paper demonstrate the significantly higher incidence of all cytogenetically visible parameters: CAs, PCDs and MNi in individuals exposed to low radiation doses of radionuclides (ranged from 0 to approximately 10 mSv per year) than in unexposed persons. Different authors have also reported increments of CA and MN incidence in health workers occupationally exposed to IR (Ballardin et al. 2007; Barquinero et al. 1993; Bigatti et al. 1988; Bonassi et al. 1997; Bouraoui et al. 2013; Jovičić et al. 2009; Maffei et al. 2002; Ropolo et al. 2012; Saberi et al. 2013; Sari-Minodier et al. 2007; Thierens et al. 2000; Touil et al. 2002). Similar observations, confirmed by application of FISH procedure, revealed a significant difference (p < 0.001) in incidence between the two groups (exposed and controls) for PCDs as well as for CAs (Jovičić et al. 2010).
Our results also revealed no difference between genders, age and smoking status for the CAs and PCDs. This finding could be expected for at least some of the analyzed CAs (dicentrics and rings) since their formation is caused almost exclusively by ionizing radiation (Ainsbury et al. 2011; Rozgaj et al. 2002). Cytogenetic study of healthy unexposed persons also reported that age and gender were not significant predictors of chromosomal aberrations (Stephan and Pressl 1999). Regarding PCD, authors (Vig et al. 1989) related PCD of acrocentric chromosomes and aneuploidy of some chromosomes and showed that a gender- and age-dependent increase in PCD rates of spontaneous aneuploidy of the X chromosome was correlated with PCD (Spremo-Potparevic et al. 2004). All CBMN parameters were significantly higher in females, smokers and subjects older than 45 years. It is well known that the micronucleus prevalence is affected by age, gender, diet, smoking and other lifestyle factors (Antunes et al. 2014; Fenech and Bonassi 2011; Nefic and Handzic 2013).
Correlation analysis revealed positive correlations between CA and PCD parameters as well as some of the parameters characterizing MNi and PCDs in the exposed group. Similarly to the results presented here, some other researchers (Jovičić et al. 2010; Major et al. 1999) reported PCD in individual chromosomes in subjects that already had CAs in their metaphases, and they suggested that the two phenomena (CA and PCD) are not mutually exclusive or dependent on each other. Duration of exposure (DOE) and PCD occurrence were also positively correlated. Positive correlation was also found between DOE and total number of aberrant cells (tAC) and acentric fragments (ACE)—regarding parameters of CAs and total number of micronuclei ƩMN and parameters of micronuclei distribution MN1 and MN2. This finding suggests that several genotoxic actions are probably more likely to induce both well-established cytogenetic changes and PCD than short-term exposures. However, no correlation was established between physical dosimetry data (expressed by ED1 and ED5 in the exposed group) and PCD, or other analyzed cytogenetic parameters (CAs and MNi). These data refer only to external exposure, while the examined subjects were exposed to radionuclides, which could have entered the body, and therefore, the biological endpoints, found at a significantly higher level in PBL of exposed subjects in comparison with unexposed ones, were better markers of exposure. So, our finding of a lack of correlation between physical and biological dosimetry data may be explained by possible exposure to amounts of radionuclides small enough not to be recorded by personal TLDs, but genotoxic enough to cause changes in genetic material that can be seen at the cytogenetic level. The present data also suggest that personal protection is not at a satisfactory level and adequate personal protection could prevent PCD induction (Major et al. 1999). Since little attention has been devoted to studying PCD phenomenon, the question could be raised whether PCD may be considered as an artifact, or could be regarded as biological expression of the cellular response to the action of genotoxic agents, such as IR. Bühler et al. (1987) suggested that the PCD phenomenon was not an accidental finding. If this is the case, the incidence of PCDs should be different in the control and the exposed group. The results of our study confirmed the previous statement. PCD incidence was significantly lower in the control group than in the exposed group. Furthermore, the applied ROC analysis clearly marked one of the three PCD parameters, PCD5–10, as the best for separation of exposed from unexposed persons.
Our results as well as results of other authors (Major et al. 1999) showed significant influence of exposure to various genotoxic agents (IR and genotoxic chemicals) on premature dividing of centromeres. These findings could be explained by the DNA structure of human centromeric regions and its influence on the timing of centromeric separation. According to research of Shueller et al. (2001) as well as some earlier findings (Way and Willard 1987; Willard 1985), human centromeric regions are rich in repetitive sequences. It has been proposed that centromere separation is a function of the timing of replication of the centromere, which splits into two units, presumably after undergoing some sort of maturation. This suggestion is supported by the fact that the mammalian centromeres replicate during late S phase (Broccoli et al. 1989) but do not separate into two units until late metaphase. Mutations in this satellite region due to action of genotoxic agent, such as IR, can cause gain or loss of entire repeat units and slippage during replication (Sankaranarayanan et al. 2013). This could alter replication time of centromeres and cause early replication and consequently early separation of centromeric regions seen in already metaphase of the cell cycle (Vig 1984; Vig and Zinkowski 1986). Higher prevalence of metaphases with split centromeres can also arise as the result of molecular checkpoint defects in the cell cycle influenced by highly potent mutagenic agents, such as IR (Gollin 2005; Matsuura et al. 2000; Michor et al. 2005; Morgan et al. 1995; Resta et al. 2006).
Conclusion
Most human radiation exposure nowadays involves chronic or highly fractionated doses below 1 Gy and occurs most often in occupational settings. The presence of an unstable CA is the best criterion of health consequences and radiation damage (Jovičić et al. 2009), and standardized techniques, such as CA analysis and the CBMN test, are often used in dose assessment. However, detection and identification of other phenomena related to IR exposure may clarify recent problems in radiobiology concerning the biological response to low doses of IR and its consequences. One of these phenomena is certainly premature centromere division.
Since PCD may be observed as a phenomenon representing the manifestation of chromosomal instability in the exposed persons, our study led us to consider PCD as a possible parameter of genotoxic risk for individuals occupationally exposed to low doses of IR.
Studies of PCD could allow better insight into molecular events underlying chromosomal instability considering that cell cycle progression requires control mechanisms which could be associated with the origin of PCD.
The results presented here were obtained from preliminary studies on a relatively small sample by applying only conventional cytogenetic techniques. However, further studies that will include a larger number of subjects, application of different molecular techniques and in vitro investigations should be performed in order to understand better the described phenomenon and its association with established biomarkers of radiation exposure.
References
Ainsbury EA, Bakhanova E, Barquinero JF, Brai M, Chumak V, Correcher V et al (2011) Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat Prot Dosim 147(4):573–592
Antunes AC, Martins V, Cardoso J, Santos L, Monteiro Gil O (2014) The cytokinesis-blocked micronucleus assay: dose estimation and inter-individual differences in the response to γ radiation. Mutat Res 760:17–22
Ballardin M, Antonelli A, Cipollini M, Fallahi P, Scarpato R, Tomei A et al (2007) Induction of chromatid-type aberrations in peripheral lymphocytes of hospital workers exposed to very low doses of radiation. Mutat Res 626(1–2):61–68
Barquinero JF, Barrios L, Caballin MR, Miro R, Subias A, Egozeue J (1993) Cytogenetic analyses of lumphocytes from hospital workers occupationally exposed to low levels of ionizing radiation. Mutat Res 286(2):275–279
Bigatti P, Lamberti L, Ardito G, Armellino F (1988) Cytogenetic monitoring of hospital workers exposed to low-level ionizing radiation. Mutat Res 204(2):343–347
Bonassi S, Forni A, Bigatti P, Canevarolo N, De Ferrari M, Lando C et al (1997) Chromosome aberrations in hospital workers: evidence from surveillance studies in Italy (1963–1993). Am J Ind Med 31(3):353–360
Bouraoui S, Mougou S, Drira A, Tabka F, Bouali N, Mrizek N et al (2013) A cytogenetic approach to the effects of low levels of ionizing radiation (IR) on the exposed Tunisian hospital workers. Int J Occup Med Environ Health 26:144–154
Broccoli D, Paweletz N, Vig BK (1989) Sequence of centromere separation: characterization of multicentric chromosomes in a rat cell line. Chromosoma 98(1):13–22
Bühler EM, Fessier R, Beutler C, Gargano G (1987) Incidental finding of double minutes (DM), single minutes (SM), homogenously staining regions (HSR), premature chromosome condensation (PCC) and premature centromere division (PCD)? Ann Genet (Paris) 30:75–79
Engin AB, Ergum MA, Yurtcu E, Kan D, Sahin G (2005) Effect of ionizing radiation on the pteridine metabolic pathways and evaluation of its cytotoxicity in exposed hospital staff. Mutat Res 585:184–192
Fenech M, Bonassi S (2011) The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 26(1):43–49
German U, Ben-Shachar B, Weinstein M (2000) Twenty five years of TL-dose measurements at the NRC-NEGEV. In: Proceedings of IRPA-10; Hiroshima, Japan: International Radiation Protection Assocation: CD ROM paper p3b–168
Gollin SM (2005) Mechanisms leading to chromosomal instability. Semin Canc Biol 15:33–42
Hoeijmakers JHJ (2001) Genome maintenance mechanisms for preventing cancer. Nature 411:366–374. doi:10.1038/35077232
International Atomic Energy Agency (IAEA) (2001) Cytogenetic analysis for radiation dose assessment. International Atomic Agency Technical Report Series 405. Vienna: IAEA
Jovičić D, Milačić S, Milić N, Bukvić N, Vukov DT (2009) Chromosomal aberrations in subjects exposed to ionizing radiation. J Environ Pathol Toxicol Oncol 28:75–82
Jovičić D, Milačić S, Vukov TD, Rakić B, Stevanović M, Drakulić D et al (2010) Detection of premature segregation of centromeres in persons exposed to ionizing radiation. Health Phys 98:717–727
Ko M, Lao XY, Kapadia R, Elmore E, Redpath JL (2006) Neoplastic transformation in vitro by doses of ionizing radiation: role of adaptive response and bystander effects. Mutat Res 597:11–17
Litmanovic T, Altras MM, Dotana A, Avivi L (1998) Asynchronous replication of homologous alfa-satellite DNA loci in man is associated with nondisjunction. Cytogen Cell Genet 81:26–35
Madan K, Lindhout D, Palan A (1987) Premature centromere division (PCD): a dominantly inherited cytogenetic anomaly. Hum Genet 77:193–196
Maffei F, Angelini S, Forti GC, Lodi V, Violante FS, Mattioli S et al (2002) Micronuclei frequencies in hospital workers occupationaly exposed to low levels of ionizing radiation: influence of smoking status and other factors. Mutagenesis 17(5):405–409
Major J, Jakab MG, Tompa A (1999) The frequency of induced premature centromere division in human populations occupationally exposed to genotoxic chemicals. Mutat Res 445:241–249
Matsuura S, Ito E, Tauchi H, Kenshi K, Ikeuchi T, Tadashi K (2000) Chromosomal instability syndrome of total premature chromatid separation with mosaic variegated aneuploidy is defective in mitotic-spindle checkpoint. Am J Hum Genet 67:483–486
Michor F, Iwasa Y, Vogelstein B, Lengauer C, Nowak MA (2005) Can chromosomal instability initiate tumorigenesis? Rev Semin Canc Biol 15:43–49
Miller JH, Jin S, Morgan WF, Yang A, Wan Y, Aypar U et al (2008) Profiling mitochondrial proteins in radiation-induced genome-unstable cell lines with persistent oxidative stress by mass spectrometry. Radiat Res 169(6):700–706. doi:10.1667/RR1186.1
Morgan WF (2003) Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res 159(5):567–580
Morgan WF, Day JD, Kaplan MI, McGhee EM, Limoli CL (1995) Genomic instability induced by ionizing radiation. Radiat Res 146:247–258
Mothersill C, Seymour CB (2004) Radiation-induced bystander effects-implications for cancer. Nat Rev Cancer 4(2):158–164
Nefic H, Handzic I (2013) The effect of age, gender, and lifestyle factors on micronucleus frequency in peripheral blood lymphocytes of the Bosnian population. Mutat Res 753:1–11
Preston RJ (2005) Bystander effects: genomic instability, adaptive response and cancer risk assessment for radiation and chemical exposures. Toxicol Appl Pharmacol 207:550–556
Resta N, Susca FC, Di Giacomo MC, Stella A, Bukvic N, Bagnulo R et al (2006) Homozygous frameshift mutation in the ESCO2 gene: evidence of intertissue and interindividual variation in Nmd efficiency. J Cell Physiol 209:67–73
Ropolo M, Balia C, Roggieri P, Lodi V, Nucci MC, Violante FS et al (2012) The micronucleus assay as biological dosimeter in hospital workers exposed to low doses of ionizing radiation. Mutat Res 747(1):7–13. doi:10.1016/j.mrgentox.2012.02.014
Rozgaj R, Kosuba V, Simic DT (2002) The frequency of dicentrics and acentrics and the incidence of rogue cells in radiation workers. Mutagenesis 17:135–139
Ruskovsky SR, Petrenko YW, Bezrukov VF (2003) Premature centromere division as a biomarker of genotoxic influences. In: abstract book of the sixth international symposium and exibition on environmental contamination in Central and Eastern Europe and the commonwealth of independent status. Prague, Czech Republic, pp 1–4
Saberi A, Salari E, Latifi SM (2013) Cytogenetic analysis in lymphocytes from radiation workers exposed to low levels of ionizing radiation in radiotherapy, CT-scan and angiography units. Mutat Res 750(1–2):92–95. doi:10.1016/j.mrgentox.2012.10.001
Sankaranarayanan K, Taleei R, Rahmanian S, Nikjoo H (2013) Ionizing radiation and genetic risks. XVIII. Formation mechanisms underlying naturally occurring DNA deletions in the human genome and their potential relevance for bridging the gap between induced DNA double-strand breaks and deletions in irradiated germ cells. Mutat Res 753:114–130
Sari-Minodier I, Orsière T, Auquier P, Martin F, Botta A (2007) Cytogenetic monitoring by use of micronucleu assay among hospital workers exposed to low doses of ionizing radiation. Mutat Res 629(2):111–121
Shueller MG, Higgins A, Rudd MK, Gustashaw K, Willard HF (2001) Genomic and genetic definition of functional human centromere. Science 294:109–115
Spremo-Potparevic B, Zivkovic L, Djelic N, Bajic V (2004) Analysis of premature centromere division (PCD) of the X chromosome in Alzheimer patients through cell cycle. Exp Gerontol 39:849–854
Stephan G, Pressl S (1999) Chromosomal aberrations in peripheral lymphocytes from healthy subjects as detected in the first cell division. Mutat Res 446:231–237
Thierens H, Vral A, Morthier R, Aousalah B, De Ridder L (2000) Cytogenetic monitoring of hospital workers occupationally exposed to ionizing radiation using micronucleus centromere assay. Mutagenesis 15(3):245–249
Touil N, Aka PV, Buchet JP, Thierens H, Kirsch-Volders M (2002) Assesment of genotoxic effects related to low level exposure to ionizing radiation using biomarkers for DNA damage and repair. Mutagenesis 17(3):223–232
Ullrich RL, Ponnaiya B (1998) Radiation-induced instability and its relation to radiation carcinogenesis. Int J Radiat Biol 74(6):747–754
Vig BK (1984) Sequence of centromere separation: orderly separation of multicentric chromosomes in mouse L-cells. Chromosoma 90:39–45
Vig BK, Wodnicki J (1974) Separation of sister centromeres in some chromosomes from cultured human leukocytes. J Hered 65:149–152
Vig BK, Zinkowski RP (1986) Sequence of centromere separation: a mechanism for orderly separation of dicentrics. Cancer Genet Cytogenet 22:347–359
Vig BK, Keith L, Sternes L, Paweletz N (1989) Centromere structure and function in neoplasia. Cancer Genet Cytogen 43:151–178
Way JS, Willard HF (1987) Nucleotide sequence heterogeneity of alpha satellite repetive DNA: a survey of alphoid sequences from different human chromosomes. Nucl Acid Res 15:7549–7569
Willard HF (1985) Chromosome specific organization of human satellite DNA. Am J Hum Genet 37:524–532
Acknowledgments
We express thanks to all donors who kindly gave their blood to perform this study. We also acknowledge Ana and Ivan Sreckovic for technical support in processing of the manuscript and figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pajic, J., Rakic, B., Jovicic, D. et al. A cytogenetic study of hospital workers occupationally exposed to radionuclides in Serbia: premature centromere division as novel biomarker of exposure?. Int Arch Occup Environ Health 89, 477–484 (2016). https://doi.org/10.1007/s00420-015-1086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-015-1086-2